Introduction
Cyclooxygenases (COX) are inflammatory regulators
that mediate the production of prostaglandins from arachidonic
acid. Two COX isoforms have been identified (1). COX-1 is constitutively expressed and
maintains homeostatic level of prostaglandins, whereas COX-2 is
induced by growth factors, tumor promoters, and cytokines (2). To date, COX-2 has been found to be
highly expressed in many types of solid cancers including breast,
prostate, colon and lung (3,4) and
to contribute to tumorigenesis via the inhibition of apoptosis,
increased angiogenesis and invasiveness (4). Moreover, in many of these cancer
types, an elevated COX-2 expression was correlated with a poor
response to therapy and decreased survival (5).
Concerning hematological malignancies, Bernard et
al (6) highlighted the recent
interest in studying and modulating COX-2 expression in cells of
hematopoietic origin. They described the different studies that
were conducted on the impact of COX-2 expression in hematological
diseases and showed that chronic lymphocytic leukemia, chronic
myeloid leukemia, Hodgkin’s lymphoma, non-Hodgkin’s lymphoma and
multiple myeloma all highly express COX-2 and that elevated COX-2
expression is often correlated with decreased survival of patients
with hematological malignancies (6). As for acute myeloid leukemia, COX-2
has been shown to be expressed in various cell lines and functional
genetic variations of COX-2 have been recently correlated to
susceptibility to acute myeloid leukemia (7,8).
However, there are scarce data focused on the effect of COX-2
expression in erythroleukemia cells, a subtype of acute myeloid
leukemia with a very poor response and survival to current
available therapeutic agents (9).
The interest on COX-2 activity in hematological
malignancies has also been reinforced by the use of NSAIDs and
COX-2 selective inhibitors in vitro, to induce proliferation
arrest and apoptosis in leukemia cells (6,10,11).
Furthermore, it was also shown that targeting COX-2 reduced
toxicity toward low-dose chemotherapy with vinblastine and extended
survival in an erythroleukemia model of juvenile mice with Friend
disease (12).
Despite this evident interest of COX-2 modulation in
leukemia cells, literature lacks detailed data on the role of COX-2
in myeloid leukemia onset and management and particularly in cases
of erythroleukemia.
Berberis libanotica (Bl), specifically the
roots, has been used in traditional herbal Lebanese remedies for
rheumatic and neuralgic diseases (13,14).
B. vulgaris and B. stata are the two most studied
species of Berberis (15–18).
Alkaloids constitute the major class of compounds reported to exist
in Berberis species and represent a very wide range of
secondary metabolites with important biological activities
(19,20). Various herbal alkaloids exhibit
in vitro and in vivo anti-proliferative and
anti-metastatic effects on various types of cancers. Alkaloids,
such as camptothecin (21) and
vinblastine (22), have already
been successfully developed into anticancer drugs. Berberine, a
major alkaloid characterizing Berberis species, has been
intensively investigated for its pharmacological properties. It was
shown to inhibit the migration of melanoma cancer cells (23), and the growth of human tongue
squamous carcinoma tumors in a murine xenograft model (24), enhance tumor necrosis
factor-related apoptosis-inducing ligand in breast cancer (25), and exert a cytotoxic effect against
many cell lines (23,26,27).
To date, the biological and phytochemical properties of Bl
extracts have only been investigated in three studies reporting the
inhibition of adult T-cell leukaemia viability via ethanol fraction
(28), the inhibition of key
enzymes linked to Alzheimer’s disease (13), and the anti-neoplastic effects on
prostate cancer stem/progenitor cells (29).
In this overall context, the aim of this study was
to describe and understand the relationship between COX-2
expression and apoptosis rate in erythroleukemia cells after
apoptosis induction by Bl extract. To achieve this goal we
used erythroleukemia cells lines expressing COX-2 (HEL cell line)
or not (K562 cell line). Moreover, we made use of COX-2 cDNA to
overexpress COX-2 in K562 cells. Then, to understand the mechanisms
implicated in the effect of Bl extract, we studied
intracellular signalling pathways.
Materials and methods
Materials
RPMI-1640, fetal calf serum (FCS) and penicillin
streptomycin were supplied by Gibco BRL (Cergy Pontoise, France).
Human antibody against caspase-9 was purchased from Cell Signaling
Technology (Ozyme, France), poly-ADP-ribose polymerase (PARP),
p-Akt antibodies were purchased from Santa Cruz Biotechnology
(Tebu-Bio, Le Perray en Yvelines, France), cyclooxygenase-2 (COX-2)
and β-actin were respectively purchased from Cayman Chemical
(Bertin Pharma, Montigny le Bretonneux, France) and Sigma-Aldrich
(Saint Quentin Fallavier, France).
Berberis libanotica extraction and
HPLC
Berberis libanotica was collected from Ehden,
North of Lebanon, in November 2012 at an altitude up to 1521 m.
Botanical identity was authenticated by Professor S. Safi, Biology
Department, Faculty of Science II, Lebanese University, Fanar,
Lebanon. Powdered root material (10 g) was extracted with ethanol
(100 ml) for 48 h under magnetic stirring at room temperature, then
it was filtered using Whatman paper number 1, and concentrated
using a rotator evaporator. The extraction method used has been
reported (30). The filtred
extract was dried with liquid nitrogen and stored at 4°C.
HPLC was done on a Waters Alliance 2690 using a
reversed-phase C18 X-Terra® 5 μm (150×4.6 mm) and using
a photodiode-array detector (Waters 996). TLC was performed on
pre-coated silica gel aluminium sheets (kieselgel 60 F254, 0.20 mm,
Merck). Visualisation of plates was carried out under UV light (254
and 365 nm) and using Draggendorf reagent. Compounds were
identified by HPLC (RP-18 column,
ACN-H2O-H3PO4, flow rate, 1
ml/min) with CHCL3/MeOH/NH4OH
(80/20/0.5).
Cells lines and cell culture
The HEL and K562 human erythroleukemia cell lines
were kindly provided, respectively, by Professor J.P. Cartron
(INSERM U76, Paris, France) and Dr I. Dusanter-Fourt (INSERM U567,
CNRS UMR 8104, Paris, France). Cells were seeded at 105
cells/ml in 75-cm2 tissue culture flasks and grown in
RPMI-1640 medium (Gibco BRL, Cergy-Pontoise, France) supplemented
with 10% fetal calf serum (Gibco BRL), 1% sodium pyruvate, 1% HEPES
(N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid), 100 U/ml
penicillin and 100 μg/ml streptomycin (Gibco BRL). Cultures were
maintained in a humidified atmosphere with 5% CO2 at
37°C. Cells were allowed to grow for 24 h in culture medium prior
to exposure or not to 40–300 μg/ml Bl extract or 20 and 40
μM berberine (Sigma-Aldrich) for 6–72 h.
COX-2 cDNA transfection
COX-2 transfection-ready cDNA was purchased from
OriGene Technologies (Rockville, MD, USA) and was transfected to
K562 cells by electroporation using the AMAXA Nucleofactor system
(Lonza). Stable K562 cell line was obtained after transfection and
selection with 0.5 mg/ml of neomycin (G418, Sigma-Aldrich). COX-2
expression was confirmed by western blotting.
Cell proliferation and lactate
dehydrogenase (LDH) assays
Measurement of cell proliferation was determined
using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay. HEL, K562 and K562 (COX-2+) cells
were cultured and plated, respectively, at 105 cells/ml
in 10% FCS medium in 96-well culture plates and grown 24 h before
treatment or not (time 0) with 50–300 μg/ml Bl extract for
24–72 h. MTT tests were carried out daily as previously described
(31) and experiments were
performed in three independent assays.
The cytotoxicity of Bl extract on the three
cell lines was determined by measuring the activity of LDH released
from the cytosol of damaged cells using a Cytotoxicity Detection
kit PLUS (LDH) (Roche Diagnostics GmbH). The assay was conducted
following the manufacturer’s instructions. Briefly, medium
supernatants of control and treated cells were collected and equal
volume of reaction mixture was added. The reaction mixtures were
incubated for 20 min at room temperature in the dark. Following
incubation, the absorbance was determined at 490 nm using an ELISA
plate reader. The percentage of cytotoxicity was calculated
according to the manufacturer’s instructions.
Mitochondrial membrane potential
(Δψm)
Δψm was estimated using
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazole
carbocyanide iodide (JC-1, Molecular Probes). JC-1 is a fluorescent
compound that exists as a monomer at low concentrations. At higher
concentrations, it forms aggregates. Fluorescence of the JC-1
monomers is green, whereas that of aggregates is red. Mitochondria
with intact membrane potential concentrate JC-1 into aggregates,
which fluoresces red, whereas de-energized mitochondria cannot
concentrate it and are stained green (31,32).
HEL, K562 and K562 (COX-2+) cells were
grown for 24 h before treatment with 50–300 μg/ml Bl extract
for 6 and 24 h. Control cells were grown in medium containing the
same amount of vehicle as treated cells. Then cells were incubated
in 1 ml of medium containing JC-1 (1 μg/ml) for 30 min at 37°C and
images were taken with a confocal laser microscope (Zeiss LSM 510
Meta).
Protein expression
After treatment, HEL, K562 and K562
(COX-2+) cells were washed and lysed in RIPA lysis
buffer (50 mM HEPES pH 7.5, 150 mM NaCl, 1% deoxycholate, 1% NP-40,
0.1% SDS, 20 μg/ml aprotinin) containing protease inhibitors
(Complete™ Mini, Roche Diagnostics, Meylan, France). Briefly, as
previously described (33),
proteins (10–100 μg) were separated by electrophoresis on
SDS-polyacrylamide gels, transferred to PVDF membranes (Amersham
Pharmacia Biotech, Saclay, France) and probed with respective human
antibodies against caspase-9 (Cell Signaling Technology, Ozyme,
France), poly-ADP-ribose polymerase (PARP), p-Akt (Santa Cruz
Biotechnology) and COX-2 (Cayman Chemical, Bertin Pharma). After
incubation with secondary antibodies (Dako France S.A.S., Trappes,
France), blots were developed using the ECL Plus Western Blotting
Detection system (Amersham Pharmacia Biotech) and G:BOX system
(Syngene, Ozyme, Saint Quentin en Yvelines, France). Membranes were
then reblotted with anti-β-actin (Sigma-Aldrich) used as a loading
control.
