Introduction
Nasopharyngeal carcinoma (NPC), which is a type of
malignant tumor that originates from the epithelium of the
nasopharynx, is relatively common in China, South-East Asia, North
Africa, Alaska and parts of the Mediterranean basin. During the
progression of NPC, distant metastasis and early cervical lymph
node metastasis may occur, representing a serious clinical problem
(1,2). The main treatments for NPC are
radiotherapy and adjuvant chemotherapy; however, the total 5-year
survival rate is <40% and these treatments are associated with a
series of side-effects and complications. Consequently, patient
tolerance and compliance is not good. Therefore, effective and safe
novel therapeutics for NPC are urgently required (3–5).
Cancer gene therapy is a new strategy that shows
great potential for the treatment of tumors. This approach depends
on the introduction of hereditary material into cells to generate a
biological effect. Recombinant adenovirus expression vectors are
frequently used for this purpose. NPC is characterized by a
multistep process of molecular and genetic changes in oncogenes and
tumor suppressor genes. Therefore, the efficacy of single
gene-mediated NPC therapy is limited and a multigene-based
combination approach may be more effective.
RGD peptides are a class of short peptides
containing arginine-glycine-aspartic acid (Arg-Gly-Asp), which
function mainly in the role of cell adhesion to fibronectin.
Extracellular matrix proteins and adhesion proteins in the blood
containing RGD sequences, together with the integrins which serve
as their receptors, constitute a major recognition system for cell
adhesion. RGD sequences have high affinity for αvβ3 integrins,
which are usually expressed at high levels in tumor cells and
vascular endothelial cells in tumors (6); consequently, RGD containing proteins
have been used as carriers to deliver drugs or genes into tumor
cells (7). Some studies have used
RGD-modified polymers or liposome as non-viral vectors for
delivering genetic material to improve the efficiency of cancer
gene therapy, including anti-angiogenic therapy (8,9). A
class of RGD peptides called RGD-4C has been shown to bind
specifically to αvβ3 integrins by phage display technology. In this
study, the capsid protein encoded by the adenoviral vectors was
transformed to express RGD-4C to facilitate targeted adherence to
tumor cells and improve the efficiency of infection.
Inhibitor of growth 4 (ING4), a member of the ING
tumor suppressor family that was first discovered by Shiseki et
al (10), is attracting
increasing attention as novel candidate tumor suppressor gene.
ING4, which is the best characterized member of the ING protein
family (11) has been shown to
interact with different structures, including histone modification
complexes such as histone deacetylase (HDAC) and histone
acetyltransferase (HAT). Structural and biochemical analyses have
shown that ING4 is composed of a flexible nuclear localization
sequence (NLS), an N-terminal domain, and a plant homeodomain (PHD)
finger that is formed from a homodimer with obvious affinity for
H3K4me3. The N-terminal domain enables the formation of homodimers
by permitting independent folding, which leads to the formation of
a coiled structure (12). Several
studies have demonstrated that ING4 expression is commonly
decreased or lost at the RNA and protein levels in human tumors
such as in HNSCC, hepatocellular carcinoma, melanoma, ovarian and
brain cancers (13–16). ING4 has been shown to inhibit tumor
cell growth and to induce cell apoptosis in different tumor types
such as hepatocellular, lung and pancreatic carcinomas (17–19).
Furthermore, non-physiological overexpression of ING4 inhibits cell
proliferation and induces G2/M cell cycle arrest (20). ING4 can also inhibit the activity
NF-κB and HIF-1α, and interact with liprin α1 to suppress tumor
angiogenesis, invasion and metastasis.
Phosphatase and tensin homolog deleted on chromosome
ten (PTEN) is another tumor suppressor, the activity of which is
lost through various mechanisms in many diverse forms of cancer
(21–23). PTEN is a lipid phosphatase and
dual-specificity protein that blocks phosphatidylinositol 3 kinase
(PI3K) signaling by converting phosphatidylinositol-(3,4,5)-triphosphate (PIP3) into
phosphatidylinositol (4,5)-bisphosphate (PIP2). This negatively
regulates PIP3-dependent processes such as the activity of protein
kinase B (AKT) and phosphatidylinositol-dependent kinase-1 (PDK1)
to inhibit cell growth and metabolism, cell cycle progression and
migration. Accumulating evidence shows that PTEN has significant
PIP3-independent functions. PTEN protein phosphatase activity is
important for the inhibition of cellular migration mediated by PTEN
(24). Furthermore, several
studies have confirmed that PTEN is able to exit and exist outside
the cell (25,26).
These observations implicate ING4 and PTEN as
promising tumor suppressors that can negatively modulate tumor
growth via diverse pathways. On the basis of the antitumor
characteristics of ING4 and PTEN, we speculated that combination
therapy comprising ING4 and PTEN would lead to an intensive
antitumor effect; however, the therapeutic potential of a
combination of ING4 and PTEN for NPC has not yet been reported.
Therefore, in this study, we constructed an RGD-modified
bicistronic ING4 and PTEN recombinant adenoviral vector
(Ad.RGD-ING4-PTEN). We analyzed its combined therapeutic effect on
human NPC cells in vitro and in vivo in an athymic
nude mouse xenografted tumor model and also explored its potential
molecular mechanisms.
Materials and methods
All animals received humane care according to the
guidelines of the Guidebook for the Care and Use of Laboratory
Animals (16). The study protocol
was approved by the Animal Research Ethics Committee at the First
Affiliated Hospital of Soochow University (Suzhou, China).
Adenoviruses, cell lines, reagents and
mice
The Ad.RGD-ING4-PTEN and Ad.RGD-green fluorescent
protein (GFP) replication-incompetent Ad5E1- and E3-deleted
adenoviruses and the QBI-293A human embryonic kidney cell line were
kindly provided by Dr Jicheng Yang (Cell and Molecular Biology
Institute, College of Medicine, Soochow University, Suzhou, China).
