Introduction
Non-Hodgkin’s lymphomas (NHLs) are common
hematologic malignancies representing ~5.3% of all cancers in the
United States and >50% of all blood cancers (1). B-cell lymphomas are the most common
types of NHLs. Despite the considerable research progress on
lymphomas, as well as the improved treatment regimens, the survival
statistics remain poor, especially for the aggressive forms of
NHLs. Currently, combination therapy is the preferred treatment
modality for lymphoma, albeit with significant adverse side
effects, particularly for the more aggressive types, such as
Burkitt’s lymphoma (BL) and mantle cell lymphoma (MCL). The
majority of patients with BL respond poorly to the CHOP treatment
(2). Similarly, many patients with
MCL have a poor response to CHOP, have high rates of relapse, with
a median survival rate of 3–5 years (3). Finding drugs that specifically target
BL and MCL while sparing normal cells is a major focus of current
research. Because treatment failure depends on a complex interplay
of factors including tumor biology, pharmacokinetics and
pharmacogenomics (4,5), the use of natural products, such as
Huachansu (HCS), comprising many bioactive components (6) may prove to be more efficacious than a
single agent or single agent combinations.
In traditional Chinese medicine (TCM), the use of
Chansu was initially recorded in the Tang Dynasty, >1,000 years
ago. Described in the Chinese pharmacopoeia as a detoxicant and an
anodyne, its pharmacological attributes include detoxification and
resuscitation as well as the ability to reduce swelling and pain
associated with infections and malignant cell growth. Its wide use
as a local anesthetic, a cardiotonic, and a diuretic has also been
recorded. More recently, studies have suggested that Chansu
inhibits vasodilation (increased vasoconstriction) and increases
vascular resistance and blood pressure via its inherent
anti-inflammatory effects and through inhibition of Na+,
K+-ATPase (7). In
addition, formulations of Chansu have been used for the treatment
of various cancers including hepatic, pancreatic, gastric, lung,
skin, and esophageal cancers in oncology clinics in China (8–10).
Among the different formulations, Huachansu, an injectable form of
Chansu, is one of the most popular formulations and has been
extensively used in the treatment of various solid tumors including
hepatocellular and non-small cell lung cancer in China (11).
As a form of traditional Chinese medicine approved
by the State of Food and Drug Administration (SFDA), HCS and its
bioactive components, cardiac glycosides (mainly bufadienolides),
exhibited significant inhibitory activity against various human
cancer cells, such as human colon cancer cells 26-L5; leukemia
cells (K562, U937 and HL-60); hepatocellular carcinoma SMMC-7721,
Bel-7402 and HepG2 cells; prostate cancer LNCaP, PC3 and DU145
cells; endometrial (HHUA and HEC-1), and ovarian (SK-OV-3) cancer
cells (12–14). Mechanistically, the
antiproliferative effect of HCS in the various cancer cells was
mediated through cell cycle alteration and induction of apoptosis
by modulating apoptosis-related proteins such as Bax, Bcl-2, Fas,
Fas-L, survivin, and mitochondria-mediated pathways (15–18),
as well as angiogenesis by inhibiting expression of VEGF and EGFR
proteins (19,20). Taken together, these studies
support the notion that HCS and bufadienolides have the ability to
suppress the proliferation of various solid tumors and, possibly,
have great potential as anticancer agents. However, the effect of
HCS on the growth of malignancies of hematopoietic origin,
particularly lymphomas is limited. Case reports from China have
demonstrated that HCS alone or in combination with CHOP enhances
the response rate of patients with NHLs (21,22)
supporting its role in the management of NHLs.
In the present study, we investigated the potential
anti-tumor activity and the associated molecular mechanisms of HCS
in NHL cells. The antiproliferative effect of HCS and its fraction
was evaluated on a number of different NHL cell lines including
human Burkitt’s B-cell lymphoma, such as Raji, Ramos, Namalwa and
mantle cell lymphoma, SP53 cells. A transcriptome analysis of Ramos
cells treated with HCS revealed HCS altered several interesting
oncogenes, including MAP kinase. Given that a number of studies
have demonstrated that pharmacologically targeting MAPK and
inhibiting its activity decrease proliferation in a variety of
tumor types (23), including NHL,
HCS may have great potential to be developed as a targeted
anticancer agent for NHL, particularly B-cell lymphomas.
Materials and methods
Chemical, reagents and antibodies
Huachansu was manufactured by Anhui JinChan
Biochemistry Sharing Inc. (Anhui, China). DMSO was purchased from
Sigma (St. Louis, MO, USA). ZDEVD was purchased from BD Pharmingen
(Franklin Lakes, NJ, USA). ZDEVD was dissolved in DMSO and stored
at −20°C. In all cases, the final concentration of DMSO was
<0.1% (vol/vol). PrestoBlue reagent was purchased from
Invitrogen (Frederick, MD, USA). Caspase-3 (E8, sc-7272), caspase-9
(F-7, sc-17784), survivin (D-8, sc-17779), p21 (sc-187), Mcl-1
(sc-12756) were purchased from Santa Cruz (Santa Cruz, CA, USA);
anti-cdk2 (BD-610145) and anti-cdk4 (BD-610147) were purchased from
BD Transduction Laboratories (Franklin Lakes, NJ, USA); Rb (4H1,
CS-9309), phospho-Rb (ser308) (CS-2181), p38MAPK (CS-9212S) were
purchased from Cell Signaling (Danvers, MA, USA), pMAPK (V803A) was
purchased from Promega (Madison, WI, USA) and β-actin (S-A5441) was
purchased from Sigma; RNase A was purchased from Invitrogen
(Carlsbad, CA, USA); Propidium iodide was purchased from BD
Pharmingen (San Diego, CA, USA).
Fractionation of HCS
To obtain the water and lipid soluble fractions, HCS
was subjected to a solid phase extraction using Sep-Pak C18
Cartridge (Waters Corp., Milford, MA, USA). Briefly, an aliquot of
HCS (1 ml) was applied to a preconditioned Sep-Pak solid phase
extraction cartridge (1 ml/50 mg, Waters Corp.). The eluate was
collected and defined as water soluble fraction. The column was
then washed with 1 ml of water and lipid soluble compounds were
eluted with 1 ml of ethyl acetate, after which the effluent was
collected (lipid soluble fraction). The water-soluble fraction was
used as is and the lipid fraction was dried down in a stream of
liquid nitrogen and reconstituted to an equivalent volume of DMSO
(1 ml) as that of the water soluble fraction.
Cell culture
Human Burkitt’s B-cell lymphoma cells, Raji, Ramos
and Namalwa cells were purchased from American Tissue and Culture
Collection (ATCC, Manassas, VA, USA). Mantle cell lymphoma cell
line, SP53, was kindly provided by Dr James You at The University
of Texas MD Anderson Cancer Center. All cells were cultured in
RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS;
Hyclone, Logan, UT, USA), 1 mM L-glutamine, and 50 IU/ml penicillin
and 50 μg/ml streptomycin. Human peripheral blood mononuclear cells
(PBMCs) were purchased from Astarte Biologics (Redmond, WA, USA)
and cultured in RPMI-1640 medium. Cells were cultured at 37°C with
5% CO2 in a humid atmosphere.
Cell viability and proliferation
The effect of HCS or bufalin on the growth of
lymphoma cells was assessed by the PrestoBlue assay. Raji, Ramos,
Namalwa and SP53 cells were seeded at a density of 1×104
cells per well in 96-well plates in RPMI medium and incubated for
24 h. Following incubation, media was replaced and the cells were
treated with the indicated concentrations of HCS (0.195–50 μl/ml)
or different fraction of HCS, i.e., the water or lipid soluble
fractions, or bufalin (1.95–1,000 nM) for 72 h. The cell viability
was then measured using the PrestoBlue reagent according to the
manufacturer’s instructions. Briefly, 20 μl of the PrestoBlue
reagent was added to each well containing 200 μl media. After 1 h
of incubation at 37°C, the absorbance was read at a wavelength of
590 nm (Ex/Em, 560/590 nm) using a V-Max Micro-plate Reader by
Molecular Devices, Inc. (Sunnyvale, CA, USA). Experiments were
repeated at least three times.
