1. Introduction
Mucous epithelia cover the inner surfaces of our
body and are essential for interaction with the outside world
(e.g., respiration, uptake and digestion of food, excretion,
reproduction, visual and auditory systems). These delicate
epithelia are exposed to a myriad of noxious agents and thus rely
on multiple mucosal protection and defense mechanisms (1), including the: i) mucosal barrier
(i.e., the extracellular mucous gel, the apical glycocalyx, and the
polarized epithelium connected by tight junctions), ii) secretion
of antimicrobial peptides, iii) rapid repair by cell migration
(restitution), iv) continuous self-renewal by differentiation from
stem and precursor cells, and v) an acute inflammatory response.
For the structure and function of the extracellular viscoelastic
mucous gel, i.e., the mucus, both secretory mucins (MUC2, MUC5AC,
MUC5B, MUC6, MUC19) as well as trefoil factor family (TFF) peptides
play key roles (2–5). Protection of the stomach epithelium
is a unique problem because of the acidic pH of the gastric juice.
Thus, the mucous gels covering the gastric surface mucosa and its
glands, respectively, have a special composition designed to
optimally fulfill their physiological function(s). Here, the
function of TFF2 for the gastric mucus barrier is discussed.
TFF2, a gastroduodenal mucus constituent
and more
TFF2 (originally termed ‘pancreatic spasmolytic
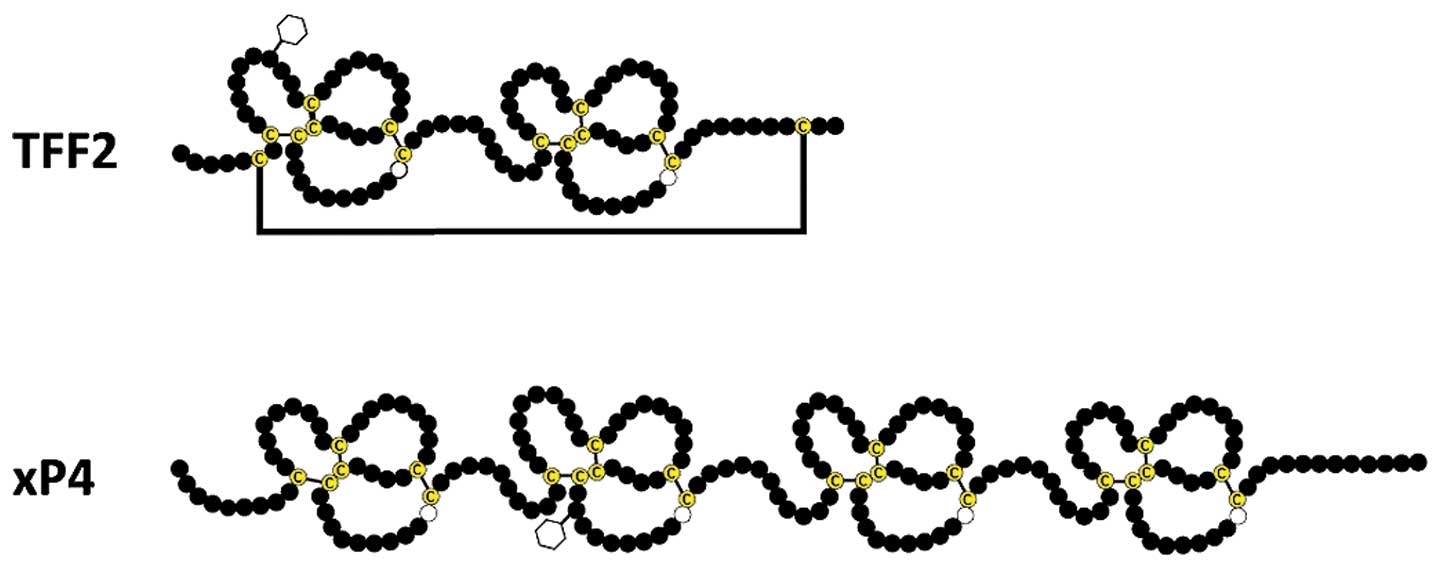
peptide') is a 106 amino acids containing secretory peptide
conserved from birds to human containing two TFF domains (reviewed
in refs 4–5). Of special note, the TFF domain
(7), in the past often also
referred to as ‘trefoil domain', is not related to the ‘β-trefoil
fold' found in certain lectins. Each of the two TFF domains
contains one conserved tryptophan and 6 conserved cysteine residues
forming 3 disulfide bridges. Furthermore, a seventh disulfide
bridge links Cys6 and Cys104 (Fig. 1) (8,9).
This is probably the reason why TFF2 is stable in the gastric juice
(4). Human gastric TFF2 is
N-glycosylated containing a fucosylated N,N'-diacetyllactosediamine
(LacdiNAc) oligosaccharide (10);
in contrast, the porcine, murine, and rat TFF2 lack N-glycosylation
sites. Thus far, the functional significance of the fucosylated
LacdiNAc structure in human TFF2 is not known but it might
influence microbial colonization (e.g., by Helicobacter
pylori) particularly of the antrum (10). Neither is it known currently, if
the fucosylated LacdiNAc structure is recognized by galectins, in
particular by galectin-3. Interestingly, porcine pancreatic TFF2
forms non-covalently linked dimers in the solid phase (9,11) as
well as in solution (12); the
latter being unusually resistant even to boiling SDS (12).
TFF2 is typically co-secreted with the mucin MUC6
from gastric fundic and antral glands (mucous neck and antral gland
cells, respectively) as well as duodenal Brunner's glands (13–17)
and represents a characteristic constituent of the gastroduodenal
mucus (18). Of special note, in
the gastric fundic glands TFF2 mRNA and protein do not co-localize.
TFF2 transcripts are detectable in mucous neck cell progenitors
only. This has been shown for human (19), mouse (20), and rat (21). TFF2 also appears as a constituent
of the gastric juice with dramatic diurnal variations (22). In the pig, in contrast to human,
TFF2 is also abundantly secreted from exocrine pancreatic glands
(acinar cells) (14). Furthermore,
TFF2 is expressed in salivary glands (21,23).
Other than in mucous epithelial glands, TFF2 is also
expressed in the immune and the central nervous systems (CNS). For
example, TFF2 is found in macrophages and lymphocytes (24,25)
as well as in the anterior pituitary and the developing brain
(26).
TFF2 is an early response gene after gastric mucosal
injury (27). During various
chronic inflammatory conditions, TFF2 is expressed ectopically in
the ulcer-associated cell lineage (28–30).
Furthermore, pathological expression of TFF2 occurs in several
metaplastic and neoplastic epithelia (compilations in refs.
4,17,30,31),
most prominent being the spasmolytic polypeptide-expressing
metaplasia (SPEM), which is found at the base of gastric fundic
units as a consequence of dysregulated trans-differentiation of
zymogenic cells as well as arrest of mucous neck cell
trans-differentiation into zymogenic cells (32,33).
SPEM gives rise to intestinal metaplasia and is even more strongly
associated with gastric cancer than is intestinal metaplasia
(34).
In the past, TFF2 was considered as a protective
rapid response peptide (27,35)
responsible for epithelial repair due to its relatively weak
motogenic effects in vitro and moderate protective or
healing effects in vivo (compilations in refs. 4,31).
Detailed comparative studies demonstrated that only luminal, but
not parenteral TFF2 was protective in one out of two colitis models
in vivo (36). This is in
line with a report that delivery of TFF2 by genetically modified
Lactococcus lactis prevents and heals acute colitis in mice
(37). The weak motogenic effect
is a chemotactic effect and is dependent on the ERK1/2 pathway
(38,39). Furthermore, also (anti)apoptotic
and angiogenic effects have been reported for TFF2 (4,5,31).
However, all attempts have so far failed to convincingly
demonstrate a typical transmembrane receptor for TFF2. Currently,
TFF2 is considered a low affinity ligand for the chemokine receptor
CXCR4 (40,41). Furthermore, integrin β1 as well as
a large transmembrane glycoprotein with similarity to
CRP-Ductin/DMBT1/gp-340/hensin have been identified as TFF2 binding
proteins in the porcine stomach (42). Interestingly, intravenously
administered TFF2 was taken up by mucous neck cells, parietal
cells, and pyloric gland cells and subsequently appeared in the
mucus layer (43). This could be
an indication for receptor-mediated transcytosis.
In contrast to the abundant synthesis of TFF2 in the
stomach, Tff2-deficient (Tff2KO) mice show surprisingly
moderate gastric phenotypes. Tff2KO mice had increased
susceptibility to Helicobacter felis-induced gastritis
(25), they showed accelerated
progression of gastritis to dysplasia in the antrum (44), and laser-induced photodamage of the
gastric surface epithelium resulted in an attenuated alkalization
of the surface pH in these animals (45). Furthermore, Tff2KO mice
showed an altered expression of genes implicated in the immune
system (46), they were
hyperresponsive to interleukin-1β stimulation (25), exhibited increased susceptibility
to Yersinia enterocolitica infection (47), and displayed reduced gut
immunopathology after oral infection with Toxoplasma gondii
(48). Taken together,
TFF2KO mice show both gastric and immunological
phenotypes.
The extracellular gastric mucus
barrier
The gastric mucus-bicarbonate-phospholipid barrier
is a viscoelastic gel acting as the first line of defense against
chemical, physical, and biological insults (1,49,50).