Caspase-3 activity
Caspase-3 activity was assayed using
Quantikine® human active caspase-3 (R&D Systems) as
previously described (34). HEL,
K562 and K562 (COX-2+) cells were treated or not with
50–300 μg/ml Bl extract for 24 and 48 h, and then incubated
with 10 μM biotin-ZVKD-fmk inhibitor for 1 h at 37°C. Caspase-3
activity was measured in accordance with the manufacturer’s
protocol (R&D Systems). Briefly, cells were harvested, washed
in PBS and resuspended in extraction buffer containing protease
inhibitors. Standards and sample extracts containing covalently
linked active caspase-3-ZVKD-biotin were added to a microplate
pre-coated with monoclonal antibody specific for caspase-3. Then,
streptavidin conjugated to horseradish peroxidase was added to the
wells. The amount of active caspase-3 was quantified by colorimetry
at 450 nm after addition of HRP substrate.
Apoptosis quantification: DNA
fragmentation
HEL, K562 and K562 (COX-2+) cells were
seeded at 105 cells/ml in 75-cm2 tissue
culture flasks and then treated or not with 50–300 μg/ml Bl
extract for 24 and 48 h, or with 20 and 40 μM berberine for 48 h.
Apoptosis was quantified on pooled cells (floating and adherent)
using ‘cell death’ enzyme-linked immunosorbent assay (ELISA) (Cell
Death Detection ELISAPlus, Roche Diagnostics). Cytosol extracts
were obtained according to the manufacturer’s protocol and
apoptosis was measured as previously described (35).
Subcellular protein fractionation
HEL and K562 (COX-2+) cells were
incubated alone or with 300 μg/ml Bl extract for 12, 24 and
48 h, or with 20 and 40 μM berberine for 48 h. Cytosolic and
nuclear fractions were obtained using the Subcellular Protein
Fractionation kit according to the manufacturer’s protocol (Thermo
Fischer Scientific, Rockford, IL, USA) as previously described
(31).
Electromobility shift assay (EMSA)
EMSA experiments were performed using DIG Gel Shift
kit (Roche Diagnostics) (36).
Briefly, nuclear extracts were prepared from HEL and K562
(COX-2+) cells treated or not with 300 μg/ml Bl
extract for 12, 24 and 48 h, or with 20 and 40 μM berberine for 48
h. NF-κB binding reactions were carried out with 10 μg nuclear
proteins incubated with digoxigenin (DIG) labeled NF-κB probe
according to the manufacturer’s protocol. The samples were loaded
on a 5% native polyacrylamide gel in Tris-borate-EDTA buffer. After
transfer to nylon membranes and incubation with anti-DIG antibody
conjugated with alkaline phosphatase, gel mobility shift was
visualized by incubation with CSPD® chemiluminescence
reagent and G:BOX system (Syngene). Quantification of each band was
performed by densitometry analysis software in respect of band
intensity and band area. Results are expressed relative to controls
in arbitrary units.
Statistical analysis
Data are expressed as the arithmetic means ±
standard deviation (SD) of separate experiments. The statistical
significance of results obtained from in vitro studies was
evaluated by the two tailed unpaired Student’s t-test, with
p<0.05 being considered as statistically significant.
Results and Discussion
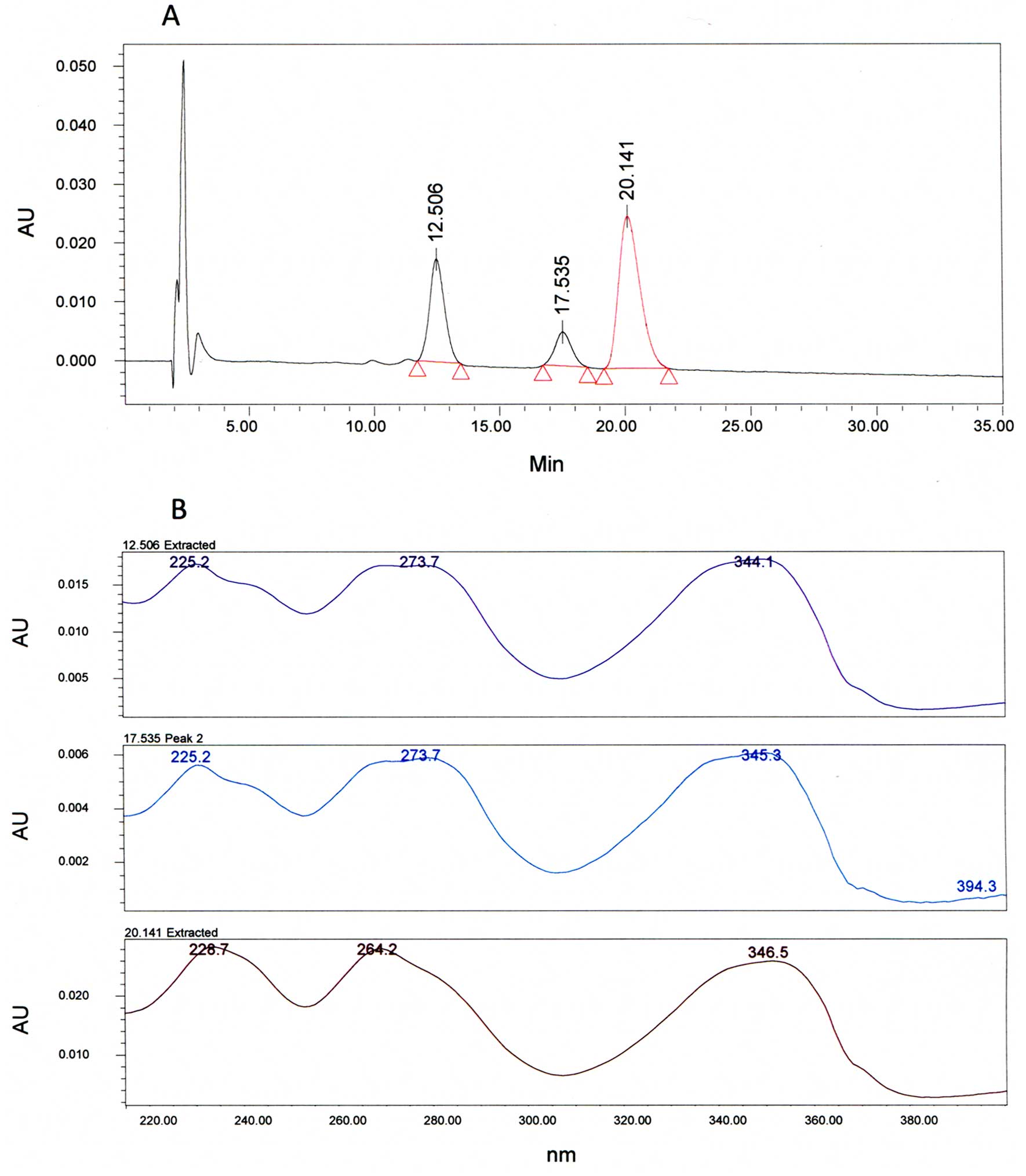
The HPLC chromatogram show three main peaks at
tR = 12.5, 17.5 and 20.1 min (Fig. 1A). By comparing the control, we
found that the peak with a tR = 20 min matches to
berberine. The two other peaks matched with alkaloids derived from
berberine regarding close similarities between their UV spectrum
and the UV spectrum of the control (Fig. 1B).
In light of the reported chemopreventive and
chemosensitive effects of natural products on various tumor cells
and animal models, we postulated that our Bl extract may
mediate its effects through apoptosis induction with suppression of
cell survival pathways. Our study is the first report on the
specific examination of intrinsic apoptosis and Akt/NF-κB/COX-2
pathways in human erythroleukemia cells upon Bl extract
exposure.
In this study, we aimed to establish cellular models
of erythroleukemia cells in which we could study the effect of
COX-2 expression. As COX-2 has been widely implicated in apoptosis
resistance in various cancer cells (4,37–39)
including leukemia cells (6–8,40),
we further checked for the first time whether COX-2 expression
could modulate apoptosis induction by Bl extract in
erythroleukemia cells.
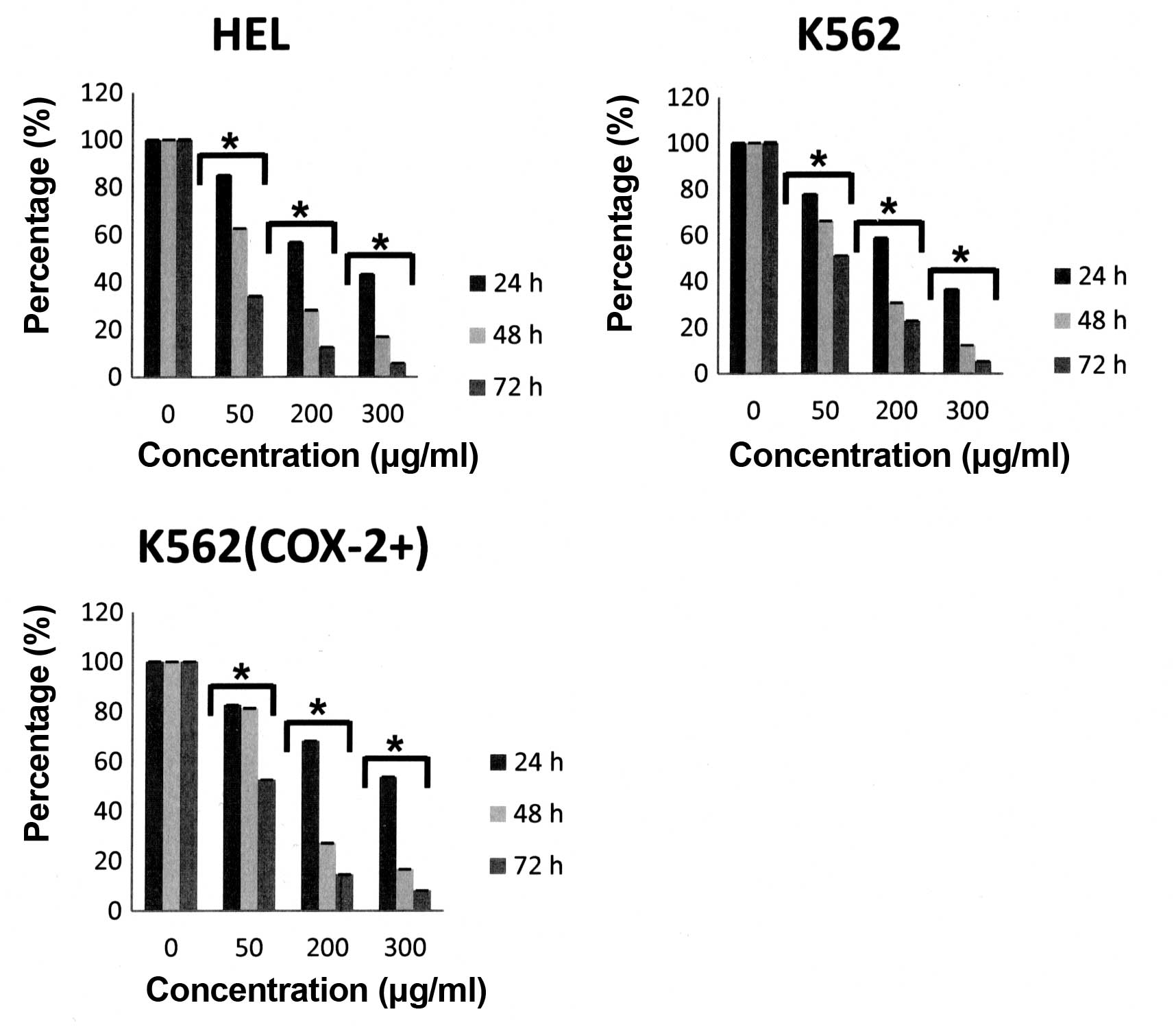
We first demonstrated that, under our experimental
conditions, a decrease in proliferation was observed as early as 24
h after Bl extract treatment in a dose- and time-dependent
manner for the three cell lines (Fig.