Cell cycle, apoptosis and cell-death
For cell cycle analysis, Ramos cells
(2.5×106) grown in 100-mm dishes were treated with HCS
(5 and 25 μl/ml) or bufalin (10 and 50 nM) for 24 h. Cells were
centrifuged, the pellets were resuspended and washed in 1× PBS, and
fixed overnight in 70% ethanol at 4°C. They were then washed with
1× PBS and resuspended in staining solution (PBTB) containing PBS,
0.5% BSA, 0.005% Tween-20, 10 μg/ml propidium iodide (PI) and 1
μg/ml of DNase-free RNase. Cells were incubated in the dark for 30
min at 37°C prior to analysis by fluorescence-activated
cell-sorting analysis (FACS) using a FACSCalibur flow cytometer
(Becton-Dickinson). The percentage of cells in each phase of the
cell cycle was estimated from the DNA histogram content. Apoptotic
cell death was further measured by Annexin V surface staining.
Briefly, cells (2.5×106) were double stained with
fluorescein isothiocyanate (FITC) conjugated Annexin V and PI
according to the manufacturer’s instructions (BD Biosciences, San
Diego, CA, USA). Fluorescence was detected by the FACSCalibur flow
cytometer and analyzed using CellQuest software program
(Becton-Dickinson). To determine the effect of caspase-3 inhibitor
on HCS induced apoptosis, cells were treated with HCS in the
presence or absence of the caspase-3 inhibitor,
benzyloxycarbonyl-Asp-Glu-Val-Asp-fluoromethylketone (ZDEVD-fmk)
(20 μM) followed by Annexin V staining. For DNA laddering, the DNA
was extracted, resolved on a 1% agarose gel, photographed using the
Chemidoc-Transilluminator XRS instrument (Bio-Rad, Hercules, CA,
USA) and the image captured using the Quantity One software
system.
Immunoblotting
Cytosolic extracts were prepared from Ramos cells
treated with HCS with and without ZDEVD as well as bufalin (10 and
50 nM) for 24 h. Briefly, cells were washed in PBS and then
resuspended in 50 μl of lysis buffer [20 mM HEPES
(N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic
acid), pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA
(ethylenediaminetetraacetic acid), and 1 mM dithiothreitol (DTT)].
After sonication on ice for 3 min with a sonicator 3000 (Misonex
Inc., Farmingdale, NY, USA), the protein concentrations were
determined by the Bradford assay. Immunoblot assays were performed
as per standard procedure. Briefly, equal amounts (50 μg) of
protein were subjected to sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) followed by transfer to PVDF membranes.
Membranes were probed with the indicated antibodies. Secondary
antibodies consisting of horseradish peroxidase (HRP)-conjugated
goat anti-mouse IgG and anti-rabbit IgG (1:500 vol/vol) were
purchased from Santa Cruz. Detection was performed by the enhanced
chemiluminescence method from GE Healthcare (Little Chalfont,
Buckinghamshire, UK).
Transmission electron microscopy
Ramos cells (5×106) were seeded in 100-mm
dishes. The cells were then incubated at 37°C, 5% CO2
for ~12–24 h. Following treatment with HCS (0, 25 μl/ml) for 24 h,
the cells were harvested by centrifugation at 3,000 rpm for 2 min.
After two washes with 1× PBS, the pellet was resuspended in
fixative (2% paraformaldehyde and 3% gluteraldehyde) and stored at
4°C. Samples were fixed with a solution containing 3%
glutaraldehyde plus 2% paraformaldehyde in 0.1 M cacodylate buffer,
pH 7.3, for 1 h. After fixation, the samples were washed and
treated with 0.1% Millipore-filtered cacodylate buffered tannic
acid, postfixed with 1% buffered osmium tetroxide for 30 min, and
stained en bloc with 1% Millipore-filtered uranyl acetate. The
samples were dehydrated in increasing concentrations of ethanol,
infiltrated, and embedded in LX-112 medium. The samples were
polymerized in a 60°C oven for 2 days. Ultrathin sections were cut
in a Leica Ultracut microtome (Leica, Deerfield, IL, USA), stained
with uranyl acetate and lead citrate in a Leica EM Stainer, and
examined in a JEM 1010 transmission electron microscope (Jeol, USA,
Inc., Peabody, MA, USA) at an accelerating voltage of 80 kV.
Digital images were obtained using AMT Imaging System (Advanced
Microscopy Techniques Corp., Danvers, MA, USA).
Gene expression
Ramos cells (2.5×106) were seeded
overnight in 100-mm dishes and were then treated with 25 μl/ml of
HCS for 24 h. Total RNA was extracted using an RNeasy kit (Qiagen,
Valencia, CA, USA). Gene expression analysis was performed at the
The University of Texas MD Anderson Sequence and Microarray Core
facility, using the Affymetrix Gene chip 1.0 ST. Gene expression
analysis was normalized by β-actin expression and set to 1 for the
control DMSO-treated cells.
Statistical analysis
Student’s t-test was used to determine the
statistical differences between various experimental groups; a
value of P≤0.05 was considered statistically significant.
Results
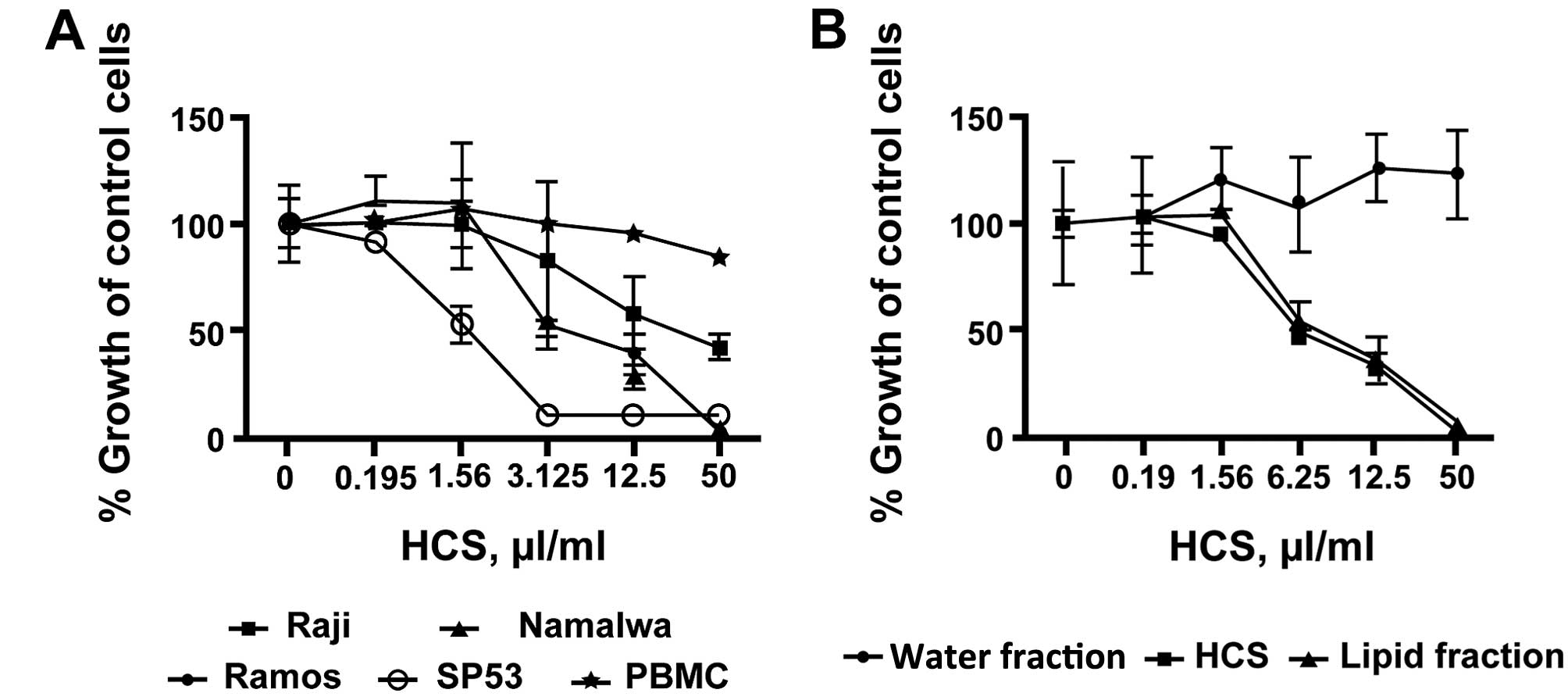
HCS and its lipid fraction inhibit
proliferation of lymphoma cell lines but not human PBMCs
To investigate the effects of HCS on cell
proliferation in lymphomas, we treated three human Burkitt’s
non-Hodgkin’s lymphoma cell lines, Raji, Ramos and Namalwa as well
as mantle cell lymphoma SP53 cells with various concentrations of
HCS. HCS markedly inhibited cell proliferation in all three cell
lines tested in a dose- and time-dependent manner, with
IC50 of inhibition ranging from 3.125 μl/ml for Ramos
and Namalwa to 25 μl/ml for Raji cells (Fig. 1A). A comparable level of
antiproliferative effect, IC50 of ~1.5 μl/ml, was
achieved when mantle cell lymphoma SP53 cells were treated with HCS
(Fig. 1A). In contrast to the
lymphoma cells, HCS did not affect the proliferation of human PBMC
(Fig. 1A). Because a number of
bioactive components either water or lipid soluble are present in
HCS, we tested for antiproliferation of the aqueous and lipid
fractions in the Ramos cells. In Fig.