The secretory gel-forming mucins MUC5AC and MUC6 are major
constituents of the gastric cell surface mucus barrier (3). These heavily O-glycosylated, expanded
glycoconjugates confer viscous properties due to the enormous
length of mainly linear mucin homo-oligomers and their extreme
hydration (2). MUC5AC is secreted
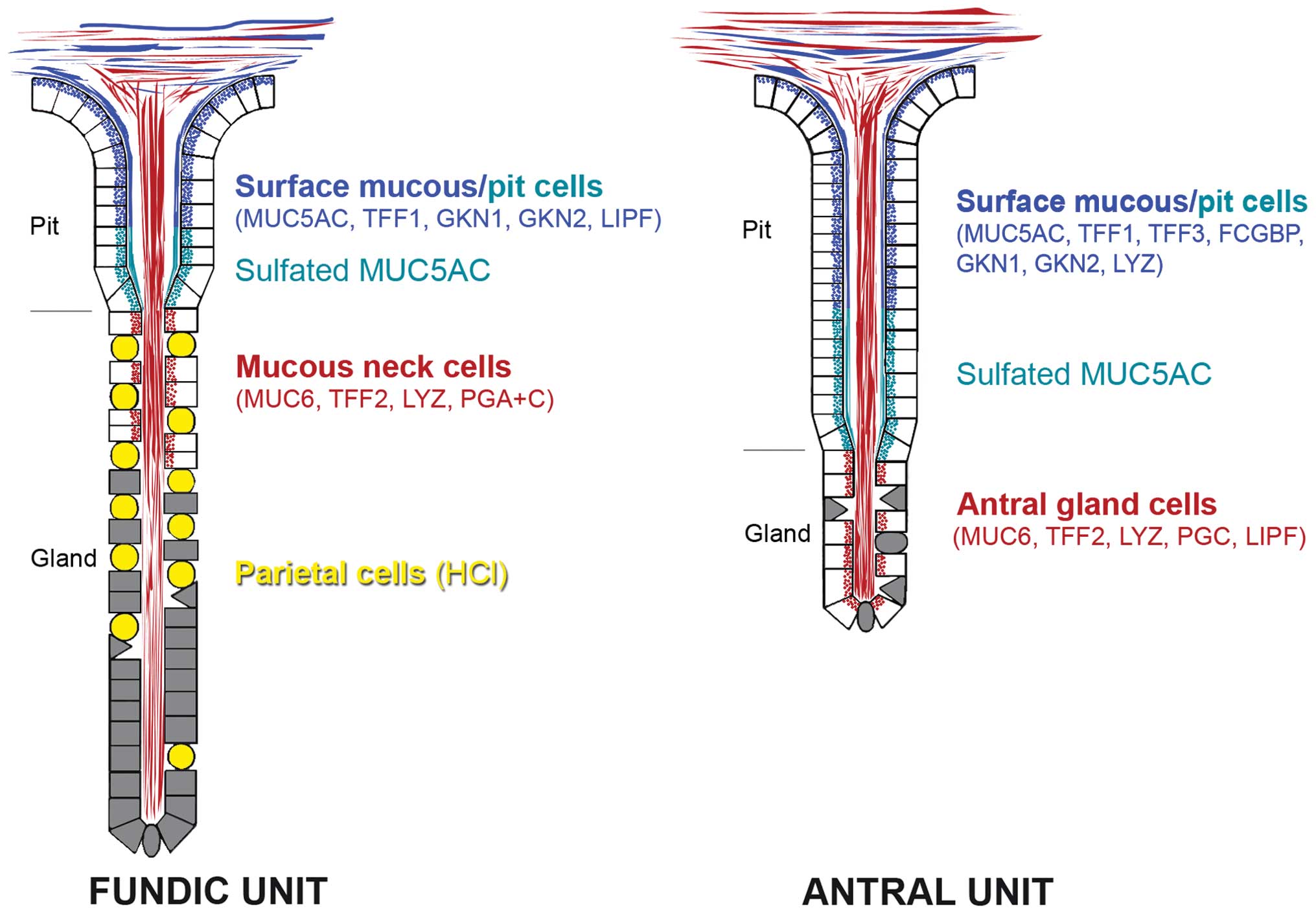
by surface mucous and pit cells of the gastric mucosa (Fig. 2) (51–53).
Of special note, MUC5AC in the mid and lower pit regions is
sulfated (Fig. 2) (54). In contrast, MUC6 is typically
secreted by the gastric glands, i.e., mucous neck cells in fundic
glands as well as antral gland cells (Fig. 2) (52,53).
Interestingly, MUC6 is aberrantly expressed in surface mucous cells
in H. pylori-infected patients (55). Gastric MUC6 is characterized by the
specific carbohydrate moiety GlcNAcα1→4Galβ1→R at non-reducing
terminals (56). A key enzyme for
formation of this epitope is the
α1,4-N-acetylglucosaminyltransferase (α4GnT) (57). This enzyme plays an essential role
for gastric protection as mice lacking this enzyme
(A4gntKO) typically show gastric mucosal inflammation
and spontaneously develop adenocarcinoma exclusively in the gastric
antrum through a hyperplasia-dysplasia-carcinoma sequence in the
absence of H. pylori infection (58). The GlcNAcα1→4Galβ1→R moiety is
recognized by the lectin GSA-II binding to the terminal GlcNAc
(59), the monoclonal antibody
HIK1083 (56), and paradoxical
concanavalin A staining (60). Of
note, GSA-II prevented the subsequent binding of HIK1083 (59).
Gastric mucins MUC5AC and MUC6 also differently
regulate colonization with H. pylori (61). For example, H. pylori
colonizes the MUC5AC layers of the gastric mucus (62–64).
In contrast, the MUC6 layers are barely colonized because the
terminal α1,4-GlcNAc of the MUC6 carbohydrate moiety has an
antimicrobial activity against H. pylori by inhibiting the
biosynthesis of cholesteryl-α-D-glucopyranoside, an essential cell
wall constituent of H. pylori (58,65).
Furthermore, the membrane-tethered mucin MUC1, which
is also capable of intracellular signaling, is found on the apical
side of surface mucous cells and in the mucous neck cell zone
(66). In addition to tethering
the mucus to the epithelial surface, MUC1 is a sensor of the
environment including microorganisms and it can be shed from the
cell surface (67). Of note,
Muc1KO mice have thinner gastric mucus layers (68). MUC13 is another membrane-tethered
mucin expressed in the surface epithelium as well as in gastric
glands (69).
In addition to the gel-forming mucins MUC5AC and
MUC6 as well as a variety of ions (e.g., H+,
Na+, Ca2+, Mn2+, Cu2+,
Cl−, HCO3−), gastric mucus also
contains a complex mixture of additional proteins such as sIgA,
IgG, IgM (67), TFF peptides
(5), gastrokines (70), IgG-Fc-binding protein (FCGBP)
(19), DMBT1/gp-340 (71), galectins (72), β-defensins (73), cathelicidin LL-37/hCAP18 (74), lysozyme (19), and the extremophilic gastric lipase
(75), which binds optimally to
lipid-water interphases at low pH. On top, the luminal surface of
the gastric mucus seems to be coated with a hydrophobic film of
surfactant phospholipids (1,76,77).
We are only at the beginning in the understanding of how these
proteins interact and form a viscous gel matrix. Certainly, a
complex interplay of different transient and non-transient
interactions is involved to build up a complex network (78).
The viscous gastric mucus barrier has multiple
physiological functions: this biofilm lubricates the passage of
undigested food, protects the epithelium from mechanical damage and
pepsin digestion, it is essential for maintaining a pH gradient
towards the acidic gastric juice, and it supports and also
restricts the adhesion and colonization of microorganisms (such as
H. pylori) (49).
In rats, the mucus covering the gastric mucosa has
been described as being composed of two layers, i.e., a loosely
adherent (outer) layer, which can be removed by gentle suction, and
a firmly adherent (inner) layer attached to the epithelium
(68,79). The thickness of the latter has been
reported to be approximately 80 and 154 μm in the corpus and
antrum, respectively (79). The
loosely adherent layer had a similar thickness and is not present
in all rats (79). One possibility
is that it may be rubbed off after food intake. Mice have a
significantly thinner firmly adherent mucus layer compared with
rats (68). This inner mucus layer
can only be removed after application of a strong force and it is
penetrable to beads the size of bacteria (80). In contrast to the colon, only small
amounts of the murine gastric outer mucus layer could be removed by
gentle aspiration (80). Muc5ac is
a major component in both the firmly and loosely adherent mucus
layers in mice (68).
In human as well as in the rat (54), the firmly adherent, water-insoluble
gastric mucus layer (mean thickness: 44 μm) is composed of an
alternating laminated array of two types of mucin, i.e., MUC5AC and
MUC6 (18,62,81,82).
Furthermore, there is also soluble mucus mixed with the luminal
gastric juice (49,83).
The firmly adherent gastric mucus layer, and not the
loosely adherent layer, is important for the maintenance of a pH
gradient across the mucus layer (84). There are numerous reports of a pH
gradient across the gastric mucus barrier with near neutral pH at
the mucosal surface (50,84,85).
It is generally accepted that the buffering of the H+
from the gastric juice occurs in the mucus via
HCO3−, which is actively released from
gastric surface mucous cells via an apical
Cl−/HCO3− exchanger (50).
Another interesting point discussed repeatedly is,
how can hydrochloric acid, i.e., H+ and Cl−
ions, be secreted across the mucus layer in order to reach the
gastric juice (50). In the past,
temporary canals have been reported in the mucous layer (86); there are also studies which failed
to show these channels (50).
However, ultrastructural studies of the excretory flow of gastric
glands clearly described the merging of zymogenic contents with
MUC6 and the development of laminated mucus structures at the
surface (54). Based on the
penetrability using beads (80) as
well as on in vivo studies (50), there is clear evidence that the
firmly adherent mucus layer is freely permeable to ions and small
molecules.
2. TFF2 and its function in the gastric
mucus barrier
TFF2 interacts with the mucin MUC6
TFF2 is an integral part of the laminated gastric
mucus barrier. This has been clearly demonstrated by
immunohistochemistry where TFF2 is associated with the gland mucin
layers, i.e., MUC6, and not with layers representing the surface
cell mucin MUC5AC (18). The
association of TFF2 with mucins was also observed after size
exclusion chromatography of both human and porcine gastric mucosa
extracts. Here, TFF2 exclusively appeared in the high-molecular
mass mucus fraction (10,12,87).
Furthermore, after anion exchange chromatography and non-reducing
non-denaturing agarose gel electrophoresis TFF2 was predominantly
associated with the mucin MUC6 in the porcine stomach forming an
ultra-high molecular mass complex hardly entering the gel (12). Of special note, the porcine
TFF2-MUC6 complex had an even higher Mr than MUC5AC
(12), which is in line with a
previous report that also native human MUC6 appeared to be of
larger size than MUC5AC (52).