2) without significative difference between K562 and K562
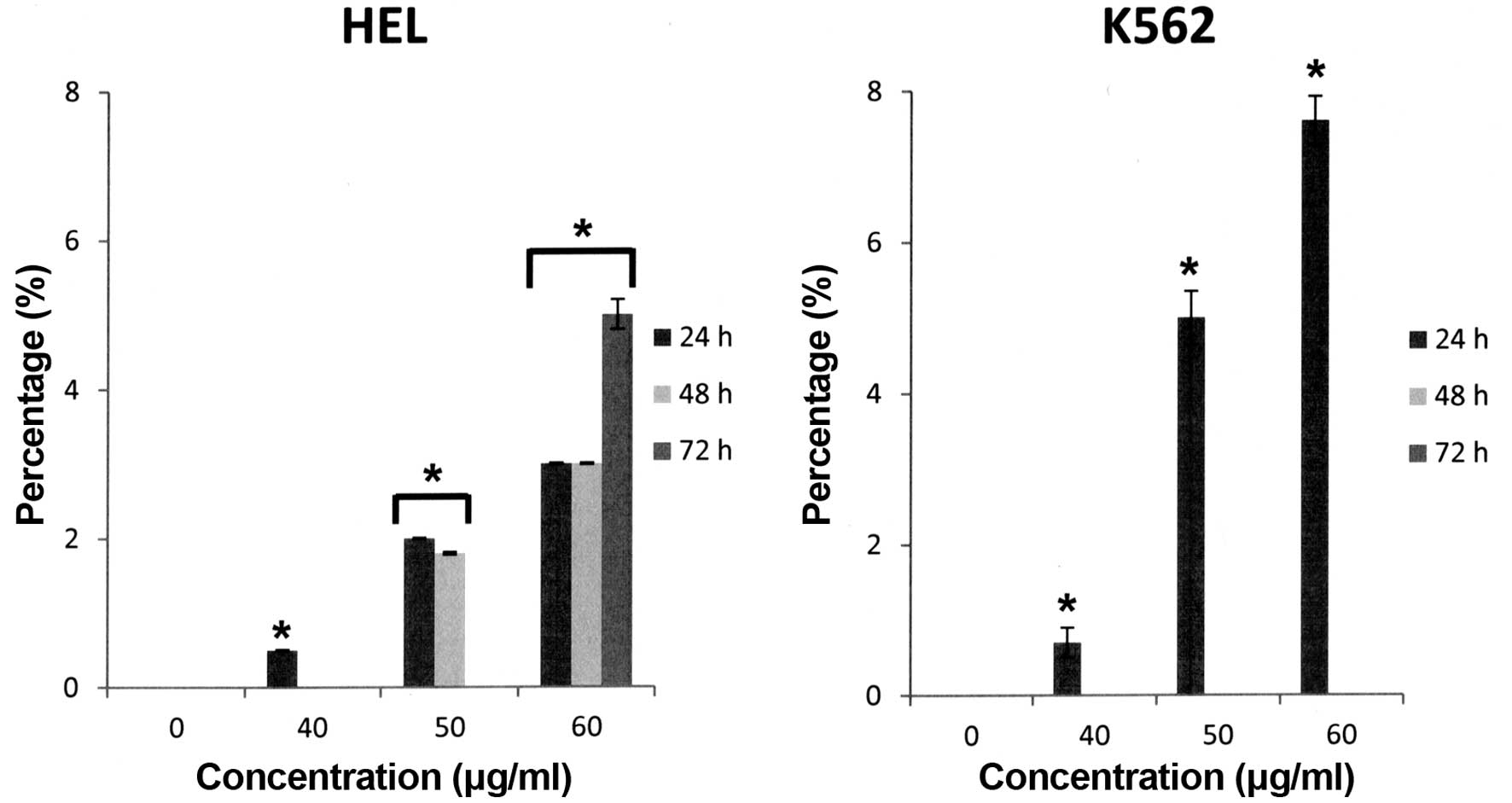
(COX-2+) cells. Furthermore, Bl extract
engendered only a very low cytotoxicity on HEL cells (5.2% at 60
μg/ml for 72 h versus control, p<0.05) and K562 cells (7.8% at
60 μg/ml for 72 h versus control, p<0.05) (Fig. 3).
Apoptosis is characterized by chromatin condensation
and DNA fragmentation, and is mediated by caspases (41). Mitochondria are involved in a
variety of key events, including release of caspase activators,
changes in electron transport, loss of mitochondrial membrane
potential (Δψm), and participation of both pro- and anti-apoptotic
Bcl-2 family proteins (42).
Alterations in mitochondrial structure and function have been shown
to play a crucial role in caspase-9-dependent apoptosis (43). Caspase-9 cleaves and activates
caspase-3, the executioner caspase, which cleaves PARP and
activates endonucleases leading to DNA fragmentation (43). Mitochondria have, apart from their
function in respiration, an important role in the
apoptotic-signalling pathway. It is well known that the
modification of Δψm depends on the nature of the stimulus and the
cell system and the collapse of Δψm is an early step in the
apoptotic cascade (44). To
determine potential mechanisms by which Bl extract inhibited
the human erythroleukemia cell proliferation, we analyzed the
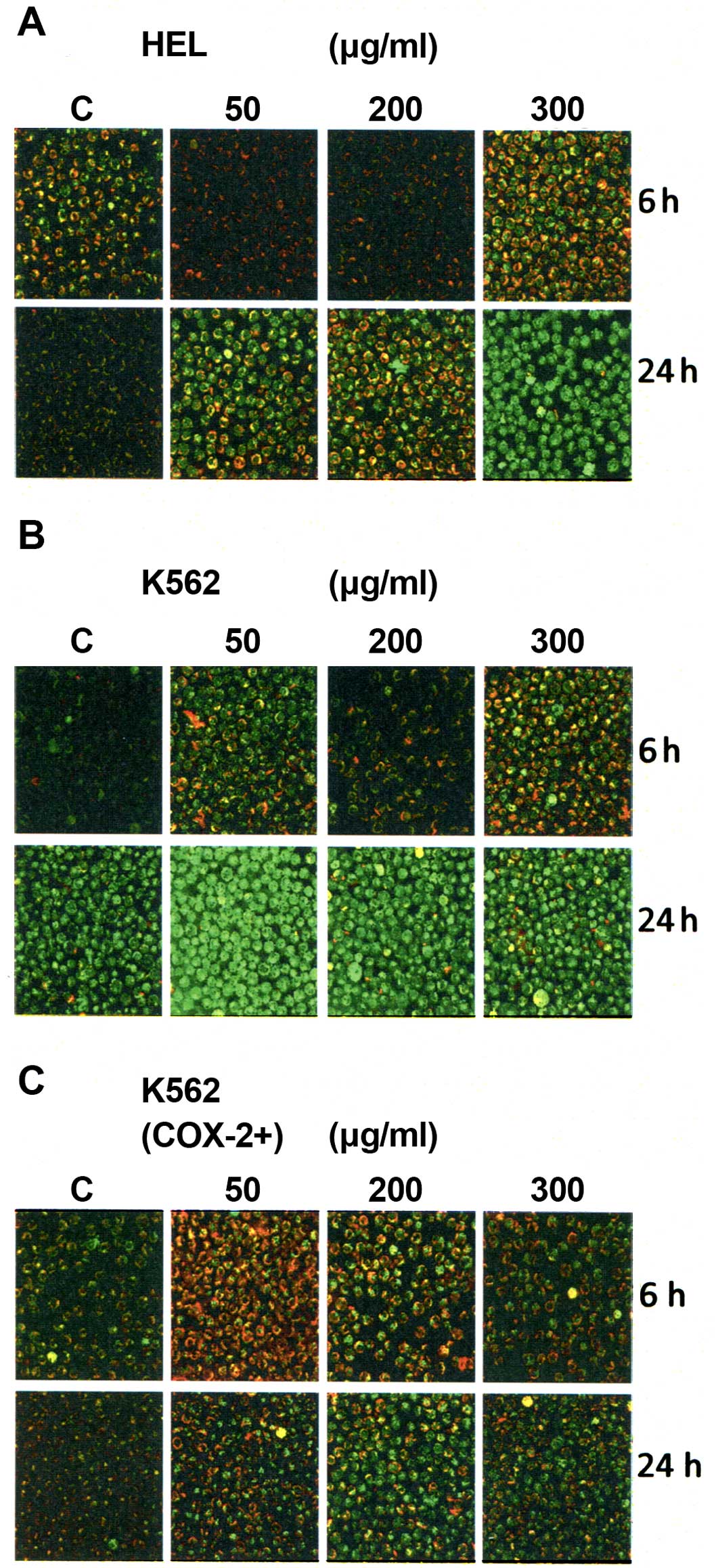
effect of Bl extract on Δψm. Δψm was analyzed after 6- and
24-h treatment with Bl extract using JC-1. We found that
Bl extract decreased Δψm in a dose- and time-dependent
manner for the three cell lines (Fig.