1B, the lipid fraction exhibited antiproliferative activity
comparable to the parent HCS whereas the aqueous fraction showed no
antiproliferative activity in these cells, suggesting that the
lipid fraction is responsible for the antiproliferative effect in
HCS.
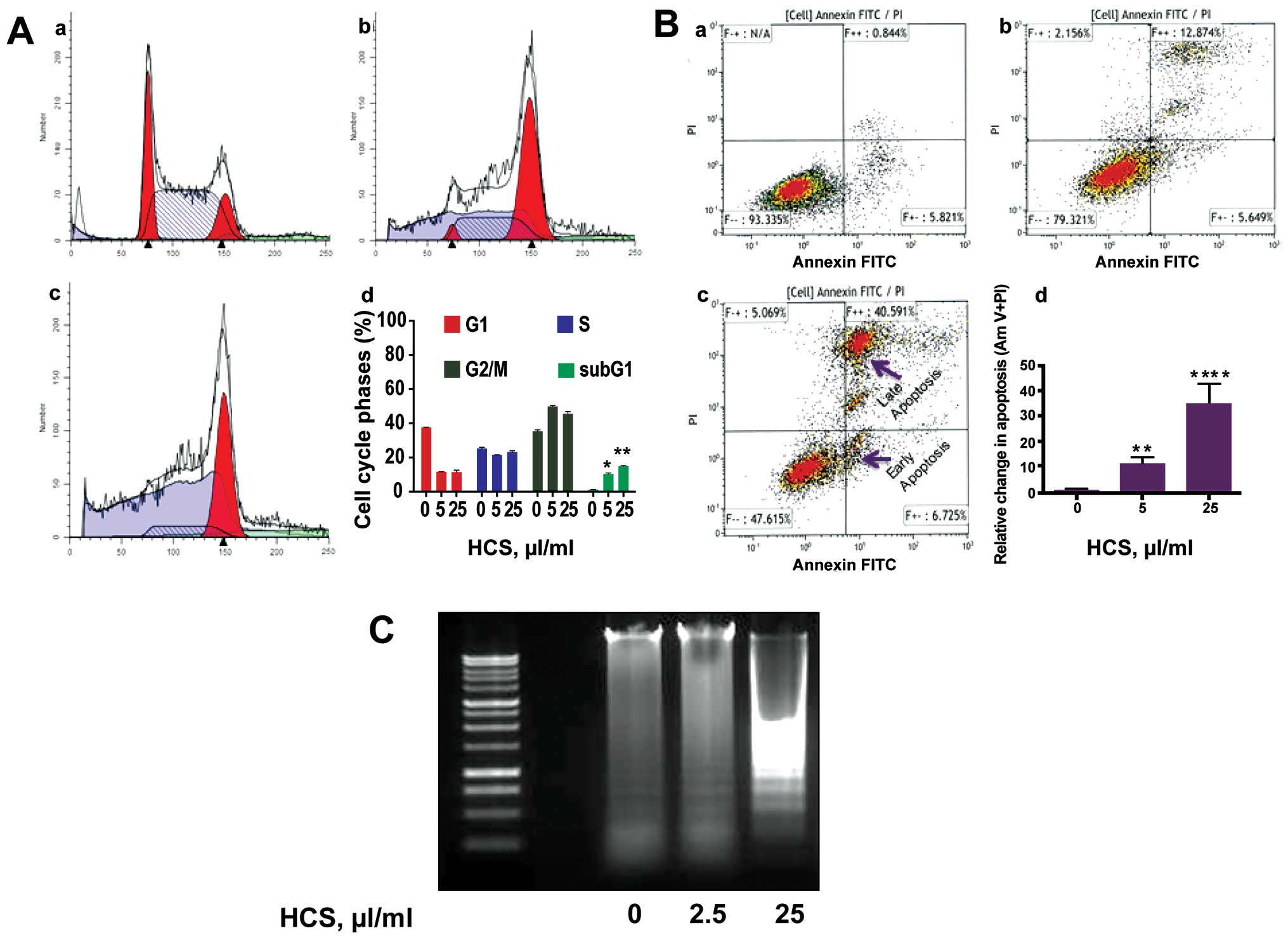
Effects of HCS on the cell cycle and cell
death in Ramos cells
We next investigated the type of cell death elicited
by HCS in Ramos cells, which is most sensitive to HCS treatment.
After treatment with increasing concentrations (0, 5 and 25 μl/ml)
of HCS for 24 h, cells were subjected by Annexin V/propidium iodide
(PI) staining followed by flow cytometry analysis. HCS treatment
resulted in a dose-dependent increase in sub-G1 phase arrest
(Fig. 2A-b and -c) compared to
vehicle treated cells (Fig. 2A-a).
Compared to control cells, there was a ~10-fold increase in sub-G1
phase cells in HCS (25 μl/ml) treated samples, suggesting the
induction of apoptosis (Fig.
2A-d). The induction of apoptosis by HCS in Ramos cells was
further evidenced by increased both early and late apoptotic cell
population with Annexin V- and PI staining, in a dose-dependent
manner (Fig. 2B-b and c) compared
to control cells (Fig. 2B-a). In
fact, HCS 25 μl/ml increased late stage apoptosis by almost 35-fold
compared to vehicle treated cells (Fig. 2B-d). The formation of
internucleosomal DNA fragments is frequently used as a marker for
cells undergoing programmed cell death. Fig. 2C is indicative of the DNA laddering
pattern triggered, in a dose-dependent manner, by HCS
treatment.
HCS modulates key regulatory proteins
critical for cell growth in lymphoma cells
We next investigated how cell cycle and apoptotic
cell death regulatory proteins were impacted by HCS. Immunoblotting
analyses showed an increase of the cyclin-dependent kinase
inhibitor p21CIP1, a key regulator of the cell cycle, at
a low concentration of 12.5 μl/ml. In contrast, there was a
dose-dependent decrease in phosphorylated Rb (pRb) protein and
survivin (Fig. 3A). Additionally,
HCS resulted in a dose-dependent increase of caspase-3, as this
caspase is cleaved from the procaspase to an active caspase when
cells undergo caspase-dependent apoptotic cell death. To further
confirm the role of caspase-3 in HCS-induced cell death, Ramos
cells were treated with HCS and the caspase-3 specific inhibitor
ZDEVD-FMK for 24 h, followed by detection of active caspase-3 by
western blot analysis. Fig. 3B
shows that HCS alone induced formation of active caspase-3, in a
dose-dependent manner, whereas ZDEVD alone inhibited active
caspase-3 formation. The combination of HCS and ZDEVD abrogated the
HCS effect alone, as evidenced by the marked decrease in the 17-kDa
fragment of caspase-3 in the ZDEVD. In line with this, the results
of Annexin V staining of Ramos cells treated with ZDEVD alone and
in combination with HCS mirrored the active caspase-3 cell death
depicted in Fig. 3B, as the
combination of ZDEVD and HCS significantly reduced the cells
undergoing the early phase apoptosis and partially abrogated late
phase apoptotic cell death (Fig. 3C, V
and VI) compared to that caused by HCS alone (Fig. 3C, II and III), whereas ZDEVD alone
did not affect cell death (Fig. 3C,
IV) compared to that of control group (Fig. 3C, I).
