Introduction
Gastric cancer is the fourth most common cancer
worldwide and is the second most common cause of cancer-related
deaths. Due to lack of specific symptoms, gastric cancer patients
are often diagnosed at advanced stage. Advanced gastric cancer has
a poor prognosis (1) due to its
high rate of metastasis. The identification of predictive markers
for cancer progression and prognosis would facilitate evaluating
the clinical outcome and potential treatment stratification for
patients with gastric cancer. More importantly, identification of
such markers with biological functions that are critical in gastric
tumorigenesis and progression will also provide targets for cancer
specific treatment.
Rho GTPases are sensitive molecular switches
existing either in an inactive, GDP-bound form or an active
GTP-bound form. They are endowed with GTP hydrolytic activity,
mainly involved in cytoskeleton rearrangements and cell motility,
but also involved in cell proliferation, transformation and
differentiation (2). The exchange
of GDP to GTP and thus the activation of Rho GTPases are catalyzed
by guanine nucleotide exchange factors (GEFs), which act as
downstream factors for many extracellular growth factors. Thus, Rho
GTPases are key integrating molecules from different extracellular
signals. When activated, Rho GTPases can interact and activate a
large panel of effectors that are responsible for regulating
critical cellular functions. Members of the Rho small GTPases
family, prototype RhoA, Rac1 and Cdc42, are involved in the
regulation of a variety of cellular processes, such as organization
of the microfilament network, cell-cell contact, and malignant
transformation and also perform essential and specialized functions
during organization of the actin cytoskeleton.
Ras-related C3 botulinum toxin substrate 1 (Rac1) is
a member of the small molecule G-protein Rho family (Ras homologue)
and is an important class of intracellular signaling molecules.
RAC1 activity, as a modulator of the cytoskeleton, is critical for
a number of normal cellular activities including phagocytosis,
mesenchymal-like migration, axonal growth, adhesion and
differentiation of multiple cell types. Rac1 also plays key roles
in transmitting upstream signaling, such as receptors for
extracellular growth factors (3)
and receptor tyro-sine kinases (RTKs) (4), into downstream cascades and, thus,
modulates cellular functions. Upon activation, Rac1 interacts with
various specific effectors to coordinate the activation of a
multitude of signaling cascades that influence diverse
physiological outcomes. For example, Pak1 binds to Rac1 in a
GTP-dependent manner, after which activated Pak1 regulates cellular
functions such as cytoskeletal dynamics, cell adhesion and
transcription (5). Rac1 signals
can also activate MAPK family member including p38, p42/44 and
c-Jun N-terminal kinase (JNK) (6–9).
Rac1 was found being able to elevate cytosolic and nuclear levels
of β-catenin, thus, activated Wnt signaling cascades (10).
Although Rac1 as a cytoskeleton organizer has been
documented well, more and more recent findings provoked broad
functions of Rac1 in human cancers. Rac1 regulates a diverse
spectrum of cellular functions including tumorigenesis (11), angiogenesis (12,13)
and metastasis (11,14,15).
Overexpression of Rac1 occurs in several different types of tumors
including breast (16,17), colon (18), bladder (19), and gastric cancer (20,21).
Rac1 overexpression was observed in metastatic gastric (21), breast (15), prostate (22), bladder upper urinary tract cancer
(19). Rac1 promoted prostate
cancer progression and recurrence upon activation by VAV3, a GEF
for Rac1 activation (14). Rac1 is
also involved in sustained cell growth (23) and therapy resistance (24) through crosstalk with other
signaling pathways. Rac1 regulates vasculogenesis and angiogenesis,
two distinct but related steps in vasculature. Thus, Rac1 has been
shown closely related with tumor angiogenesis and microvascular
density (13). As Rac1 is
intimately involved in broad range of cellular functions in human
cancers and emerging as a potential target for anticancer
therapies, we explored the clinical correlation of Rac1 in gastric
cancer specimens and its biological impacts on gastric cancer
malignancy. Our studies suggest that Rac1 is correlated with an
aggressive phenotype in gastric cancer and may serve as a target
against gastric cancer.
Materials and methods
Patients and specimens
Gastric cancer tissues, confirmed by pathological
diagnosis, were obtained from 92 patients who under went radical
resection for gastric cancer between 2006 and 2008 at the
Department of Surgery, Ruijin Hospital, Shanghai, China. The
corresponding non-tumor gastric tissue was obtained at least 6 cm
from the tumor. All tissue samples were formalin-fixed and
paraffin-embedded. Clinicopathological and survival data of all
patients were collected. TNM staging was classified based on the
criteria of the American Joint Committee on Cancer (AJCC, 7th
edition) for gastric cancer. The mean age of the patients at
initial surgery was 63 years (range, 37–84 years); 62 men and 30
women were included in the present study. The mean duration of
follow-up was 39 months (range, 1–73 months). The AJCC tumor stage
distribution and vital status of the patients are shown in Table I. The study was approved by the
Shanghai Jiao Tong University Medical School Institutional Review
Board. Informed written consent to participate in the study was
obtained from each of the patients before the entry into the
study.
 | Table IDemographic and clinicopathological
parameters of enrolled patients. |
Table I
Demographic and clinicopathological
parameters of enrolled patients.
| Clinicopathological
characteristics | N | % |
|---|
| Age (years) |
| Median | 63 | |
| Range | 37–84 | |
| Gender |
| Male | 62 | 67.4 |
| Female | 30 | 32.6 |
| Local invasion |
| T1/2 | 25 | 27.2 |
| T3 | 53 | 57.6 |
| T4 | 14 | 15.2 |
| Lymph node
metastasis |
| Negative | 23 | 25.0 |
| Positive | 69 | 75.0 |
| Metastasis |
| M0 | 82 | 89.1 |
| M1 | 10 | 10.9 |
| TNM staging |
| I | 11 | 12.0 |
| II | 28 | 30.4 |
| III | 43 | 46.7 |
| IV | 10 | 10.9 |
| Lauren’s type |
| Intestinal | 54 | 58.7 |
| Diffuse | 38 | 41.3 |
|
Differentiation |
| Well,
moderate | 57 | 62.0 |
| Poor,
undifferentiated | 35 | 38.0 |
Immunohistochemistry staining
Immunohistochemistry (IHC) staining was performed
using a highly sensitive streptavidin-biotin-peroxidase detection
system with gastric cancer tissue microarrays. Rabbit anti-Rac1 at
a dilution of 1:50 (Abcam, Cambridge, MA, USA) was used as
previously reported (25). Mayer’s
haematoxylin was used for counterstain. The slides were evaluated
by a single board-certified pathologist (R.R.T.) who remained
blinded to the clinical data using standard light microscopy.
Immunohistochemistry assessment
Expression status of Rac1 was determined by using a
semi-quantitative scoring system based on the percentage of
positive cells. The percentage of positive cells was divided into
four grades (percentage scores): <5% (0), 5–25% (1), 25–50% (2),
50–75% (3) and >75% (4). All analyses were performed with the
SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Correlation
between Rac1 expression and clinical parameters was analyzed using
the Pearson correlation coefficient analysis. Overall survival was
calculated with the Kaplan-Meier method, and differences were
compared by the log-rank test. P-value <0.05 was considered as
statistically significant.
Plasmids and transfection
The GFP-fused wild-type Rac1 (Rac1) and GFP-empty
vector (vector) (26) were
purchased from Addgene (Cambridge, MA, USA). For plasmid
transfection, cells were seeded into 6-well plate and allowed to
grow 24 h prior to transfection. Cells in each well were
transfected with 4 μg plasmid using Lipofectamine 2000
(Invitrogen). Clones stably transfected with either Rac1 or GFP
vectors were selected through G418 culture.
Gastric cancer cell lines and cell
culture
Gastric cancer cell lines AGS, NCI-N87, MKN45,
MKN28, SGC-7901, KATO III, BGC823, MGC803, SNU-1 and SNU-16 were
preserved in our institute. The cells were grown in RPMI-1640
medium containing 10% fetal bovine serum (FBS), penicillin and
streptomycin (Gibco-BRL, Gaithersburgh, MD, USA). Cell
proliferation was assessed by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) (Sigma,
St. Louis, MO, USA) assay. Rac1 inhibitor NSC23766 was obtained
from Merck Biosciences (Darmstadt, Germany). Final concentration of
NSC23766 in treating cells was 100 μM (27).
