Introduction
Multiple myeloma is a malignant proliferative
disease of plasma cells in the bone marrow. While drugs such as
lenalidomide and bortezomib have improved survival, myeloma remains
largely incurable. Efforts are underway to optimize existing
chemotherapeutic strategies and discover new agents. It is
important to control the disease in the long term while maintaining
the patient’s quality of life because it is still difficult to
cure. The development of less-cytotoxic agents would be desirable
for this purpose.
Cotylenin A, which has a diterpenoid tricarbocyclic
skeleton, has been shown to induce the differentiation of myeloid
leukemia cell lines and leukemic cells isolated from acute myeloid
leukemia patients in primary culture (1–3).
Administration of cotylenin A significantly prolonged the survival
of mice that had been inoculated with retinoid-sensitive and
-resistant NB4 cells, and no appreciable adverse effects were
observed in the experiments (4).
Combined treatment with IFNα and cotylenin A
preferentially induced apoptosis in human lung cancer cells while
sparing normal lung epithelial cells and significantly inhibited
the growth of human lung cancer cells as xenografts without
apparent adverse effects, suggesting that this combination may have
therapeutic value in treating human cancer (5–7).
These findings suggest that cotylenin A may be useful in the
therapy for leukemia and some other malignancies.
In the present study, we examined the antitumor
effects of cotylenin A and identified drugs that could be
administered in combination with cotylenin A to inhibit the growth
of myeloma cells to develop a novel therapeutic strategy against
multiple myeloma.
Materials and methods
Cells and culture
Human multiple myeloma cell lines RPMI-8226, KMS-11,
KMS-26, KMS-12 PE and KMS-12 BM were obtained from JCRB Cell Bank
(Osaka, Japan), and cultured in RPMI-1640 supplemented with 10%
fetal bovine serum. Matrigel-coated dishes were prepared according
to the manufacturer’s instructions (BD Biosciences, San Jose, CA,
USA).
Materials
Cotylenin A was purified from a stock ethyl acetate
extract obtained from the culture filtrate of Cladosporium
fungus sp. 501-7W by flash chromatography on silica gel with
>99% purity (8). Vincristine
and other anticancer agents,
3-(4,5-di-methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT), and propidium iodide were purchased from Sigma-Aldrich (St.
Louis, MO, USA). Anti-XIAP, anti-survivin and anti-LC3 monoclonal
antibodies were purchased from Cell Signaling Technology (Danvers,
MA, USA). Anti-P62 monoclonal antibody was obtained from MBL
(Nagoya, Japan).
Assay of cell growth
The cells were seeded at 1×105 cells/ml
in a 24-well multidish. After culture with or without the test
compounds for indicated times, viable cells were examined by a
modified MTT assay (9).
Annexin V binding assay
Cells were labeled with PE-labeled Annexin V (BD
Biosciences) for 30 min on ice, as described previously (10). After staining, cells were washed
and analyzed by flow cytometry (BD FACSCalibur, San Jose, CA,
USA).
Flow cytometric analysis of cell cycle
distribution
The cell cycle was analyzed using propidium
iodide-stained nuclei (11). The
histogram of DNA content was analyzed by flow cytometry (BD
FACSCalibur) using CellQuest software (BD Immunocytometry Systems,
San Jose, CA, USA) to determine the cell cycle distribution
(sub-G1, G1, S and G2/M).
Protein profiler array
RPMI-8226 cells were treated with or without
cotylenin A and/or vincristine for 48 h. All immuno detection steps
were performed using a Proteome Profiler Array (R&D Systems,
Minneapolis, MN, USA) in accordance with the manufacturer’s
instructions. Briefly, the cells were collected and lysed in lysis
buffer. The array was incubated overnight with the diluted lysates
(500 μg/250 μl) at 4°C on a rocking platform shaker. Primary
(reconstituted detection antibody cocktail) and secondary
[streptavidin-horseradish peroxidase (HRP) (1:2000)] antibodies
were added to each array.
Western blot analysis
Cells were packed after being washed with cold
phosphate-buffered saline (PBS) and then lysed at a concentration
of 1×107 cells/ml in lysis buffer (Wako, Osaka, Japan).
Equal amounts of protein were separated on 10% SDS-polyacrylamide
gels. The proteins were electrophoresed on gels and transferred to
an Immobilon-P membrane (Millipore, Bedford, MA, USA) using rabbit
anti-XIAP (1:1000) and rabbit anti-survivin (1:1000) antibodies.
All Western blots shown are representative of at least 3
independent experiments.
Transwell chamber invasion assay
The invasiveness of RPMI-8226 cells was evaluated by
a transwell chamber assay. Matrigel (50 μg/ml, BD Biosciences) was
melted at 4°C, and diluted to 1:8 by serum-free RPMI-1640 medium.
The upper side of the polycarbonate filter of the Transwell chamber
was coated with matrigel. Cells (2×105) were suspended
in 800 μl of serum-free medium that contained 1 mg/ml bovine serum
albumin to maintain the osmotic pressure, and placed in the upper
chamber. The lower chamber was filled with 10% serum-medium (1000
μl). Cells were then cultured for 24 h at 37 °C in 5%
CO2. Cells on the upper surface of the filter were
removed using a cotton swab. The invading cells on the lower
surface of the filter were fixed with formaldehyde (4%) and stained
with 0.1% crystal violet in 2% methanol. Invasiveness was
determined by counting cells in five microscopic fields per well,
and the extent of invasion was expressed as the average number of
cells per microscopic field.
Colony-forming assay
Cells (1×104 per dish) were plated into
1.1 ml of a semisolid methylcellulose medium with 0.8%
methylcellulose and 20% fetal bovine serum in triplicate for 14
days. A solution of 0.2 ml of PBS containing various concentrations
of cotylenin A or vincristine was added to the semisolid medium.
Colonies were photographed under an inverted microscope. Colonies
in enlarged photographs were measured and counted. Colonies were
classified as large (>0.7 mm in diameter), medium (0.4–0.7 mm)
or small (0.2–0.4 mm).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from cells using TRI reagent
(Sigma-Aldrich). Total RNA (1 μg) was converted to first-strand
cDNA primed with random hexamer in a reaction volume of 20 μl using
an RNA PCR kit (Takara Bio, Tokyo, Japan), and 4 μl of this
reaction mixture was used as a template in the polymerase chain
reaction (9). The primers used
were as previously described (12).