The human NPC CNE cell line was purchased from the American Type
Culture Collection (Shanghai, China). The CNE and QBI-293A cells
were cultured in RPMI-1640 (Gibco, Shanghai, China) supplemented
with 10% fetal bovine serum (Hyclone, Shanghai, China). The reverse
transcriptase MuMLV and TRIzol were purchased from Invitrogen
(Shanghai, China).
The following reagents were used in this study:
3-(4,5-dimeth-ylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
kit (Sigma, Shanghai, China); Annexin V-PE/7-AAD apoptosis
detection kit and propidium iodide (PI) staining kit (BD
Biosciences, Shanghai, China); antibodies specific for ING4, P21,
Bax, Bcl-2, VEGF, CD34 and β-actin (Santa Cruz, Shanghai, China);
antibodies specific for PTEN, survivin and caspase-3 (Cell
Signaling, Shanghai, China); PageRuler Prestained Protein Ladder
(Fermentas, Shanghai, China); Dylight 800-Labeled Antibody (KPL,
Shanghai, China); UltraSensitive™ SP kit (Maixin, Fuzhou, China);
SuperEnhanced chemiluminescence detection kit (Applygen
Technologies, Beijing, China).
Male BALB/c athymic nude mice (aged 4 weeks) were
purchased from Shanghai Experimental Animal Center (Shanghai,
China) and were raised in a specific pathogen-free environment in
the Laboratory Animal Center of Soochow University according to the
Regulations for the Administration of Affairs Concerning
Experimental Animals. All experimental protocols were approved by
the Institutional Animal Care and Use Committee.
Analysis of the adenoviral infection
efficiency with and without RGD modification
The infection efficiency of adenoviruses with and
without RGD modification was investigated to assess the optimal
multiplicity of infection (MOI) for maximal adenoviral infection
and transgene expression in CNE tumor cells. CNE NPC cells were
infected with Ad-GFP, Ad-ING4, Ad-PTEN, Ad-ING4-PTEN, Ad.RGD-GFP,
Ad.RGD-ING4, Ad.RGD-PTEN or Ad.RGD-ING4-PTEN at various MOIs (0, 1,
10, 25, 50, 100 and 200). After 48 h, the infection efficiency was
assessed according to GFP expression observed by fluorescence
microscopy. Following infection of CNE cells with Ad-GFP, Ad-ING4,
Ad-PTEN, Ad-ING4-PTEN, Ad.RGD-GFP, Ad.RGD-ING4, Ad.RGD-PTEN or
Ad.RGD-ING4-PTEN (MOI, 50), GFP expression was analyzed by flow
cytometry.
ING4/PTEN transgene expression in CNE
tumor cells
The adenovirus-mediated transcriptional expression
of ING4 and PTEN in CNE cells was determined by RT-PCR and western
blot analysis. Total RNA was extracted from Ad.RGD-GFP-,
Ad.RGD-ING4-, Ad.RGD-PTEN- or Ad.RGD-ING4-PTEN-infected and
uninfected CNE cells using TRIzol. First-strand cDNA was
reverse-transcribed using RNA as the template and oligo d(T)18 as
the primer. RT-PCR was carried out using cDNA as the template and
ING4-F (5′-GCGTCGACATGGATGATGGGATGTATTTGGAAC-3′), ING4-R
(5′-GCAAGCTTCTATTTCTTCTTCCGTTCTTGGGAG-3′), PTEN-F
(5′-GCGGTACCATGACAGCCATCATCAAAGAG-3′), PTEN-R
(5′-CGAAGCTTTCAGACTTTTGTAATTTGTGT-3′), GAPDH-F
(5′-TGATGACATCAAGAAGGTGGTGAA-3′) and GAPDH-R
(5′-TCCTTGGAGGCCATGTGGGCC-3′) as primers. All of the RT-PCR
products were analyzed by 2% agarose gel electrophoresis. Total
cellular lysates derived from Ad.RGD-GFP, Ad.RGD-ING4, Ad.RGD-PTEN
and Ad.RGD-ING4-PTEN-infected and uninfected CNE cells were
resolved by 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE), then transferred onto a poly-vinylidene
fluoride membrane. The membrane was blocked with 5% skimmed milk
solution and then the incubated with the appropriate primary
antibody [mouse anti-ING4 (1:1,000), anti-PTEN (1:1,000), β-actin
(1:1,000)]. The membrane was washed with TBST three times and
incubated with Dylight 800-Labeled Antibody. Finally, the membrane
was photographed on a fluorescence imager. The experiment was
repeated three times.
Cell viability assay
The in vitro cytotoxic effect of
Ad.RGD-ING4-PTEN on the CNE cells was assessed by MTT assay. The
CNE cells were dispensed into 96-well culture plates at
1×104 cells per well and then treated with (Ad.RGD,
Ad.RGD-ING4, Ad.RGD-PTEN or Ad.RGD-ING4-PTEN) or without the
adenovirus (PBS control) at the optimal MOI of 50 for the indicated
time periods (0–4 days) after 24-h incubation at 37°C. The
viability of CNE cells was evaluated using an MTT kit according to
the manufacturer’s protocol before treatment and at different four
points after treatment.
Flow cytometric analysis of the cell
cycle and apoptosis
Cell cycle analysis of CNE cells was performed by
flow cytometry following PI staining. CNE cells (1×105)
were cultured with (Ad.RGD-ING4-PTEN, Ad.RGD-ING4, Ad.RGD-PTEN or
Ad.RGD) or without adenovirus (PBS control) at the optimal MOI of
50. After 72 h, cells were collected, washed twice in cold PBS and
fixed in 70% cold alcohol (>12 h at 4°C). Cells were then washed
in cold PBS again and stained with PI solution at 37°C for 30 min
in the dark. Apoptosis was evaluated by Annexin V-PE/7-AAD double
staining following the manufacturer’s instructions. In brief,
adenovirus-treated cells were collected, washed in cold PBS and
incubated with 5 μl Annexin V-PE, 5 μl 7-AAD and 100 μl 1X binding
buffer for 15 min at room temperature in the dark. A further 400-μl
1X binding buffer was then added to the cells and apoptosis was
analyzed by flow cytometry.