In contrast, gel electrophoresis under reducing
denaturing conditions easily released monomeric TFF2 from MUC6
(12). However, after non-reducing
denaturing gel electrophoresis both a high molecular mass TFF2
heteromer as well as monomeric TFF2 were observed (12). This indicates that in the porcine
stomach TFF2 is bound to MUC6 in part covalently by disulfide
bridges and in part non-covalently. Thus, a lectin activity of TFF2
was predicted to be responsible for its non-covalent binding to
MUC6, particularly to its characteristic structure
GlcNAcα1→4Galβ1→R (12). Of note,
based on its crystal structure a lectin activity cross-linking
mucins have already been discussed for TFF2 (9). In particular, two hydrophobic clefts
have been identified, one within each TFF domain, and all conserved
residues are localized in their vicinity; two monosaccharides have
been suggested to fit into each groove with the highly conserved
Trp45 and Trp94 (Fig. 1) being very important residues in
these binding pockets (11).
Non-covalent lectin interactions of TFF2
and MUC6
Based on these results, the sugar epitope
responsible for TFF2 binding was characterized in more detail. With
the help of TFF2 fusion proteins, the carbohydrate specificity was
narrowed down in a porcine gastric mucin preparation to
GlcNAcα1→4Galβ1→4GlcNAcβwith only minor cross-reactivity to
GlcNAcα1→4Galβ, which points to an extended glycotope that
comprises more than a common disaccharide (88). The GlcNAcα1→4Galβ1→4GlcNAcβ
glycotope has been described previously as part of core 2 mucin
structures from human and porcine stomach (56,89)
as well as from duodenal Brunner's glands, which also secrete MUC6
(90).
Lectin binding using TFF2 fusion proteins has been
reported as being Ca2+-independent (88). This would be unusual as many
lectins are calcium-dependent (91). However, studies with TFF2 purified
from porcine pancreas suggest that its lectin activity is modulated
by Ca2+. For example, binding of 125I-labeled
TFF2 to FPLC-purified gastric mucus preparations from
Tff2KO mice immobilized on a CNBr-activated Sepharose™
4B column depends on Ca2+ (Stürmer and Hoffmann,
unpublished data). Furthermore, binding of 125I-labeled
TFF2 to FPLC-purified gastric mucus preparations after gel
electrophoresis and western blotting is reduced in the absence of
Ca2+ (Richter, Stürmer and Hoffmann, unpublished
data).
Possible covalent interactions of TFF2
and MUC6
Other than as a lectin, TFF2 is probably also
covalently bound via disulfide bridges to the cysteine-rich domains
of MUC6 at least in the porcine gastric mucus (12). The latter would be reminiscent to
the formation of TFF1-GKN2 and TFF3-FCGBP heteromers (87,92,93)
and could particularly cross-link MUC6 dimers via an
inter-molecular TFF2 bridge. However, TFF2 contains an even number
of cysteine residues forming 7 disulfide bridges; thus, at least
one disulfide bridge must be opened in order to enable formation of
a heteromer. Of special note, the linkage between Cys6
and Cys104 (Fig. 1) has
been reported to be particular sensitive to reduction (94) making this disulfide bridge the most
likely candidate. Thus, the question arises, which cysteine
residues of MUC6 could form a heteromer with TFF2.
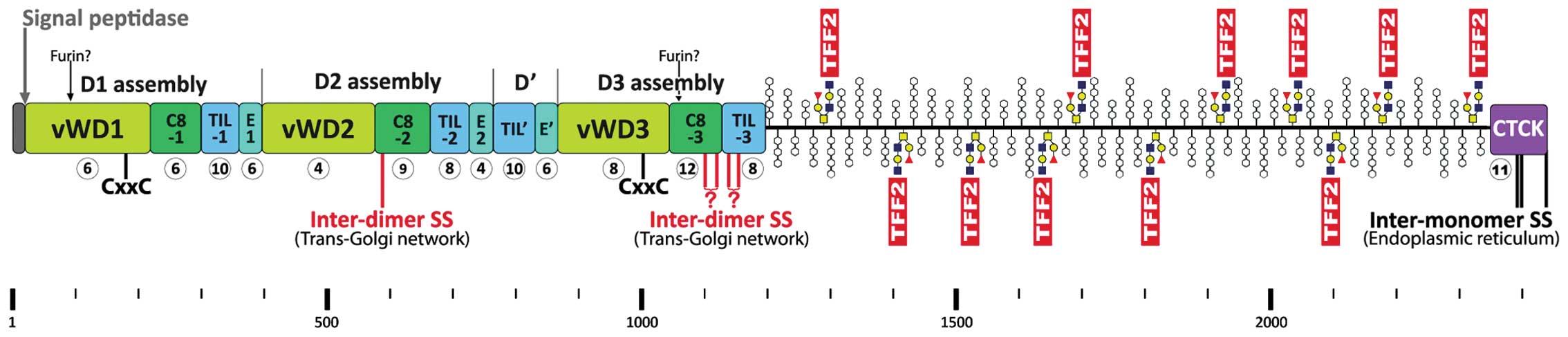
Human MUC6 is a 2439 amino acid residue long protein
(pre-pro numbering, Fig. 3) with a
mosaic structure typical of the human secretory mucins MUC2,
MUC5AC, MUC5B, and MUC19 and the frog integumentary mucin FIM-B.1
(2,95–97).
The cysteine-rich domains have similarities to von Willebrand
factor (vWF). MUC6 consists of a cysteine-rich N-terminal domain
(D1D2D'D3), a highly O-glycosylated domain rich in proline,
threonine and serine residues (PTS), and a C-terminal cystine knot
(CTCK) domain (Fig. 3) (95). Native MUC6 is known to form large
oligomeric complexes (52) and
both the N-terminal as well as the C-terminal cysteine-rich domains
are essential for its oligomerization (98).
The N-terminal D1D2D'D3 domain of MUC6 (Fig. 3) contains an odd number of Cys
residues (i.e., 97), triggers MUC6 oligomerization (98) and shows striking similarity to the
assembly domain of vWF (particularly conserved Cys and Trp
residues). In vWF, this domain is responsible for dimeric N-to-N
assembly between juxtaposed D3 domains via two homophilic
inter-molecular disulfide bridges, i.e.,
Cys1142-Cys1142 and
Cys1099-Cys1099 (99,100). In contrast to the standard
formation of disulfide bridges, which is restricted to the
endoplasmic reticulum, D3 assembly occurs via a rearrangement of
disulfide bonds in the acidic environment of the trans-Golgi
network. This process depends on an intrinsic oxidoreductase
activity of vWF (CxxC motifs in D1, D2, and D3) (99,101). However, the D1D2D'D3 assembly
domain of MUC6 shows some characteristic features, which
distinguish it from vWF (Fig. 3).
For example, the sub-domain C8-2 in MUC6 lacks two conserved Cys
residues, but contains an additional Cys residue at position 587,
which is not conserved in vWF and the secretory mucins MUC2,
MUC5AC, MUC5B, and MUC19. Thus, C8-2 contains an odd number of Cys
residues (i.e., 9), so that one can expect that this domain is
capable of forming an inter-molecular disulfide bridge at
Cys587 characteristic of MUC6 (Fig. 3). Furthermore, Cys1100
in C8-3 of MUC6 corresponds to the homologous Cys1099 of
vWF, which forms a homophilic inter-molecular disulfide bridge
essential for assembly of vWF (99). However, C8-3 in MUC6 (and also
MUC2, MUC5AC, MUC5B, and MUC19) contains an additional Cys residue
(Cys1118) when compared with vWF resulting in an even
number (i.e., 12). Thus, one could conclude, that the C8-3 MUC6 is
capable of forming either two (if Cys1100 forms an
inter-molecular disulfide bridge) or no inter-molecular disulfide
bridge (if Cys1100 does not form an inter-chain bridge).
Additionally, the TIL-3 domain is somewhat changed in MUC6.
However, Cys1153 of MUC6 is homologous to
Cys1142 in vWF, Cys1130 in MUC2, and
Cys1199 in porcine submaxillary mucin (PSM)/Muc19; the
latter three cysteine residues have been shown to form a homophilic
inter-molecular disulfide bridge essential for assembly of vWF
(99), MUC2 (102), and PSM/Muc19 (103), respectively. Because TIL-3 in
MUC6 contains an even number (i.e., 8) of Cys residues it is not
clear if TIL-3 is capable of forming inter-molecular disulfide
bridges in MUC6 (similar situation as in C8-3). Taken together,
Cys587 in C8-2 of MUC6 (Fig. 3) is a prime candidate for an
inter-chain disulfide bridge, either homophilic or heterophilic
with TFF2 as a cross-linker (Fig.
3). Further potential inter-molecular disulfide bridges could
originate from C8-3 and TIL-3 (either homophilic or heterophilic
with TFF2; Fig. 3).
The C-terminal cysteine-rich domain of MUC6 is much
shorter than that of the other secretory mucins and vWF (104) and it has been shown to be
responsible for dimerization (98). It consists only of a CTCK domain
containing an odd number of Cys residues (i.e., 11) with striking
similarity to vWF (105). It is
known that three intra-chain disulfide bridges of the CTCK domain
are responsible for C-to-C dimerization of vWF in the endoplasmic
reticulum (106). A similar
situation has been reported for PSM/Muc19 (107). Thus, it is expected that the
homologous residues (i.e., Cys2393, Cys2395,
and Cys2437) in MUC6 are also involved in dimerization
of MUC6 (Fig. 3). Whether
cross-linking via TFF2 plays a role in this process is a matter of
speculation.
Function of TFF2 for MUC6 assembly in the
secretory pathway and for mucus rheology
Based on multiple and characteristic structural
similarities, the biosynthesis of MUC6 is generally expected to
share many typical features already observed in both vWF (100) and secretory mucins (2,96,108). Furthermore, TFF2 and MUC6 are
co-expressed in the same cells, and it is expected that TFF2 and
MUC6 share the same secretory pathway and end up in the same
secretory vesicles.
The primary translation product of MUC6 is probably
co-translationally cleaved by the signal peptidase and then
dimerizes in the endoplasmic reticulum (ER; pH ~7.5) via disulfide
bridges in the CTCK domain (Fig.