Soft agar and Matrigel 3D culture
Soft agar colony formation assay was performed by
using 0.3% agar in complete medium with cells as the feeder layer
and 0.6% agar in complete medium as the bottom layer. 3D Matrigel
culture was performed using Matrigel matrix (BD Biosciences, San
Jose, CA, USA).
Reverse transcription and quantitative
real-time PCR (qRT-PCR)
Total RNA was isolated from cultured cells using the
RNeasy mini kit (Qiagen) and cDNA was synthesized with oligo (dT)
primers by using of a SuperScript First-strand cDNA Synthesis kit
(Invitrogen) according to the manufacturer’s protocols. Gene
expression was assessed by qRT-PCR using an Applied Biosystems 7500
Fast Sequence detection system (Life Technologies, Carlsbad, CA,
USA). The PCR reaction mixture consisted of QuantiTect SYBR-Green
PCR Master Mix (2× QuantiTect SYBR-Green kit, contains HotStart
Taq® DNA polymerase, QuantiTect SYGB-Green PCR buffer,
dNTP mix, SYGB I, Rox passive reference dye and 5 mM
MgCl2) (Qiagen), 0.5 μmol/l of each primer and cDNA. The
transcript of the housekeeping gene, glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) gene was used as endogenous control to
normalize expression data. The comparative Ct (threshold cycle)
method was used to calculate the relative changes in gene
expression. The primers used to amplify Rac1 were:
5′-ATGTCCGTGCAAAGTGGTATC-3′ (forward) and
5′-CTCGGATCGCTTCGTCAAACA-3′ (reverse). Primers to amplify GAPDH
were 5′-AATGGGCAGCCGTTAGGAAA-3′ (forward) and
5′-GCCCAATACGACCAAATCAGAG-3′ (reverse).
Immunofluorescence imaging
Immunofluorescence images were visualized using
Olympus BX50 microscope (Olympus Opticol Co., Tokyo, Japan), images
were taken using Nikon Digital Sight DS-U2 (Nikon, Tokyo, Japan),
and NIS elements F3.0 software was used (Nikon). Confocal images
were taken by inverted Zeiss LSM 710 confocal microscope (40× oil
lens) (Carl Zeiss, Oberkochen, Germany). Zen 2009 Light Edition
(Carl Zeiss) was used for the measurement of images.
Cell migration and invasion assays
Cell migration was analyzed by a Transwell chamber
assay. Cell invasion assays were performed using BD BioCoat™
Matrigel™ Invasion Chambers. FCS (10%) was used as the
chemoattractant. Cells on the lower surface of the insert were
fixed and stained followed by counting under a light microscope.
Cells were visualized using Olympus BX50 microscope (Olympus
Opticol Co.), images were taken using Nikon Digital Sight DS-U2
(Nikon), and NIS elements F3.0 software was used (Nikon).
Cell lysis and western blot analysis
Cells were incubated for 4.5 h after transfection,
washed once with PBS, and incubated an additional 1.5 h in DMEM
lacking serum. Cells were washed twice with cold PBS and lysed with
150–200 μl HEPES lysis buffer [50 mM HEPES (pH 7.0), 150 mM NaCl,
1% Triton X-100, 10% glycerol, 50 mM NaF and 1 mM
Na3VO4] supplemented with protease inhibitors
(5 μg/ml leupeptin, 5 μg/ml aprotinin, 1 μg/ml pepstatin and 100 μM
PMSF). Lysates were cleared at maximum speed in a microcentrifuge
at 4°C, and the protein concentration of each sample was determined
using the Bio-Rad protein reagent. For western blots, proteins were
resolved on 12% SDS-PAGE gels and transferred to PVDF membrane.
Antibodies against Rac1 and GAPDH were purchased from Abcam.
Activity of Rac1 assay
The activation of Rac1 was measured using Rac1
Activation Assay Biochem kit (Cytoskeleton, Denver, CO, USA).
Briefly, cell lysates were collected using lysis buffer from the
kit. The activated forms of Rac1 were combined by PAK-PBD affinity
beads. Beads were centrifuged and activate GTPases were pulled-down
in the bead pallets. Bound GTPases were eluted by SDS buffer and
analyzed by SDS-PAGE and western blotting. Rac1 levels were
analyzed by the specific antibodies.
In vivo tumorigenesis and metastasis
Male BALB/c nu/nu nude mice (Institute of Zoology
Chinese Academy of Sciences, Shanghai, China), were housed at a
specific pathogen-free environment in the Animal Laboratory Unit,
School of Medicine, Shanghai Jiao Tong University, China. Mice
received humane care and the study protocols were approved by the
Animal Care and Use Committee and conducted in accordance with the
Guide for the Care and Use Laboratory Animals of Ruijin Hospital,
Shanghai Jiaotong University School of Medicine. Cells
(1×106) were subcutaneously injected into 4-week-old
male BALB/c mice. Tumor length (L) and width (W) were measured and
tumor volume was calculated by the equation: Volume =
(W2 × L)/2 (28). Mice
were sacrificed 28 days after injection under anesthesia. Ten mice
were used in each group. To produce peritoneal spreading
experimental metastasis, 2×106 cells were injected into
5-week-old male BALB/c nude mice intraperitoneally. After 6 weeks,
the mice were sacrificed under anesthesia. Ten mice were used in
each group. Macrometastatics were visualized and counted.
Statistical analysis
The statistical analyses were performed using SPSS
13.0 software (SPSS Inc., Chicago, IL, USA). Values represent mean
± standard deviation (SD) of samples measured in triplicate. Each
experiment was repeated three times, unless otherwise indicated.
The significance of differences between experimental groups was
analyzed using the Student’s t-test and two-tailed
distribution.