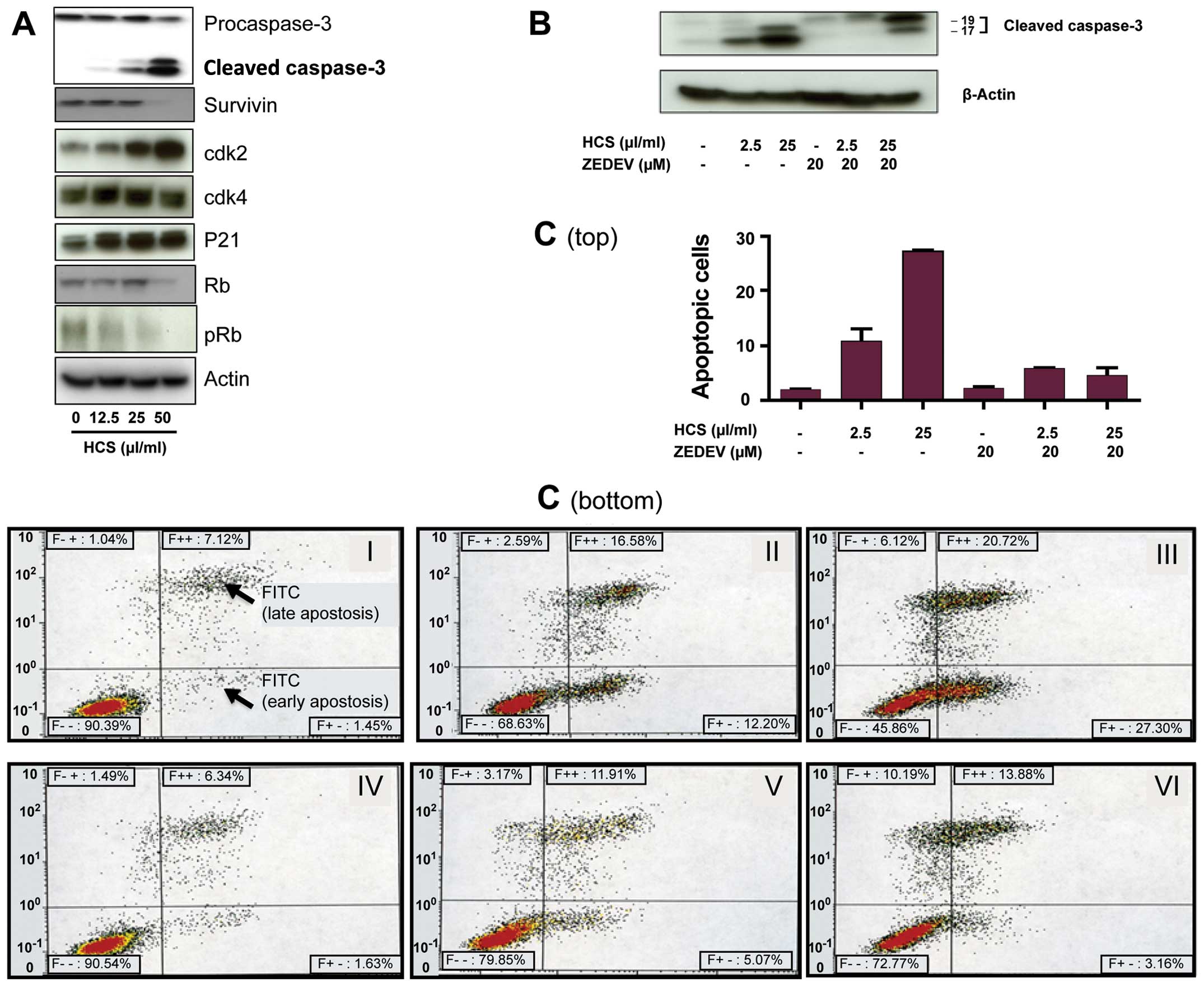 | Figure 3Modulation of apoptotic and cell
cycle regulatory proteins in Ramos cells treated with HCS. (A)
Ramos cells were treated with HCS (0, 12.5, 25 and 50 μl/ml) for 24
h, followed by western blot analysis to detect caspase-3, cdk2,
cdk4, p21CIP1, survivin, Rb and pRb proteins. (B) Cells
were treated with the indicated concentrations of HCS with or
without the caspase inhibitor, ZDEVD, for 24 h and immunoblotted
with the caspase-3 antibodies. (C) The effect of ZDEVD on HCS
induced apoptotic cell death by Annexin V staining. ZDEVD markedly
blocked the early phase of apoptosis induced by HCS while
moderately modulated the late phase of apoptotic or necrotic cells
(top, bar graph of early phase apoptosis; bottom, histogram). Each
experiment was performed in duplicate and repeated twice
independently. Data are presented as mean ± SD. |
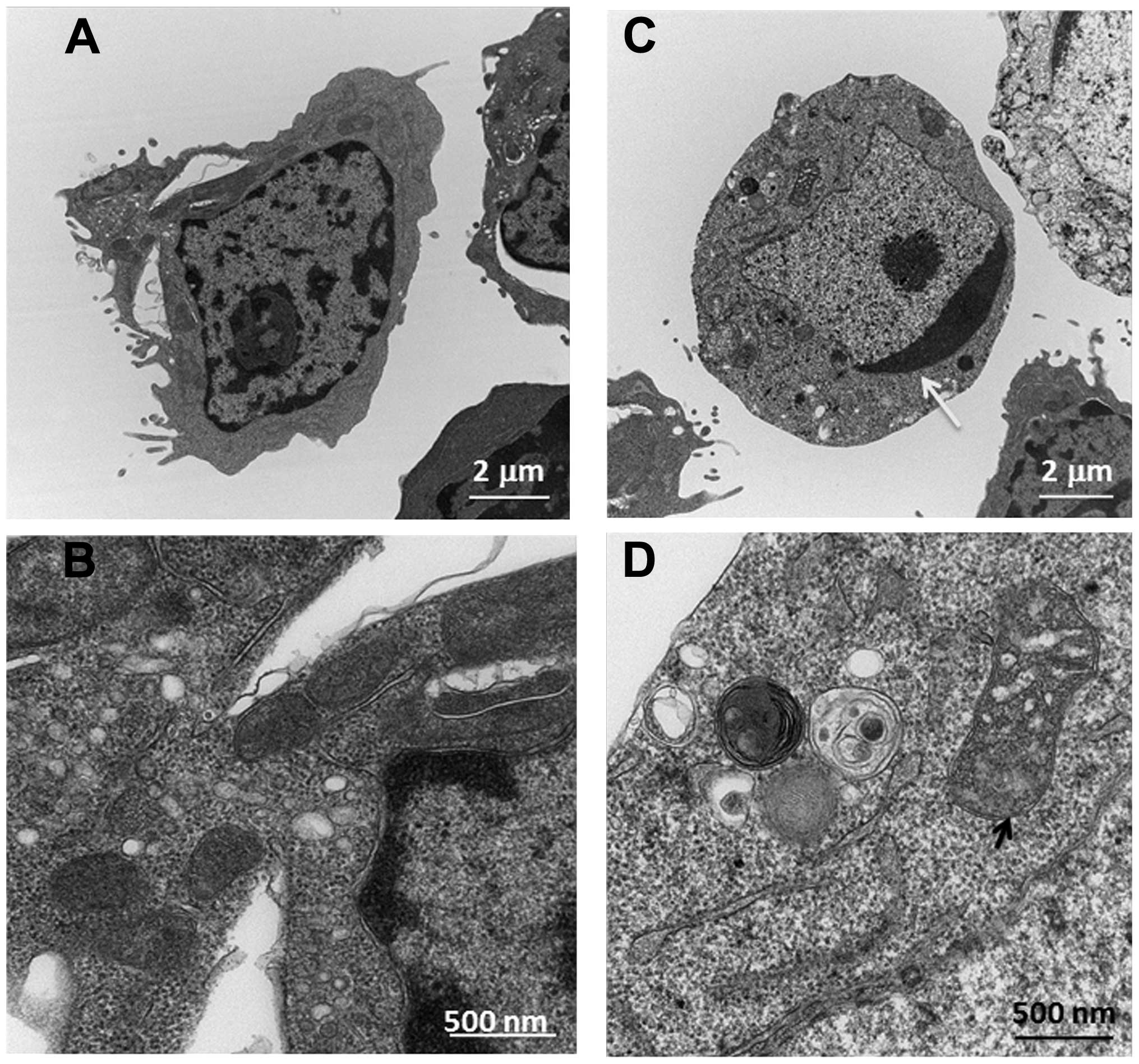
Ultrastructural changes of Ramos cells by
transmission electron microscopy
To further confirm that HCS mediated cell death
through apoptosis in Ramos cells, we determined the ultrastructural
changes of Ramos cells after being treated with HCS using
transmission electron microscopy (TEM). As shown in Fig. 4, HCS (25 μl/ml) induced marked
ultra-structural changes in cellular organelles, especially
condensed chromatin in the nucleus and distorted mitochondria (see
arrows in Fig. 4C and D) compared
to that of control treated cells (Fig.