Transplantation of myeloma cells into
SCID mice and treatment
Six-week-old female (Fox Chase SCID C.B-17/Icr-scid
Jcl) mice were subcutaneously inoculated with 6×106
KMS-26 cells. The adjusted 6×107 cells/ml were mixed
with an equal volume of Matrigel (BD Biosciences) and 0.2 ml of the
mixture was subcutaneously injected into the lower back of each
animal using a 26-gauge needle. Mice were given intraperitoneal
injections of 0.2 ml of PBS with 0.5 mg/kg vincristine and/or 5
mg/kg cotylenin A three times per week for 3 weeks. The first
treatment was given 4 days after the inoculation of tumor cells.
Tumor volume was measured with vernier calipers. We performed
experiments according to national legislation on laboratory animal
protection and our protocol was approved by the animal ethics
committee of Shimane University.
Statistical analysis
Pairs of data were compared using Student’s t-test.
For the experiment in vivo, the significance of differences
among the four groups was assessed using a one-way analysis of
variance and the Kruskal-Wallis test. P-values of <0.05 were
considered to reflect statistical significance.
Results
Combined effects of cotylenin A and
various drugs on the growth of myeloma cells
Since IFNα has been shown to have beneficial effects
in the treatment of multiple myeloma (13,14),
and combined treatment with cotylenin A and IFNα synergistically
inhibited growth both in vitro and in vivo in various
human cancer cells (6,7), multiple myeloma might be a reasonable
target for combined treatment with cotylenin A and IFNα. Therefore,
we examined the anti-proliferative effects of cotylenin A and IFNα
in 5 myeloma cell lines. RPMI-8226, KMS-12 PE and KMS-26 cells were
highly sensitive to the combined treatment. However, KMS-12 BM
cells did not respond to IFNα even in the presence of cotylenin A,
and KMS-11 cells were less sensitive to the combined treatment.
These results suggest that this treatment may be useful only in
certain patients with multiple myeloma.
Next, we screened various anticancer agents with
small molecules to identify the most potent drug with respect to
its co-operative effect with cotylenin A on the growth of multiple
myeloma cells. To measure the effects of various drugs on the
growth of myeloma RPMI-8226 cells, the number of viable cells was
determined by the MTT assay after 6 days of exposure to various
concentrations of drugs with or without 2 μg/ml of cotylenin A. The
growth-inhibiting effects of the drugs were examined by determining
the concentrations of drugs required to reduce the cell number to
one-half of that in untreated cells (IC50). While the
sensitivity to anticancer agents, such as doxorubicin, camptotecin,
cisplatin, 5-fluorouracil, methotrexate or gemcitabine, was not
affected by cotylenin A, the sensitivity to vincristine and other
microtubule-disturbing agents was significantly enhanced (Table I).
 | Table IPotentiation of the growth-inhibitory
activities of various anticancer agents in RPM-I8226 myeloma cells
by cotylenin A. |
Table I
Potentiation of the growth-inhibitory
activities of various anticancer agents in RPM-I8226 myeloma cells
by cotylenin A.
| Anticancer agent
(ng/ml) | Growth inhibition
(IC50) | Ratio (−/+) |
|---|
|
|---|
| − Cotylenin A | + Cotylenin A |
|---|
| Doxorubicin | 5.5±0.6 | 5.3±0.5 | 1.03 |
| Cisplatin | 246±30.2 | 237±28.4 | 1.03 |
| Camptotecin | 1.42±0.16 | 1.38±0.14 | 1.03 |
| Methotrexate | 2.76±0.3 | 1.81±0.2 | 1.52 |
| Gemcitabine | 4.3±0.4 | 3.1±0.3 | 1.39 |
| 5-Fluorouracil | 56.5±5.1 | 57.8±6.3 | 0.98 |
| Vincristine | 6.3±0.6 | 1.6±0.1 | 3.94 |
| Vinblastine | 0.91±0.11 | 0.42±0.05 | 2.17 |
| Paclitaxel | 16.4±1.9 | 7.7±0.9 | 2.13 |
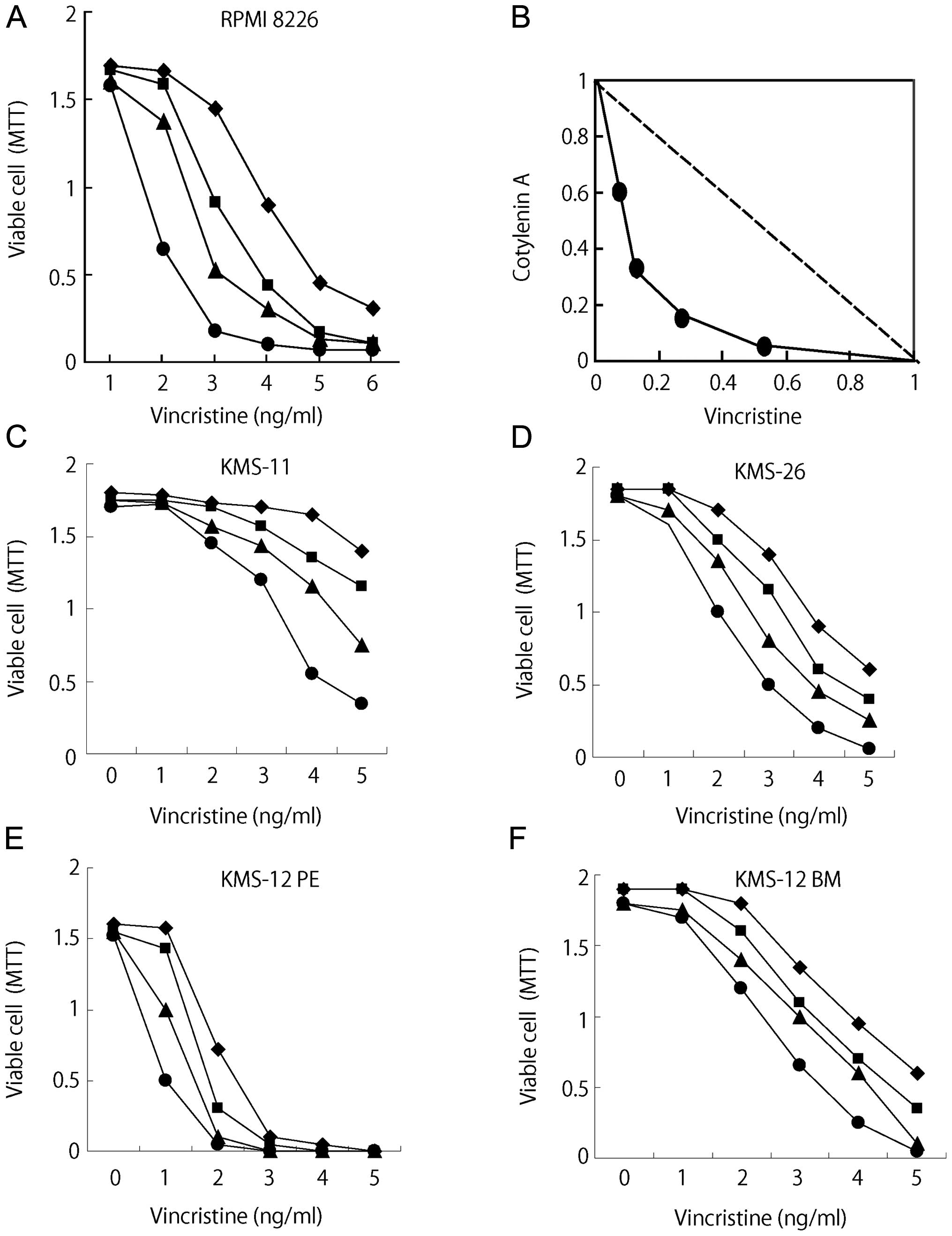
Cotylenin A had synergistic effects with vincristine
(Fig. 1A) and the results were
confirmed by an isobologram analysis (15). Fig.