3) similar to vWF (100,106) as well as MUC2 and other
gel-forming mucins (96,98). Generally, folding and initial
polymerization of mucins occur in the ER, which requires the
protein disulfide isomerase AGR2 (109,110). AGR2 has been reported to be
involved in ER stress and the unfolded protein response (111). Of note, AGR2 is expressed in the
stomach specifically together with MUC6 and TFF2 in mucous neck and
antral gland cells, respectively and Agr2KO mice develop
severe glandular hyperplasia at the expense of chief but also of
pit and parietal cells (112). If
the protein disulfide isomerase ERp57 and the calnexin-calreticulin
cycle play a role for correct folding of MUC6 and/ or TFF2 is not
known currently.
As the next steps in the secretory pathway, N- and
O-glycosylations of MUC6 are completed in the Golgi and
multimerization via the N-terminal D1D2D'D3 domain occurs in the
trans-Golgi network (pH ~6.0). Multimerization of both vWF as well
as the mucins MUC2 and PSM/Muc19 is known to require an acidic pH
and Ca2+ as well as an intrinsic oxidore-ductase
activity (CxxC motifs) (103,113–115). Thus, the CxxC motifs in the vWD1
and vWD3 domains (Fig. 3) are
expected to catalyze N-to-N multimerization of MUC6. However,
multimerization of vWF occurs via N-terminal dimerization (100,114), whereas both MUC2 and PSM/Muc19
form N-terminal trimers (96,102,116). This fundamental difference in the
multimerization between vWF and MUC2 leads to linear thread-like
structures in vWF and two-dimensional net-like sheets in MUC2,
respectively. It is not known currently, how MUC6 multimerizes. Of
special note, the N-terminal assembly domain of human MUC6 differs
characteristically by some cysteine residues when compared with
conserved positions in both vWF and the secretory mucins MUC2,
MUC5AC, MUC5B (Fig. 3):
particularly Cys587 (C8-2) is present in MUC6 only,
whereas some conserved cysteine residues in the C8-1 and vWD2
domains are lacking in MUC6. The latter also lacks the E3 and CysD
domains and C-terminal cysteine-rich regions (96). Furthermore, in contrast to the
human secretory mucins MUC2, MUC5AC, MUC5B, and MUC19,
His395 essential for pH-dependent multimerization of vWF
(117) is not conserved in MUC6.
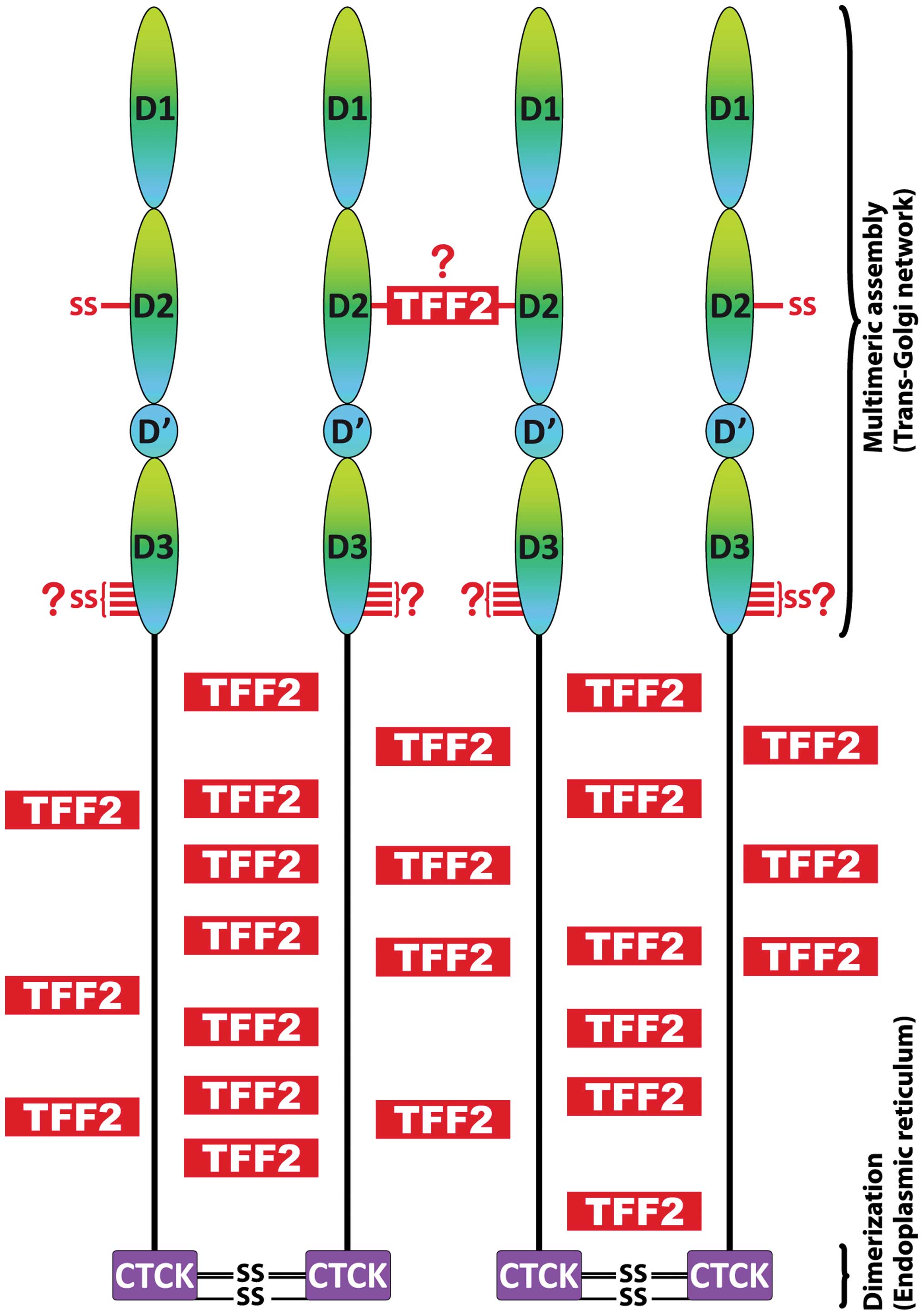
On the other hand, TFF2 is synthesized together with MUC6
connecting the PTS domains via its lectin activity (Fig. 3) and probably also forming
disulfide bridge(s) with MUC6, e.g., at Cys587 (perhaps
with the help of the CxxC motifs in vWD1 and vWD3; Fig. 3). Non-covalent cross-linking of the
PTS domains via TFF2 could play a major role for zipping up a
dimeric MUC6 bouquet (Fig. 4), a
reaction with functional analogy to vWF (100). Taken together, the
multimerization of MUC6 is expected to be different from both vWF
and the mucins MUC2 and PSM/Muc19; for MUC6, a complex branched
rather than a simple linear structure is expected. TFF2 probably
plays a role here.
It should be mentioned that MUC6 as well as the
secretory mucins MUC2, MUC5AC, and MUC5B contain 2 potential furin
cleavage sites at conserved positions (in MUC6 after positions 88
and 1054, respectively; Fig. 3).
It is not known currently whether MUC6 is processed by this
Ca2+-dependent protease.
The last steps in the secretory pathway of
MUC6/TFF2 are storage in secretory granules (diameter of mucin
granules: <1 μm) and mainly regulated exocytosis after
extracellular stimulation with a secretagogue, such as ATP
(110). Secretory granules from a
variety of cells are typical acidic Ca2+ stores (pH
~5.5–5.9; total Ca2+ bound to a matrix, 20–150 mM; free
Ca2+, 10–100 μM) (118,119). In contrast, resting
Ca2+ levels in the cytosol are only ~100 nM. Progressive
acidification along the secretory pathway is maintained by a
vesicular H+-ATPase and Ca2+ uptake probably
occurs via a SERCA-type Ca2+-ATPase (119,120). Furthermore, there are probably
also channels for K+ and Cl− import; whereas
Ca2+ release is probably operated by IP3/ryanodine
receptors (118,120). It has been demonstrated that both
vWF as well as secretory mucins are stored in a highly condensed
and well-organized packaging in the corresponding secretory
granules in the presence of Ca2+ at pH <6.0. vWF
assembles into helices forming the tubules of the Weibel-Palade
bodies (100,114); whereas secretory mucins are
tightly packed forming a condensed polyanionic matrix with
Ca2+ as counter-ions embedded in a fluid phase (108,115,121,122). Particular for MUC2 a detailed
mechanistic model has been proposed for packaging and unfolding
(115). In the case of MUC6,
non-covalent cross-linking of the PTS domains via the lectin
activity of TFF2 would be a perfect design enforcing extremely
dense packaging of MUC6 within the secretory granules (Fig. 4). Furthermore, the many acidic
residues of TFF2, often clustered, could also serve as additional
Ca2+ binding sites. During exocytosis, rapid unfolding
of condensed mucins is triggered by an exchange of Ca2+
against Na+ from the extracellular fluid thus doubling
the number of counter-ions and causing the mucin polymer to expand
up to 600-fold in its volume by osmotic swelling within 20–30 msec
(108,122,123,124). Of special note,
HCO3− plays a crucial role for the extremely
rapid transition into the expanded hydrated phase because it
efficiently competes for Ca2+ binding (125). In many organs, this process is
linked with the CFTR-channel, which also transports
HCO3−. However, in the human stomach the CFTR
is expressed at low levels only and gastric surface epithelial
cells secrete abundantly bicarbonate via an apical
Cl−/HCO3− exchanger (50) allowing expansion of MUC6. Gastric
HCO3− secretion is stimulated by E-type
prostaglandins (50), which is in
agreement with their general protective function - here, in
particular due to their role for proper MUC6 exocytosis.
Upon release and expansion of MUC6, TFF2 still
stays attached also at lower Ca2+ concentrations
allowing the formation of three-dimensional networks. Generally,
the assembly and the structure of the TFF2-MUC6 network are
expected to be quite different when compared with MUC2. TFF2 seems
to be an important link peptide enabling a dense MUC6 network,
which is typical of glands (fundic glands, antral glands, Brunner's
glands). This would also explain why native MUC6 appeared to be of
larger size than MUC5AC (12,52)
despite the fact that the MUC6 monomer is much smaller than that of
MUC5AC. The formation of large TFF2-MUC6 complexes probably
supports the proper excretory flow from the gastric glands
(54). Furthermore, the large
TFF2-MUC6 complexes probably ensure that MUC5AC and MUC6 do not mix
homogeneously, but rather form a layered mucus structure.