4), shown by the incorporation of JC-1 monomers into
mitochondria (green fluorescence), compared with cytosolic JC-1
aggregate formation at high membrane potentials in control cells
(red fluorescence). Furthermore, this assay showed a dominant
effect of 50 μg/ml Bl extract on early intrinsic apoptosis
of K562 cells for 24-h treatment (Fig.
4B) compared to K562 (COX-2+) cells (Fig. 4C).
Caspase-3 is a key executioner of apoptosis, its
activation is mediated by the initiator caspases such as caspase-8
and caspase-9 (45). In our study,
we showed that intrinsic apoptosis pathway was implicated in
Bl extract-induced apoptosis in human erythroleukemia cells.
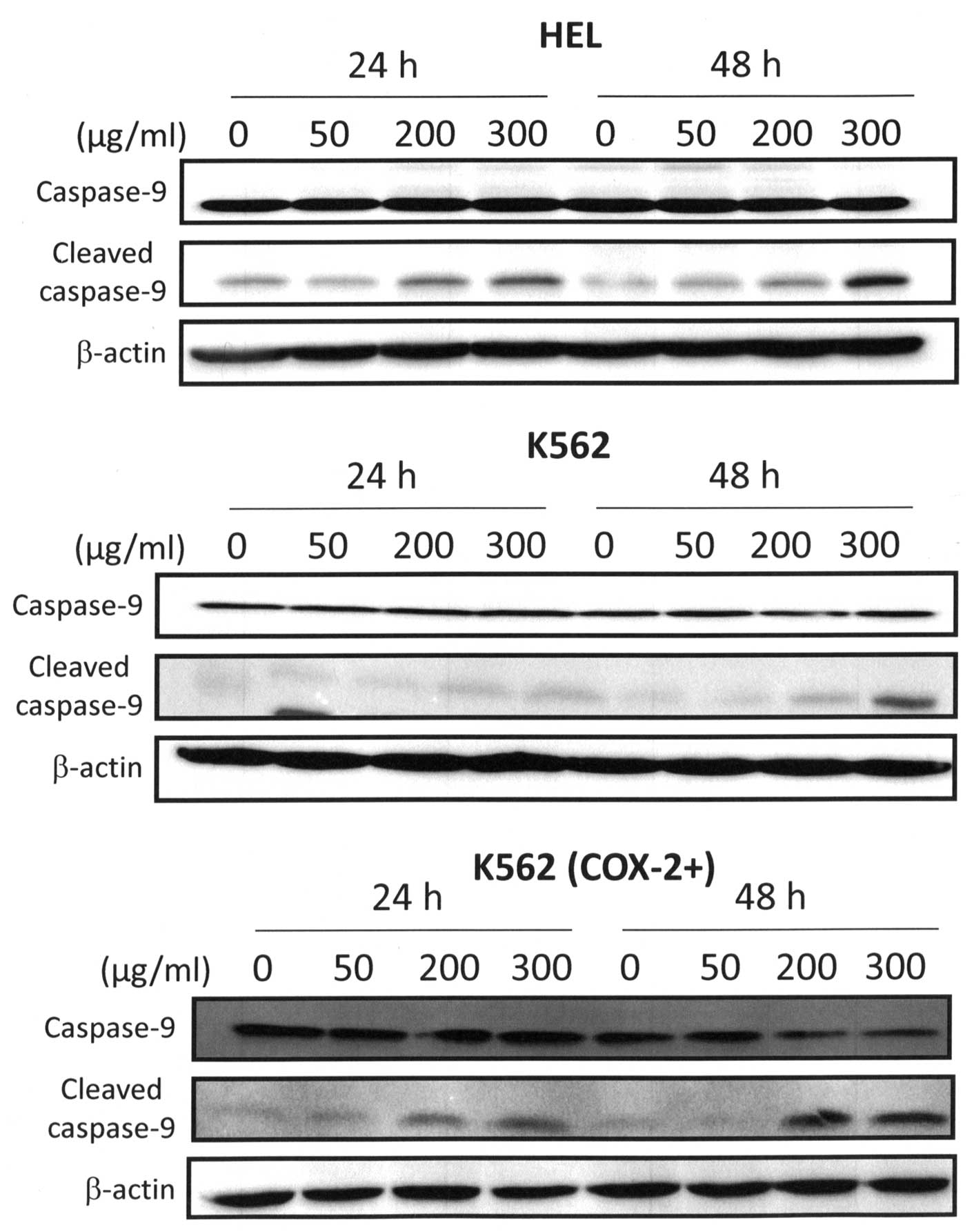
Indeed, we showed that Bl extract induced an activation of
caspase-9 especially at 300 μg/ml after 48-h treatment as shown in
Fig. 5. Bl extract induced
a cleavage of caspase-9 at 48 h but the expression of cleaved
fragment of caspase-9 after 300 μg/ml Bl extract treatment
for HEL and K562 (COX-2+) cells was more important than
in K562 cells. Consequently, 300 μg/ml Bl extract induced
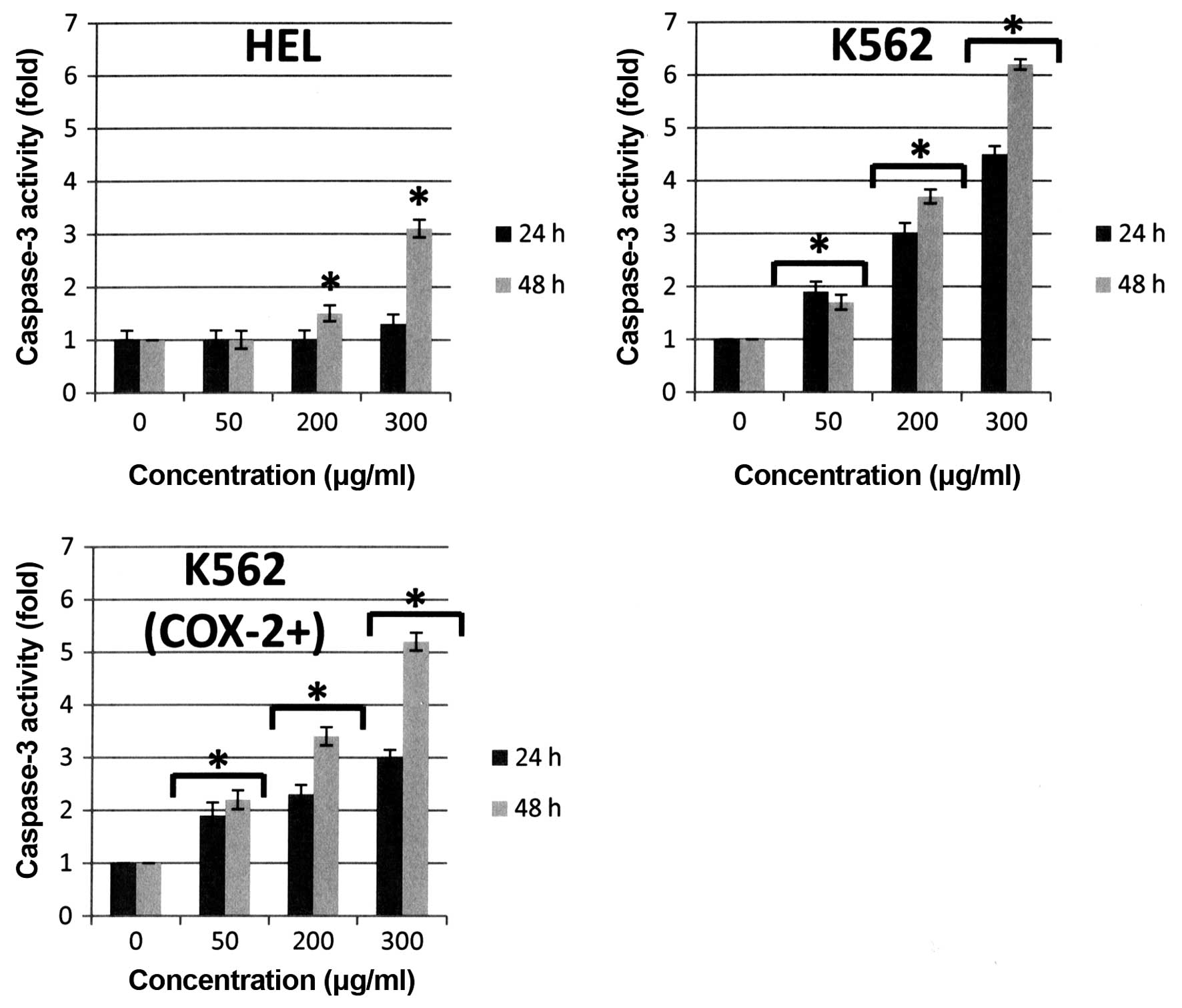
activation of executive caspase-3 activity in the three cell lines
(+3-fold versus control at 48 h for HEL cells, +6-fold versus
control at 48 h for K562 cells, and +5-fold versus control at 48 h
for K562 (COX-2+) cells, p<0.05) (Fig. 6). These observations were directly
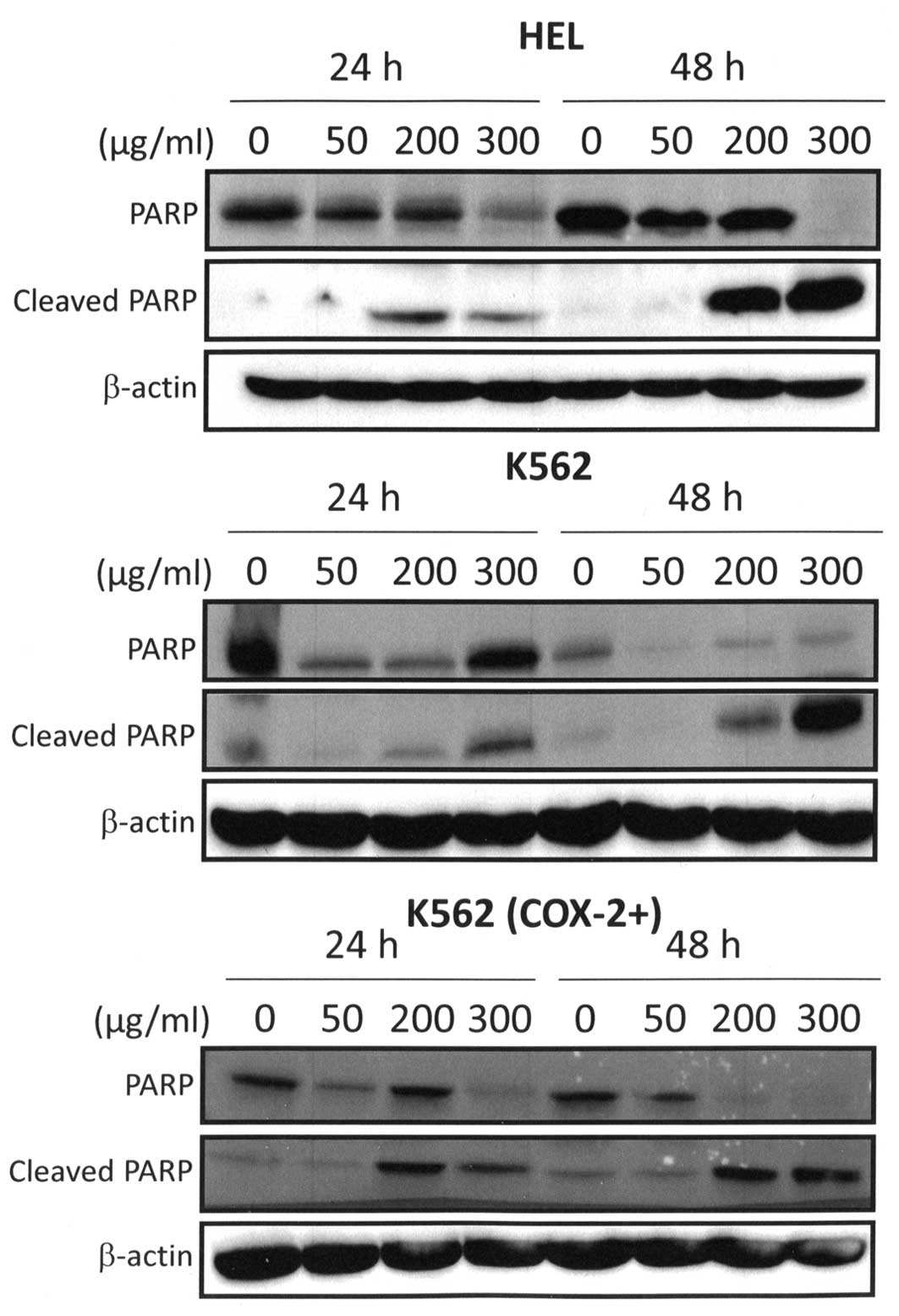
correlated with PARP cleavage because western blot analysis
detected the cleaved form of PARP in 300 μg/ml Bl extract
treated cells (Fig. 7). Cleaved
fragment of PARP was expressed starting at 24 h for 300 μg/ml
Bl extract treatment and strongly maintained after 48-h
treatment (Fig. 7). PARP is a
nuclear enzyme involved in the repair of DNA damage (46). Moreover, it is known that PARP is a
substrate for caspases such as caspase-3 and is typically cleaved
and inactivated during the apoptotic process (47).