1B shows isoboles for the combination of vincristine with
cotylenin A that were isoeffective (IC50: concentration
of the drug required for 50% inhibition of cell growth) for
inhibition of the proliferation of RPMI-8226 cells. These isoboles
indicate that the combination of these drugs had synergistic
effects. The synergistic effects of cotylenin A and vincristine
were also observed in other myeloma cell lines, although the
different myeloma cell lines had different sensitivities to
vincristine (Fig. 1C–F).
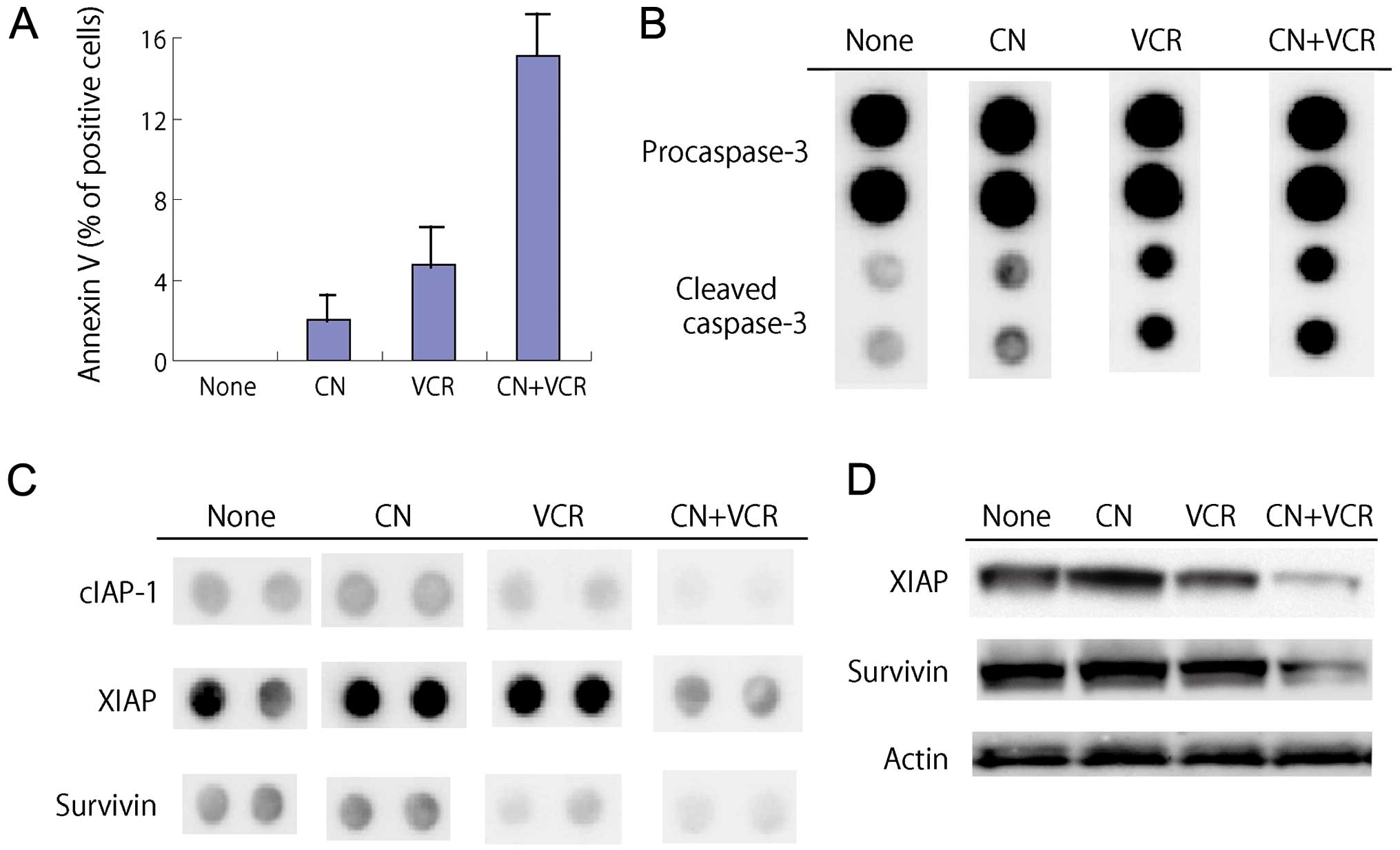
Induction of apoptosis in RPMI-8226 cells
treated with cotylenin A and vincristine
When RPMI-8226 cells were exposed to 2 ng/ml
vincristine in the presence of cotylenin A for 4 days, a
morphological analysis showed shriveled cells, chromatin
condensation, nuclear fragmentation and cytoplasmic blebbing,
although these morphological changes were hardly observed in cells
treated with 2 ng/ml vincristine alone. The induction of apoptosis
was confirmed by an analysis of the DNA histogram (cells in sub-G1
phase) and the expression of Annexin V (Table II, Fig. 2A). A proteome profiler analysis of
myeloma cells was performed to elucidate the effects of the
combined treatment on apoptotic pathways.