4A and B), further suggesting that the antiproliferative effect
of HCS is being mediated through the induction of apoptosis.
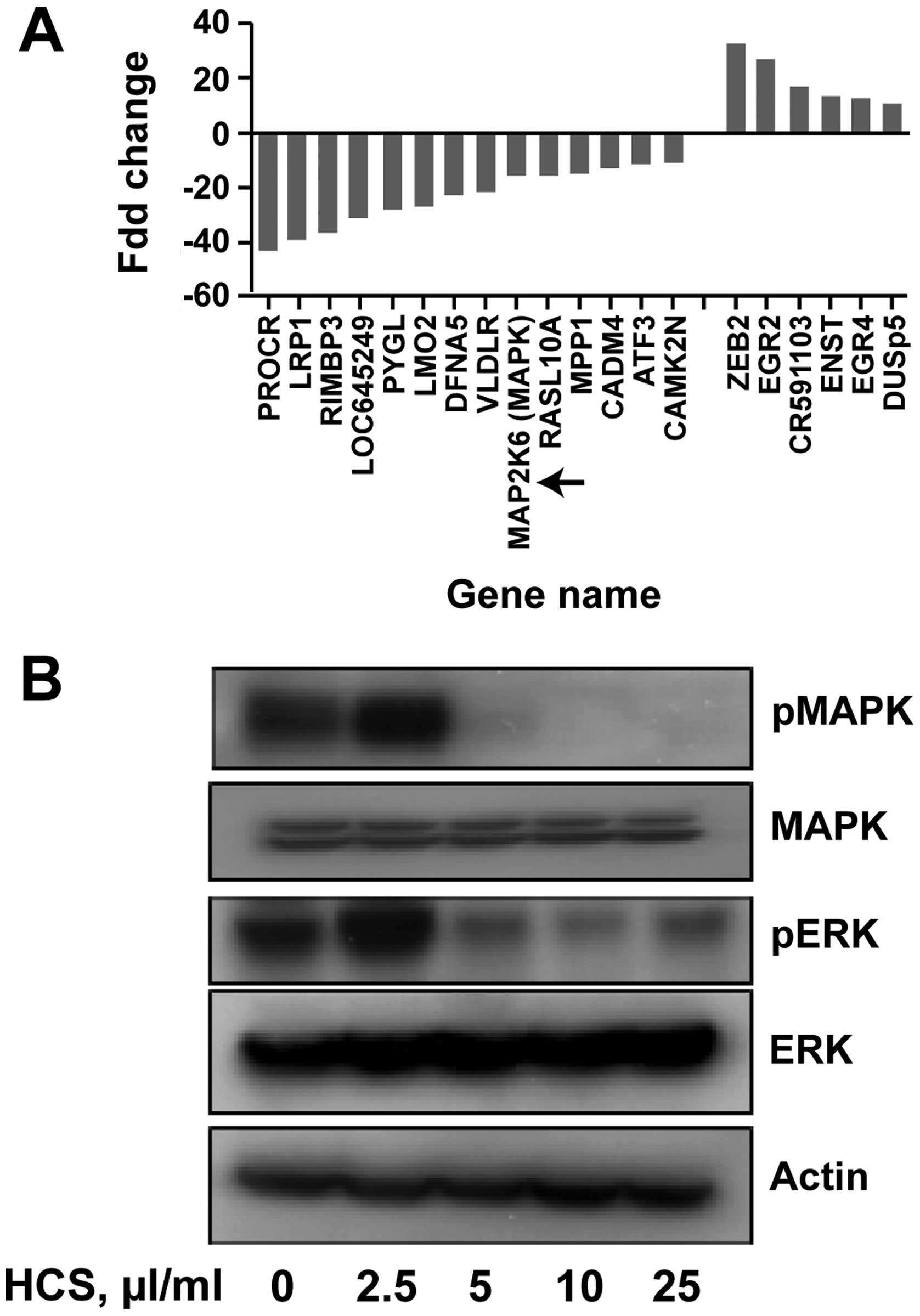
HCS affects MAP kinase gene and protein
expression
To determine the molecular mechanisms associated
with HCS-induced cell death on Ramos cells at the transcriptional
level, we treated Ramos cells with 25 μl/ml of HCS for 24 h and the
alteration of gene expression was assessed using an Affymetrix
chip. All genes showing 10–40-fold up- or down-regulation are
summarized in Fig. 5A. Among these
highly regulated genes, the mRNA expression of MAP2K6 was reduced
by 19-fold compared to that of vehicle treated Ramos cells. To gain
further insights into whether HCS also regulated the MPA kinase
translationally, we treated Ramos cells with HCS for 24 h and
determined the protein expression of MAP kinase by immunoblotting.
As shown in Fig. 5B, expression of
phosphorylated MAPK and ERK was suppressed in a
concentration-dependent manner, with no changes in the total MAPK
and ERK, potentially implicating HCS in the inhibition of the MAP
kinase signaling pathway. Together, these findings suggest that, in
addition to the caspase-3-mediated cell death, HCS might also
induce cell death in lymphomas through inhibition of MAP kinase
signaling.
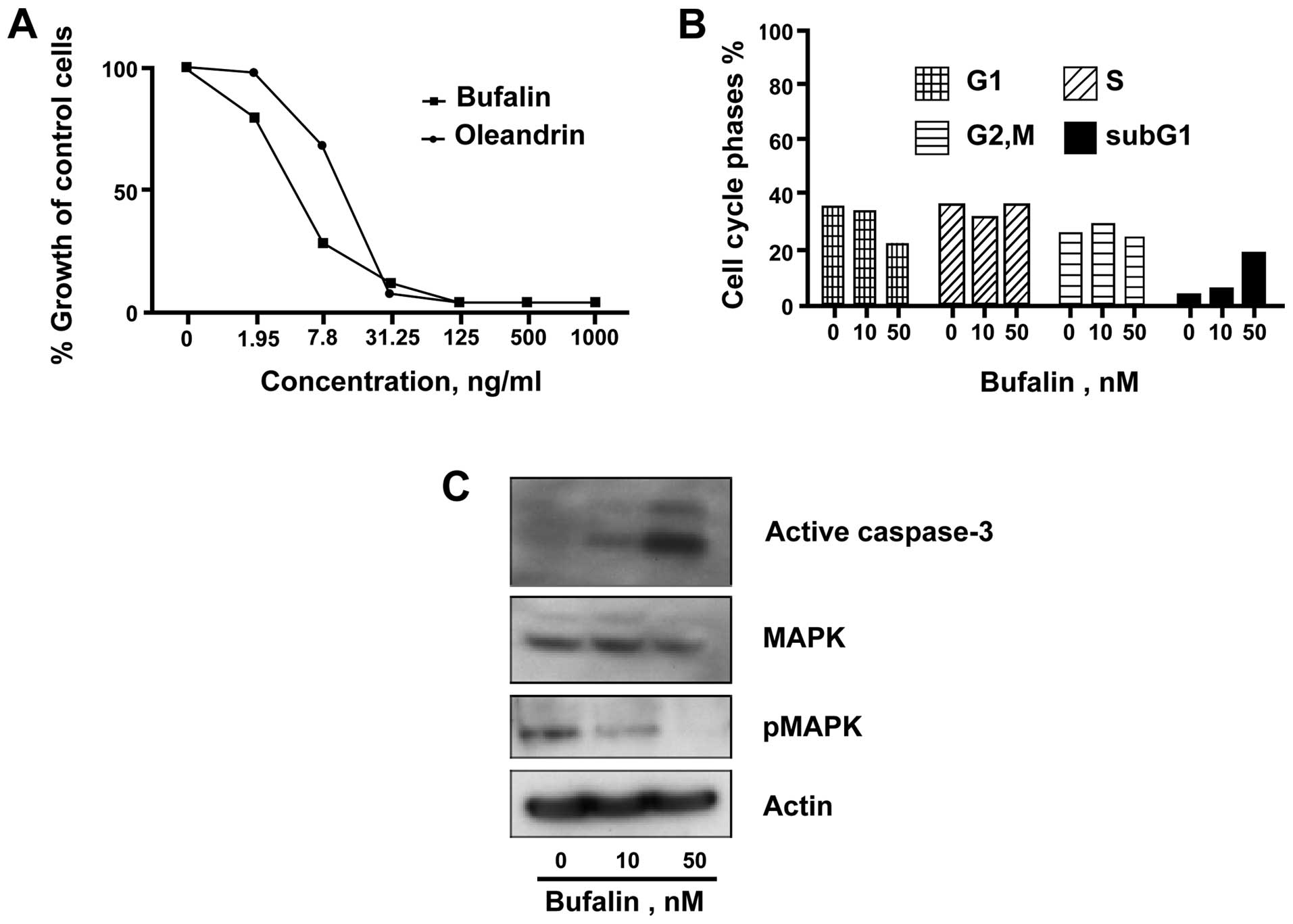
Cardiac glycosides, the major component
of HCS, inhibited Ramos cells by induction of apoptosis
Bufalin, the major cardiac glycoside present in HCS,
has been shown to have relatively potent anticancer activity in
various solid tumors (24). To
test its antiproliferative effect in NHL, we treated Ramos cells
with bufalin and another cardiac glycoside, oleandrin, for 24 and
72 h and assayed for cell cycle and proliferation, respectively.