Western blot analysis
CNE cells (1×107) were infected with
(Ad.RGD-ING4-PTEN, Ad.RGD-ING4, Ad.RGD-PTEN or Ad.RGD) or without
adenovirus (PBS control) at the optimal MOI of 50. After 48 h, the
cells were collected, washed with cold PBS and lysed in 1 ml lysis
buffer. Total cellular proteins were isolated and the protein
concentration was determined spectrophotometrically in BCA protein
assays. To explore the molecular mechanism involved in
Ad.RGD-ING4-PTEN enhancement of growth inhibition and apoptosis and
changes in the cell cycle, CNE cell lysates (100 μg per lane) were
subjected to western blot analysis as described previously using
primary antibodies specific for survivin, caspase-3, Bcl-2, Bax and
P21.
Animal studies
Male athymic nude mice were hypodermically injected
on the right anterior axilla with 5×106 CNE cells and
then monitored daily for tumor growth. Tumor dimensions were
measured with calipers and the volume was calculated according to
the following formula: tumor size = ab2/2, where a and b
are the larger and smaller dimensions, respectively. When the tumor
reached ~200 mm3, CNE cell xenografted tumor-bearing
mice (n=5 per group) were intratumorally injected with PBS (PBS
control) or 1×109 pfu of Ad.RGD-ING4-PTEN, Ad.RGD-ING4,
Ad.RGD-PTEN and Ad.RGD (a total of 6 doses on alternate days).
Tumor volume was then measured every other day and tumor-bearing
mice were sacrificed by cervical dislocation 20 days after
treatment. All of the xenografted tumors were weighed, fixed with
10% neutral formalin and embedded in paraffin for hematoxylin and
eosin (H&E) staining and immunohistochemical analysis.
Immunohistochemical analysis
CD34, VEGF, survivin, caspase-3, Bcl-2, Bax and P21
expression by treated and untreated CNE s.c. xenografted tumors was
investigated by immunohistochemistry using the UltraSensitive™ SP
kit according to the manufacturer’s instructions. Microvessel
density (MVD) was determined by CD34 immunostaining as previously
described by Weidner (27). Any
endothelial cell cluster that was immunoreactive for CD34 and was
clearly separated from adjacent microvessels was defined as a
single countable vessel. The mean number of microvessels or
integral optical density (IOD) of immunohistochemical intensity
counted in five randomly selected fields of view under high-power
microscopy (×200) was calculated by Image-Pro Plus 6.0 software
(Media Cybernetics, Bethesda, MD, USA).
Evaluation of synergistic
interaction
The interactive effects of ING4 and PTEN in CNE
cells following RGD-modified adenovirus-mediated ING4 and PTEN
coexpression were evaluated in terms of the Q-value calculated
according to the formula (28), Q
= F(A + B)/FA + (1 − FA)FB, where F(A+B) represents the fraction of
cells affected by Ad.RGD-ING4-PTEN treatment compared with the
untreated control group, FA represents the fraction of cells
affected by Ad.RGD-ING4 alone, and FB represents the fraction of
cells affected by Ad.RGD-PTEN alone. A value of Q>1.15 indicates
a synergistic effect between ING4 and PTEN, Q<0.85 indicates an
antagonistic effect and Q between 0.85 and 1.15 indicates an
additive effect.
Statistical analysis
All data are presented as the mean values ± SD. The
significance of the difference between groups was evaluated by
one-way and two-way repeated measures analysis of variance (ANOVA)
and multiple comparisons with SPSS 10.0 software. A value of
P<0.05 was considered statistically significant.
Results
Infection efficiency of adenoviruses with
and without RGD modification
More than 95% of CNE tumor cells transfected with
Ad.RGD-GFP, Ad.RGD-ING4, Ad.RGD-PTEN, and Ad.RGD-ING4-PTEN at 50
MOI or Ad-GFP, Ad-ING4, Ad-PTEN, and Ad-ING4-PTEN at 100 MOI showed
GFP expression, with no abnormalities in the cell form observed.
Flow cytometry showed that the infection rate of RGD-modified
adenoviruses at 50 MOI was >96% compared with 75% for unmodified
adenoviruses. Therefore, we selected 50 MOI RGD-modified
adenoviruses as an optimal dose for transfection of CNE human NPC
cells in this study.
Ad.RGD-ING4-PTEN transgene
expression
RT-PCR and western blot analysis of infected and
uninfected CNE cells revealed transgene expression of both ING4 and
PTEN at the transcriptional and translational levels in the
Ad.RGD-ING4-PTEN-infected CNE cells (Fig. 1). ING4 and PTEN expression was also
found in Ad.RGD-ING4- and Ad.RGD-PTEN-infected CNE cells,
respectively, but neither ING4 nor PTEN expression was detected in
Ad.RGD-GFP-infected or uninfected CNE cells.