Results
Rac1 mRNA is overexpressed in human
gastric cancer tissues
We first searched publicly available Oncomine
database for the general information of Rac1 expression in gastric
cancer tissues from previous publications. As we expected, Rac1
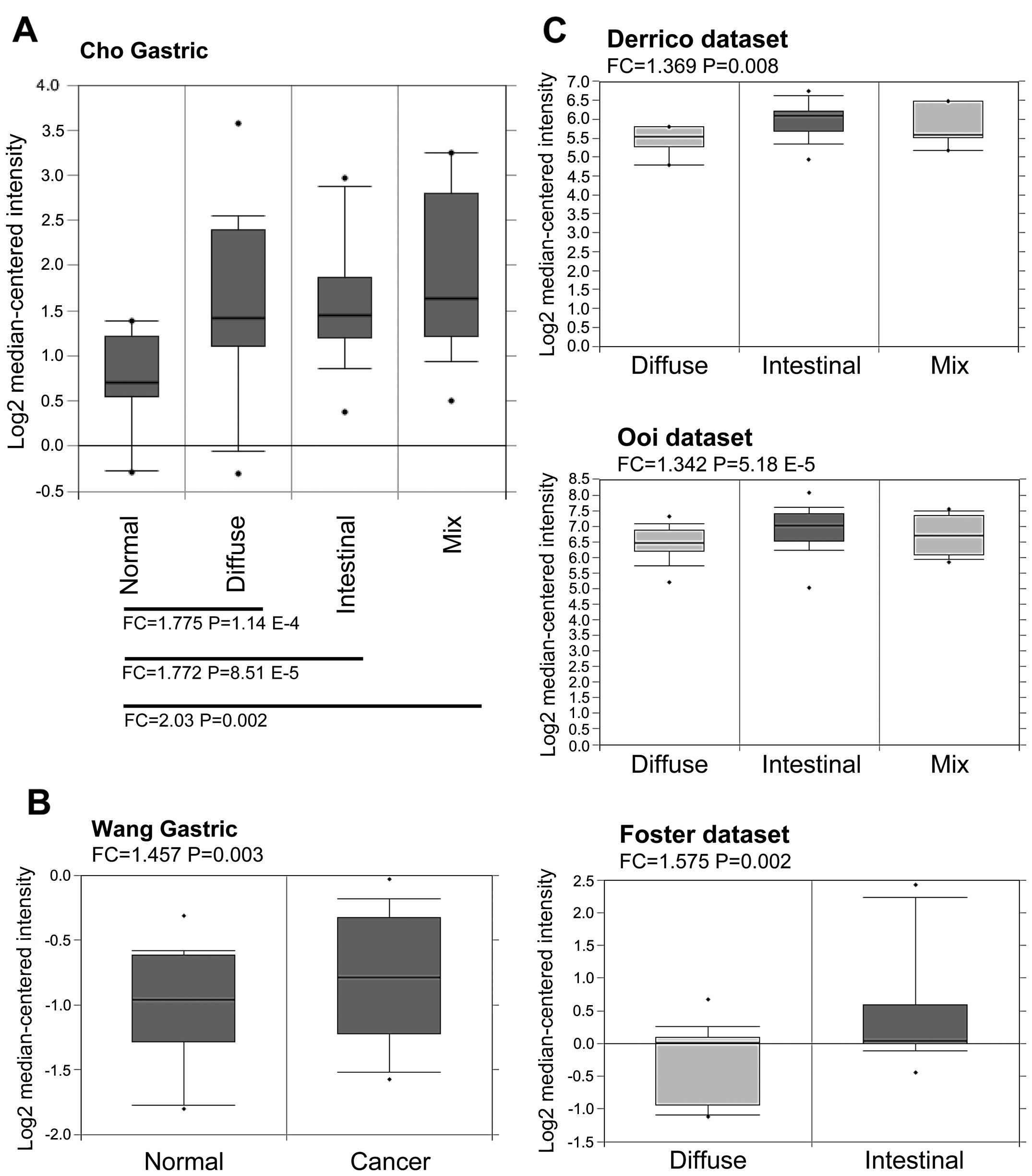
mRNA showed elevated levels compared with normal tissues. As shown
in Fig. 1A and B, Rac1 mRNA levels
were found to be higher in all the subtypes of gastric cancer
compared with paired non-tumor tissues or normal gastric mucosal
tissues in Cho et al (29)
and Wang et al (30)
datasets. Furthermore, by comparing Rac1 mRNA levels within
different subtypes based on Lauren’s classification, all the
available datasets showed Rac1 mRNA levels were higher in
intestinal-type than diffuse- and mix-types in D’Errico et
al (31), Ooi et al
(32), and Foster et al
(33) data-sets (Fig. 1C). These data suggest that Rac1
mRNA levels are elevated in gastric cancer tissues.
Rac1 protein is overexpressed in human
gastric cancer tissues
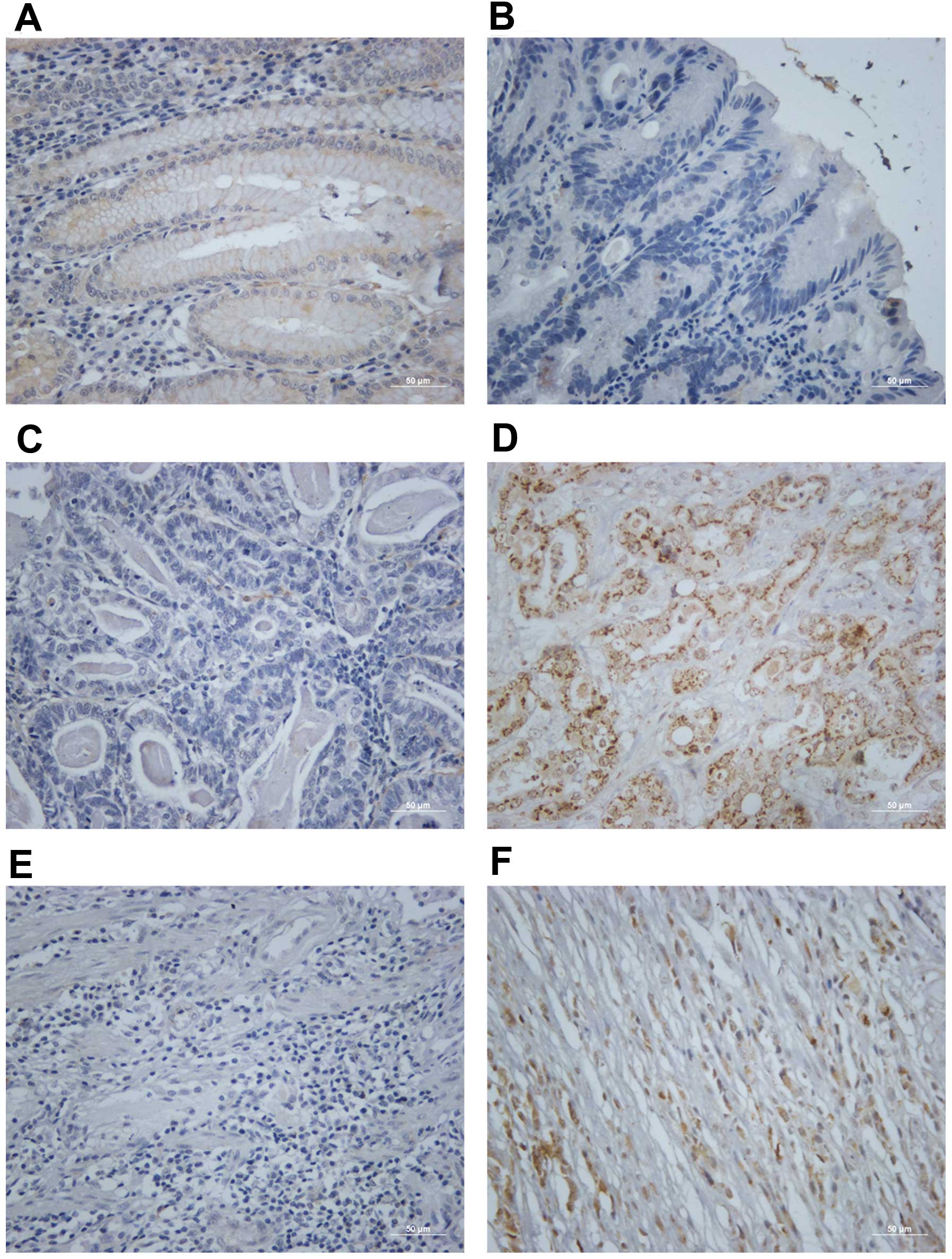
We performed immunohistochemistry staining to
examine the Rac1 protein expression in gastric cancer tissues.
Gastric tumor and paired non-tumor tissues from 92 patients were
stained. Rac1 positivity was observed in several crypt and isthmus
epithelial cells (Fig. 2A) but not
the surface epithelium (Fig. 2B)
in normal gastric tissues. Positive Rac1 staining was 72.8% (67 of
92) in gastric cancer tissues, and 27.2% (25 of 92) of the samples
showed negative staining. Rac1 positivity was cytoplasmic, and in
the membrane of the cancer cells (Fig.
2D and F) compared with negative staining (Fig. 2C and E). A significant correlation
was found between positive expression of Rac1 with gender (female,
P<0.001), local invasion (T3/4, P<0.001), lymph node
metastasis (P<0.001), distant metastasis (M1, P<0.001), late
TNM stage (III/IV, P=0.024), Lauren’s classification (intestinal
type, P<0.001) and differentiation status (well and moderate,
P<0.001) (Table II). These
data suggest that Rac1 is highly expressed in a subset of human
gastric cancer which shows more malignant characteristics according
to clinicopathological features.
 | Table IIProtein expression and
clinicopathological characteristics. |
Table II
Protein expression and
clinicopathological characteristics.