The proposed role of TFF2 for MUC6 assembly and
rheology fits well with several experimental observations. For
example, TFF2 increased dramatically the viscosity and elasticity
of porcine gastric mucin solutions in vitro (126). These solutions showed
non-Newtonian pseudo-plastic behavior, i.e., the viscosity
decreased when increasing the applied shear rate (126). Such a behavior is typical of an
entangled network. Of note, the viscous response was pH-dependent
with highest viscosity at low pH (126). This is typical of gastric mucus
(127) and is an indication for
the involvement of hydrogen bonds. Furthermore, systemically
administered TFF2 in vivo also increased the viscosity of
stomach secretions after uptake and probably transcytosis to the
mucosal surface (128). Also the
effects on gastric pH regulation (45) and proton permeation through gastric
mucus (129) fit well with a role
of TFF2 for MUC6 assembly and stabilization of the mucus
barrier.
TFF2 orthologs in the amphibian
gastrointestinal tract
In the Xenopus laevis stomach epithelium,
two peptides consisting of four TFF modules arranged in tandem are
expressed in mucous neck and antral gland cells (xP4.1 and xP4.2)
(130). They are considered to be
the X. laevis functional homologs of mammalian TFF2
(31). Interestingly, xP4.1 is
N-glycosylated (as is human TFF2) and expressed in all regions of
the stomach; whereas xP4.2 lacks the N-glycosylation site (131) and is expressed with a decreasing
gradient from the fundus to the antrum (31). Thus far, it is not known if xP4.1
contains the LacdiNAc oligosaccharide as human TFF2. However, both
xP4.1 and xP4.2 contain 4×6 cysteine residues typical of the four
TFF domains and, in contrast to TFF2, lack the additional cysteine
residues at the N- and C-termini typical of TFF2 (Fig. 1). Thus, there is no additional
disulfide bridge in xP4 equivalent to
Cys6-Cys104 of TFF2 (Fig. 1), which could be opened and used
for a covalent cross-linking of a MUC6 homolog. As a consequence,
the xP4 peptides are expected to only bind non-covalently via a
lectin activity to the mucin.
3. Conclusions and future perspectives
TFF2 and the gastric barrier
Cross-linking of mucins, by both covalent and
non-covalent interactions, plays a key role for the assembly of the
laminated structure and the rheological properties of gastric
mucus. Sugar-lectin interactions are known to stabilize mucin films
in order to sustain their exceptional resistance to extreme salt
conditions and to a broad pH range (132). The latter is particularly
important for the gastric mucus, where the secreted hydrochloric
acid is transported through temporary canals (86) and zymogenic secretions form
initially droplet-like structures which finally merge with the
gland mucus (54). Thus, TFF2 is
expected to play a major role for cross-linking MUC6 and
stabilizing particularly the mucus barrier of gastric glands as a
‘link peptide'. However, many details still await experimental
elucidation, particularly the N-to-N assembly of MUC6.
Unfortunately, the data basis describing this process (98) is by far not comparable with that
for MUC2, PSM/Muc19 or vWF.
As gastric mucus regulates the colonization with
H. pylori (61), it is not
to surprising that TFF2 is probably also involved in this process
(25). TFF2 also has a protective
function against the progression of premalignant lesions in H.
pylori-infected mice (44).
This is in line with the observation that epigenetic silencing of
TFF2 by H. pylori infection leads to gastric tumor
development (133). The human
stomach is also the host for a variety of non-H. pylori
microbiota (134) and it will be
interesting in the future to determine whether TFF2 plays a role in
the colonization of these microorganisms.
More roles for TFF2 and TFF modules as
lectins in mucous epithelia, the immune system, the CNS, and during
fertilization
TFF2 is also ectopically secreted from a variety of
mucous epithelia during stone diseases, such as nephrolithiasis,
hepatolithiasis, dacryolithiasis, and cholecystolithiasis (135,136). Thus, TFF2, together with MUC6,
would be a prime candidate for initiating the complex process of
stone formation. Furthermore, pH-dependent lectin activities have
also been reported for TFF1 and TFF3 (137). Thus far, the carbohydrate
specificities of TFF1 and TFF3 have not been determined. However,
there are indications that the binding characteristics of these
peptides are different. A general lectin activity of TFF domains
could functionally explain the occurrence of such modules in
various mosaic proteins, such as certain zona pellucida
proteins (ZP1, ZPB), intestinal sugar-degrading enzymes
(sucrase-isomaltase, α-glucosidase, maltase-glucoamylase), and some
frog integumentary mucins (FIM-A.1, FIM-C.1) (compilations in refs.
31,138). Clearly, a function for the
3D-structure and rheology of mucus can easily be inferred for the
TFF domains in frog integumentary mucins. However, a molecular
function of TFF domains also beyond the structure of mucus and
stone formation can be expected, e.g., during fertilization or for
the extracellular degradation of saccharides. Interestingly, all
TFF domains analyzed thus far are encoded by single exons
establishing this domain as an evolutionary conserved shuffled
lectin module.
Furthermore, the lectin activity could also explain
the multiple and diverse biological effects of TFF2 [e.g.,
motogenic, proliferative, (anti)apoptotic, and angiogenic effects]
(reviewed in refs. 4,5,31,41,104) by binding to a plethora of
transmembrane glycoproteins, such as receptors, e.g., CXCR4
(40,41), integrins (42), and a CRP-Ductin/DMBT1/gp-340-like
glycoprotein (42). Such
transmembrane glycoproteins interacting with TFF2 (and possibly
also with TFF1 and TFF3) are expected to occur particularly in the
immune and central nervous systems, e.g., the body weight is
regulated by hypothalamic TFF2 (139). In particular, the relatively high
dissociation constants of lectin interactions explain now
conclusively why relatively high TFF2 concentrations were necessary
for biological activity (40,41).
Furthermore, similar to galectins (140,141), TFF2 is also perfectly designed to
form various two- and three-dimensional cross-linked lattices with
trans-membrane glycoproteins at the cell surface. Thus, TFF2, and
probably also TFF1 and TFF3, are reminiscent to galectins in some
ways, both occurring in the digestive tract as well as the immune
and the nervous systems and regulating a variety of different
biological processes not related to mucus.
Acknowledgements
The author would like to thank D. Lorenz for
excellent secretarial assistance, F. Richter for help in computer
searches, and Dr J. Lindquist for critically reading the
manuscript.
References
|
1
|
Laine L, Takeuchi K and Tarnawski A:
Gastric mucosal defense and cytoprotection: Bench to bedside.
Gastroenterology. 135:41–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thornton DJ, Rousseau K and McGuckin MA:
Structure and function of the polymeric mucins in airways mucus.
Annu Rev Physiol. 70:459–486. 2008. View Article : Google Scholar
|
|
3
|
Senapati S, Sharma P, Bafna S, Roy HK and
Batra SK: The MUC gene family: Their role in the diagnosis and
prognosis of gastric cancer. Histol Histopathol. 23:1541–1552.
2008.PubMed/NCBI
|
|
4
|
Kjellev S: The trefoil factor family -
small peptides with multiple functionalities. Cell Mol Life Sci.
66:1350–1369. 2009. View Article : Google Scholar
|
|
5
|
Hoffmann W: TFF peptides. Handbook of
Biologically Active Peptides. 2nd edition. Kastin A: Elsevier; San
Diego: pp. 1338–1345. 2013, View Article : Google Scholar
|
|
6
|
Thim L: Trefoil peptides: From structure
to function. Cell Mol Life Sci. 53:888–903. 1997. View Article : Google Scholar
|
|
7
|
Wright NA, Hoffmann W, Otto WR, Rio MC and
Thim L: Rolling in the clover: Trefoil factor family (TFF)-domain
peptides, cell migration and cancer. FEBS Lett. 408:121–123. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thim L: A new family of growth factor-like
peptides. ‘Trefoil' disulphide loop structures as a common feature
in breast cancer associated peptide (pS2), pancreatic spasmolytic
polypeptide (PSP), and frog skin peptides (spasmolysins). FEBS
Lett. 250:85–90. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gajhede M, Petersen TN, Henriksen A,
Petersen JFW, Dauter Z, Wilson KS and Thim L: Pancreatic
spasmolytic polypeptide: First three-dimensional structure of a
member of the mammalian trefoil family of peptides. Structure.
1:253–262. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanisch FG, Ragge H, Kalinski T, Meyer F,
Kalbacher H and Hoffmann W: Human gastric TFF2 peptide contains an
N-linked fucosylated N,N'-diacetyllactosediamine (LacdiNAc)
oligosaccharide. Glycobiology. 23:2–11. 2013. View Article : Google Scholar
|
|
11
|
Petersen TN, Henriksen A and Gajhede M:
Structure of porcine pancreatic spasmolytic polypeptide at 1.95 A
resolution. Acta Crystallogr D Biol Crystallogr. 52:730–737. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stürmer R, Müller S, Hanisch FG and
Hoffmann W: Porcine gastric TFF2 is a mucus constituent and differs
from pancreatic TFF2. Cell Physiol Biochem. 33:895–904. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tomasetto C, Rio MC, Gautier C, Wolf C,
Hareuveni M, Chambon P and Lathe R: hSP, the domain-duplicated
homolog of pS2 protein, is co-expressed with pS2 in stomach but not
in breast carcinoma. EMBO J. 9:407–414. 1990.PubMed/NCBI
|
|
14
|
Rasmussen TN, Raaberg L, Poulsen SS, Thim
L and Holst JJ: Immunohistochemical localization of pancreatic
spasmolytic polypeptide (PSP) in the pig. Histochemistry.
98:113–119. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hanby AM, Poulsom R, Singh S, Elia G,
Jeffery RE and Wright NA: Spasmolytic polypeptide is a major antral
peptide: Distribution of the trefoil peptides human spasmolytic
polypeptide and pS2 in the stomach. Gastroenterology.