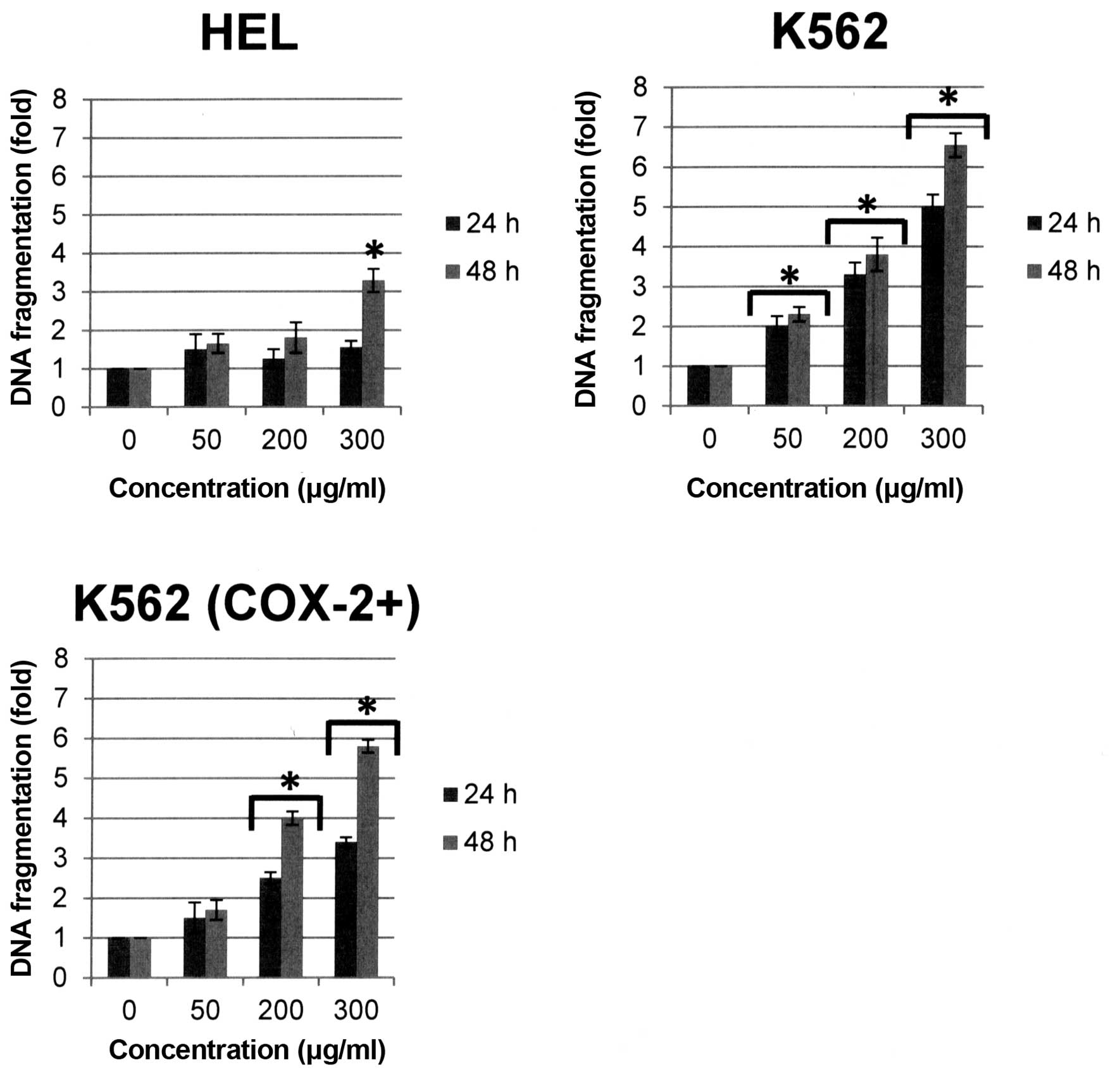
DNA fragmentation occurs simultaneously with this
phenomenon and is considered as a major marker of apoptotic cells.
DNA fragmentation was observed in human erythroleukemia cells after
Bl extract treatment. Quantitative determination of
cytoplasmic histone-associated DNA fragments (mono and
oligonucleosomes) was performed by ELISA in our study. Results
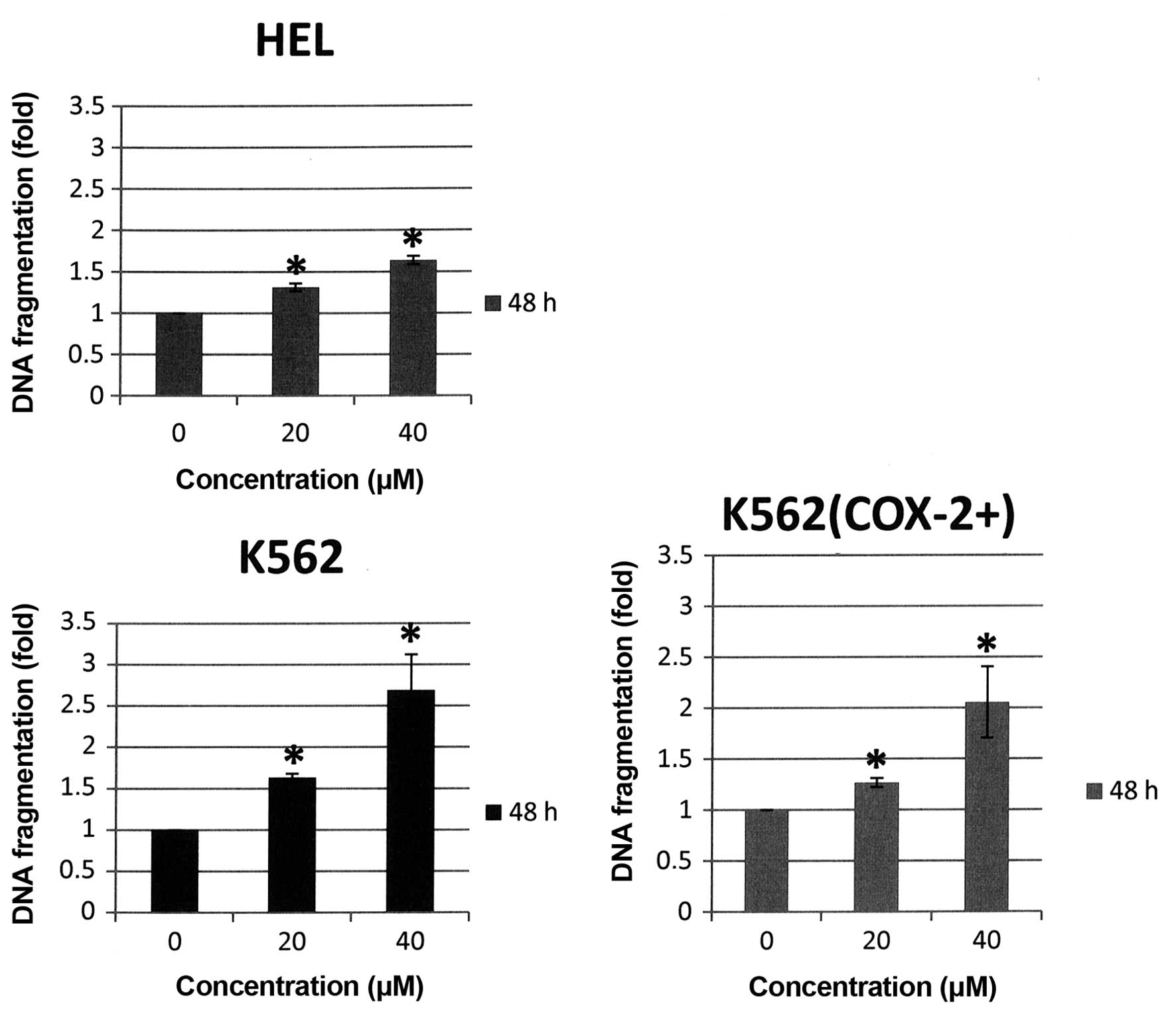
showed that DNA fragmentation was strongly induced in the three
cell lines after 48-h Bl extract treatment [+3.3-, +6.5 and
+5.8-fold versus control respectively for HEL, K562 and K562
(COX-2+), p<0.05] (Fig.