Increased tumor suppression by ING4 and
PTEN coexpression
To investigate whether combined ING4 and PTEN
treatment led to an enhanced antitumor effect, we coexpressed the
ING4 and PTEN tumor suppressor genes by RGD-modified
adenovirus-mediated co-transfer and evaluated its combined effect
on CNE human NPC cells. The CNE tumor cells were infected with
Ad.RGD-ING4-PTEN, Ad.RGD-ING4, Ad.RGD-PTEN or Ad.RGD at the optimal
MOI of 50. The viability of CNE tumor cells infected in
vitro with Ad.RGD-ING4-PTEN was tested daily before and for 4
days after treatment using MTT assays. As shown in Fig. 2A, compared with the Ad.RGD and PBS
control groups, adenovirus-mediated ING4 and/or PTEN expression
obviously suppressed CNE cell growth in vitro in a
time-dependent manner with peak inhibition at day 4 after infection
(P<0.05). Moreover, combined ING4 and PTEN coexpression induced
additive antitumor activity against CNE tumor cells compared with
the Ad.RGD-ING4- and Ad.RGD-PTEN-treated groups (P<0.05; Q=1.01
and 1.06 at days 3 and 4 after treatment, respectively). To further
investigate whether the combination of ING4 with PTEN enhanced
antitumor efficacy in vivo, athymic nude mice (n=5 per
group) bearing s.c. xenografted CNE tumors were intratumorally
injected with Ad.RGD-ING4-PTEN, Ad.RGD-ING4, Ad.RGD-PTEN or Ad.RGD
(100 μl, 1×109 pfu/l on alternate days). The tumor
volume (Fig. 2B) was recorded on
alternate days. Xenografted tumors were isolated at 20 days after
treatment and the weight (Fig. 2C)
was measured. Compared with the Ad.RGD-ING4- and
Ad.RGD-PTEN-treated groups, the growth of xenografted tumors in
nude mice was observably retarded in the Ad.RGD-ING4-PTEN-treated
group (P<0.05; Qvolume=0.98 and 1.02 at days 15 and
20 after treatment, respectively, and Qweight=1.07),
indicating that Ad.RGD-ING4-PTEN administration significantly
suppressed CNE xenografted tumor growth in vivo eclipsing
the additive effect.
 | Figure 2Ad.RGD-ING4-PTEN induced enhanced
tumor suppression in CNE human nasopharyngeal carcinoma cells. (A)
The cytotoxic effect of Ad.RGD-ING4-PTEN on CNE human
nasopharyngeal carcinoma cells in vitro. CNE cells were
treated with Ad.RGD-ING4-PTEN, Ad.RGD-ING4, Ad.RGD-PTEN or Ad.RGD
(blank adenovirus control) at the optimal MOI of 50 (PBS served as
a control) for the indicated time periods (0–4 days). Cell survival
was assessed at days 0, 1, 2, 3 and 4 by MTT assay.
*P<0.05 vs. PBS and Ad.RGD groups;
#P<0.05 vs. Ad.RGD-ING4 and Ad.RGD-PTEN groups
(Q=1.01 and 1.06 at days 3 and 4 after treatment, respectively);
one-way repeated measures ANOVA and multiple comparisons, n=3
replicates per condition. (B and C) Ad.RGD-ING4-PTEN enhanced the
antitumor effect on xenografted CNE tumors in vivo. Athymic
nude mice bearing subcutaneously xenografted tumors were
intratumorally injected with Ad.RGD-ING4-PTEN, Ad.RGD-ING4,
Ad.RGD-PTEN, Ad.RGD or PBS (a total of 6 doses on alternate days).
The CNE xenografted tumor volume (B) was measured before and after
treatment and xenografted tumors were isolated at 20 days after
treatment and tumor weight (C) was measured. *P<0.05
vs. PBS and Ad.RGD groups; #P<0.05 vs. Ad.RGD-ING4
and Ad.RGD-PTEN groups (Qvolume=0.98 and 1.02 at days 15
and 20 after treatment, respectively, and Qweight=1.07),
one-way and two-way repeated measures ANOVA and multiple
comparisons (n=5 mice per condition). Data shown are representative
of three independent experiments. |
Alteration in cell cycle distribution and
enhanced induction of apoptosis by ING4 and PTEN coexpression
To explore the mechanism by which Ad.RGD-ING4-PTEN
causes enhanced tumor suppression in CNE tumor cells, the cell
cycle profiles and apoptosis of the CNE tumor cells treated for 72
h with Ad.RGD-ING4-PTEN, Ad.RGD-ING4, Ad.RGD-PTEN, Ad.RGD (MOI of
50) or PBS were investigated by flow cytometric analysis of PI
staining, and Annexin V-PE/7-AAD double staining, respectively. As
shown in Fig. 3A and C, compared
with the PBS (7.73%) and Ad.RGD (9.27%) control groups, a
significant increase in the G2/M phase population was observed in
the Ad.RGD-ING4 (24.00%), Ad.RGD-PTEN (22.90%) and Ad.RGD-ING4-PTEN
(38.77%) groups (P<0.05). In contrast, a significant reduction
in the G0/G1 phase population was observed in the Ad.RGD-ING4
(50.94%), Ad.RGD-PTEN (55.17%) and Ad.RGD-ING4-PTEN (40.87%) groups
compared with the PBS (73.57%) and Ad.RGD (70.4%) control groups
(P<0.05). Compared with the single Ad.RGD-ING4- and
Ad.RGD-PTEN-treated groups, the G2/M phase and G0/G1 phase
populations of CNE tumor cells in vitro were significantly
increased and decreased, respectively, in the
Ad.RGD-ING4-PTEN-treated group (P<0.05; QG2/M=0.936;
QG0/G1=0.861). In addition, Ad.RGD-ING4-PTEN treatment
resulted in early apoptosis in 40.20% of CNE tumor cells, whereas
early apoptosis was detected in only 2.27, 3.57, 23.9 and 20.27% of
CNE tumors cells in the PBS, Ad.RGD, Ad.RGD-ING4 and Ad.RGD-PTEN
treated groups, respectively. Compared with the single Ad.RGD-ING4-
and Ad.RGD-PTEN-treated groups, Ad.RGD-ING4-PTEN treatment more
efficiently induced apoptosis with an additive effect (P<0.05;
Q=1.042) (Fig. 3B and D).