| Protein expression
(n) | |
|---|
|
| |
|---|
| Cases (n=92) | Negative
(n=25) | Positive
(n=67) | P-value |
|---|
| Age (years) |
| 60 | 38 | 6 | 32 | 0.092b |
| ≥60 | 54 | 19 | 35 | |
| Gender |
| Male | 62 | 19 | 43 | <0.001b |
| Female | 30 | 6 | 24 | |
| Local invasion |
| T1/2 | 25 | 14 | 11 | <0.001a |
| T3 | 53 | 7 | 46 | |
| T4 | 14 | 4 | 10 | |
| Lymph node
metastasis |
| Negative | 23 | 15 | 8 | <0.001b |
| Positive | 69 | 10 | 59 | |
| Metastasis |
| M0 | 82 | 24 | 58 | <0.001b |
| M1 | 10 | 1 | 9 | |
| TNM staging |
| I/II | 39 | 15 | 24 | 0.024b |
| III/IV | 53 | 10 | 43 | |
| Lauren’s type |
| Intestinal | 54 | 12 | 42 | <0.001b |
| Diffuse | 38 | 13 | 25 | |
|
Differentiation |
| Well,
moderate | 57 | 14 | 43 | <0.001b |
| Poor,
undifferentiated | 35 | 11 | 24 | |
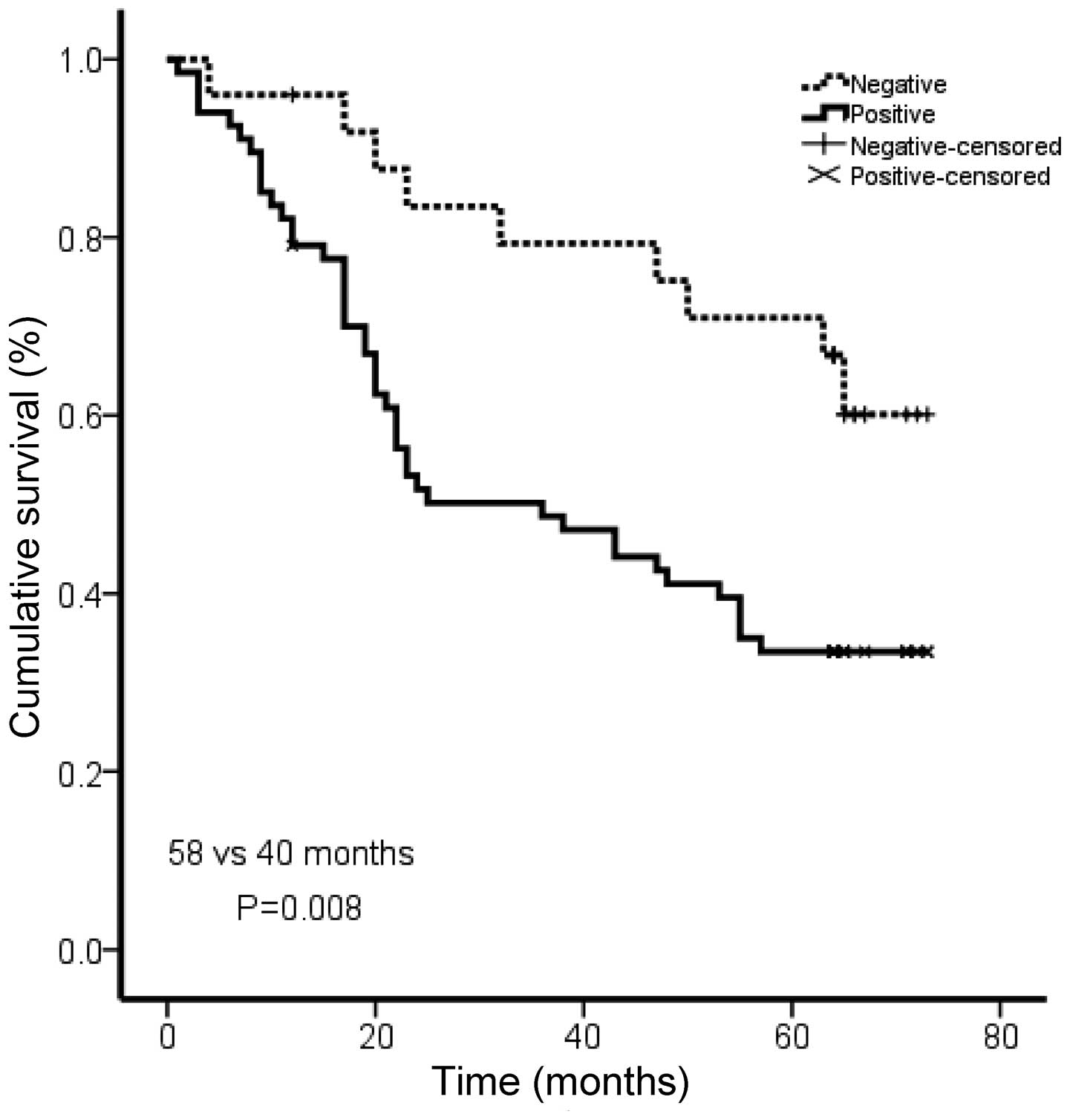
Rac1 expression levels are correlated
with shorter survival in gastric cancer patients
To investigate the prognostic significance of Rac1
in gastric cancer, we analyzed the correlation of Rac1 with
patients’ survival using Kaplan Meier analysis. The patients in the
Rac1-positive group showed shorter overall survival than those in
Rac1-negative group (medium survival, 40.0 vs. 58.0 months,
P=0.008; Fig. 3). Our data
suggested an association between Rac1 expression and a short
overall survival in gastric cancer patients.
Expression of Rac1 in gastric cancer cell
lines
We then analyzed the Rac1 expression in human
gastric cancer cell lines and immortalized normal gastric
epithelial cell line GES-1. We first performed qRT-PCR and
immunoblotting to analyze the Rac1 mRNA and protein levels in
gastric cancer cell lines and GES-1. In consistent with database
analysis, we showed that Rac1 mRNA (Fig. 4A) and protein (Fig. 4B) levels were highly expressed in
all gastric cancer cell lines compared to GES-1. These data
demonstrate that Rac1 is highly expressed in gastric cancer cell
lines.
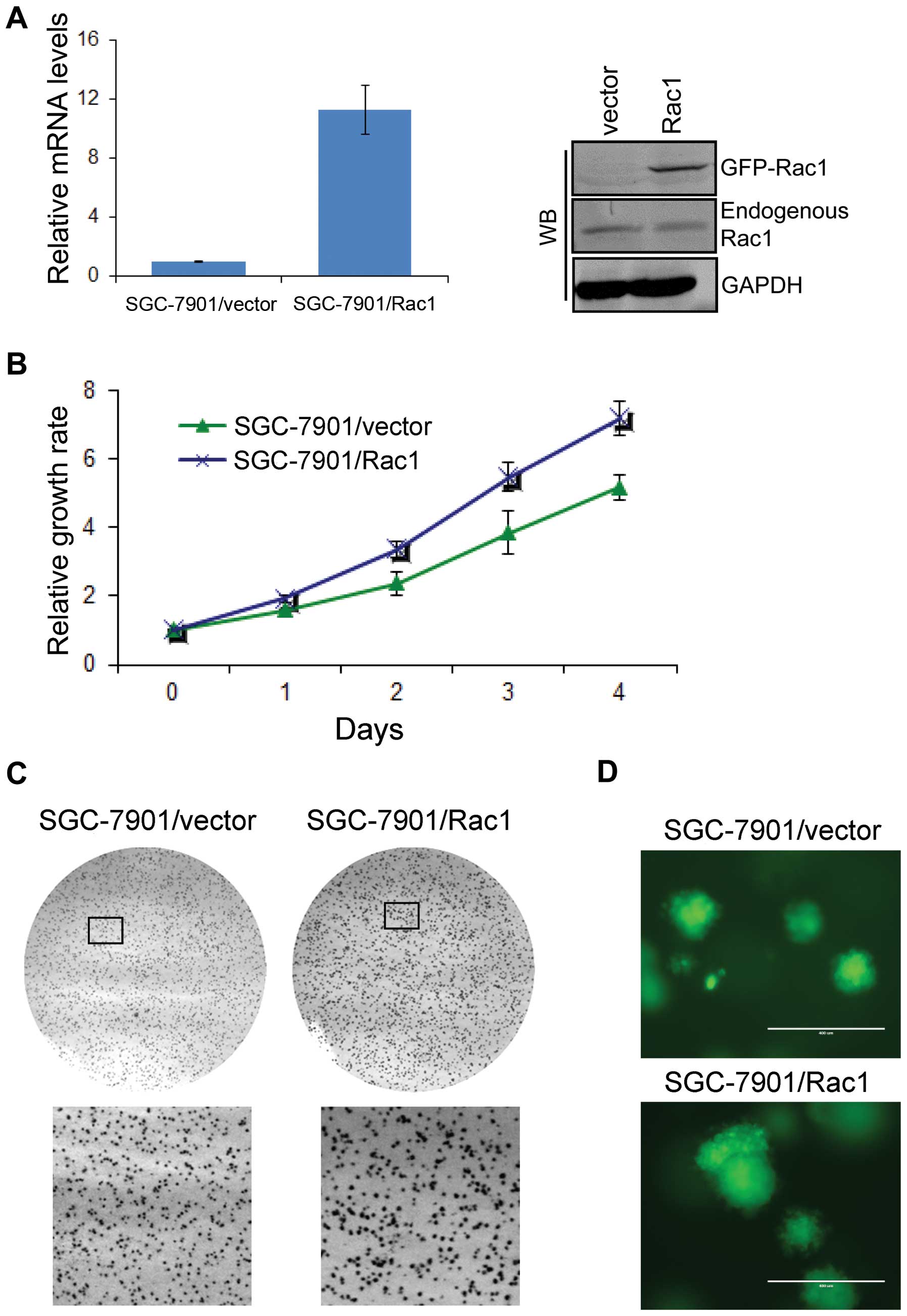
Overexpression of Rac1 in gastric cancer
cells increases cell proliferation in monolayer and 3D culture
We next explored the role of Rac1 in gastric cancer
cells. To do so, we generated Rac1 overexpression models using
SGC-7901 (Fig. 5A) and BGC823 cell
line (data not shown), which expressed moderate and low levels of
Rac1, respectively. Analyzed by quantitative RT-PCR and western
blotting, Rac1 mRNA (Fig. 5A, left
panel) and protein (Fig. 5A, right
panel) levels were increased in Rac1 stable transfectants compared
with vector-control transfectants. To investigate the role of Rac1
in the growth of gastric cancer cells, we first performed a cell
proliferation assay in monolayer culture. As shown in Fig. 5B, Rac1 over-expression
significantly increased cell growth in monolayer culture. We then
performed soft agar and Matrigel 3D culture. As expected, we found
that Rac1 overexpression promoted the growth of SGC-7901 (Fig. 5C) and BGC823 (data not shown)
gastric cancer cells in soft agar as suggested by larger colonies.