The proliferation analysis showed a marked growth inhibition of
Ramos cells with an IC50 of 5±0.15 ng/ml and 15±0.18
ng/ml for bufalin and oleandrin, respectively (Fig. 6A). The cell cycle analysis showed a
dose-dependent increase in the sub-G1 cell population, following
treatment of Ramos cells with bufalin at a relatively low
concentration of 50 nM (Fig. 6B).
Similar to the effect of HCS, bufalin treatment of Ramos cells
increased formation of activated caspase-3 as well as decreased
pMAPK levels (Fig. 6C).
Discussion
Nature abounds with drugs that are exploited by
humans for the treatment of a variety of ailments (25,26).
Many anti-cancer agents are derived from natural sources, primarily
from plants (27). Animal-derived
anticancer drugs are also available, including ARA-C, modeled after
compounds from the Caribbean sponge, used to treat leukemia and
lymphoma (28). The drug TM 601 is
derived from the Israeli yellow scorpion and attacks malignant
glioma tumors, without harming healthy cells (29). ET 743, which comes from sea
squirts, is being tested for treatment of ovarian cancer and soft
tissue sarcoma (30–32). A number of marine natural products
and related compounds have progressed on to clinical trials
(33). Herein, we report that HCS
inhibited cell proliferation, and induced cell death through
apoptosis in human NHL cells.
In these investigations, we found that HCS, at
clinically achievable doses, significantly inhibited the
proliferation of a number of NHL cells, especially Ramos. The
antiproliferative effect of HCS in NHL appears to be mediated
through induction of apoptosis of Ramos cells via activation of
caspase-3 pathway. Intriguingly, HCS also downregulated the MAP
kinase translationally and transcriptionally in Ramos cells. Given
that the MAP kinase pathway has been recognized as one of the most
important oncogenic pathways in aggressive B-cell lymphoma
(34,35), HCS certainly warrants further
investigation.
As a hot water extract of toad skin, HCS contains
multiple biologically active components. To ascertain the most
active components that are responsible for HCS-mediated anticancer
activity, we separated HCS into lipid- or water-soluble components
using reverse phase solid phase extraction method. As depicted in
Fig. 1B, the lipid component
inhibited proliferation comparable to the HCS mixture, whereas the
water-soluble fraction had no measurable effect. These results
indicated that the lipid-fraction of HCS is primarily responsible
for its antiproliferative activity. We, and others, have reported
that cardiac glycosides (bufadeinolides), especially, bufalin, the
major bioactive component of HCS are responsible for HCS-mediated
anticancer activity in various solid tumors (36). However, whether bufalin is also
capable of inhibiting the proliferation of NHL, particular
Burkitt’s B-cell lymphoma, is lacking. In this study, our results
showed that, at as low as 5 ng/ml, bufalin inhibited proliferation
of Ramos cells by 50% suggesting that it has potent anticancer
effect in NHL cells. Additionally, bufalin inhibited proliferation
of Ramos cells through induction of apoptosis, activation of the
caspase-3 pathways, and inhibition of the MAP kinase pathway
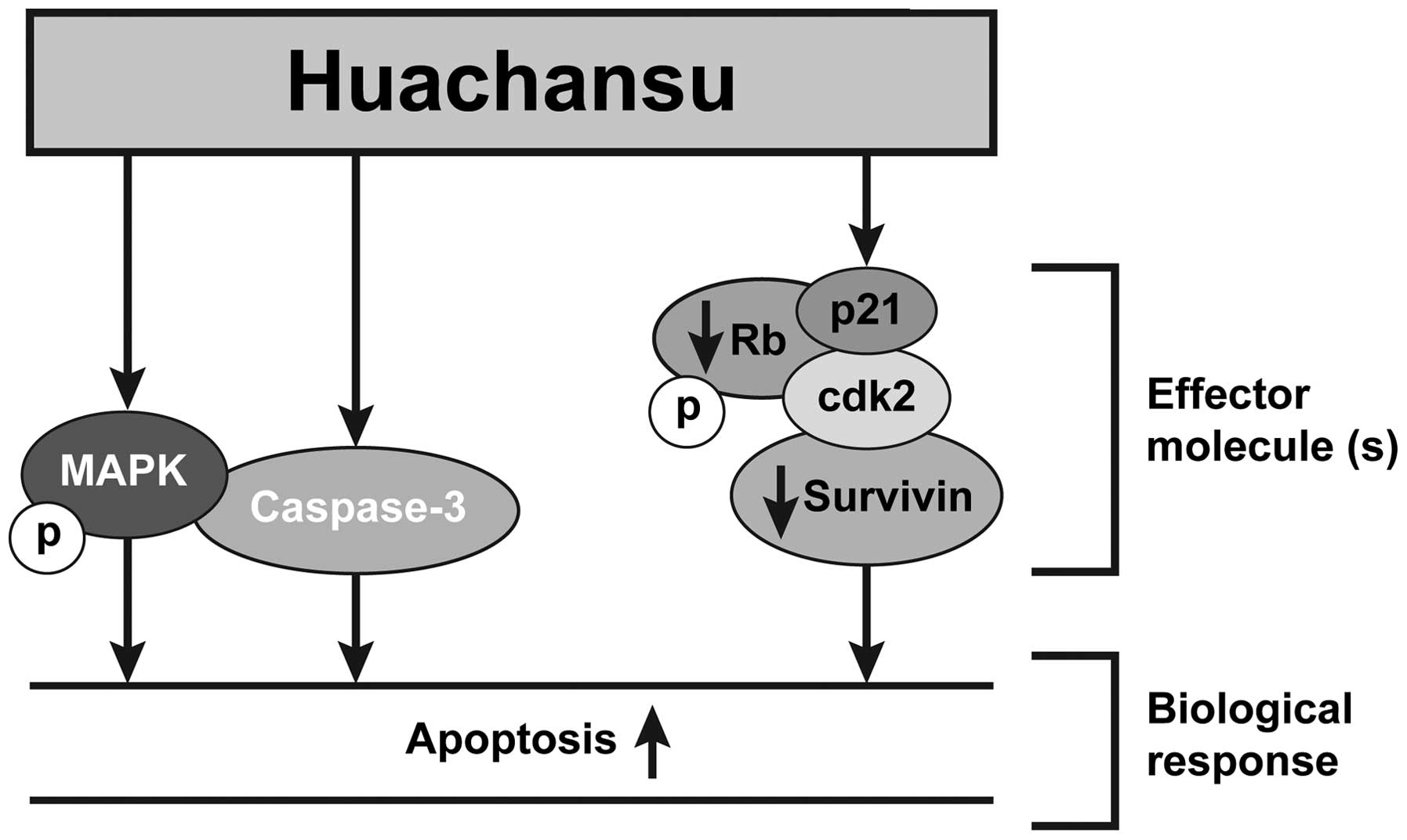
(Fig. 7, model), suggesting that
bufalin could be a main bioactive component responsible for HCS
anticancer activity in aggressive B-cell lymphomas.