 | Table IIEffects of cotylenin A and
vincristine on apoptosis of myeloma cells. |
Table II
Effects of cotylenin A and
vincristine on apoptosis of myeloma cells.
| Treatment | Apoptosis (% of
cells in sub-G1 phase) |
|---|
| None | 6.1±1.2 |
| Cotylenin A
(μg/ml) |
| 1 | 7.3±1.3 |
| 2 | 7.7±1.8 |
| 3 | 9.6±1.7 |
| 6 | 12.9±2.3 |
| Vincristine
(ng/ml) |
| 1 | 7.1±1.9 |
| 2 | 12.1±2.8 |
| 3 | 20.9±3.2 |
| 6 | 64.4±8.9 |
| Cotylenin A (2
μg/ml) and Vincristine (2 ng/ml) | 87.5±9.6 |
The expression of cleaved caspase-3 was increased in
treatment with vincristine and further enhanced by cotylenin A
(Fig. 2B). Significant alterations
in protein expression were noted in cells treated with cotylenin A
and vincristine: the levels of anti-apoptotic proteins such as
cIAP, XIAP and survivin were markedly decreased (Fig. 2C). These changes were confirmed by
Western blot analysis (Fig. 2D).
The expression of anti-apoptotic proteins, Bcl-2 or Bcl-X, was
hardly affected by treatment.
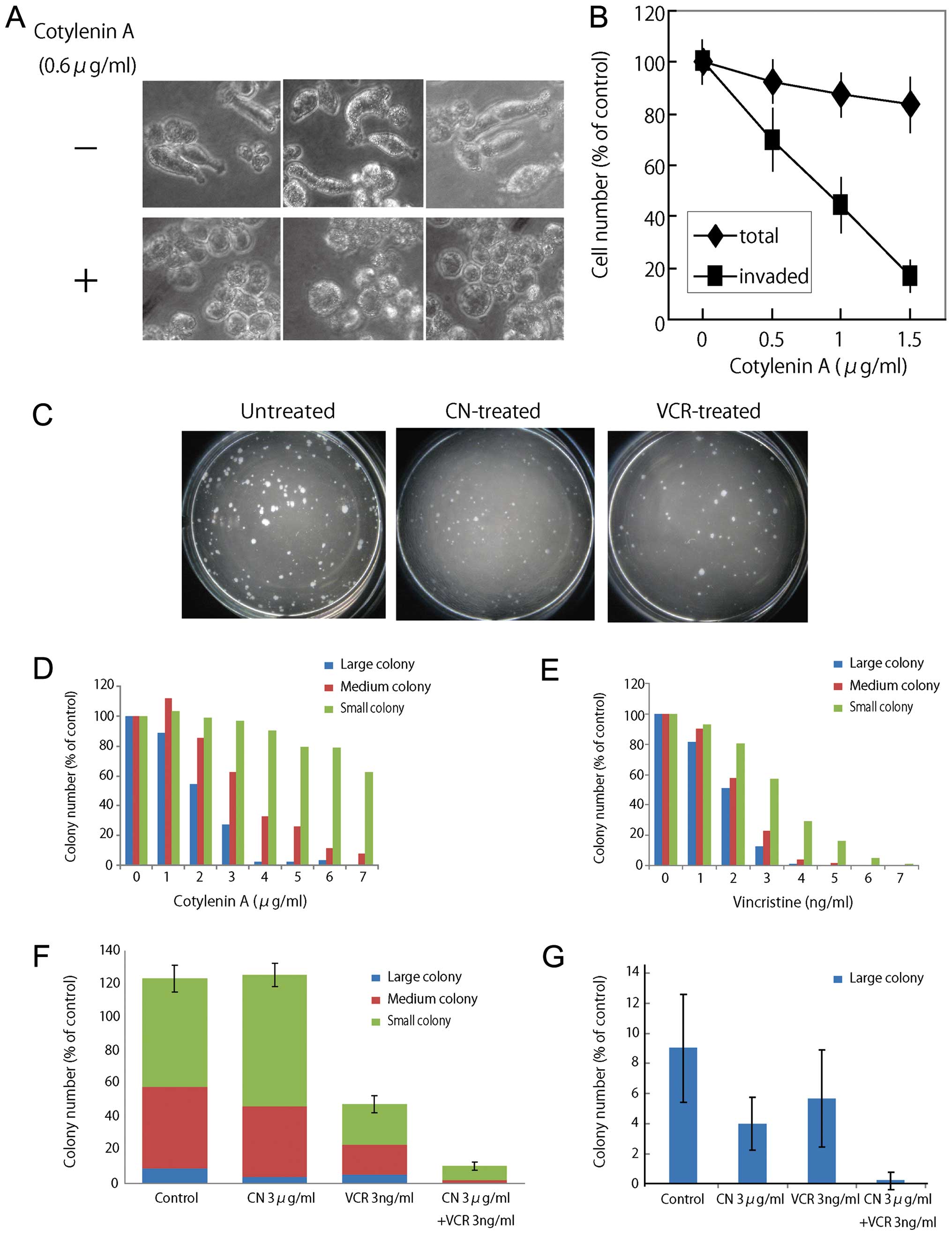
Morphologic changes and reduction of
invasive activity in cells treated with cotylenin A
When RPMI-8226 cells were cultured on a
Matrigel-coated dish, most of the cells had elongated shapes and
were migrating. However, treatment with a low concentration of
cotylenin A (0.6 μg/ml) induced morphological changes without
inhibiting growth. Most of the treated cells had round shapes
within 3 days (Fig. 3A). An
invasion assay revealed that cotylenin A effectively inhibited the
invasive activity of RPMI-8226 cells without inhibiting growth
(Fig. 3B).