Similarly, Rac1 overexpressing cells formed bigger spheres in
Matrigel 3D culture (Fig. 5D).
These data suggest that Rac1 overexpression increases cell
proliferation in monolayer and 3D condition.
Overexpression of Rac1 in gastric cancer
cells increases cell migration and invasion
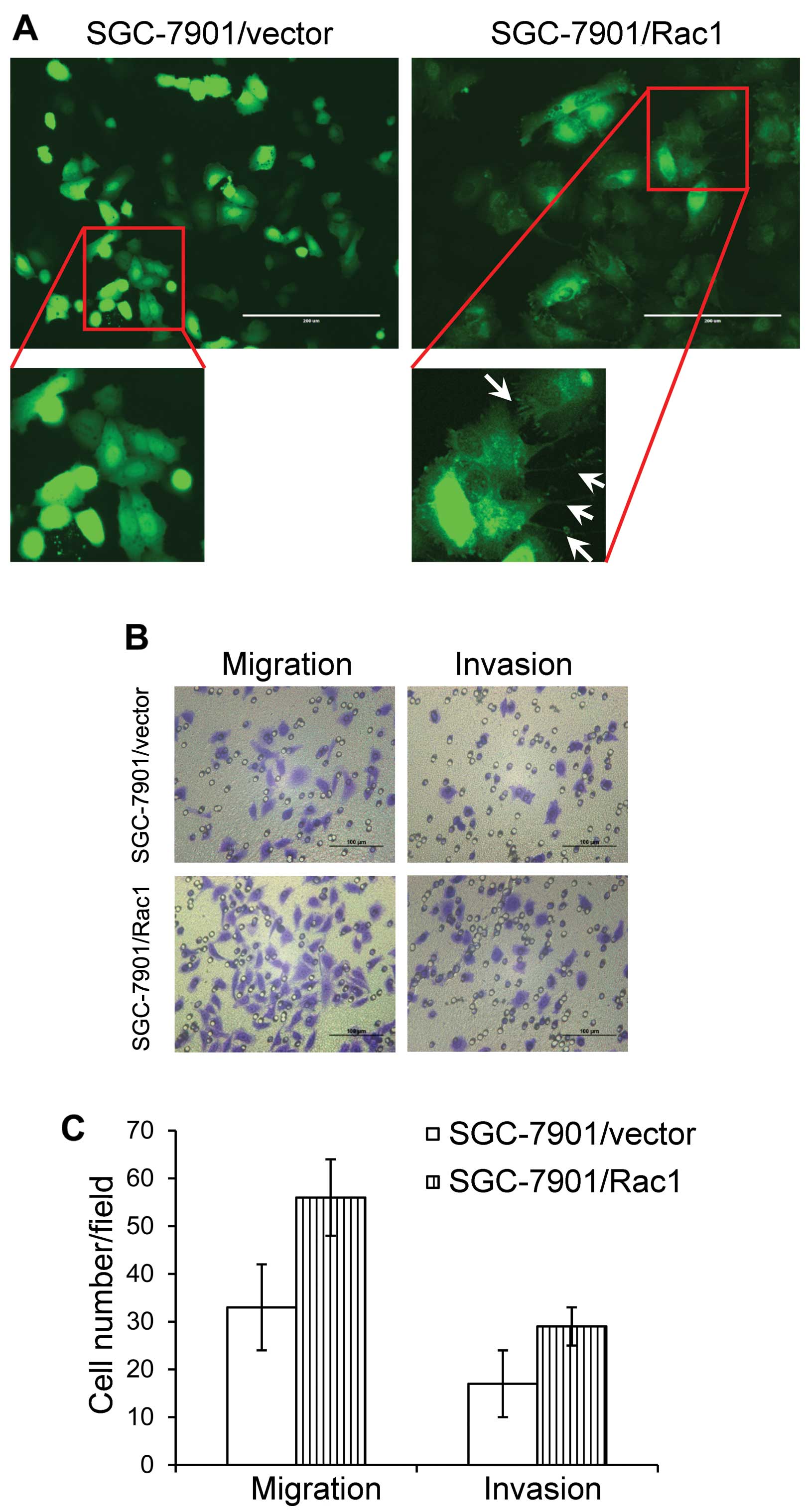
We observed a morphological alteration in
Rac1-overexpressing cells, with abundant cell protrusions and
membrane ruffling (Fig. 6A,
arrows). This phenomenon suggested a highly aggressive feature. As
Rac1 expression was positively correlated with the aggressiveness
in gastric cancer specimens, we next asked whether overexpression
of Rac1 affected the ability of gastric cancer migration and
invasion. Boyden chamber assays were used to investigate the in
vitro ability of migration and invasion in Rac1-overexpression
and vector-control cells. As expected, Rac1-overexpression induced
more cells to migrate through the Boyden chambers (Fig. 6B and C). Likewise, more cells in
Rac1-overexpression group invaded through Matrigel-coated Boyden
chambers than those in vector-control cells (Fig. 6B and C). These data suggest that
Rac1 promotes gastric cancer cell metastasis in vitro.