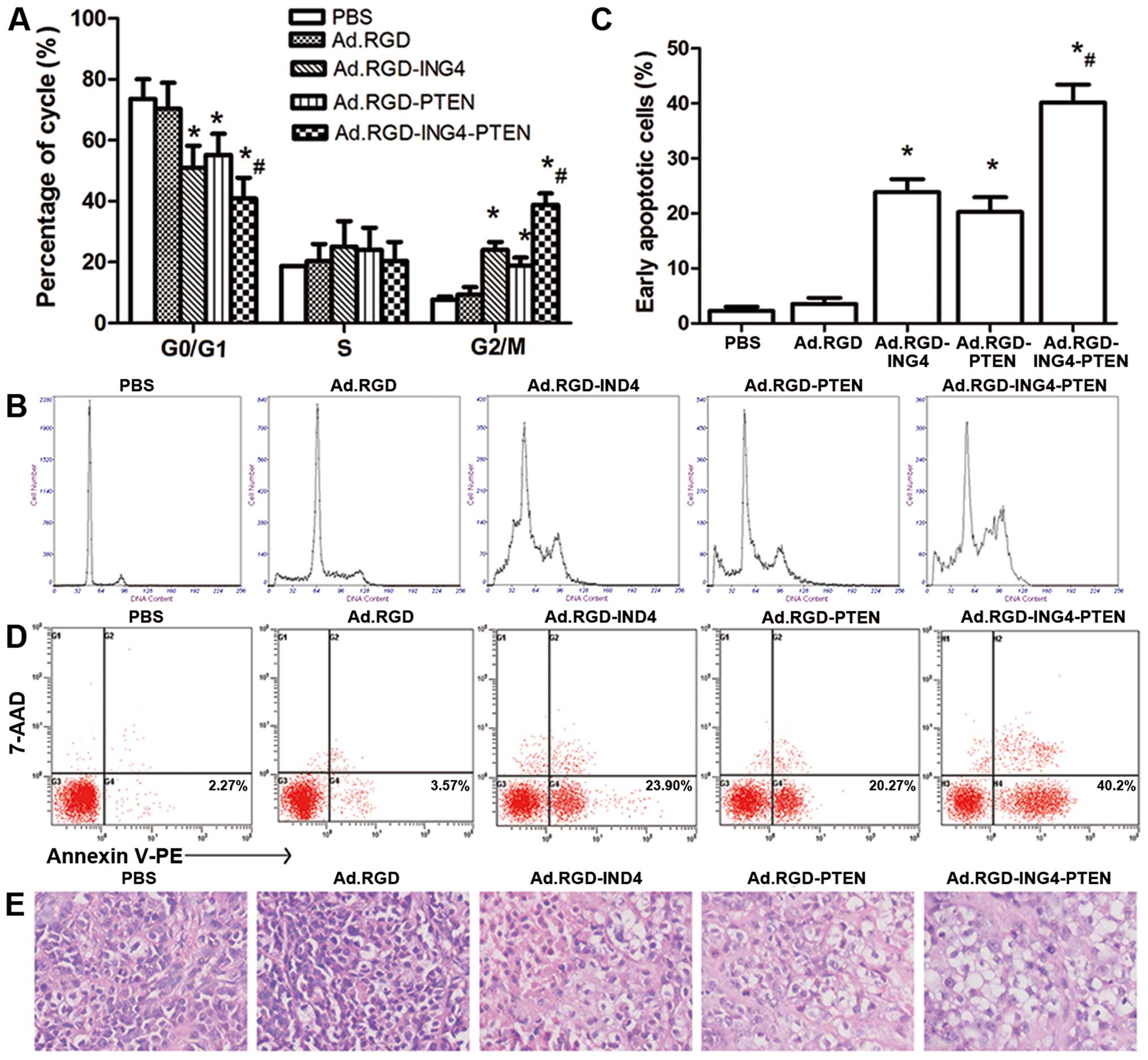 | Figure 3Ad.RGD-ING4-PTEN enhances G2/M phase
arrest and apoptosis. (A and B) Cell cycle analysis by flow
cytometry in vitro. The CNE human NPC cells were treated for
72 h with Ad.RGD-ING4-PTEN, Ad.RGD-ING4, Ad.RGD-PTEN, Ad.RGD at the
optimal MOI of 50 and PBS. *P<0.05 vs. PBS and Ad.RGD
groups; #P<0.05 vs. Ad.RGD-ING4 and Ad.RGD-PTEN
groups (QG2/M=0.936; QG0/G1=0.861), one-way
repeated measures ANOVA and multiple comparisons, n=3 replicates
per condition. (C and D) Flow cytometric analysis of apoptosis
in vitro. CNE tumor cells were treated for 72 h with
Ad.RGD-ING4-PTEN, Ad.RGD-ING4, Ad.RGD-PTEN, Ad.RGD at the optimal
MOI of 50 and PBS. The Annexin V single-positive cells in the total
cell population represent early apoptotic cells.
*P<0.05 vs. PBS and Ad.RGD groups;
#P<0.05 vs. Ad.RGD-ING4 and Ad.RGD-PTEN groups
(Q=1.042), one-way repeated measures ANOVA and multiple
comparisons, n=3 replicates per condition. (E) The morphology of
tumor tissues in the five different groups (×400). |
To further evaluate the induction of apoptosis in
vivo, we observed the cellular morphology and karyomorphism of
the treated and untreated s.c. xenografted CNE tumors by light
microscopy (Fig. 3E). The
Ad.RGD-ING4-PTEN group displayed degeneration, and necrosis of
tumor cells, as wells as nuclear dissolution. Tumor cell
degeneration and necrosis were also observed in the Ad.RGD-ING4 and
Ad.RGD-PTEN groups but compared to the Ad.RGD-ING4-PTEN group, the
effects were less marked. In the PBS and Ad.RGD groups, tumor cell
degeneration and necrosis were not obviously detected.