Inhibition of colony formation and
expression of pluripotency-associated transcription factors by
cotylenin A
A colony-forming assay indicated that cotylenin A
preferentially inhibited the formation of large colonies whereas
vincristine similarly inhibited the formation of colonies of
different sizes (Fig. 3C–E). The
combined treatment with cotylenin A and vincristine effectively
suppressed colony formation by myeloma cells (Fig. 3F), and especially the formation of
large colonies (Fig. 3G).
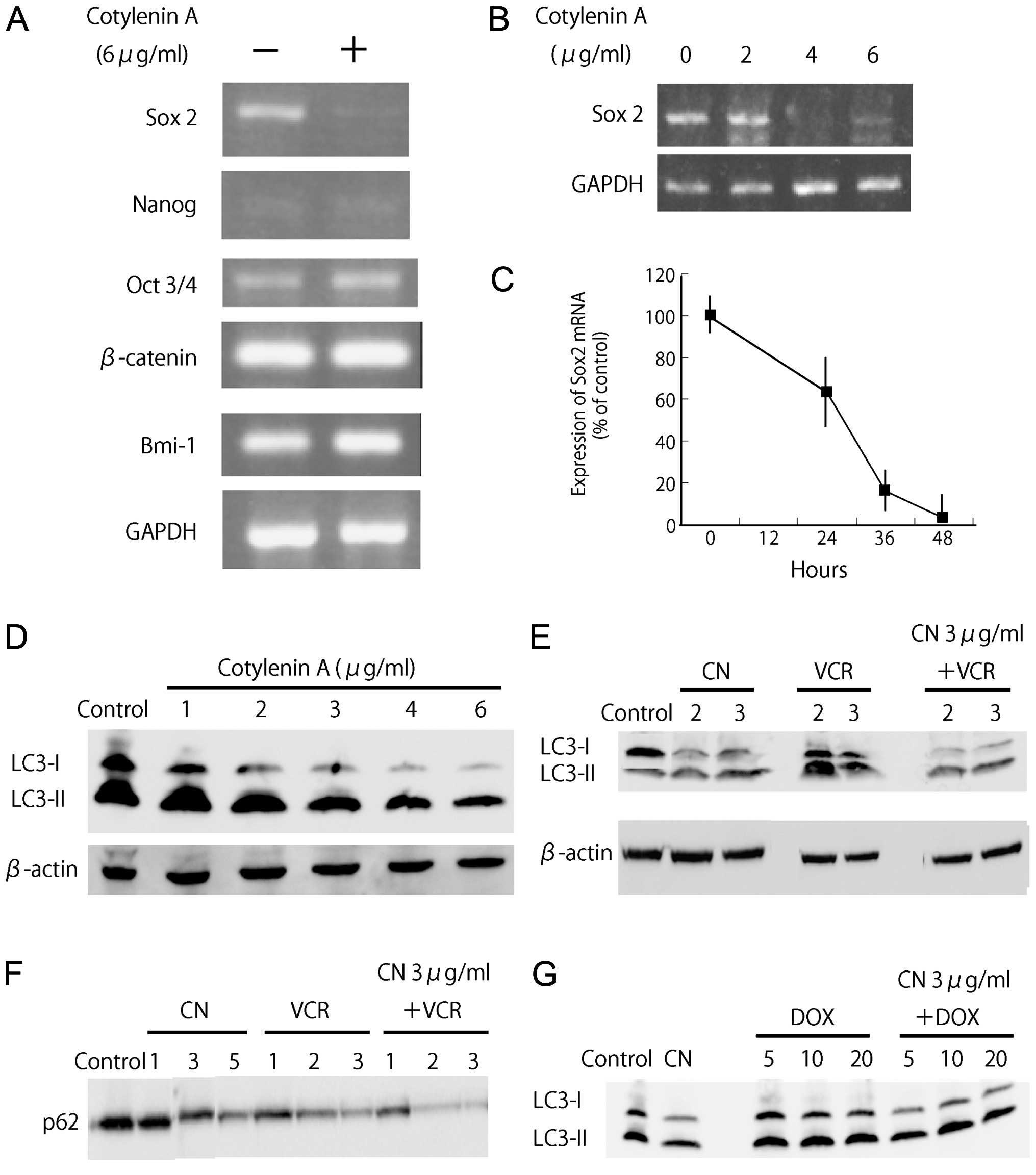
Since Wnt/β-catenin signaling molecules have been
implicated in the maintenance of pluripotency-associated
transcription factors (16), we
evaluated the mRNA expression of β-catenin as well as the mRNA
expression of pluripotency-associated transcription factor
(Fig. 4A and B). Expression of
Sox2 mRNA was significantly suppressed by treatment with cotylenin
A. The reduction of Sox2 mRNA expression was observed within 24 h
(Fig. 4C).
Effect of vincristine or doxorubicin on
autophagy induced by cotylenin A
Autophagy is a survival pathway that is responsible
for the breakdown of damaged organelles and protein aggregates,
although there is an ongoing controversy regarding whether the
autophagy pathway primarily represents a pro-survival or pro-cell
death mechanism with cancer therapy (17). Microtubules are required for
autophagosomal biogenesis and degradation, and vinca alkaloids
disrupt autophagosome maturation/degradation by preventing the
movement of autophagosomes and their fusion with lysosomes
(18). Several studies have
demonstrated that the treatment of cancers with agents that induce
autophagy in combination with agents that block autophagosome
maturation/degradation results in synergistic apoptotic cell death
(19–21). Myeloma cells require a basal level
of autophagy for survival and the pharmacologic or genetic
inhibition of autophagy caused myeloma cells to die (22). Therefore, we examined the effects
of vincristine and doxorubicin on autophagic processes to
understand how cotylenin A preferentially sensitizes myeloma cells
to vincristine.
As described above, treatment with cotylenin A alone
inhibited cell growth in a concentration-dependent manner, but did
not significantly induce apoptosis. Since cotylenin A might induce
autophagy in RPMI-8226 cells, we examined the levels of LC3-I and
-II in cells treated with cotylenin A. Upon the initiation of
autophagy, LC3 relocates from the cytosol to autophagosome
membranes, where it plays a role in autophagosome enlargement, and
cytosolic LC3 (LC3-I, 18 kDa) undergoes C-terminal proteolytic
processing to a 16 kDa isoform, LC3-II. Fig. 4D shows that cotylenin A reduced the
accumulation of LC3-I in a dose-dependent manner. This finding
suggests that cotylenin A induced autophagosomes. Vincristine also
induced the accumulation of LC3-II, whereas doxorubicin hardly
affected the accumulation of LC3-II (Fig. 4E and G). Cells treated with
cotylenin A and vincristine showed a synergistic increase in the
levels of LC3-II compared to those treated with either of the
agents alone (Fig. 4E). A
synergistic increase was not observed in cells treated with
cotylenin A and doxorubicin (Fig.