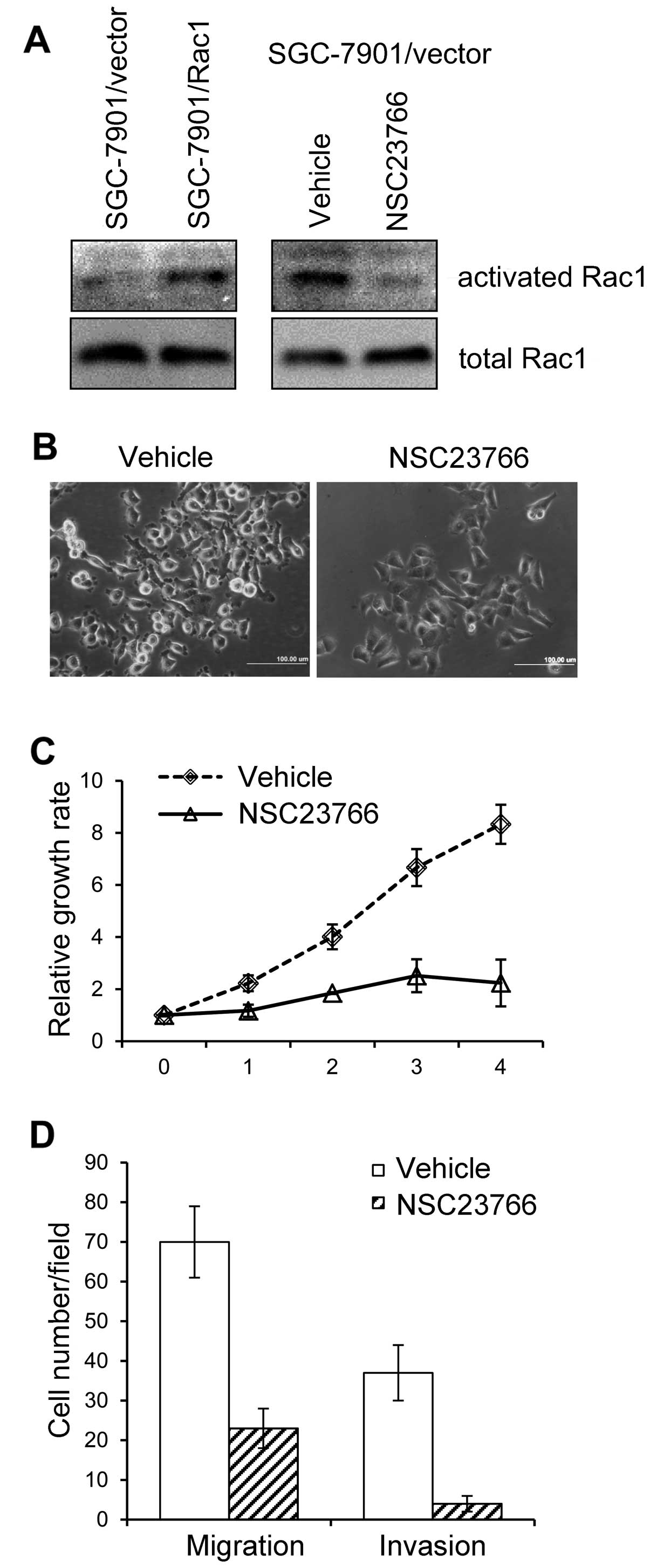
Inhibition of Rac1 activity abrogates the
effects of Rac1-overexpression
Previous findings have suggested that the activity
Rac1 is essential in mediating downstream effects on cell
proliferation and migration/invasion. We first tested whether Rac1
activity was increased in SGC-7901 cells overexpressing Rac1. By
using Rac1 activity assays, we found that GTP-bound-Rac1 (activated
form of Rac1) was increased in Rac1-overexpressing cells compared
with that in vector-control cells (Fig. 7A, left panel). We next asked
whether Rac1 activity was responsible for the increased
proliferation and migration/invasion in gastric cancer cells. To
this end, we used a chemical (NSC23766) that specifically inhibited
Rac1 activity (34). When treated
with NSC23766, the level of GTP-bound-Rac1 in SGC-7901/Rac1 cells
dramatically dropped (Fig. 7A,
right panel). Of note, Rac1 inhibitor NSC23766 induced an obvious
morphological change in SGC-7901/Rac1 cells. As shown in Fig. 7B, NSC23766 treatment inhibited the
formation of membrane ruffling and protrusions. We then asked
whether Rac1 inhibition rendered cellular function alterations. As
expected, NSC23766 treatment inhibited Rac1-overexpressing cell
growth in 4-day observation period (Fig. 7C). Furthermore, Rac1 inhibition
suppressed cell migrating and invading ability (Fig. 7D). These data suggest that the
inhibitor of Rac1 activity abolishes Rac1-overexpression inducing
cell morphological alterations and cellular functions.
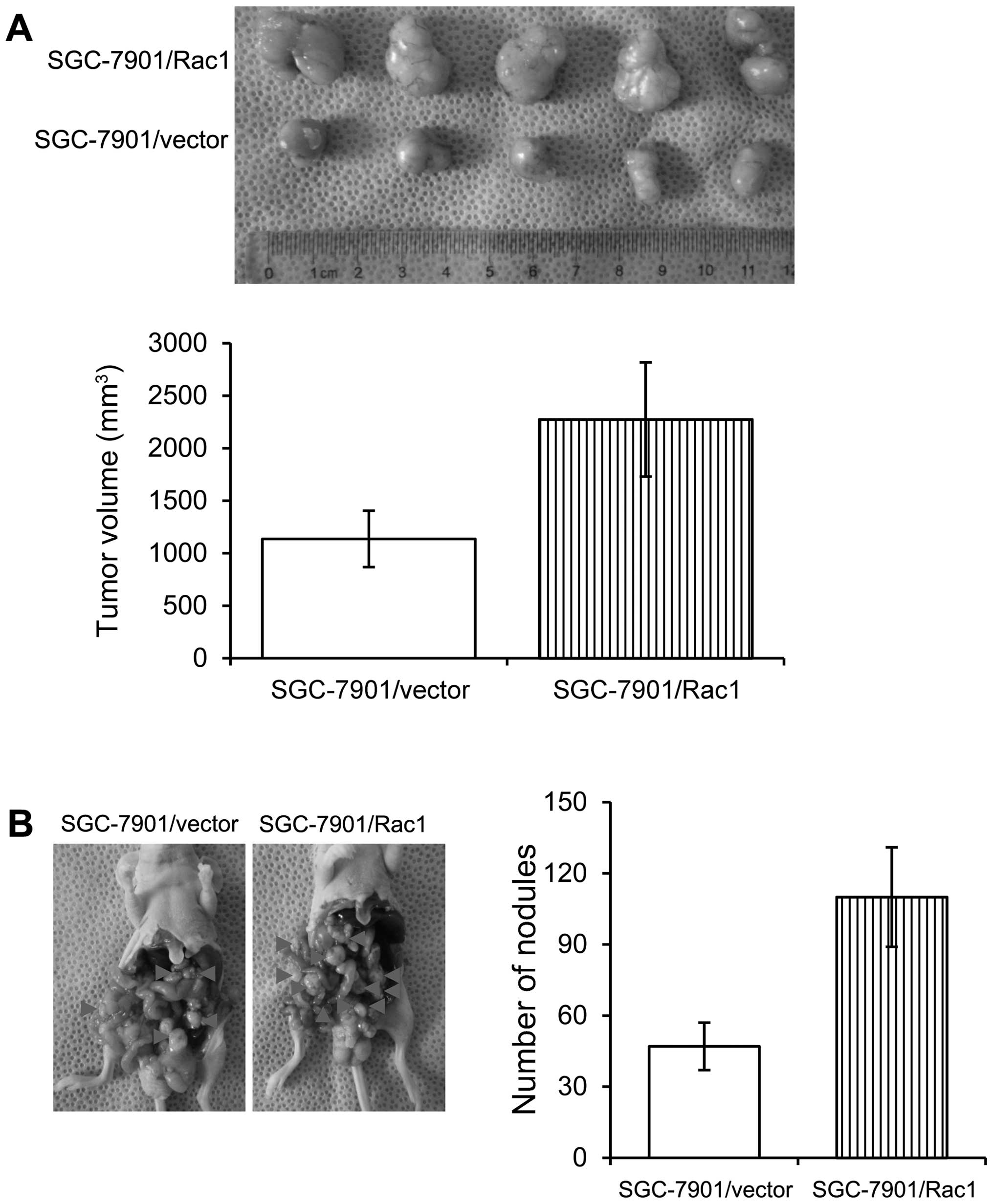
Overexpression of Rac1 in gastric cancer
cells increases in vivo tumorigenesis and metastasis
In order to examine the effects of Rac1 on the in
vivo growth of gastric cancer cells, we employed two
experimental models. Control and Rac1-overexpression SGC-7901 cells
were injected subcutaneously into nude mice and tumor growth was
examined. Mice injected with control and Rac1-overexpression cells
formed similar size tumors within 37 days (Fig. 8A). As peritoneal spreading and
metastasis are common in gastric cancer and are pivotal factors for
its poor prognosis, we used a nude mouse model to investigate the
influence of Rac1 levels on peritoneal metastasis. Consistent with
in vitro observations, we found that Rac1-overexpression
cells formed less metastatic nodules than control cells (Fig. 8B). Our data suggest that Rac1
overexpression increased in vivo tumorigenesis and
metastasis.