Ad.RGD-ING4-PTEN cooperatively regulates
the intrinsic and extrinsic apoptotic pathways
The potential molecular mechanism responsible for
the Ad.RGD-ING4-PTEN-induced increase in antitumor activity was
further investigated in western blot and immunohistochemical
analyses of the expression of cell cycle- and apoptosis-related
proteins such as P21, Bcl-2, Bax, survivin and caspase-3 both in
vitro and in vivo. As shown in Fig. 4, the expression of P21 and Bax in
the Ad.RGD-ING4, Ad.RGD-PTEN and Ad.RGD-ING4-PTEN groups was
obviously increased, whereas Bcl-2 and survivin was decreased.
Cleaved caspase-3 was also detected in these groups, but not in the
PBS and Ad.RGD groups. Furthermore, Ad.RGD-ING4-PTEN treatment
obtained an additive effect on the altered expression of P21,
Bcl-2, Bax, survivin and cleaved caspase-3, which are involved in
the activation of the intrinsic and extrinsic apoptotic pathways.
These observations indicated that Ad.RGD-ING4-PTEN additively
suppresses CNE cell growth and induces apoptosis in a manner that
is closely related with the adenovirus-mediated ING4 and PTEN
coexpression. This effect is likely to be mediated by cooperative
regulation of the intrinsic and extrinsic apoptotic pathways
(P<0.05, QP21=0.912, QSurvivin=0.928,
QBcl-2=1.016, QBax=1.161, Qcleaved
caspase-3=0.927 and QBcl-2/Bax=1.158).
 | Figure 4Ad.RGD-ING4-PTEN regulates the
intrinsic and extrinsic apoptotic pathways. (C) Western blot
analysis of cell cycle- and apoptosis-related proteins. The CNE
human nasopharyngeal carcinoma cells were treated for 48 h with
Ad.RGD-ING4-PTEN, Ad.RGD-ING4, Ad.RGD-PTEN or Ad.RGD at the optimal
MOI of 50 or PBS. Total cellular lysates were subjected to western
blot analysis of P21, survivin, Bcl-2, Bax, caspase-3 and β-actin
expression. Protein expression was normalized to the control
β-actin. (A, B and D) Immunohistochemical analysis of cell cycle-
and apoptosis-related proteins. Representative images of
immunohistochemical detection of P21, survivin, Bcl-2, Bax, and
cleaved caspase-3 in xenografted CNE human nasopharyngeal carcinoma
tumors (×200). The immunostaining intensity of P21, survivin,
Bcl-2, Bax, and cleaved caspase-3 was quantified as integral
optical density (IOD) by Image-Pro Plus 6.0 software.
*P<0.05 vs, PBS and Ad.RGD groups,
#P<0.05 vs. Ad.RGD-ING4 and Ad.RGD-PTEN groups
(QP21=0.912, QSurvivin=0.928,
QBcl-2=1.016, QBax=1.161, Qcleaved
caspase-3=0.927 and QBcl-2/Bax=1.158), one-way
repeated measures (ANOVA) and multiple comparisons, n=5 replicates
per condition, n=5 observations per representative section. Data
shown are representative of three independent experiments. |
Enhanced reduction of MVD by
Ad.RGD-ING4-PTEN
To examine the combined effect of RGD-modified
adenovirus-mediated ING4 and PTEN coexpression on tumor
angiogenesis in vivo, we evaluated the MVD in s.c.
xenografted CNE human NPC tumors on the basis of CD34
immunohistochemical analysis. The CD34-positive expression was
mainly manifested as brownish yellow or brownish granules in
vascular endothelial cells of CNE human NPC xenografted tumors
(Fig. 5A). Compared with the PBS
and Ad.RGD control groups, the CD34 expression of vascular
endothelial cells in the Ad.RGD-ING4, Ad.RGD-PTEN and
Ad.RGD-ING4-PTEN groups was weaker (Fig. 5A and B; P<0.05), indicating that
Ad.RGD-ING4-PTEN treatment down-regulates CD34 expression in s.c.
xenografted CNE human NPC tumor vessels. Furthermore, the MVD
(Fig. 5B) in the Ad.RGD-ING4,
Ad.RGD-PTEN and Ad.RGD-ING4-PTEN groups was obviously less than
that in the PBS and Ad.RGD groups (P<0.05). In addition,
Ad.RGD-ING4-PTEN exhibited an overlapping effect on downregulation
of CD34 and reduction in MVD in the xenografted CNE human NPC
tumors (P<0.05; QCD34=0.882 and
QMVD=1.031). These observations closely correlated with
the enhanced growth inhibition of Ad-ING4-PTEN-modified CNE NPC
xenografted tumors in the athymic nude mouse model.
Ad.RGD-ING4-PTEN suppresses the
expression of the proangiogenic factor VEGF
To investigate whether Ad.RGD-ING4-PTEN affects the
production of proangiogenic factors, we estimated vascular
endothelial growth factor (VEGF) expression in vivo in the
s.c. xenografted CNE human NPC tumors by immunohistochemical
analysis. As shown in Fig. 5A and
C, VEGF expression in the Ad.RGD-ING4, Ad.RGD-PTEN and
Ad.RGD-ING4-PTEN groups was obviously decreased compared with the
PBS and Ad.RGD groups. In addition, Ad.RGD-ING4-PTEN treatment
obtained an overlapping effect on the altered expression of VEGF,
indicating that Ad.RGD-ING4-PTEN gene therapy is capable of
suppressing the production of VEGF resulting in the inhibition of
tumor growth (P<0.05, QVEGF=1.132).