4G). p62 protein is a marker for autophagic flux, since the
autophagy pathway normally degrades this protein. The accelerated
degradation of p62 protein was also found in response to the
combination treatment (Fig. 4F).
These results indicate that there is a significant difference
between the autophagic responses to vincristine and
doxorubicin.
Effects of pre- and post-treatment with
cotylenin A and vincristine on the growth of myeloma cells
To determine whether simultaneous treatment gives
the best results, we examined the effects of pre- and
post-treatment with cotylenin A on the vincristine-induced
inhibition of the growth of RPMI-8226 cells. The results may
provide useful information regarding the best schedule for combined
treatment with cotylenin A and vincristine against xenografts of
human myeloma cells. For pretreatment, cells were treated with 2
μg/ml of cotylenin A for 3 days, washed in fresh medium, and
incubated with 2 ng/ml of vincristine for 3 days. For
posttreatment, cells were treated with 2 ng/ml of vincristine for 3
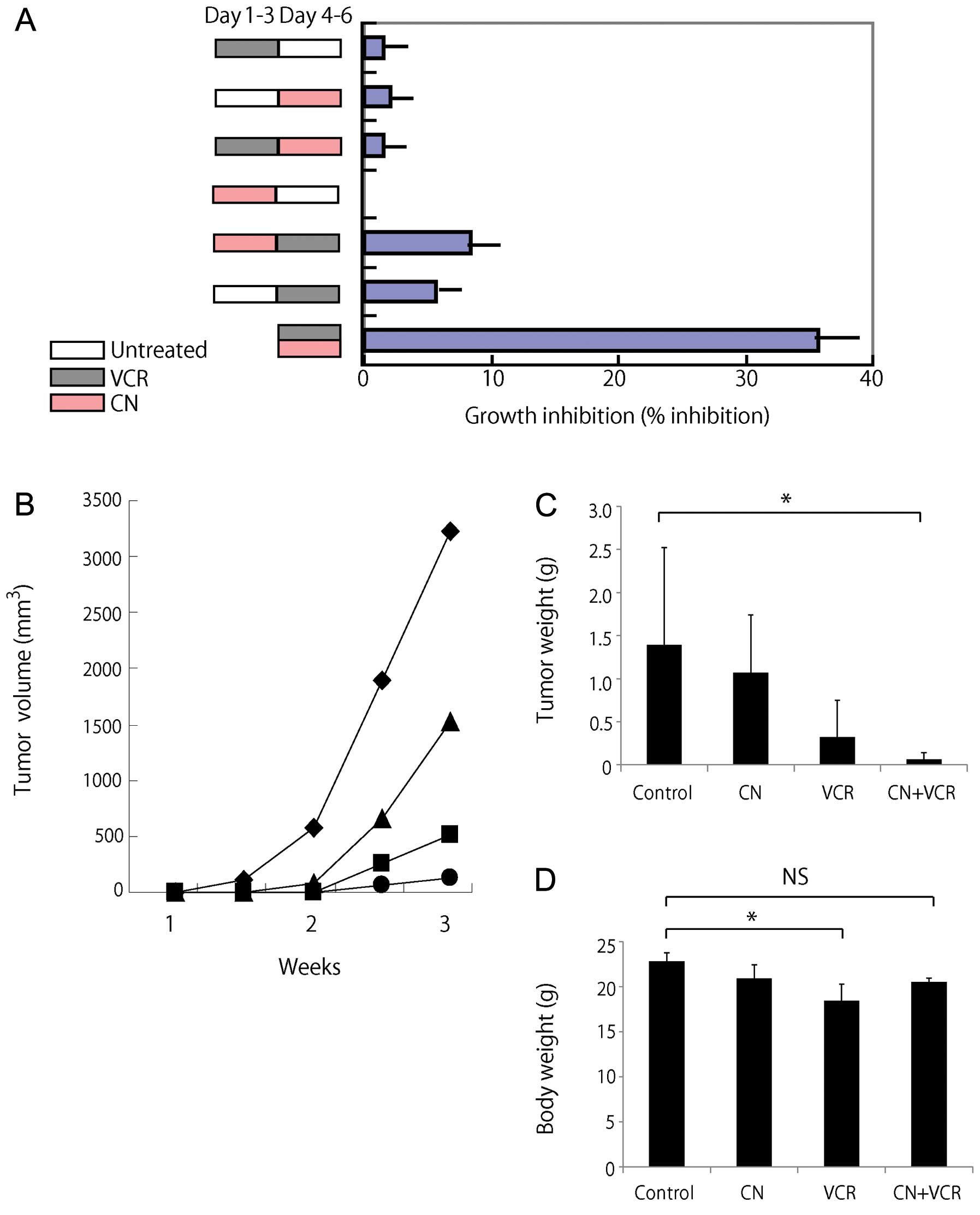
days and then with 2 μg/ml of cotylenin A. Fig. 5A shows that simultaneous treatment
is more effective than pre- or post-treatment with cotylenin A,
although pretreatment with cotylenin A gave better results than
posttreatment. Similar results were obtained when other myeloma
cells were treated with cotylenin A and vincristine.
Effects of cotylenin A and vincristine on
in vivo growth of myeloma cells as xenografts
To determine the potential for cotylenin A in
treating myeloma, we treated KMS-26 xenograft-bearing SCID mice
with cotylenin A alone or in combination with vincristine. We
treated mice with cotylenin A at 5 mg/kg body weight, as described
previously (4,6). This dose of cotylenin A was
well-tolerated without a loss of body weight. Cotylenin A alone
inhibited the growth of KMS-26 xenograft tumors with a day 21 (end
of treatment period) T/C value (the mean volume of xenograft tumor
in treated mice/that in untreated control mice) of 76.8% (Fig. 5B and C). Our in vitro
studies (Fig. 5A) suggest that
simultaneous treatment with cotylenin A and vincristine is more
effective therapeutically than treatment with vincristine alone.