105:1110–1116. 1993.PubMed/NCBI
|
|
16
|
Hanby AM, Poulsom R, Elia G, Singh S,
Longcroft JM and Wright NA: The expression of the trefoil peptides
pS2 and human spasmolytic polypeptide (hSP) in ‘gastric metaplasia'
of the proximal duodenum: Implications for the nature of ‘gastric
metaplasia'. J Pathol. 169:355–360. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Poulsom R: Trefoil peptides. Baillieres
Clin Gastroenterol. 10:113–134. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ota H, Hayama M, Momose M, El-Zimaity HMT,
Matsuda K, Sano K, Maruta F, Okumura N and Katsuyama T:
Co-localization of TFF2 with gland mucous cell mucin in gastric
mucous cells and in extracellular mucous gel adherent to normal and
damaged gastric mucosa. Histochem Cell Biol. 126:617–625. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kouznetsova I, Kalinski T, Meyer F and
Hoffmann W: Self-renewal of the human gastric epithelium: New
insights from expression profiling using laser microdissection. Mol
Biosyst. 7:1105–1112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Quante M, Marrache F, Goldenring JR and
Wang TC: TFF2 mRNA transcript expression marks a gland progenitor
cell of the gastric oxyntic mucosa. Gastroenterology.
139:2018–2027.e2012. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeffrey GP, Oates PS, Wang TC, Babyatsky
MW and Brand SJ: Spasmolytic polypeptide: A trefoil peptide
secreted by rat gastric mucous cells. Gastroenterology.
106:336–345. 1994.PubMed/NCBI
|
|
22
|
Semple JI, Newton JL, Westley BR and May
FE: Dramatic diurnal variation in the concentration of the human
trefoil peptide TFF2 in gastric juice. Gut. 48:648–655. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kouznetsova I, Gerlach KL, Zahl C and
Hoffmann W: Expression analysis of human salivary glands by laser
microdissection: Differences between submandibular and labial
glands. Cell Physiol Biochem. 26:375–382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cook GA, Familari M, Thim L and Giraud AS:
The trefoil peptides TFF2 and TFF3 are expressed in rat lymphoid
tissues and participate in the immune response. FEBS Lett.
456:155–159. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kurt-Jones EA, Cao L, Sandor F, Rogers AB,
Whary MT, Nambiar PR, Cerny A, Bowen G, Yan J, Takaishi S, et al:
Trefoil family factor 2 is expressed in murine gastric and immune
cells and controls both gastrointestinal inflammation and systemic
immune responses. Infect Immun. 75:471–480. 2007. View Article : Google Scholar :
|
|
26
|
Hinz M, Schwegler H, Chwieralski CE, Laube
G, Linke R, Pohle W and Hoffmann W: Trefoil factor family (TFF)
expression in the mouse brain and pituitary: Changes in the
developing cerebellum. Peptides. 25:827–832. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wong WM, Playford RJ and Wright NA:
Peptide gene expression in gastrointestinal mucosal ulceration:
Ordered sequence or redundancy? Gut. 46:286–292. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Poulsom R and Wright NA: Trefoil peptides:
A newly recognized family of epithelial mucin-associated molecules.
Am J Physiol. 265:G205–G213. 1993.PubMed/NCBI
|
|
29
|
Wright NA: Aspects of the biology of
regeneration and repair in the human gastrointestinal tract. Philos
Trans R Soc Lond B Biol Sci. 353:925–933. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Longman RJ, Thomas MG and Poulsom R:
Trefoil peptides and surgical disease. Br J Surg. 86:740–748. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hoffmann W and Jagla W: Cell type specific
expression of secretory TFF peptides: Colocalization with mucins
and synthesis in the brain. Int Rev Cytol. 213:147–181. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schmidt PH, Lee JR, Joshi V, Playford RJ,
Poulsom R, Wright NA and Goldenring JR: Identification of a
metaplastic cell lineage associated with human gastric
adenocarcinoma. Lab Invest. 79:639–646. 1999.PubMed/NCBI
|
|
33
|
Nam KT, Lee HJ, Sousa JF, Weis VG, O'Neal
RL, Finke PE, Romero-Gallo J, Shi G, Mills JC, Peek RM Jr, et al:
Mature chief cells are cryptic progenitors for metaplasia in the
stomach. Gastroenterology. 139:2028–2037.e9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goldenring JR, Nam KT, Wang TC, Mills JC
and Wright NA: Spasmolytic polypeptide-expressing metaplasia and
intestinal metaplasia: time for reevaluation of metaplasias and the
origins of gastric cancer. Gastroenterology. 138:2207–2210.
2210.e22012010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Playford RJ: Peptides and gastrointestinal
mucosal integrity. Gut. 37:595–597. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Poulsen SS, Kissow H, Hare K, Hartmann B
and Thim L: Luminal and parenteral TFF2 and TFF3 dimer and monomer
in two models of experimental colitis in the rat. Regul Pept.
126:163–171. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vandenbroucke K, Hans W, Van Huysse J,
Neirynck S, Demetter P, Remaut E, Rottiers P and Steidler L: Active
delivery of trefoil factors by genetically modified Lactococcus
lactis prevents and heals acute colitis in mice. Gastroenterology.
127:502–513. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Graness A, Chwieralski CE, Reinhold D,
Thim L and Hoffmann W: Protein kinase C and ERK activation are
required for TFF-peptide-stimulated bronchial epithelial cell
migration and tumor necrosis factor-α-induced interleukin-6 (IL-6)
and IL-8 secretion. J Biol Chem. 277:18440–18446. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chwieralski CE, Schnurra I, Thim L and
Hoffmann W: Epidermal growth factor and trefoil factor family 2
synergistically trigger chemotaxis on BEAS-2B cells via different
signaling cascades. Am J Respir Cell Mol Biol. 31:528–537. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dubeykovskaya Z, Dubeykovskiy A,
Solal-Cohen J and Wang TC: Secreted trefoil factor 2 activates the
CXCR4 receptor in epithelial and lymphocytic cancer cell lines. J
Biol Chem. 284:3650–3662. 2009. View Article : Google Scholar :
|
|
41
|
Hoffmann W: Trefoil factor family (TFF)
peptides and chemokine receptors: A promising relationship. J Med
Chem. 52:6505–6510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Thim L and Mørtz E: Isolation and
characterization of putative trefoil peptide receptors. Regul Pept.
90:61–68. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Poulsen SS, Thulesen J, Nexø E and Thim L:
Distribution and metabolism of intravenously administered trefoil
factor 2/porcine spasmolytic polypeptide in the rat. Gut.
43:240–247. 1998. View Article : Google Scholar
|
|
44
|
Fox JG, Rogers AB, Whary MT, Ge Z, Ohtani
M, Jones EK and Wang TC: Accelerated progression of gastritis to
dysplasia in the pyloric antrum of TFF2−/− C57BL6 ×
Sv129 Helicobacter pylori-infected mice. Am J Pathol.
171:1520–1528. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xue L, Aihara E, Podolsky DK, Wang TC and
Montrose MH: In vivo action of trefoil factor 2 (TFF2) to speed
gastric repair is independent of cyclooxygenase. Gut. 59:1184–1191.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Baus-Loncar M, Schmid J, Lalani N,
Rosewell I, Goodlad RA, Stamp GWH, Blin N and Kayademir T: Trefoil
factor 2 (TFF2) deficiency in murine digestive tract influences the
immune system. Cell Physiol Biochem. 16:31–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shah AA, Mihalj M, Ratkay I, Lubka-Pathak
M, Balogh P, Klingel K, Bohn E, Blin N and Baus-Loncar M: Increased
susceptibility to Yersinia enterocolitica infection of Tff2
deficient mice. Cell Physiol Biochem. 30:853–862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
McBerry C, Egan CE, Rani R, Yang Y, Wu D,
Boespflug N, Boon L, Butcher B, Mirpuri J, Hogan SP, et al: Trefoil
factor 2 negatively regulates type 1 immunity against Toxoplasma
gondii. J Immunol. 189:3078–3084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Allen A: Gastrointestinal mucus. Section
6: The gastrointestinal system. Handbook of physiology. III.
Schultz SG: Am Physiol Soc; Bethesda, MD: pp. 359–382. 1989
|
|
50
|
Allen A and Flemström G: Gastroduodenal
mucus bicarbonate barrier: Protection against acid and pepsin. Am J
Physiol Cell Physiol. 288:C1–C19. 2005. View Article : Google Scholar
|
|
51
|
De Bolós C, Garrido M and Real FX: MUC6
apomucin shows a distinct normal tissue distribution that
correlates with Lewis antigen expression in the human stomach.
Gastroenterology. 109:723–734. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nordman H, Davies JR, Lindell G, de Bolós
C, Real F and Carlstedt I: Gastric MUC5AC and MUC6 are large
oligomeric mucins that differ in size, glycosylation and tissue
distribution. Biochem J. 364:191–200. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hoffmann W: Self-renewal of the gastric
epithelium from stem and progenitor cells. Front Biosci (Schol Ed).
5:720–731. 2013. View
Article : Google Scholar
|
|
54
|
Sawaguchi A, Ishihara K, Kawano Ji J,
Oinuma T, Hotta K and Suganuma T: Fluid dynamics of the excretory
flow of zymogenic and mucin contents in rat gastric gland processed
by high-pressure freezing/freeze substitution. J Histochem
Cytochem. 50:223–234. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Byrd JC, Yan P, Sternberg L, Yunker CK,
Scheiman JM and Bresalier RS: Aberrant expression of gland-type
gastric mucin in the surface epithelium of Helicobacter
pylori-infected patients. Gastroenterology. 113:455–464. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ishihara K, Kurihara M, Goso Y, Urata T,
Ota H, Katsuyama T and Hotta K: Peripheral α-linked
N-acetylglucosamine on the carbohydrate moiety of mucin derived
from mammalian gastric gland mucous cells: Epitope recognized by a
newly characterized monoclonal antibody. Biochem J. 318:409–416.