Discussion
Rac1 is one of the Rho-family small guanine
nucleotide triphosphate hydrolases. Rac1 regulates cellular
functions such as cell proliferation, survival and invasiveness. In
human cancers, Rac1 is considered as an oncogene which promotes
malignant transformation and progression. Rac1 is the most studied
Rac family protein in human cancers. Rac1 is a multi-functional
protein that serves not only as a scaffolding protein but also as a
special regulator of downstream effectors modulating a number of
cellular responses. Rac1 is found to play a pivotal role in
progression of cancer. In the present study, we evaluated the
expression and clinical relevance of Rac1 in human gastric cancer
specimens. We further explored the role of Rac1 in gastric cancer
by using ectopic-overexpression models. In line with the clinical
conclusions, we showed that Rac1 promoted cell proliferation and
metastasis by in vitro and in vivo studies.
Metastasis has a major impact on the morbidity and
mortality of patients with cancer (35). In the case of gastric cancer, vast
majority of the patients are diagnosed at advanced stage.
Identifying biomarkers that are functionally critical in metastasis
is urgent in treating gastric cancer patients. Our studies
discovered that high Rac1 levels were well correlated with gastric
cancer malignancy and predicted poor survival in gastric cancer
patients as assessed in clinical specimens. Our findings are
consistent with previous studies which suggested a correlation of
Rac1 with gastric cancer malignancy (20,21).
We also found that Rac1 was highly expressed in intestinal-type
gastric cancer and positively correlated with tumor
differentiation, local invasion and lymph node metastasis.
Noteworthy, these pathological parameters reflect tumor malignancy
and determine, at least partially, the outcome of patients. A
robust line of studies have provided evidence supporting the
crucial role of Rac1 in human cancers. Rac1 expression was
associated with invasion and metastasis in urothelial carcinoma
(19). Rac1 promoted colon cancer
progression through promotion of cell proliferation, survival and
migration (36). Rac1 expression
is correlated with tumorigenesis, aggressiveness and treatment
resistance in a number of cancers such as lung (37,38),
breast cancer (39,40) and melanoma (23,41).
These clinical investigations have shown the close correlation of
Rac1 with human cancers.
Tumor cell invasion and migration are driven by
continuous remodeling of the actin cytoskeleton which also provides
cell shape maintaining cellular structure and polarization. The
classic Rac1 activity is as a cytoskeleton organizer and is
required for lamellipodia formation. Rac1 plays an important role
in the pathobiology of various malignancy-related processes that
promote tumor progression. Studies from diverse types of cancers
have suggested the essential role of Rac1 in regulating tumor
metastasis. Rac1 can be activated by multiple upstream signals and
induce invadopodia formation and cause metastasis in breast cancer
(15). Activated Rac1 promotes the
assembly of cell surface integrin protein molecules in the surface
of the head of the cells and passes regulative signals to the actin
cytoskeleton, thus, inducing actin filaments to aggregate in the
plasma membrane and form sheets of pseudopodia, leading to cell
membrane multipolarization, which eventually affects the movement
of cell migration. In our studies, Rac1-overexpressing gastric
cancer cells exhibited obvious morphogenic changes showing more and
longer cell lamellipodia protrusions, as well as enhanced cell
migration and invasion. The current findings suggested that Rac1
activation occurred in the leading edge of the tumor. In agreement
with the previous findings that Rac1 signaling is not required for
the mode of amoeboid motility (42); we found that Rac1-overexpression
caused more mesenchymal phenotype features such as extended
protrusions in multiple directions. Our further findings that
Rac1-overexpressing cells had elevated Rac1 activity, in line with
previous findings, illustrated the role of Rac1 as a key regulator
of cell mobility in gastric cancer.
Recently, a study on head and neck squamous cell
carcinomas (HNSCC) showed that Rac1 is abundantly expressed in
HNSCC tumor tissues and associated with lower early response rate
as well as higher risk of tumor recurrences, while normal cells
largely lack the Rac1 expression (43). They further found that inhibition
of Rac1 activity could be useful in overcoming treatment resistance
and could be proposed for HNSCC patients with primary or secondary
chemo-radioresistance (43). In
addition, Kaneto et al (38) discovered that Rac1 inhibition was a
potential therapeutic target for gefitinib-resistant non-small cell
lung cancer (NSCLC). In agreement with the study of Kaneto et
al, another group found that Rac1 suppression could suppress
NSCLC stem cells and thus inhibit their tumorigenic activity
(44). These investigations have
provided insight into Rac1 signaling as therapeutic targets for
human cancer treatment. In our experimental cell model, we found
similar effects when Rac1-overexpressing cells were treated with
NSC23766, a specific inhibitor of Rac1 activity.
Rac1-overexpressing gastric cancer cell aggressiveness, as shown by
proliferation, migration and invasion, was largely compromised by
NSC23766. Rac1 appears to be a promising and relevant target for
the development of novel anticancer drugs. Indeed, emerging
evidence indicates that Rac1 regulates tumor cell motility,
survival, and more importantly tumor angiogenesis (13). Because novel inhibitors targeting
Rac1 activity have been developed by many groups (45) and Rac1 plays an essential role in
gastric cancer cellular functions, Rac1 is a potential target for
gastric cancer treatment.
In summary, our findings show the significant
correlation of Rac1 in the patient outcome and the antitumor effect
of Rac1 inhibition in gastric cancer cells. Our initial data
support a central role of Rac1 in gastric tumorigenesis and
progression. Rac1 shows potential as a biomarker for targeted
therapy in gastric cancer treatment.
Acknowledgements
This study was supported by grants from the National
Science Foundation of China (81372645), Shanghai Natural Science
Foundation from municipal government (13ZR1425900), Shanghai Jiao
Tong University School of Medicine Science and Technology
Foundation (13XJ10035) and Fong Shu Fook Tong Foundation to J.
Zhang. This study was also partially supported by the Chinese
National High Tech Program (2012AA02A504, 2012AA02A203), the
National Natural Science Foundation of China (81172329, 81372644)
to Y. Yu.
References
|
1
|
Brenner H, Rothenbacher D and Arndt V:
Epidemiology of stomach cancer. Methods Mol Biol. 472:467–477.
2009. View Article : Google Scholar
|
|
2
|
Ridley AJ: Rho GTPases and actin dynamics
in membrane protrusions and vesicle trafficking. Trends Cell Biol.
16:522–529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karpel-Massler G, Westhoff MA, Zhou S, et
al: Combined inhibition of HER1/EGFR and RAC1 results in a
synergistic antiproliferative effect on established and primary
cultured human glioblastoma cells. Mol Cancer Ther. 12:1783–1795.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Menard L, Parker PJ and Kermorgant S:
Receptor tyrosine kinase c-Met controls the cytoskeleton from
different endosomes via different pathways. Nat Commun. 5:39072014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumar R, Gururaj AE and Barnes CJ:
p21-activated kinases in cancer. Nat Rev Cancer. 6:459–471. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Valente AJ, Yoshida T, Clark RA,
Delafontaine P, Siebenlist U and Chandrasekar B: Advanced oxidation
protein products induce cardiomyocyte death via
Nox2/Rac1/superoxide-dependent TRAF3IP2/JNK signaling. Free Radic
Biol Med. 60:125–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shepelev MV, Chernoff J and Korobko IV:
Rho family GTPase Chp/RhoV induces PC12 apoptotic cell death via
JNK activation. Small GTPases. 2:17–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Foldynova-Trantirkova S, Sekyrova P,
Tmejova K, et al: Breast cancer-specific mutations in CK1epsilon
inhibit Wnt/beta-catenin and activate the Wnt/Rac1/JNK and NFAT
pathways to decrease cell adhesion and promote cell migration.