Therefore, we examined the combined effects of cotylenin A and
vincristine on the in vivo growth of KMS-26 cells (Fig. 5B and C). Vincristine alone greatly
inhibited tumor growth with a T/C value of 22.7%. Combined
treatment caused tumor stasis at day 21 with a T/C value of 4.6 %.
In this model, cotylenin A exhibited single-agent activity in
xenografts of KMS-26 cells, along with increased antitumor effects
in combination with vincristine. A statistical analysis revealed
that the difference was significant (P<0.05, versus control).
The effects of single-agent treatments were not statistically
significant in this experiment among the 4 groups. Vincristine
caused significant decrease in the body weight of the mice.
However, the weight loss in the combined-treated group was lower
than that in mice treated with vincristine alone (Fig. 5D).
Discussion
The phenotypes of cancer stem cells in multiple
myeloma are still controversial (23,24).
In the present study, we demonstrated that cotylenin A
preferentially inhibited the population that formed large colonies,
suggesting that cells have high self-renewal activity. The
pluripotency-associated transcription factor Sox2 was effectively
down-regulated by treatment with cotylenin A. Expression of Sox2
was important for the development of tumors, as shown in
vivo experiments. Spisek et al reported that Sox2 was
highly expressed in the clonogenic compartment of plasma cells and
that anti-Sox2 T cells help to prevent disease progression in
patients with monoclonal gammopathy (25). These results suggest that cotylenin
A is a useful drug for cancer stem cell-targeted therapy against
multiple myeloma.
Multiple myeloma remains incurable for the vast
majority of patients, which suggests that the cancer stem cells
that mediate relapse are relatively resistant to chemotherapy.
Cotylenin A effectively inhibits clonogenic growth and the
expression of Sox2 in myeloma cells. The inhibition of clonogenic
growth was coupled with accelerated differentiation of the cells
into mature CD138+ cells (26). Cotylenin A effectively and
concentration-dependently decreased CD138− cells in an
RPMI-8226 cell population (data not shown). The sensitivity to
vincristine was effectively enhanced by cotylenin A, and combined
treatment with cotylenin A and vincristine significantly inhibited
tumor growth in xenografts. Cotylenin A had no apparent effects on
mice (body weight or behavior), and reduced the vincristine-induced
toxicity in tumor-bearing mice. Vincristine is frequently used for
the treatment of multiple myeloma (27,28).
These results suggest that the combination of cotylenin A and
vincristine could be useful for the treatment of multiple
myeloma.
While cotylenin A significantly enhanced the
sensitivity to vincristine and other microtubule-disturbing agents,
cotylenin A did not affect the sensitivity to other anticancer
agents such as doxorubicin, camptotecin, cisplatin, 5-fluorouracil,
methotrexate and gemcitabine. These differences might be attributed
to the effects on autophagic responses. Cotylenin A and vincristine
concentration-dependently induced autophagy, and combined treatment
with cotylenin A and vincristine further induced autophagy
(formation of LC3-II and degradation of p62 protein). However,
doxorubicin did not affect the autophagic responses, and did not
enhance the autophagy induced by cotylenin A. Combined treatment
with vincristine and cotylenin A also induced apoptosis (activation
of caspase-3, accumulation of subG1 fraction and induction of
Annexin V). Myeloma cells exhibit low-level autophagy under basal
conditions, and uncontrolled autophagy may reduce cellular
viability under some circumstances (29). Further studies will be needed to
better understand the cell death induced by vincristine plus
cotylenin A.
A receptor of fusicoccins, including cotylenin A,
has been reported to be a member of a family of 14-3-3 proteins
that are found in a huge array of signaling and regulatory pathways
(30). The 14-3-3 proteins bind to
discrete phosphoserine-containing motifs present in many signaling
molecules. The 14-3-3 proteins are associated with dynamic
nucleocytoplasmic shuttling. However, further studies will be
needed to explain how the sensitization to vincristine is related
to the effects of cotylenin A on the 14-3-3 signaling pathway.
The administration of cotylenin A significantly
prolonged the survival of mice that had been inoculated with cells
of the human promyelocytic leukemia cell line NB4 (4). Cotylenin A co-operatively inhibited
tumor growth in xenografts with IFNα (6), rapamycin (9) or cetuximab (31). Treatment with cotylenin A had no
apparent adverse effects on mice, and reduced the adverse effects
of vincristine on tumor-bearing mice. A novel fusicoccin
derivative, closely related to cotylenin A, significantly inhibited
the growth of pancreatic cancer MIAPaCa-2 cells as xenografts
without apparent adverse effects (32). These results suggest that 14-3-3
proteins may be novel targets in cancer therapy.
In conclusion, we found that cotylenin A and
vincristine synergistically inhibited the growth of and induced
apoptosis in myeloma cells in vitro and in vivo. Our
results suggest that the combination of cotylenin A and vincristine
may have therapeutic value in treating multiple myeloma.
References
|
1
|
Yamamoto-Yamaguchi Y, Yamada K, Ishii Y,
Asahi KI, Tomoyasu S and Honma Y: Induction of the monocytic
differentiation of myeloid leukaemia cells by cotylenin A, a plant
growth regulator. Br J Haematol. 112:697–705. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamada K, Honma Y, Asahi KI, Sassa T, Hino
KI and Tomoyasu S: Differentiation of human acute myeloid leukaemia
cells in primary culture in response to cotylenin A, a plant growth
regulator. Br J Haematol. 114:814–821. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Honma Y and Cotylenin A: Cotylenin A - a
plant growth regulator as a differentiation-inducing agent against
myeloid leukemia. Leuk Lymphoma. 43:1169–1178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Honma Y, Ishii Y, Sassa T and Asahi K:
Treatment of human promyelocytic leukemia in the SCID mouse model
with cotylenin A, an inducer of myelomonocytic differentiation of
leukemia cells. Leuk Res. 27:1019–1025. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Honma Y and Akimoto M: Therapeutic
strategy using phenotypic modulation of cancer cells by
differentiation-inducing agents. Cancer Sci. 98:1643–1651. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Honma Y, Ishii Y, Yamamoto-Yamaguchi Y,
Sassa T and Asahi K: Cotylenin A, a differentiation-inducing agent,
and IFN-alpha cooperatively induce apoptosis and have an antitumor
effect on human non-small cell lung carcinoma cells in nude mice.