1996. View Article : Google Scholar
|
|
57
|
Nakayama J, Yeh JC, Misra AK, Ito S,
Katsuyama T and Fukuda M: Expression cloning of a human α1,
4-N-acetylglucosaminyl-transferase that forms GlcNAα1→4Galβ→R, a
glycan specifically expressed in the gastric gland mucous cell-type
mucin. Proc Natl Acad Sci USA. 96:8991–8996. 1999. View Article : Google Scholar
|
|
58
|
Karasawa F, Shiota A, Goso Y, Kobayashi M,
Sato Y, Masumoto J, Fujiwara M, Yokosawa S, Muraki T, Miyagawa S,
et al: Essential role of gastric gland mucin in preventing gastric
cancer in mice. J Clin Invest. 122:923–934. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yang DH, Tsuyama S, Hotta K, Katsuyama T
and Murata F: Expression of N-acetylglucosamine residues in
developing rat fundic gland cells. Histochem J. 32:187–193. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Nakayama J, Katsuyama T and Fukuda M:
Recent progress in paradoxical concanavalin A staining. Acta
Histochem Cytochem. 33:153–157. 2000. View Article : Google Scholar
|
|
61
|
Skoog EC, Sjöling Å, Navabi N, Holgersson
J, Lundin SB and Lindén SK: Human gastric mucins differently
regulate Helicobacter pylori proliferation, gene expression and
interactions with host cells. PLoS One. 7:e363782012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shimizu T, Akamatsu T, Sugiyama A, Ota H
and Katsuyama T: Helicobacter pylori and the surface mucous gel
layer of the human stomach. Helicobacter. 1:207–218. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Van den Brink GR, Tytgat KM, Van der Hulst
RW, Van der Loos CM, Einerhand AWC, Büller HA and Dekker J: H.
pylori colocalises with MUC5AC in the human stomach. Gut.
46:601–607. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hidaka E, Ota H, Hidaka H, Hayama M,
Matsuzawa K, Akamatsu T, Nakayama J and Katsuyama T: Helicobacter
pylori and two ultrastructurally distinct layers of gastric mucous
cell mucins in the surface mucous gel layer. Gut. 49:474–480. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kawakubo M, Ito Y, Okimura Y, Kobayashi M,
Sakura K, Kasama S, Fukuda MN, Fukuda M, Katsuyama T and Nakayama
J: Natural antibiotic function of a human gastric mucin against
Helicobacter pylori infection. Science. 305:1003–1006. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jonckheere N and Van Seuningen I: The
membrane-bound mucins: From cell signalling to transcriptional
regulation and expression in epithelial cancers. Biochimie.
92:1–11. 2010. View Article : Google Scholar
|
|
67
|
McGuckin MA, Lindén SK, Sutton P and
Florin TH: Mucin dynamics and enteric pathogens. Nat Rev Microbiol.
9:265–278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Phillipson M, Johansson ME, Henriksnäs J,
Petersson J, Gendler SJ, Sandler S, Persson AEG, Hansson GC and
Holm L: The gastric mucus layers: Constituents and regulation of
accumulation. Am J Physiol Gastrointest Liver Physiol.
295:G806–G812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Williams SJ, Wreschner DH, Tran M, Eyre
HJ, Sutherland GR and McGuckin MA: Muc13, a novel human cell
surface mucin expressed by epithelial and hemopoietic cells. J Biol
Chem. 276:18327–18336. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Menheniott TR, Kurklu B and Giraud AS:
Gastrokines: Stomach-specific proteins with putative homeostatic
and tumor suppressor roles. Am J Physiol Gastrointest Liver
Physiol. 304:G109–G121. 2013. View Article : Google Scholar
|
|
71
|
Kang W, Nielsen O, Fenger C, Madsen J,
Hansen S, Tornoe I, Eggleton P, Reid KBM and Holmskov U: The
scavenger receptor, cysteine-rich domain-containing molecule gp-340
is differentially regulated in epithelial cell lines by phorbol
ester. Clin Exp Immunol. 130:449–458. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Nio-Kobayashi J, Takahashi-Iwanaga H and
Iwanaga T: Immuno-histochemical localization of six galectin
subtypes in the mouse digestive tract. J Histochem Cytochem.
57:41–50. 2009. View Article : Google Scholar :
|
|
73
|
O'Neil DA, Cole SP, Martin-Porter E,
Housley MP, Liu L, Ganz T and Kagnoff MF: Regulation of human
β-defensins by gastric epithelial cells in response to infection
with Helicobacter pylori or stimulation with interleukin-1. Infect
Immun. 68:5412–5415. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hase K, Murakami M, Iimura M, Cole SP,
Horibe Y, Ohtake T, Obonyo M, Gallo RL, Eckmann L and Kagnoff MF:
Expression of LL-37 by human gastric epithelial cells as a
potential host defense mechanism against Helicobacter pylori.
Gastroenterology. 125:1613–1625. 2003. View Article : Google Scholar
|
|
75
|
Aloulou A and Carrière F: Gastric lipase:
An extremophilic interfacial enzyme with medical applications. Cell
Mol Life Sci. 65:851–854. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Mauch F, Bode G, Ditschuneit H and
Malfertheiner P: Demonstration of a phospholipid-rich zone in the
human gastric epithelium damaged by Helicobacter pylori.
Gastroenterology. 105:1698–1704. 1993.PubMed/NCBI
|
|
77
|
Lichtenberger LM: The hydrophobic barrier
properties of gastrointestinal mucus. Annu Rev Physiol. 57:565–583.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Taylor C, Allen A, Dettmar PW and Pearson
JP: The gel matrix of gastric mucus is maintained by a complex
interplay of transient and nontransient associations.
Biomacromolecules. 4:922–927. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Atuma C, Strugala V, Allen A and Holm L:
The adherent gastrointestinal mucus gel layer: Thickness and
physical state in vivo. Am J Physiol Gastrointest Liver Physiol.
280:G922–G929. 2001.PubMed/NCBI
|
|
80
|
Ermund A, Schütte A, Johansson ME,
Gustafsson JK and Hansson GC: Studies of mucus in mouse stomach,
small intestine, and colon. I Gastrointestinal mucus layers have
different properties depending on location as well as over the
Peyer's patches. Am J Physiol Gastrointest Liver Physiol.
305:G341–G347. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ota H and Katsuyama T: Alternating
laminated array of two types of mucin in the human gastric surface
mucous layer. Histochem J. 24:86–92. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ho SB, Takamura K, Anway R, Shekels LL,
Toribara NW and Ota H: The adherent gastric mucous layer is
composed of alternating layers of MUC5AC and MUC6 mucin proteins.
Dig Dis Sci. 49:1598–1606. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Hanisch FG, Chai W, Rosankiewicz JR,
Lawson AM, Stoll MS and Feizi T: Core-typing of O-linked glycans
from human gastric mucins. Lack of evidence for the occurrence of
the core sequence Gal1-6GalNAc. Eur J Biochem. 217:645–655. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Phillipson M, Atuma C, Henriksnäs J and
Holm L: The importance of mucus layers and bicarbonate transport in
preservation of gastric juxtamucosal pH. Am J Physiol Gastrointest
Liver Physiol. 282:G211–G219. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Schreiber S and Scheid P: Gastric mucus of
the guinea pig: Proton carrier and diffusion barrier. Am J Physiol.
272:G63–G70. 1997.PubMed/NCBI
|
|
86
|
Johansson M, Synnerstad I and Holm L: Acid
transport through channels in the mucous layer of rat stomach.
Gastroenterology. 119:1297–1304. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kouznetsova I, Laubinger W, Kalbacher H,
Kalinski T, Meyer F, Roessner A and Hoffmann W: Biosynthesis of
gastrokine-2 in the human gastric mucosa: Restricted spatial
expression along the antral gland axis and differential interaction
with TFF1, TFF2 and mucins. Cell Physiol Biochem. 20:899–908. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Hanisch FG, Bonar D, Schloerer N and
Schroten H: Human trefoil factor 2 is a lectin that binds
α-GlcNAc-capped mucin glycans with antibiotic activity against
Helicobacter pylori. J Biol Chem. 289:27363–27375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Rossez Y, Maes E, Lefebvre Darroman T,
Gosset P, Ecobichon C, Joncquel Chevalier Curt M, Boneca IG,
Michalski J-C and Robbe-Masselot C: Almost all human gastric mucin
O-glycans harbor blood group A, B or H antigens and are potential
binding sites for Helicobacter pylori. Glycobiology. 22:1193–1206.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Van Halbeek H, Gerwig GJ, Vliegenthart JF,
Smits HL, Van Kerkhof PJ and Kramer MF: Terminal α(1→4)-linked
N-acetylglucosamine: A characteristic constituent of duodenal-gland
mucous glycoproteins in rat and pig. A high-resolution
1H-NMR study. Biochim Biophys Acta. 747:107–116. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Gabius HJ: Ca2+: mastermind and
active player for lectin activity (including a gallery of lectin
folds). The Sugar Code: Fundamentals of Glycosciences. Gabius HJ:
Wiley-VCH; Weinheim: pp. 269–278. 2009
|
|
92
|
Westley BR, Griffin SM and May FE:
Interaction between TFF1, a gastric tumor suppressor trefoil
protein, and TFIZ1, a brichos domain-containing protein with
homology to SP-C. Biochemistry. 44:7967–7975. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Albert TK, Laubinger W, Müller S, Hanisch
F-G, Kalinski T, Meyer F and Hoffmann W: Human intestinal TFF3
forms disulfide-linked heteromers with the mucus-associated FCGBP
protein and is released by hydrogen sulfide. J Proteome Res.
9:3108–3117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Otto WR, Rao J, Cox HM, Kotzian E, Lee CY,
Goodlad RA, Lane A, Gorman M, Freemont PA, Hansen HF, et al:
Effects of pancreatic spasmolytic polypeptide (PSP) on epithelial
cell function. Eur J Biochem. 235:64–72. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Rousseau K, Byrne C, Kim YS, Gum JR,
Swallow DM and Toribara NW: The complete genomic organization of
the human MUC6 and MUC2 mucin genes. Genomics. 83:936–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Bäckström M, Ambort D, Thomsson E,
Johansson ME and Hansson GC: Increased understanding of the
biochemistry and biosynthesis of MUC2 and other gel-forming mucins
through the recombinant expression of their protein domains. Mol
Biotechnol. 54:250–256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Joba W and Hoffmann W: Similarities of
integumentary mucin B.1 from Xenopus laevis and prepro-von
Willebrand factor at their amino-terminal regions. J Biol Chem.