Breast Cancer Res. 12:R302010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Zhang F, Chen F, et al: MEKK3
regulates IFN-gamma production in T cells through the
Rac1/2-dependent MAPK cascades. J Immunol. 186:5791–5800. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Esufali S and Bapat B: Cross-talk between
Rac1 GTPase and dysregulated Wnt signaling pathway leads to
cellular redistribution of beta-catenin and TCF/LEF-mediated
transcriptional activation. Oncogene. 23:8260–8271. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goc A, Abdalla M, Al-Azayzih A and
Somanath PR: Rac1 activation driven by 14-3-3zeta dimerization
promotes prostate cancer cell-matrix interactions, motility and
transendothelial migration. PLoS One. 7:e405942012. View Article : Google Scholar
|
|
12
|
Vader P, van der Meel R, Symons MH, et al:
Examining the role of Rac1 in tumor angiogenesis and growth: a
clinically relevant RNAi-mediated approach. Angiogenesis.
14:457–466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma J, Xue Y, Liu W, et al: Role of
activated rac1/cdc42 in mediating endothelial cell proliferation
and tumor angiogenesis in breast cancer. PLoS One. 8:e662752013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin KT, Gong J, Li CF, et al: Vav3-rac1
signaling regulates prostate cancer metastasis with elevated Vav3
expression correlating with prostate cancer progression and
posttreatment recurrence. Cancer Res. 72:3000–3009. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin CW, Sun MS, Liao MY, et al:
Podocalyxin-like 1 promotes invadopodia formation and metastasis
through activation of Rac1/Cdc42/cortactin signaling in breast
cancer cells. Carcinogenesis. 35:2425–2435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schnelzer A, Prechtel D, Knaus U, et al:
Rac1 in human breast cancer: overexpression, mutation analysis, and
characterization of a new isoform, Rac1b. Oncogene. 19:3013–3020.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fritz G, Just I and Kaina B: Rho GTPases
are over-expressed in human tumors. Int J Cancer. 81:682–687. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu G, Wang Y, Huang B, et al: A Rac1/PAK1
cascade controls beta-catenin activation in colon cancer cells.
Oncogene. 31:1001–1012. 2012. View Article : Google Scholar
|
|
19
|
Kamai T, Shirataki H, Nakanishi K, et al:
Increased Rac1 activity and Pak1 overexpression are associated with
lymphovascular invasion and lymph node metastasis of upper urinary
tract cancer. BMC Cancer. 10:1642010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhan H, Liang H, Liu X, Deng J, Wang B and
Hao X: Expression of Rac1, HIF-1alpha, and VEGF in gastric
carcinoma: correlation with angiogenesis and prognosis. Onkologie.
36:102–107. 2013. View Article : Google Scholar
|
|
21
|
Wu YJ, Tang Y, Li ZF, et al: Expression
and significance of Rac1, Pak1 and Rock1 in gastric carcinoma. Asia
Pac J Clin Oncol. 10:e33–e39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kato T, Kawai K, Egami Y, Kakehi Y and
Araki N: Rac1-dependent lamellipodial motility in prostate cancer
PC-3 cells revealed by optogenetic control of Rac1 activity. PLoS
One. 9:e977492014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bauer NN, Chen YW, Samant RS, Shevde LA
and Fodstad O: Rac1 activity regulates proliferation of aggressive
metastatic melanoma. Exp Cell Res. 313:3832–3839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jun Cho H, Kim IK, Park SM, et al: VEGF-C
mediates RhoGDI2-induced gastric cancer cell metastasis and
cisplatin resistance. Int J Cancer. 135:1553–1563. 2014. View Article : Google Scholar
|
|
25
|
Zeng W, Fu K, Quintanilla-Fend L, Lim M,
Ondrejka S and Hsi ED: Cyclin D1-negative blastoid mantle cell
lymphoma identified by SOX11 expression. Am J Surg Pathol.
36:214–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Subauste MC, Von Herrath M, Benard V, et
al: Rho family proteins modulate rapid apoptosis induced by
cytotoxic T lymphocytes and Fas. J Biol Chem. 275:9725–9733. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Deng Y, Mao Z, et al: CCN1
promotes tumorigenicity through Rac1/Akt/NF-kappaB signaling
pathway in pancreatic cancer. Tumour Biol. 33:1745–1758. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Safina A, Vandette E and Bakin AV: ALK5
promotes tumor angiogenesis by upregulating matrix
metalloproteinase-9 in tumor cells. Oncogene. 26:2407–2422. 2007.
View Article : Google Scholar
|
|
29
|
Cho JY, Lim JY, Cheong JH, et al: Gene
expression signature-based prognostic risk score in gastric cancer.
Clin Cancer Res. 17:1850–1857. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Q, Wen YG, Li DP, et al: Upregulated
INHBA expression is associated with poor survival in gastric
cancer. Med Oncol. 29:77–83. 2012. View Article : Google Scholar
|
|
31
|
D’Errico M, de Rinaldis E, Blasi MF, et
al: Genome-wide expression profile of sporadic gastric cancers with
microsatellite instability. Eur J Cancer. 45:461–469. 2009.
View Article : Google Scholar
|
|
32
|
Ooi CH, Ivanova T, Wu J, et al: Oncogenic
pathway combinations predict clinical prognosis in gastric cancer.
PLoS Genet. 5:e10006762009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Forster S, Gretschel S, Jons T, Yashiro M
and Kemmner W: THBS4, a novel stromal molecule of diffuse-type
gastric adenocarcinomas, identified by transcriptome-wide
expression profiling. Mod Pathol. 24:1390–1403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao Y, Dickerson JB, Guo F, Zheng J and
Zheng Y: Rational design and characterization of a Rac
GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA.
101:7618–7623. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bid HK, Roberts RD, Manchanda PK and
Houghton PJ: RAC1: an emerging therapeutic option for targeting
cancer angiogenesis and metastasis. Mol Cancer Ther. 12:1925–1934.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leve F and Morgado-Diaz JA: Rho GTPase
signaling in the development of colorectal cancer. J Cell Biochem.
113:2549–2559. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oleinik NV, Helke KL, Kistner-Griffin E,
Krupenko NI and Krupenko SA: Rho GTPases RhoA and Rac1 mediate
effects of dietary folate on metastatic potential of A549 cancer
cells through the control of cofilin phosphorylation. J Biol Chem.
289:26383–26394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kaneto N, Yokoyama S, Hayakawa Y, Kato S,
Sakurai H and Saiki I: RAC1 inhibition as a therapeutic target for
gefitinib-resistant non-small-cell lung cancer. Cancer Sci.
105:788–794. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Elsayed HE, Akl MR, Ebrahim HY, et al:
Discovery, optimization, and pharmacophore modeling of oleanolic
acid and analogues as breast cancer cell migration and invasion
inhibitors through targeting Brk/Paxillin/Rac1 Axis. Chem Biol Drug
Des. Jun 20–2014. View Article : Google Scholar : (Epub ahead of
print). PubMed/NCBI
|
|
40
|
Baugher PJ, Krishnamoorthy L, Price JE and
Dharmawardhane SF: Rac1 and Rac3 isoform activation is involved in
the invasive and metastatic phenotype of human breast cancer cells.
Breast Cancer Res. 7:R965–R974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Watson IR, Li L, Cabeceiras PK, et al: The
RAC1 P29S hotspot mutation in melanoma confers resistance to
pharmacological inhibition of RAF. Cancer Res. 74:4845–4852. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fackler OT and Grosse R: Cell motility
through plasma membrane blebbing. J Cell Biol. 181:879–884. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Skvortsov S, Dudas J, Eichberger P, et al:
Rac1 as a potential therapeutic target for chemo-radioresistant
head and neck squamous cell carcinomas (HNSCC). Br J Cancer.
110:2677–2687. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Akunuru S, Palumbo J, Zhai QJ and Zheng Y:
Rac1 targeting suppresses human non-small cell lung adenocarcinoma
cancer stem cell activity. PLoS One. 6:e169512011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cardama GA, Comin MJ, Hornos L, et al:
Preclinical development of novel Rac1-GEF signaling inhibitors
using a rational design approach in highly aggressive breast cancer
cell lines. Anticancer Agents Med Chem. 14:840–851. 2014.
View Article : Google Scholar :
|