Cancer Res. 63:3659–3666. 2003.PubMed/NCBI
|
|
7
|
Honma Y, Kasukabe T, Yamori T, Kato N and
Sassa T: Antitumor effect of cotylenin A plus interferon-α:
Possible therapeutic agents against ovary carcinoma. Gynecol Oncol.
99:680–688. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sassa T, Tojyo T and Munakata K: Isolation
of a new plant growth substance with cytokinin-like activity.
Nature. 227:3791970. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kasukabe T, Okabe-Kado J, Kato N, Sassa T
and Honma Y: Effects of combined treatment with rapamycin and
cotylenin A, a novel differentiation-inducing agent, on human
breast carcinoma MCF-7 cells and xenografts. Breast Cancer Res.
7:R1097–R1110. 2005. View
Article : Google Scholar
|
|
10
|
Ishii Y, Hori Y, Sakai S and Honma Y:
Control of differentiation and apoptosis of human myeloid leukemia
cells by cytokinins and cytokinin nucleosides, plant
redifferentiation-inducing hormones. Cell Growth Differ. 13:19–26.
2002.PubMed/NCBI
|
|
11
|
Niitsu N, Higashihara M and Honma Y: The
catalytic DNA topoisomerase II inhibitor ICRF-193 and all-trans
retinoic acid cooperatively induce granulocytic differentiation of
acute promyelocytic leukemia cells: Candidate drugs for
chemodifferentiation therapy against acute promyelocytic leukemia.
Exp Hematol. 30:1273–1282. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ikegame A, Ozaki S, Tsuji D, et al: Small
molecule antibody targeting HLA class I inhibits myeloma cancer
stem cells by repressing pluripotency-associated transcription
factors. Leukemia. 26:2124–2134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Quesada JR, Alexanian R, Hawkins M,
Barlogie B, Borden E, Itri L and Gutterman JU: Treatment of
multiple myeloma with recombinant alpha-interferon. Blood.
67:275–278. 1986.
|
|
14
|
Mandelli F, Avvisati G, Amadori S, et al:
Maintenance treatment with recombinant interferon alfa-2b in
patients with multiple myeloma responding to conventional induction
chemotherapy. N Engl J Med. 322:1430–1434. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marson A, Foreman R, Chevalier B, Bilodeau
S, Kahn M, Young RA and Jaenisch R: Wnt signaling promotes
reprogramming of somatic cells to pluripotency. Cell Stem Cell.
3:132–135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
White EJ, Martin V, Liu JL, Klein SR, Piya
S, Gomez-Manzano C, Fueyo J and Jiang H: Autophagy regulation in
cancer development and therapy. Am J Cancer Res. 1:362–372.
2011.PubMed/NCBI
|
|
18
|
Xie R, Nguyen S, McKeehan WL and Liu L:
Acetylated microtubules are required for fusion of autophagosomes
with lysosomes. BMC Cell Biol. 11:892010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marimpietri D, Brignole C, Nico B, et al:
Combined therapeutic effects of vinblastine and rapamycin on human
neuroblastoma growth, apoptosis, and angiogenesis. Clin Cancer Res.
13:3977–3988. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Q, Lui VW, Lau CP, Cheng SH, Ng MH,
Cai Y, Chan SL and Yeo W: Sustained antitumor activity by
co-targeting mTOR and the microtubule with temsirolimus/vinblastine
combination in hepatocellular carcinoma. Biochem Pharmacol.
83:1146–1158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adiseshaiah PP, Clogston JD, McLeland CB,
et al: Synergistic combination therapy with nanoliposomal
C6-ceramide and vinblastine is associated with autophagy
dysfunction in hepatocarcinoma and colorectal cancer models. Cancer
Lett. 337:254–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hoang B, Benavides A, Shi Y, Frost P and
Lichtenstein A: Effect of autophagy on multiple myeloma cell
viability. Mol Cancer Ther. 8:1974–1984. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Agarwal JR and Matsui W: Multiple myeloma:
A paradigm for translation of the cancer stem cell hypothesis.
Anticancer Agents Med Chem. 10:116–120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boucher K, Parquet N, Widen R, Shain K,
Baz R, Alsina M, Koomen J, Anasetti C, Dalton W and Perez LE:
Stemness of B-cell progenitors in multiple myeloma bone marrow.
Clin Cancer Res. 18:6155–6168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Spisek R, Kukreja A, Chen LC, et al:
Frequent and specific immunity to the embryonal stem
cell-associated antigen SOX2 in patients with monoclonal
gammopathy. J Exp Med. 204:831–840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leung-Hagesteijn C, Erdmann N, Cheung G,
Keats JJ, Stewart AK, Reece DE, Chung KC and Tiedemann RE:
Xbp1s-negative tumor B cells and pre-plasmablasts mediate
therapeutic proteasome inhibitor resistance in multiple myeloma.
Cancer Cell. 24:289–304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar A, Galeb S and Djulbegovic B:
Treatment of patients with multiple myeloma: An overview of
systematic reviews. Acta Haematol. 125:8–22. 2011. View Article : Google Scholar
|
|
28
|
Suzuki K: Current therapeutic strategy for
multiple myeloma. Jpn J Clin Oncol. 43:116–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Galluzzi L, Vitale I, Abrams JM, et al:
Molecular definitions of cell death subroutines: Recommendations of
the Nomenclature Committee on Cell Death 2012. Cell Death Differ.
19:107–120. 2012. View Article : Google Scholar :
|
|
30
|
Oecking C, Eckerskorn C and Weiler EW: The
fusicoccin receptor of plants is a member of the 14-3-3 superfamily
of eukaryotic regulatory proteins. FEBS Lett. 352:163–166. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Molzan M, Kasper S, Röglin L, et al:
Stabilization of physical RAF/14-3-3 interaction by cotylenin A as
treatment strategy for RAS mutant cancers. ACS Chem Biol.
8:1869–1875. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kawakami K, Hattori M, Inoue T, et al: A
novel fusicoccin derivative preferentially targets hypoxic tumor
cells and inhibits tumor growth in xenografts. Anticancer Agents
Med Chem. 12:791–800. 2012. View Article : Google Scholar : PubMed/NCBI
|