8). It is important to note that DNA fragmentation is the
strongest in K562 cells not expressing COX-2.
In summary, even if Bl extract induced
apoptosis of three human erythroleukemia cell lines, a dominant
effect of Bl extract treatment on K562 cells was observed
resulting in activation of the late markers of apoptosis with
caspase-3 activation, PARP cleavage and DNA fragmentation.
It is well known that COX-2 expression is correlated
with the activities of intracellular signalling proteins such as
NF-κB (48). Furthermore, we
showed recently that COX-2 positively regulated Akt signalling and
enhanced survival of cancer cells exposed to anticancer agents
(35). Numerous studies have shown
that COX-2 expression prevents apoptosis in cancer cells,
especially in colon (49,50) and prostate cancer (51–53).
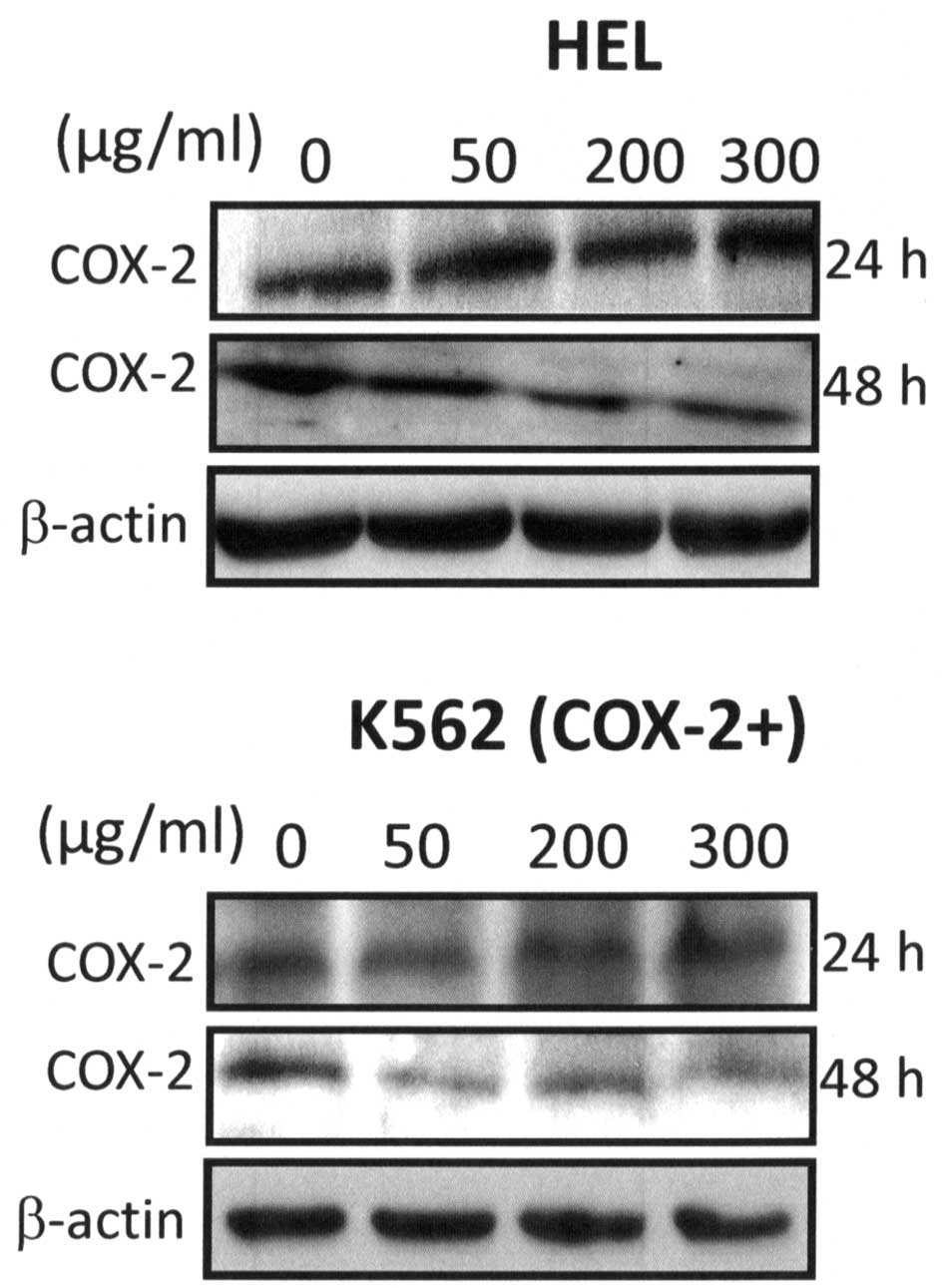
Here, we showed that Bl extract reduced significantly
expression of COX-2 by a dose-dependent manner at 48-h treatment in
HEL and K562 (COX-2+) cells (Fig. 9).
Among the cell signalling pathways that promote cell
survival, Akt is one of the most important (54). Activated Akt can also exert
anti-apoptotic effects, positively regulate NF-κB transcription,
modulate angiogenesis, promote tumor invasion/metastasis and
antagonize cell cycle arrest (55). Akt is also reported to modulate the
NF-κB transcription factor through the phosphorylation of p65 to
enhance the transcriptional activity of NF-κB (56). In turn, NF-κB activation can
regulate the expression of cell survival, proliferative, metastatic
and angiogenic gene products (57). We analyzed the effect of Bl
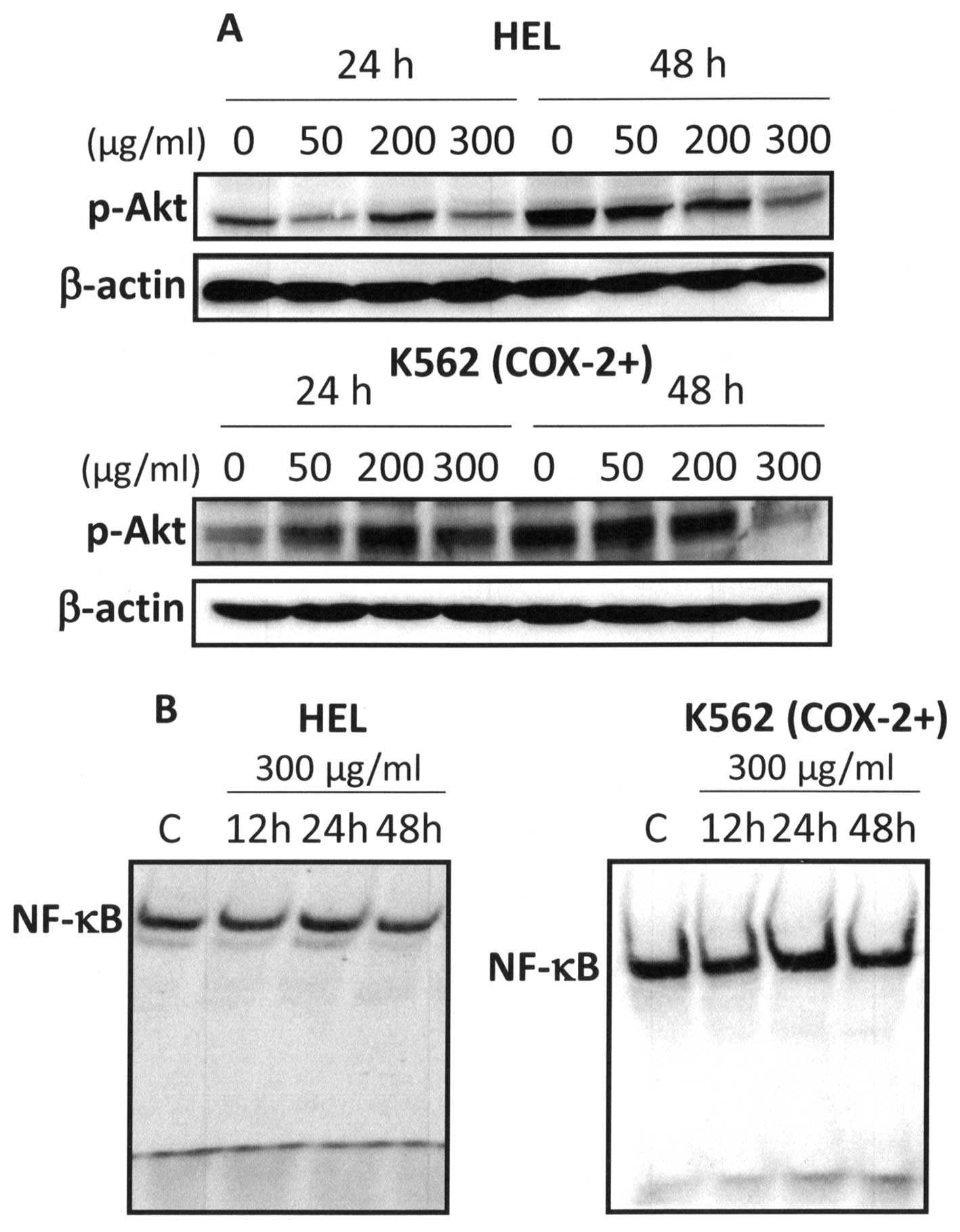
extract on two survival pathways: Akt and NF-κB. Western blot
analysis showed that 300 μg/ml Bl extract markedly inhibited
Akt phosphorylation in HEL and K562 (COX-2+) cells at
48-h treatment (Fig. 10A). Since
NF-κB activation is critical for apoptosis resistance, we examined
the effect of Bl extract on nuclear activation of NF-κB. Our
results showed that 300 μg/ml Bl extract inhibited NF-κB
activation at 12- and 48-h treatment (Fig. 10B). In regard to these results, it
is clear that the simultaneous inhibition of Akt and NF-κB
signalling can significantly contribute to the anticancer effects
of Bl extract in human erythroleukemia cells.
The results of Bl extract HPLC profile showed
that berberine is the major product (Fig. 1), thus, we then tested this
molecule on the induction of DNA fragmentation, the expression of
COX-2 and phospho-Akt (p-Akt) and the activation of NF-κB. From the
report of Bonesi et al giving the chemical composition of
Bl extract, 300 μg/ml of our Bl extract would
correspond to 40 μM of pure berberine (13). Based on these calculations, we then
tested berberine in 20 and 40 μM on human erythroleukemia cells.