The proapoptotic effect of HCS or its bioactive
components, such as cardiac glycosides, has been reported in solid
tumor derived cell lines, such as hepatocellular carcinoma HepG2
cells, and proposed to act mainly through downregulation of
mitochondria- and Fas-mediated caspase-dependent pathway (6). Active caspase-3 is a homodimer of
heterodimers and is produced by proteolysis of procaspase-3
(37). Programmed cell death
(apoptosis) can occur through caspase-dependent and -independent
pathways (38). In light of the
previous study, we reasoned that HCS might also directly activate
procas-pase-3 and cause induction of apoptosis in B-cell lymphoma
cells. Indeed, when Ramos cells were treated with HCS with and
without the caspase inhibitor, ZDEVD, a cell-permeable,
irreversible inhibitor of caspase-3/CPP32 that is known to inhibit
cell apoptosis, ZDEVD blocked formation of lower molecular forms of
active caspase-3 induced by HCS. In line with this, ZDEVD also
partially blocked the proapoptotic activity of HCS, suggesting the
activation of caspase-3 could be in part attributable to HCS
antiproliferative effect in Ramos cells. This is consistent with
previous research with HCC cells (6). In HCC cell lines treated with HCS,
the activation of caspase-9 was also observed (6,39).
In contrast, the expression of caspase-9 in the HCS treated Ramos
cells was not altered (data not shown), suggesting the HCS-induced
apoptotic effect is mediated through different molecular mechanisms
in HCC and B-cell lymphomas. More detailed biochemical in
vitro assays in cell lines and in freshly isolated tumors as
well as in vivo tumor regression analyses in animal models
need to be conducted to gain a better idea of the mode of action of
HCS.
In contrast to caspase-3 activation by HCS, MAP
kinases gene and protein were downregulated in Ramos cells treated
with HCS. MAPKs are widely expressed serine-threonine kinases that
mediate important regulatory signals in the cell. Activation of
MAPK pathway is a critical event for a number of solid tumors as
well as NHL (40). For example,
Green et al reported that the genetic alteration of the MAPK
and apoptotic pathways alone or with genetic amplification of FOXM1
as a conserved mechanism of lyphomagenesis in NHL including FLs,
DLBCLs and B-CLL (41). Three
major MAP kinase pathways, designated by their terminal kinases,
have been extensively studied: the extracellular signal-regulated
kinase (ERK1/2), c-Jun N-terminal kinase (JNK1/2), and p38 kinase
pathways. These MAP kinases are activated via a series of
sequential phosphorylations of upstream kinases, and they function
primarily to transduce signals to the cell nucleus, ultimately
affecting gene expression. The role of bufalin on MAP kinase has
been studied by a number of investigators suggesting that bufalin
induces apoptosis by activation of MAPKK1 and JNK pathways in human
leukemia U937 and HL-60 cells. In contrast, Jiang et al
reported that bufalin inhibited the phosphorylation of Akt, NF-κB,
p44/42 MAPK (ERK1/2), and p38 MAPK in A549 cells (42) suggesting MAP kinase could be
differentially regulated by bufalin depending on tumor cell
types.
The current study is the first to examine the effect
of HCS on MAPK pathways in relation to its induced cell death in
lymphomas, especially NHL. We showed that HSC at as low as 5 μl/ml
blocked almost 90% phosphorylation of MAPK and 50% phosphorylation
of ERK while no changes were observed with total MAPK expression.
Similarly, bufalin also decreased MAPK phosphorylation in a
dose-dependent manner in Ramos cells. Taken together, our data
suggest that HCS mediates cell death possibly through modulation of
the MAP kinase pathway.
In conclusion, we reported that HCS can potently
inhibit proliferation of NHL, especially Burkett’s non-Hodgkin’s
lymphoma cells. The anticancer activity of HCS appears to be linked
to the induction of apoptosis in non-Hodgkin’s lymphomas by
specific activation of caspase-3. Additionally, MAP kinases were
also notably downregulated. This is the first study suggesting the
anticancer potential of HCS in hematologic malignancies. Given that
the result of our phase I study on HCS and solid tumors has
suggested that HCS is well tolerated with minimum side effects at
doses as high as 120 ml/m2, which is almost 6-fold
higher than the dose (20 ml/m2) regularly used in
oncology clinics in China, HCS, therefore, warrants further
investigation as a novel treatment modality in NHL.
Acknowledgements
This study was supported in part by a grant from the
Gabrielle’s Angel Foundation. The TEM study was supported by the
Institutional Core grant no. CA16672 high Resolution Electron
Microscopy Facility, UTMDACC. P. Yang and L. Cohen serve as
consultants for Anhui Jinchan Biochemistry Sharing Inc.
Abbreviations:
|
HCS
|
Huachansu
|
|
NHL
|
non-Hodgkin’s lymphoma
|
|
BL
|
Burkitt’s lymphoma
|
|
MCL
|
mantle cell lymphoma
|
|
TCM
|
traditional Chinese medicine
|
|
VEGF
|
vascular endothelial growth factor
|
|
EGFR
|
epidermal growth factor receptor
|
|
MAPK
|
mitogen-activated protein kinase
|
|
ZDEVD
|
benzyloxycarbonyl-Asp-Glu-Val-Asp-fluoromethylketone
|
|
DMSO
|
dimethyl sulfoxide
|
|
PI
|
propidium iodide
|
|
PBMC
|
peripheral blood mononuclear cell
|
|
FBS
|
fetal bovine serum
|
|
FACS
|
fluorescence-activated cell-sorting
analysis
|
|
FITC
|
fluorescein isothiocyanate
|
|
HEPES
|
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic
acid
|
|
EDTA
|
ethylenediaminetetraacetic acid
|
|
SDS-PAGE
|
sodium dodecyl sulfate-polyacrylamide
gel electrophoresis
|
|
TEM
|
transmission electron microscopy
|
References
|
1
|
Evans LS and Hancock BW: Non-Hodgkin
lymphoma. Lancet. 362:139–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vose JM, Link BK, Grossbard ML, Czuczman
M, Grillo-Lopez A, Gilman P, Lowe A, Kunkel LA and Fisher RI: Phase
II study of rituximab in combination with chop chemotherapy in
patients with previously untreated, aggressive non-Hodgkin’s
lymphoma. J Clin Oncol. 19:389–397. 2001.PubMed/NCBI
|
|
3
|
Salaverria I, Perez-Galan P, Colomer D and
Campo E: Mantle cell lymphoma: From pathology and molecular
pathogenesis to new therapeutic perspectives. Haematologica.
91:11–16. 2006.PubMed/NCBI
|
|
4
|
Chen R, Chubb S, Cheng T, Hawtin RE,
Gandhi V and Plunkett W: Responses in mantle cell lymphoma cells to
SNS-032 depend on the biological context of each cell line. Cancer
Res. 70:6587–6597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Frick M, Dörken B and Lenz G: New insights
into the biology of molecular subtypes of diffuse large B-cell
lymphoma and Burkitt lymphoma. Best Pract Res Clin Haematol.
25:3–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang DL, Qi FH, Xu HL, Inagaki Y, Orihara
Y, Sekimizu K, Kokudo N, Wang FS and Tang W: Apoptosis-inducing
activity of compounds screened and characterized from cinobufacini
by bioassay-guided isolation. Mol Med Rep. 3:717–722. 2010.
|
|
7
|
Wang L, Raju U, Milas L, Molkentine D,
Zhang Z, Yang P, Cohen L, Meng Z and Liao Z: Huachansu, containing
cardiac glycosides, enhances radiosensitivity of human lung cancer
cells. Anticancer Res. 31:2141–2148. 2011.PubMed/NCBI
|
|
8
|
Gomes A, Bhattacharjee P, Mishra R, Biswas
AK, Dasgupta SC and Giri B: Anticancer potential of animal venoms
and toxins. Indian J Exp Biol. 48:93–103. 2010.PubMed/NCBI
|
|
9
|
Qi F, Li A, Inagaki Y, Kokudo N, Tamura S,
Nakata M and Tang W: Antitumor activity of extracts and compounds
from the skin of the toad Bufo bufo gargarizans Cantor. Int
Immunopharmacol. 11:342–349. 2011. View Article : Google Scholar
|
|
10
|
Qin TJ, Zhao XH, Yun J, Zhang LX, Ruan ZP
and Pan BR: Efficacy and safety of gemcitabine-oxaliplatin combined
with huachansu in patients with advanced gallbladder carcinoma.