Discussion
Radiotherapy combined with chemotherapy is the
standard treatment paradigm for NPC, but long-term survival remains
poor because of the high incidence of local recurrences and distant
metastasis. Therefore, it is important to identify a novel
treatment for NPC. Multigene-based combination therapy shows
therapeutic benefit by targeting multiple pathways. Based on
current research that has indicated the potential of ING4 and PTEN
as tumor suppressors in cancer therapy, we inferred that antitumor
activity can be enhanced by the combined expression of ING4 and
PTEN. In this study, we constructed an RGD-modified bicistronic
ING4/PTEN adenovirus (Ad.RGD-ING4-PTEN) and assessed its
therapeutic effect on CNE human NPC cells in vitro and in
vivo in a xenografted tumor model established in athymic nude
mice. We demonstrated that the infection ability of the
RGD-modified adenovirus was greater than that of the unmodified
adenovirus and that Ad.RGD-ING4-PTEN mediated enhanced growth
inhibition, apoptosis and G2/M phase arrest in CNE human NPC cells
in vitro. Moreover, Ad.RGD-ING4-PTEN additively suppressed
in vivo CNE human NPC tumor growth and angiogenesis in
xenografted nude mice.
P21 was the first discovered cyclin-dependent kinase
inhibitor (CKI) gene, and is a member of the inherently disordered
protein (IDP) family. Some studies have shown that P21 actively
regulates almost all cyclin-CDK complexes, suggesting that P21
plays a role in multiple aspects of the cell cycle and is an
important tumor suppressor gene (29,30).
A decrease in the Bcl-2/Bax ratio increases mitochondrial membrane
permeability, resulting in release of cytochrome c (Cyt-c),
which assembles into a large protein complex, the apoptosome, that
activates the caspase family of cell death proteins (31,32).
Survivin, which is a highly potent apoptosis inhibitor, is absent
in most adult tissues, but is selectively upregulated in numerous
human tumors. Survivin upregulation reduces apoptosis stimulated by
both the intrinsic and extrinsic apoptotic pathways, including FAS
ligand, overexpression of Bax and caspases-3, −7 and −8 (33). Ultimately, both pathways signal via
initiator caspases and converge at the level of the effector
caspases (e.g., caspase-3, −6, and −7) to induce cell death through
cleavage of essential cellular proteins (34). To investigate the potential
mechanism by which Ad.RGD-ING4-PTEN enhanced antitumor activity,
the expression of the cell cycle- and apoptosis-related proteins,
P21, Bcl-2, Bax, survivin and cleaved caspase-3 was investigated in
CNE human NPC cells and s.c. xenografted CNE human NPC tumors was
estimated by western blot analysis and immunohistochemical
staining. Ad.RGD-ING4-PTEN elicited a cooperative and overlapping
effect on upregulation of P21, Bax and cleaved caspase-3 and
downregulation of Bcl-2 and survivin leading to activation of the
extrinsic and intrinsic apoptotic pathways. These observations may
explain the enhanced growth inhibition and apoptosis in CNE tumor
cells and xenografted tumors induced by Ad.RGD-ING4-PTEN.
Recent studies have demonstrated that the growth and
metastasis of malignant tumors is closely related to angiogenesis,
which is therefore a potential target in cancer gene therapy. VEGF
plays a significant role in controlling the neoplastic angiogenic
process. Recent studies have confirmed that VEGF promotes the
growth of arterial, venous and lymphatic endothelial cells both
in vitro and in vivo and promotes the survival and
migration of endothelial cells (35,36).
In our study, we demonstrated that Ad.RGD-ING4-PTEN inhibits CD34
and VEGF expression and reduces MVD in s.c. xenografted CNE human
NPC tumors. These results suggest that Ad.RGD-ING4-PTEN inhibits
angiogenesis and inhibits NPC tumor growth by downregulating VEGF
expression.
In conclusion, Ad.RGD-ING4-PTEN was shown to enhance
growth inhibition and apoptosis of CNE human NPC cells and
xenografted tumors. This effect was accompanied by an overlapping
effect of the individual genes on upregulation of P21, Bax and
cleaved caspase-3 expression and downregulation of Bcl-2 and
survivin expression. Moreover, Ad.RGD-ING4-PTEN treatment
additively downregulated CD34, VEGF and MVD in s.c. xenografted CNE
human NPC tumors. The enhanced antitumor activity generated by
Ad.RGD-ING4-PTEN was closely associated with activation of the
intrinsic and extrinsic apoptotic pathways and additive inhibition
of tumor angiogenesis both in vitro and in vivo. On
the basis of this evidence, we believe that cancer gene therapy
combining two tumor suppressors, such as ING4 and PTEN, represents
an effective and novel therapeutic strategy for NPC and other
cancers.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81001016) and the Medicine
Research Foundation of the Board of Health of Suzhou City (no.
SYS201014).
References
|
1
|
Chang ET and Adami HO: The enigmatic
epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol
Biomarkers Prev. 15:1765–1777. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guigay J: Advances in nasopharyngeal
carcinoma. Curr Opin Oncol. 20:264–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Agulnik M and Epstein JB: Nasopharyngeal
carcinoma: current management, future directions and dental
implications. Oral Oncol. 44:617–627. 2008. View Article : Google Scholar
|
|
4
|
Tao Q and Chan AT: Nasopharyngeal
carcinoma: molecular pathogenesis and therapeutic developments.
Expert Rev Mol Med. 9:1–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma BB, Hui EP and Chan AT: Systemic
approach to improving treatment outcome in nasopharyngeal
carcinoma: current and future directions. Cancer Sci. 99:1311–1318.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schnell O, Krebs B, Wagner E, et al:
Expression of integrin alphavbeta3 in gliomas correlates with tumor
grade and is not restricted to tumor vasculature. Brain Pathol.