Our results showed that especially 40 μM berberine induced DNA
fragmentation of three cell lines [+1.6-fold for HEL cells,
+2.7-fold for K562 cells and +2-fold for K562 (COX-2+)
versus control, p<0.05] (Fig.
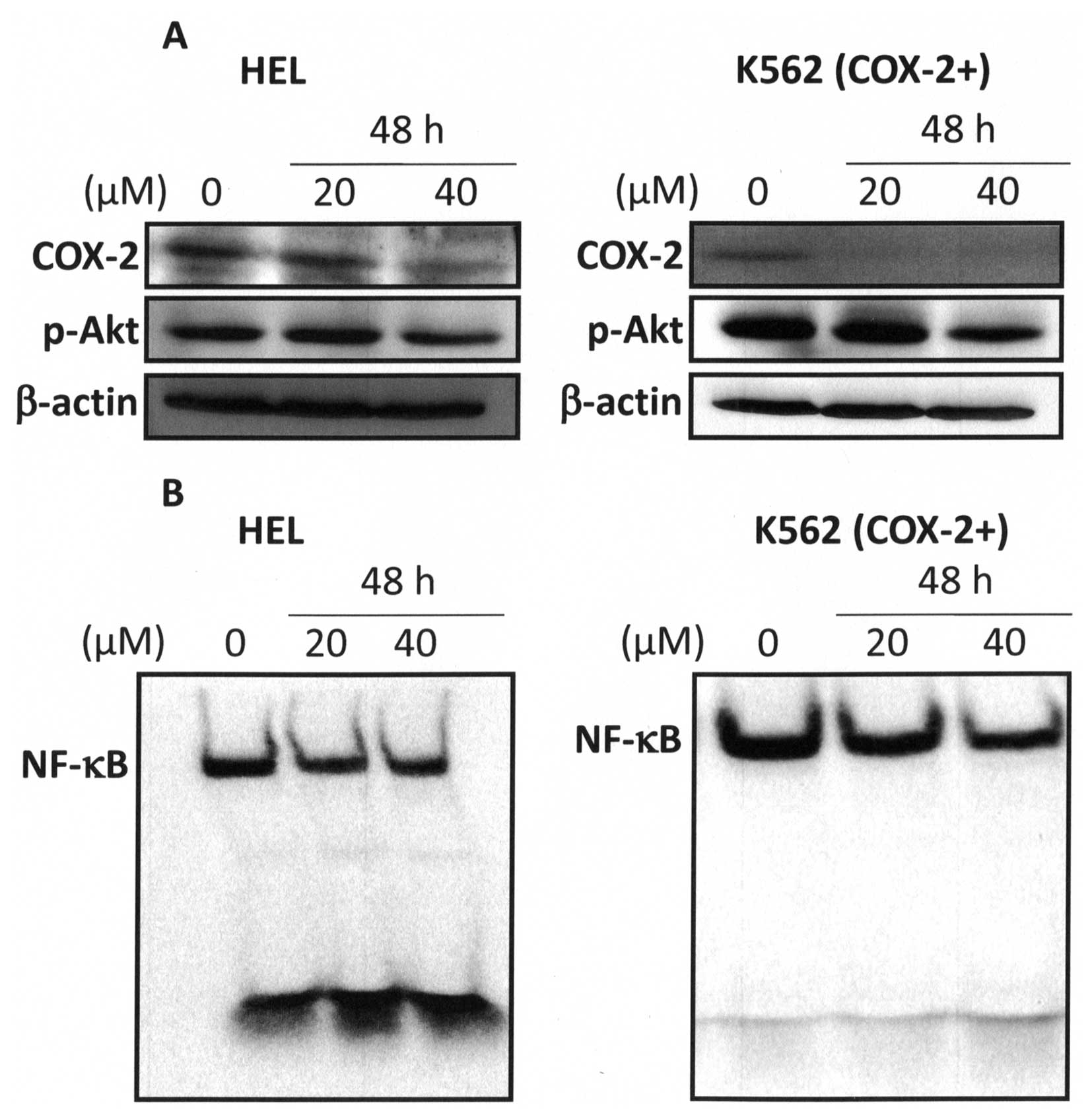
11). Whereas, western blot analysis showed that 40 μM berberine
markedly inhibited COX-2 expression and Akt phosphorylation in HEL
and K562 (COX-2+) cells at 48-h treatment (Fig. 12A). Furthermore, our results
showed that 40 μM berberine inhibited NF-κB activation at 48-h
treatment (Fig. 12B). Recently,
Fu et al demonstrated that berberine inhibited human
non-small cell lung cancer cell growth by simultaneously targeting
NF-κB/COX-2, PI3K/Akt and caspase signalling pathways (58). Furthermore, others studies showed
an antitumor effect of berberine via inhibition of NK-κB pathway
and induction of apoptosis (59,60).
Our results clearly indicate for the first time that
Bl extract exert their potent anti-proliferative and
pro-apoptotic effects through the modulation of Akt/NF-κB/COX-2
signal transduction pathways in human erythroleukemia cells and do
not act specifically on any one cellular signalling cascade. It is
obvious that Bl extract is not active against a specific
signalling cascade but it can interfere with a multitude of targets
in human erythroleukemia cells. This is quite relevant to the
changing paradigm in cancer therapy, as increasing evidence
indicates that the mono-targeted drugs, once called smart drugs,
have not had a significant impact on cancer treatment and the use
of multi-targeted drugs has become increasingly accepted, as it is
obvious that cancer is caused by dysregulation of multiple pathways
(61). Our results show that
Bl extract has a dominant effect on K562 cells do not
expressing COX-2 compared to HEL and K562 (COX-2+)
cells. Furthermore, the significance of our in vitro study
between Bl extract and berberine effects in human
erythroleukemia cells is very encouraging suggesting the relevance
of testing these compounds in xenograft animal models.
Acknowledgements
We thank Claire CARRION (UMR CNRS 7276, Platform
CIM) for the analyses of imaging. This study was supported by
grants from the French Ministry of Education and Research and from
the Lebanese University.
References
|
1
|
Smith WL, DeWitt DL and Garavito RM:
Cyclooxygenases: Structural, cellular, and molecular biology. Annu
Rev Biochem. 69:145–182. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang D and Dubois RN: Eicosanoids and
cancer. Nat Rev Cancer. 10:181–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao Y and Prescott SM: Many actions of
cyclooxygenase-2 in cellular dynamics and in cancer. J Cell
Physiol. 190:279–286. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zha S, Yegnasubramanian V, Nelson WG,
Isaacs WB and De Marzo AM: Cyclooxygenases in cancer: Progress and
perspective. Cancer Lett. 215:1–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Subbaramaiah K and Dannenberg AJ:
Cyclooxygenase 2: A molecular target for cancer prevention and
treatment. Trends Pharmacol Sci. 24:96–102. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bernard MP, Bancos S, Sime PJ and Phipps
RP: Targeting cyclooxygenase-2 in hematological malignancies:
Rationale and promise. Curr Pharm Des. 14:2051–2060. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chien MH, Ku CC, Johansson G, Chen MW,
Hsiao M, Su JL, Inoue H, Hua KT, Wei LH and Kuo ML: Vascular
endothelial growth factor-C (VEGF-C) promotes angiogenesis by
induction of COX-2 in leukemic cells via the VEGF-R3/JNK/AP-1
pathway. Carcinogenesis. 30:2005–2013. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng J, Chen S, Jiang L, You Y, Wu D and
Zhou Y: Functional genetic variations of cyclooxygenase-2 and
susceptibility to acute myeloid leukemia in a Chinese population.
Eur J Haematol. 87:486–493. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Santos FP, Bueso-Ramos CE and Ravandi F:
Acute erythroleukemia: Diagnosis and management. Expert Rev
Hematol. 3:705–718. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakanishi Y, Kamijo R, Takizawa K, Hatori
M and Nagumo M: Inhibitors of cyclooxygenase-2 (COX-2) suppressed
the proliferation and differentiation of human leukaemia cell
lines. Eur J Cancer. 37:1570–1578. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Subhashini J, Mahipal SV and Reddanna P:
Anti-proliferative and apoptotic effects of celecoxib on human
chronic myeloid leukemia in vitro. Cancer Lett. 224:31–43. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cervi D, Klement G, Stempak D, Baruchel S,
Koki A and Ben-David Y: Targeting cyclooxygenase-2 reduces overt
toxicity toward low-dose vinblastine and extends survival of
juvenile mice with Friend disease. Clin Cancer Res. 11:712–719.
2005.PubMed/NCBI
|
|
13
|
Bonesi M, Loizzo MR, Conforti F,
Passalacqua NG, Saab A, Menichini F and Tundis R: Berberis
aetnensis and B. libanotica: A comparative study on the chemical
composition, inhibitory effect on key enzymes linked to Alzheimer’s
disease and antioxidant activity. J Pharm Pharmacol. 65:1726–1735.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
El Beyrouthy M, Arnold N,
Delelis-Dusollier A and Dupont F: Plants used as remedies
antirheumatic and antineuralgic in the traditional medicine of
Lebanon. J Ethnopharmacol. 120:315–334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Imanshahidi M and Hosseinzadeh H:
Pharmacological and therapeutic effects of Berberis vulgaris and
its active constituent, berberine. Phytother Res. 22:999–1012.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zovko Koncić M, Kremer D, Karlović K and
Kosalec I: Evaluation of antioxidant activities and phenolic
content of Berberis vulgaris L. and Berberis croatica Horvat. Food
Chem Toxicol. 48:2176–2180. 2010. View Article : Google Scholar
|
|
17
|
Kim S, Choi JH, Kim JB, Nam SJ, Yang JH,
Kim JH and Lee JE: Berberine suppresses TNF-alpha-induced MMP-9 and
cell invasion through inhibition of AP-1 activity in MDA-MB-231
human breast cancer cells. Molecules. 13:2975–2985. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh J and Kakkar P: Antihyperglycemic
and antioxidant effect of Berberis aristata root extract and its
role in regulating carbohydrate metabolism in diabetic rats. J
Ethnopharmacol. 123:22–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roberts MF and Wink M: Alkaloids:
Biochemistry, Ecology, and Medicinal applications. Springer; New
York, London: 1998, View Article : Google Scholar
|
|
20
|
Aniszewski T: Alkaloids-Secrets of Life:
Alkaloid Chemistry, Biological Significance, Applications and
Ecological Role. Elsevier; Amsterdam, Oxford: 2007
|
|
21
|
Huang M, Gao H, Chen Y, Zhu H, Cai Y,
Zhang X, Miao Z, Jiang H, Zhang J, Shen H, et al: Chimmitecan, a
novel 9-substituted camptothecin, with improved anticancer
pharmacologic profiles in vitro and in vivo. Clin Cancer Res.