World J Gastroenterol. 14:5210–5216. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meng Z, Yang P, Shen Y, Bei W, Zhang Y, Ge
Y, Newman RA, Cohen L, Liu L, Thornton B, et al: Pilot study of
huachansu in patients with hepatocellular carcinoma, non-small-cell
lung cancer, or pancreatic cancer. Cancer. 115:5309–5318. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He X, Tang J, Qiao A, Wang G, Jiang M, Liu
RH and Yao X: Cytotoxic biotransformed products from cinobufagin by
Mucor spinosus and Aspergillus Niger. Steroids. 71:392–402. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yeh JY, Huang WJ, Kan SF and Wang PS:
Effects of bufalin and cinobufagin on the proliferation of androgen
dependent and independent prostate cancer cells. Prostate.
54:112–124. 2003. View Article : Google Scholar
|
|
14
|
Takai N, Ueda T, Nishida M, Nasu K and
Narahara H: Bufalin induces growth inhibition, cell cycle arrest
and apoptosis in human endometrial and ovarian cancer cells. Int J
Mol Med. 21:637–643. 2008.PubMed/NCBI
|
|
15
|
Wang J, Jin Y, Xu Z, Zheng Z and Wan S:
Involvement of caspase-3 activity and survivin downregulation in
cinobufocini-induced apoptosis in A 549 cells. Exp Biol Med
(Maywood). 234:566–572. 2009. View Article : Google Scholar
|
|
16
|
Zhang LLJ, Qian Y, Wang Y and Shen ZX:
Cinobufacini induces the apoptosis of U937 cells and its mechanism.
Tumor. 27:341–344. 2007.
|
|
17
|
Yang HYZN, Hong YW and Yu RX: The
experimental research on cinobufacini inducing apoptosis in human
leukemia cell line HL60. Fujian J Trad Chin Med. 33:43–44.
2002.
|
|
18
|
Qi F, Li A, Zhao L, Xu H, Inagaki Y, Wang
D, Cui X, Gao B, Kokudo N, Nakata M, et al: Cinobufacini, an
aqueous extract from Bufo bufo gargarizans Cantor, induces
apoptosis through a mitochondria-mediated pathway in human
hepatocellular carcinoma cells. J Ethnopharmacol. 128:654–661.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang NYLS, Zhao W, Qin SK, Liu L and Chen
HY: Study the effect of antiangiogenesis of arsenic trioxide in
combination with cinobufacini on chick embryo choriallantoic
membrane. Chin Clin Oncol. 11:864–866. 2006.
|
|
21
|
Tao WWX: Clinical observation of huachansu
injection combined with CHOP regimen in treatment of NHL. J Med
Forum. 31:89–93. 2010.
|
|
22
|
Zheng PSZY, Dai ZX, Ding XL and Sun XH:
Effect of Huachansu injection on T-lymphocyte subgroups and natural
killer cells in patients with non-Hodgkin lymphoma. Liaoning Trad
Chin Med. 37:175–176. 2010.
|
|
23
|
Zheng B, Fiumara P, Li YV, Georgakis G,
Snell V, Younes M, Vauthey JN, Carbone A and Younes A: MEK/ERK
pathway is aberrantly active in Hodgkin disease: A signaling
pathway shared by CD30, CD40, and RANK that regulates cell
proliferation and survival. Blood. 102:1019–1027. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han KQ, Huang G, Gu W, Su YH, Huang XQ and
Ling CQ: Anti-tumor activities and apoptosis-regulated mechanisms
of bufalin on the orthotopic transplantation tumor model of human
hepatocellular carcinoma in nude mice. World J Gastroenterol.
13:3374–3379. 2007.PubMed/NCBI
|
|
25
|
Tempone AG, Pimenta DC, Lebrun I,
Sartorelli P, Taniwaki NN, de Andrade HF Jr, Antoniazzi MM and
Jared C: Antileishmanial and antitrypanosomal activity of
bufadienolides isolated from the toad Rhinella jimi parotoid
macrogland secretion. Toxicon. 52:13–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cunha Filho GA, Schwartz CA, Resck IS,
Murta MM, Lemos SS, Castro MS, Kyaw C, Pires OR Jr, Leite JR, Bloch
C Jr, et al: Antimicrobial activity of the bufadienolides
marinobufagin and telocinobufagin isolated as major components from
skin secretion of the toad Bufo rubescens. Toxicon. 45:777–782.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meng Y, Whiting P, Sik V, Rees HH and
Dinan L: Ecdysteroids and bufadienolides from Helleborus torquatus
(Ranunculaceae). Phytochemistry. 57:401–407. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abdel-Aziz W, Jiang HY, Hickey RJ and
Malkas LH: Ara-C affects formation of cancer cell DNA synthesome
replication intermediates. Cancer Chemother Pharmacol. 45:312–319.
2000. View Article : Google Scholar
|
|
29
|
Mamelak AN and Jacoby DB: Targeted
delivery of antitumoral therapy to glioma and other malignancies
with synthetic chloro-toxin (TM-601). Expert Opin Drug Deliv.
4:175–186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fayette J, Coquard IR, Alberti L, Ranchère
D, Boyle H and Blay JY: ET-743: A novel agent with activity in soft
tissue sarcomas. Oncologist. 10:827–832. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Erba E, Bergamaschi D, Bassano L, Damia G,
Ronzoni S, Faircloth GT and D’Incalci M: Ecteinascidin-743
(ET-743), a natural marine compound, with a unique mechanism of
action. Eur J Cancer. 37:97–105. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simoens C, Korst AE, De Pooter CM,
Lambrechts HA, Pattyn GG, Faircloth GT, Lardon F and Vermorken JB:
In vitro interaction between ecteinascidin 743 (ET-743) and
radiation, in relation to its cell cycle effects. Br J Cancer.
89:2305–2311. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Newman DJ and Cragg GM: Marine natural
products and related compounds in clinical and advanced preclinical
trials. J Nat Prod. 67:1216–1238. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kanagal-Shamanna R, Lehman NL, O’Donnell
JP, Lim MS, Schultz DS, Chitale DA, Bueso-Ramos CE, Medeiros LJ and
Inamdar KV: Differential expression of aurora-A kinase in T-cell
lymphomas. Mod Pathol. 26:640–647. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shen H, Xu W, Luo W, Zhou L, Yong W, Chen
F, Wu C, Chen Q and Han X: Upregulation of mdr1 gene is related to
activation of the MAPK/ERK signal transduction pathway and YB-1
nuclear translocation in B-cell lymphoma. Exp Hematol. 39:558–569.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yin P, Wang Y, Qiu Y, Hou L, Liu X, Qin J,
Duan Y, Liu P, Qiu M and Li Q: Bufalin-loaded mPEG-PLGA-PLL-cRGD
nanoparticles: Preparation, cellular uptake, tissue distribution,
and anticancer activity. Int J Nanomed. 7:3961–3969. 2012.
|
|
37
|
Boatright KM and Salvesen GS: Mechanisms
of caspase activation. Curr Opin Cell Biol. 15:725–731. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rathmell JC and Thompson CB: The central
effectors of cell death in the immune system. Annu Rev Immunol.
17:781–828. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qi F, Li A, Inagaki Y, Xu H, Wang D, Cui
X, Zhang L, Kokudo N, Du G and Tang W: Induction of apoptosis by
cinobufacini preparation through mitochondria- and Fas-mediated
caspase-dependent pathways in human hepatocellular carcinoma cells.
Food Chem Toxicol. 50:295–302. 2012. View Article : Google Scholar
|
|
40
|
Schrader A, Meyer K, von Bonin F,
Vockerodt M, Walther N, Hand E, Ulrich A, Matulewicz K, Lenze D,
Hummel M, et al: Global gene expression changes of in vitro
stimulated human transformed germinal centre B cells as surrogate
for oncogenic pathway activation in individual aggressive B cell
lymphomas. Cell Commun Signal. 10:432012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Green MR, Aya-Bonilla C, Gandhi MK, Lea
RA, Wellwood J, Wood P, Marlton P and Griffiths LR: Integrative
genomic profiling reveals conserved genetic mechanisms for
tumorigenesis in common entities of non-Hodgkin’s lymphoma. Genes
Chromosomes Cancer. 50:313–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang Y, Zhang Y, Luan J, Duan H, Zhang F,
Yagasaki K and Zhang G: Effects of bufalin on the proliferation of
human lung cancer cells and its molecular mechanisms of action.
Cytotechnology. 62:573–583. 2010. View Article : Google Scholar : PubMed/NCBI
|