18:378–386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Katayama K, Furuki R, Yokoyama H, et al:
Enhanced in vivo gene transfer into the placenta using RGD
fiber-mutant adenovirus vector. Biomaterials. 32:4185–4193. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park J, Singha K, Son S, Kim J, Namgung R,
Yun CO and Kim WJ: A review of RGD-functionalized nonviral gene
delivery vectors for cancer therapy. Cancer Gene Ther. 19:741–748.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O’Neill AM, Smith AN, Spangler EA, et al:
Resistance of canine lymphoma cells to adenoviral infection due to
reduced cell surface RGD binding integrins. Cancer Biol Ther.
11:651–658. 2011. View Article : Google Scholar
|
|
10
|
Shiseki M, Nagashima M, Pedeux RM, et al:
p29ING4 and p28ING5 bind to p53 and p300, and enhance p53 activity.
Cancer Res. 63:2373–2378. 2003.PubMed/NCBI
|
|
11
|
Ythier D, Larrieu D, Brambilla C,
Brambilla E and Pedeux R: The new tumor suppressor genes ING:
genomic structure and status in cancer. Int J Cancer.
123:1483–1490. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Palacios A, Moreno A, Oliveira BL, et al:
The dimeric structure and the bivalent recognition of H3K4me3 by
the tumor suppressor ING4 suggests a mechanism for enhanced
targeting of the HBO1 complex to chromatin. J Mol Biol.
396:1117–1127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Martinka M and Li G: Role of ING4 in
human melanoma cell migration, invasion and patient survival.
Carcinogenesis. 29:1373–1379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garkavtsev I, Kozin SV, Chernova O, et al:
The candidate tumour suppressor protein ING4 regulates brain tumour
growth and angiogenesis. Nature. 428:328–332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Yu L, Wang Y, Zhang Y, Wang Y and
Zhang G: Expression of tumor suppressor gene ING4 in ovarian
carcinoma is correlated with microvessel density. J Cancer Res Clin
Oncol. 138:647–655. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng ZL, Li FJ, Gao F, Sun DS and Yao L:
Upregulation of miR-650 is correlated with down-regulation of ING4
and progression of hepatocellular carcinoma. J Surg Oncol.
107:105–110. 2013. View Article : Google Scholar
|
|
17
|
Xie Y, Sheng W, Miao J, Xiang J and Yang
J: Enhanced antitumor activity by combining an adenovirus harboring
ING4 with cisplatin for hepatocarcinoma cells. Cancer Gene Ther.
18:176–188. 2011. View Article : Google Scholar :
|
|
18
|
Zhao Y, Su C, Zhai H, Tian Y, Sheng W,
Miao J and Yang J: Synergistic antitumor effect of
adenovirus-mediated hING4 gene therapy and (125)I radiation therapy
on pancreatic cancer. Cancer Lett. 316:211–218. 2012. View Article : Google Scholar
|
|
19
|
Ling C, Xie Y, Zhao D, Zhu Y, Xiang J and
Yang J: Enhanced radiosensitivity of non-small-cell lung cancer
(NSCLC) by adenovirus-mediated ING4 gene therapy. Cancer Gene Ther.
19:697–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Unoki M, Shen JC, Zheng ZM and Harris CC:
Novel splice variants of ING4 and their possible roles in the
regulation of cell growth and motility. J Biol Chem.
281:34677–34686. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Salmena L, Carracedo A and Pandolfi PP:
Tenets of PTEN tumor suppression. Cell. 133:403–414. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leslie NR and Foti M: Non-genomic loss of
PTEN function in cancer: not in my genes. Trends Pharmacol Sci.
32:131–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leslie NR, Yang X, Downes CP and Weijer
CJ: PtdIns(3,4,5) P(3)-dependent and -independent roles for PTEN in
the control of cell migration. Curr Biol. 17:115–125. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Putz U, Howitt J, Doan A, Goh CP, Low LH,
Silke J and Tan SS: The tumor suppressor PTEN is exported in
exosomes and has phosphatase activity in recipient cells. Sci
Signal. 5:ra702012.PubMed/NCBI
|
|
26
|
Hopkins BD, Fine B, Steinbach N, et al: A
secreted PTEN phosphatase that enters cells to alter signaling and
survival. Science. 341:399–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weidner N: Current pathologic methods for
measuring intratumoral microvessel density within breast carcinoma
and other solid tumors. Breast Cancer Res Treat. 36:169–180. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang W, Qin SK, Chen BA and Chen HY:
Experimental study on antitumor effect of arsenic trioxide in
combination with cisplatin or doxorubicin on hepatocellular
carcinoma. World J Gastroenterol. 7:702–705. 2001.
|
|
29
|
Jung YS, Qian Y and Chen X: Examination of
the expanding pathways for the regulation of p21 expression and
activity. Cell Signal. 22:1003–1012. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Blain SW: Switching cyclin D-Cdk4 kinase
activity on and off. Cell Cycle. 7:892–898. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou C, Li X, Du W, et al: Antitumor
effects of ginkgolic acid in human cancer cell occur via cell cycle
arrest and decrease the Bcl-2/Bax ratio to induce apoptosis.
Chemotherapy. 56:393–402. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee JS, Jung WK, Jeong MH, Yoon TR and Kim
HK: Sanguinarine induces apoptosis of HT-29 human colon cancer
cells via the regulation of Bax/Bcl-2 ratio and caspase-9-dependent
pathway. Int J Toxicol. 31:70–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Church DN and Talbot DC: Survivin in solid
tumors: rationale for development of inhibitors. Curr Oncol Rep.
14:120–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roskoski R Jr: Vascular endothelial growth
factor (VEGF) signaling in tumor progression. Crit Rev Oncol
Hematol. 62:179–213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Canavese M and Spaccapelo R: Protective or
pathogenic effects of vascular endothelial growth factor (VEGF) as
potential biomarker in cerebral malaria. Pathog Glob Health.
108:67–75. 2014. View Article : Google Scholar : PubMed/NCBI
|



