272:1805–1810. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Leir SH and Harris A: MUC6 mucin
expression inhibits tumor cell invasion. Exp Cell Res.
317:2408–2419. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Purvis AR, Gross J, Dang LT, Huang R-H,
Kapadia M, Townsend RR and Sadler JE: Two Cys residues essential
for von Willebrand factor multimer assembly in the Golgi. Proc Natl
Acad Sci USA. 104:15647–15652. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Springer TA: von Willebrand factor, Jedi
knight of the bloodstream. Blood. 124:1412–1425. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Purvis AR and Sadler JE: A covalent
oxidoreductase intermediate in propeptide-dependent von Willebrand
factor multimerization. J Biol Chem. 279:49982–49988. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Godl K, Johansson ME, Lidell ME, Mörgelin
M, Karlsson H, Olson FJ, Gum JR Jr, Kim YS and Hansson GC: The N
terminus of the MUC2 mucin forms trimers that are held together
within a trypsin-resistant core fragment. J Biol Chem.
277:47248–47256. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Perez-Vilar J and Hill RL: Identification
of the half-cystine residues in porcine submaxillary mucin critical
for multimerization through the D-domains. Roles of the CGLCG motif
in the D1- and D3-domains. J Biol Chem. 273:34527–34534. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hoffmann W, Jagla W and Wiede A: Molecular
medicine of TFF-peptides: From gut to brain. Histol Histopathol.
16:319–334. 2001.PubMed/NCBI
|
|
105
|
Toribara NW, Ho SB, Gum E, Gum JR Jr, Lau
P and Kim YS: The carboxyl-terminal sequence of the human secretory
mucin, MUC6. Analysis of the primary amino acid sequence. J Biol
Chem. 272:16398–16403. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhou YF and Springer TA: Highly reinforced
structure of a C-terminal dimerization domain in von Willebrand
factor. Blood. 123:1785–1793. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Perez-Vilar J and Hill RL: The
carboxyl-terminal 90 residues of porcine submaxillary mucin are
sufficient for forming disulfide-bonded dimers. J Biol Chem.
273:6982–6988. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Perez-Vilar J and Mabolo R: Gel-forming
mucins. Notions from in vitro studies. Histol Histopathol.
22:455–464. 2007.PubMed/NCBI
|
|
109
|
Park SW, Zhen G, Verhaeghe C, Nakagami Y,
Nguyenvu LT, Barczak AJ, Killeen N and Erle DJ: The protein
disulfide isomerase AGR2 is essential for production of intestinal
mucus. Proc Natl Acad Sci USA. 106:6950–6955. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Adler KB, Tuvim MJ and Dickey BF:
Regulated mucin secretion from airway epithelial cells. Front
Endocrinol. 4:article 129. 2013. View Article : Google Scholar
|
|
111
|
Kaser A, Adolph TE and Blumberg RS: The
unfolded protein response and gastrointestinal disease. Semin
Immunopathol. 35:307–319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Gupta A, Wodziak D, Tun M, Bouley DM and
Lowe AW: Loss of anterior gradient 2 (Agr2) expression results in
hyperplasia and defective lineage maturation in the murine stomach.
J Biol Chem. 288:4321–4333. 2013. View Article : Google Scholar :
|
|
113
|
Mayadas TN and Wagner DD: Vicinal
cysteines in the prosequence play a role in von Willebrand factor
multimer assembly. Proc Natl Acad Sci USA. 89:3531–3535. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Huang RH, Wang Y, Roth R, Yu X, Purvis AR,
Heuser JE, Egelman EH and Sadler JE: Assembly of Weibel-Palade
body-like tubules from N-terminal domains of von Willebrand factor.
Proc Natl Acad Sci USA. 105:482–487. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Ambort D, Johansson ME, Gustafsson JK,
Nilsson HE, Ermund A, Johansson BR, Koeck PJB, Hebert H and Hansson
GC: Calcium and pH-dependent packing and release of the gel-forming
MUC2 mucin. Proc Natl Acad Sci USA. 109:5645–5650. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Perez-Vilar J, Eckhardt AE, DeLuca A and
Hill RL: Porcine submaxillary mucin forms disulfide-linked
multimers through its amino-terminal D-domains. J Biol Chem.
273:14442–14449. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Dang LT, Purvis AR, Huang RH, Westfield LA
and Sadler JE: Phylogenetic and functional analysis of histidine
residues essential for pH-dependent multimerization of von
Willebrand factor. J Biol Chem. 286:25763–25769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Chin WC, Quesada I, Nguyen T and Verdugo
P: Oscillations of pH inside the secretory granule control the gain
of Ca2+ release for signal transduction in goblet cell
exocytosis. Novartis Found Symp. 248:132–141; discussion 141–149,
277–282. 2002. View Article : Google Scholar
|
|
119
|
Dickson EJ, Duman JG, Moody MW, Chen L and
Hille B: Orai-STIM-mediated Ca2+ release from secretory
granules revealed by a targeted Ca2+ and pH probe. Proc
Natl Acad Sci USA. 109:E3539–E3548. 2012. View Article : Google Scholar
|
|
120
|
Borges R, Domínguez N, Estévez-Herrera J,
Pereda D and Machado JD: Vesicular Ca2+ mediates granule
motion and exocytosis. Cell Calcium. 51:338–341. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Perez-Vilar J, Olsen JC, Chua M and
Boucher RC: pH-dependent intraluminal organization of mucin
granules in live human mucous/goblet cells. J Biol Chem.
280:16868–16881. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Perez-Vilar J: Mucin granule intraluminal
organization. Am J Respir Cell Mol Biol. 36:183–190. 2007.
View Article : Google Scholar
|
|
123
|
Verdugo P: Mucin exocytosis. Am Rev Respir
Dis. 144:S33–S37. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Verdugo P: Supramolecular dynamics of
mucus. Cold Spring Harb Perspect Med. 2:22012. View Article : Google Scholar
|
|
125
|
Chen EY, Yang N, Quinton PM and Chin W-C:
A new role for bicarbonate in mucus formation. Am J Physiol Lung
Cell Mol Physiol. 299:L542–L549. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Thim L, Madsen F and Poulsen SS: Effect of
trefoil factors on the viscoelastic properties of mucus gels. Eur J
Clin Invest. 32:519–527. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Bansil R, Celli JP, Hardcastle JM and
Turner BS: The influence of mucus microstructure and rheology in
Helicobacter pylori infection. Front Immunol. 4:3102013. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Kjellev S, Nexø E, Thim L and Poulsen SS:
Systemically administered trefoil factors are secreted into the
gastric lumen and increase the viscosity of gastric contents. Br J
Pharmacol. 149:92–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Tanaka S, Podolsky DK, Engel E, Guth PH
and Kaunitz JD: Human spasmolytic polypeptide decreases proton
permeation through gastric mucus in vivo and in vitro. Am J
Physiol. 272:G1473–G1480. 1997.PubMed/NCBI
|
|
130
|
Jagla W, Wiede A, Kölle S and Hoffmann W:
Differential expression of the TFF-peptides xP1 and xP4 in the
gastrointestinal tract of Xenopus laevis. Cell Tissue Res.
291:13–18. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Botzler C, Oertel M, Hinz M and Hoffmann
W: Structure of the Xenopus laevis TFF-gene xP4.1, differentially
expressed to its duplicated homolog xP4.2. Biochim Biophys Acta.
1489:345–353. 1999. View Article : Google Scholar
|
|
132
|
Crouzier T, Beckwitt CH and Ribbeck K:
Mucin multilayers assembled through sugar-lectin interactions.
Biomacromolecules. 13:3401–3408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Peterson AJ, Menheniott TR, O'Connor L,
Walduck AK, Fox JG, Kawakami K, Minamoto T, Ong EK, Wang TC, Judd
LM, et al: Helicobacter pylori infection promotes methylation and
silencing of trefoil factor 2, leading to gastric tumor development
in mice and humans. Gastroenterology. 139:2005–2017. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Yang I, Nell S and Suerbaum S: Survival in
hostile territory: The microbiota of the stomach. FEMS Microbiol
Rev. 37:736–761. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Paulsen FP, Schaudig U, Fabian A, Ehrich D
and Sel S: TFF peptides and mucins are major components of
dacryoliths. Graefes Arch Clin Exp Ophthalmol. 244:1160–1170. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Rinnert M, Hinz M, Buhtz P, Reiher F,
Lessel W and Hoffmann W: Synthesis and localization of trefoil
factor family (TFF) peptides in the human urinary tract and TFF2
excretion into the urine. Cell Tissue Res. 339:639–647. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Reeves EP, Ali T, Leonard P, Hearty S,
O'Kennedy R, May FEB, Westley BR, Josenhans C, Rust M, Suerbaum S,
et al: Helicobacter pylori lipopolysaccharide interacts with TFF1
in a pH-dependent manner. Gastroenterology. 135:2043–2054.
2054.e2041–2042. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Hoffmann W and Hauser F: Biosynthesis of
frog skin mucins: Cysteine-rich shuffled modules, polydispersities
and genetic polymorphism. Comp Biochem Physiol B. 105:465–472.
1993.PubMed/NCBI
|
|
139
|
De Giorgio MR, Yoshioka M, Riedl I,
Moreault O, Cherizol R-G, Shah AA, Blin N, Richard D and St-Amand
J: Trefoil factor family member 2 (Tff2) KO mice are protected from
high-fat diet-induced obesity. Obesity. 21:1389–1395. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Sacchettini JC, Baum LG and Brewer CF:
Multivalent protein-carbohydrate interactions. A new paradigm for
supermolecular assembly and signal transduction. Biochemistry.
40:3009–3015. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Rabinovich GA, Toscano MA, Jackson SS and
Vasta GR: Functions of cell surface galectin-glycoprotein lattices.
Curr Opin Struct Biol. 17:513–520. 2007. View Article : Google Scholar : PubMed/NCBI
|