13:1298–1307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li W, Shao Y, Hu L, Zhang X, Chen Y, Tong
L, Li C, Shen X and Ding J: BM6, a new semi-synthetic vinca
alkaloid, exhibits its potent in vivo anti-tumor activities via its
high binding affinity for tubulin and improved pharmacokinetic
profiles. Cancer Biol Ther. 6:787–794. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh T, Vaid M, Katiyar N, Sharma S and
Katiyar SK: Berberine, an isoquinoline alkaloid, inhibits melanoma
cancer cell migration by reducing the expressions of
cyclooxygenase-2, prostaglandin E2 and prostaglandin
E2 receptors. Carcinogenesis. 32:86–92. 2011. View Article : Google Scholar
|
|
24
|
Ho YT, Yang JS, Lu CC, Chiang JH, Li TC,
Lin JJ, Lai KC, Liao CL, Lin JG and Chung JG: Berberine inhibits
human tongue squamous carcinoma cancer tumor growth in a murine
xenograft model. Phytomedicine. 16:887–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Refaat A, Abdelhamed S, Yagita H, Inoue H,
Yokoyama S, Hayakawa Y and Saiki I: Berberine enhances tumor
necrosis factor-related apoptosis-inducing ligand-mediated
apoptosis in breast cancer. Oncol Lett. 6:840–844. 2013.PubMed/NCBI
|
|
26
|
Liu B, Wang G, Yang J, Pan X, Yang Z and
Zang L: Berberine inhibits human hepatoma cell invasion without
cytotoxicity in healthy hepatocytes. PLoS One. 6:e214162011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng PL, Hsieh YS, Wang CJ, Hsu JL and
Chou FP: Inhibitory effect of berberine on the invasion of human
lung cancer cells via decreased productions of
urokinase-plasminogen activator and matrix metalloproteinase-2.
Toxicol Appl Pharmacol. 214:8–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Esseily F, El-Ezzy M, Gali-Muhtasib H,
Safi S, Esseily J, Diab-Assaf M, Lampronti I and Saab A: The
ethanol fraction from the stem of Berberis libanotica inhibits the
viability of adult T cell leukemia. Minerva Biotecnol. 24:129–133.
2012.
|
|
29
|
El-Merahbi R, Liu YN, Eid A, Daoud G,
Hosry L, Monzer A, Mouhieddine TH, Hamade A, Najjar F and
Abou-Kheir W: Berberis libanotica Ehrenb extract shows
anti-neoplastic effects on prostate cancer stem/progenitor cells.
PLoS One. 9:e1124532014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gritsanapan W and Mangmeesri P:
Standardized Senna alata leaf extract. J Health Res. 23:59–64.
2009.
|
|
31
|
Corbiere C, Liagre B, Terro F and
Beneytout JL: Induction of antiproliferative effect by diosgenin
through activation of p53, release of apoptosis-inducing factor
(AIF) and modulation of caspase-3 activity in different human
cancer cells. Cell Res. 14:188–196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Smiley ST, Reers M, Mottola-Hartshorn C,
Lin M, Chen A, Smith TW, Steele GD Jr and Chen LB: Intracellular
heterogeneity in mitochondrial membrane potentials revealed by a
J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci USA.
88:3671–3675. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lepage C, Léger DY, Bertrand J, Martin F,
Beneytout JL and Liagre B: Diosgenin induces death receptor-5
through activation of p38 pathway and promotes TRAIL-induced
apoptosis in colon cancer cells. Cancer Lett. 301:193–202. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Leger DY, Liagre B and Beneytout JL: Role
of MAPKs and NF-kappaB in diosgenin-induced megakaryocytic
differentiation and subsequent apoptosis in HEL cells. Int J Oncol.
28:201–207. 2006.
|
|
35
|
Bertrand J, Liagre B, Ghezali L, Beneytout
JL and Leger DY: Cyclooxygenase-2 positively regulates Akt
signalling and enhances survival of erythroleukemia cells exposed
to anticancer agents. Apoptosis. 18:836–850. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ghezali L, Leger DY, Limami Y, Cook-Moreau
J, Beneytout JL and Liagre B: Cyclopamine and jervine induce COX-2
overexpression in human erythroleukemia cells but only cyclopamine
has a pro-apoptotic effect. Exp Cell Res. 319:1043–1053. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsujii M and DuBois RN: Alterations in
cellular adhesion and apoptosis in epithelial cells overexpressing
prostaglandin endoperoxide synthase 2. Cell. 83:493–501. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dannenberg AJ, Altorki NK, Boyle JO, Dang
C, Howe LR, Weksler BB and Subbaramaiah K: Cyclo-oxygenase 2: A
pharmacological target for the prevention of cancer. Lancet Oncol.
2:544–551. 2001. View Article : Google Scholar
|
|
39
|
Fürstenberger G, Krieg P, Müller-Decker K
and Habenicht AJ: What are cyclooxygenases and lipoxygenases doing
in the driver’s seat of carcinogenesis? Int J Cancer.
119:2247–2254. 2006. View Article : Google Scholar
|
|
40
|
Ryan EP, Pollock SJ, Kaur K, Felgar RE,
Bernstein SH, Chiorazzi N and Phipps RP: Constitutive and
activation-inducible cyclooxygenase-2 expression enhances survival
of chronic lymphocytic leukemia B cells. Clin Immunol. 120:76–90.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zamzami N, Susin SA, Marchetti P, Hirsch
T, Gómez-Monterrey I, Castedo M and Kroemer G: Mitochondrial
control of nuclear apoptosis. J Exp Med. 183:1533–1544. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Green D and Kroemer G: The central
executioners of apoptosis: Caspases or mitochondria? Trends Cell
Biol. 8:267–271. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu X, Kim CN, Yang J, Jemmerson R and
Wang X: Induction of apoptotic program in cell-free extracts:
Requirement for dATP and cytochrome c. Cell. 86:147–157. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Salvesen GS and Dixit VM: Caspases:
Intracellular signaling by proteolysis. Cell. 91:443–446. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
D’Amours D, Desnoyers S, D’Silva I and
Poirier GG: Poly(ADP-ribosyl)ation reactions in the regulation of
nuclear functions. Biochem J. 342:249–268. 1999. View Article : Google Scholar
|
|
47
|
Nicholson DW: Caspase structure,
proteolytic substrates, and function during apoptotic cell death.
Cell Death Differ. 6:1028–1042. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Giri DK and Aggarwal BB: Constitutive
activation of NF-kappaB causes resistance to apoptosis in human
cutaneous T cell lymphoma HuT-78 cells. Autocrine role of tumor
necrosis factor and reactive oxygen intermediates. J Biol Chem.
273:14008–14014. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang D and Dubois RN: The role of COX-2 in
intestinal inflammation and colorectal cancer. Oncogene.
29:781–788. 2010. View Article : Google Scholar
|
|
50
|
Limami Y, Pinon A, Leger DY, Mousseau Y,
Cook-Moreau J, Beneytout JL, Delage C, Liagre B and Simon A: HT-29
colorectal cancer cells undergoing apoptosis overexpress COX-2 to
delay ursolic acid-induced cell death. Biochimie. 93:749–757. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kirschenbaum A, Liu X, Yao S and Levine
AC: The role of cyclo-oxygenase-2 in prostate cancer. Urology.
58(Suppl 1): 127–131. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee KS, Lee HJ, Ahn KS and Kim SH, Nam D,
Kim DK, Choi DY, Ahn KS, Lu J and Kim SH:
Cyclooxygenase-2/prostaglandin E2 pathway mediates icariside II
induced apoptosis in human PC-3 prostate cancer cells. Cancer Lett.
280:93–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Limami Y, Pinon A, Leger DY, Pinault E,
Delage C, Beneytout JL, Simon A and Liagre B: The
P2Y2/Src/p38/COX-2 pathway is involved in the resistance to ursolic
acid-induced apoptosis in colorectal and prostate cancer cells.
Biochimie. 94:1754–1763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen X, Thakkar H, Tyan F, Gim S, Robinson
H, Lee C, Pandey SK, Nwokorie C, Onwudiwe N and Srivastava RK:
Constitutively active Akt is an important regulator of TRAIL
sensitivity in prostate cancer. Oncogene. 20:6073–6083. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Testa JR and Bellacosa A: AKT plays a
central role in tumorigenesis. Proc Natl Acad Sci USA.
98:10983–10985. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Madrid LV, Mayo MW, Reuther JY and Baldwin
AS Jr: Akt stimulates the transactivation potential of the RelA/p65
Subunit of NF-kappa B through utilization of the Ikappa B kinase
and activation of the mitogen-activated protein kinase p38. J Biol
Chem. 276:18934–18940. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ahn KS, Sethi G and Aggarwal BB: Nuclear
factor-kappa B: From clone to clinic. Curr Mol Med. 7:619–637.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Fu L, Chen W, Guo W, Wang J, Tian Y, Shi
D, Zhang X, Qiu H, Xiao X, Kang T, et al: Berberine targets
AP-2/hTERT, NF-κB/COX-2, HIF-1α/VEGF and cytochrome-c/caspase
signaling to suppress human cancer cell growth. PLoS One.
8:e692402013. View Article : Google Scholar
|
|
59
|
Muralimanoharan SB, Kunnumakkara AB,
Shylesh B, Kulkarni KH, Haiyan X, Ming H, Aggarwal BB, Rita G and
Kumar AP: Butanol fraction containing berberine or related compound
from nexrutine inhibits NFkappaB signaling and induces apoptosis in
prostate cancer cells. Prostate. 69:494–504. 2009. View Article : Google Scholar :
|
|
60
|
Goto H, Kariya R, Shimamoto M, Kudo E,
Taura M, Katano H and Okada S: Antitumor effect of berberine
against primary effusion lymphoma via inhibition of NF-κB pathway.
Cancer Sci. 103:775–781. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mencher SK and Wang LG: Promiscuous drugs
compared to selective drugs (promiscuity can be a virtue). BMC Clin
Pharmacol. 5:32005. View Article : Google Scholar : PubMed/NCBI
|