Introduction
In the early phase of carcinogenesis, the tumor
often remains focal and restricted to the organ of origin. For the
treatment of localized cancer lesions, a focal therapeutic approach
with minimal morbidity is the ideal modality. Percutaneous chemical
ablation is an established local therapy for small hepatocellular
carcinoma, with several previous studies demonstrating comparable
safety, efficacy and long-term survival to that seen with surgical
resection (1–3). Chemical ablation methods have also
been reported to have the advantages of being inexpensive, rapid
and simple (3). Additionally, the
direct local injection of anti-oncologic or tissue-ablative agents
has been performed successfully to treat various types of tumors,
without major complications, including malignant brain tumors
(4,5), renal carcinoma (6), adrenal neoplasms (7) and prostatic hyperplasia and
adenocarcinoma (8,9).
Convection-enhanced delivery (CED) is a technique
that relies on the bulk flow established by a pressure gradient
over time to ‘push' the infused drug away from the catheter,
resulting in continuous diffusion and the widespread distribution
of the infusate within the target tissue (10–14).
In the CED method, the intratissue interstitial space between the
cells is utilized as the pathway for drug delivery. The diffusion
of the drugs usually depends on the free concentration gradient and
diffusivity of the agent itself into the tissue (10). On the other hand, intratissue fluid
convection and the bulk flow are dependent on the intratissue
pressure gradient and are involved in the diffusion of the agent.
Since the pressure-dependent bulk flow of interstitial fluid occurs
following the direct intratissue infusion of medical agents, the
convection can be used to enhance the diffusion of the drugs and
treat a much larger volume of the target tissue than that achieved
via diffusion alone (10,14).
Although the CED technique has been applied
clinically as a focal approach to treat malignant brain tumors
(4,5) and benign prostatic hypertrophy
(8) with an appropriate agent
(drug diluted in the vehicle), there are several reported
disadvantages in terms of the degree of control of drug diffusion.
The extent of infusate distribution achieved using the CED method
is affected by many factors, including the type of tissue infused
(that is, normal tissue versus tumor tissue), interstitial pressure
in the extracellular space, molecular weight of the infusate,
infusion volume and rate and diameter and type of infusion catheter
(11,12). The catheters used for CED infusion
have a lumen from which the infusate is infused, and once the
infusate is released from the lumen, the infused agent flows
unexpectedly to the path of the lowest interstitial pressure
(11). As for clinically observed
issues in brain tumor trials of CED infusion, an uneven
distribution and leakage of the drug into unexpected areas and
spaces have been reported (11,12).
The other major problem hindering effective CED-based drug delivery
is the incidence of infusate reflux or ‘backflow' along the
catheter/tissue interface of the catheter track (12,14).
This reflux causes the infusate to flow away from the target
tissue, thus reducing the chance of achieving a therapeutic drug
concentration in the target structure and increasing the risk of
off-target side effects (12).
Therefore, in order to overcome these disadvantages in the CED
technique and make local therapy more a useful modality, it is
necessary to develop a novel type of infusion device enabling the
more controlled and precise diffusion of the agent.
Based on this background, we developed a novel
method for achieving intratissue diffusion by permeating the
infusate in situ, termed the in situ permeation (ISP)
system, which is based on the principle of ISP-MW-1. The purpose of
this technique is to control the extent of intratissue diffusion
and achieve widespread distribution of the infusate. These aims
include preventing uncontrolled diffusion and backflow along the
outside of the catheter and allowing for more homogeneous drug
delivery within the target tissue, thereby overcoming the
disadvantages observed with the conventional CED system. For the
goal of attaining intratissue drug diffusion, the ISP-MW-1 system
contains a perfusion catheter connected to an injector and
aspirator, which enables the intratissue perfusion of the solute
diluted in the vehicle in the tip-inserted cavity. In comparison to
the CED system, the ISP-MW-1 system is quite different and
inventive because the various perfusion-related parameters are
controllable by changing the infusion speed and aspiration
pressure. In addition, the aspiration-based vacuum of the ISP-MW-1
device enables the removal of the target tissue-intrinsic fluid
mixed in the infused agent, which may help to reduce the
intratissue pressure and enhance the extent of agent diffusion
within the target tissue.
We herein evaluated and validated this novel in
situ permeation system and device in terms of the ability to
obtain local intratissue drug delivery by analyzing the degree of
intratissue diffusion of liquid agents. In order to further assess
the possible clinical application of the ISP-MW-1 system, we
evaluated the therapeutic utility and feasibility of this technique
for performing chemical ablation of cancer tissue in a subcutaneous
tumor model in hamsters.
Materials and methods
Construction of the in situ permeation
(ISP)-MW-1 system
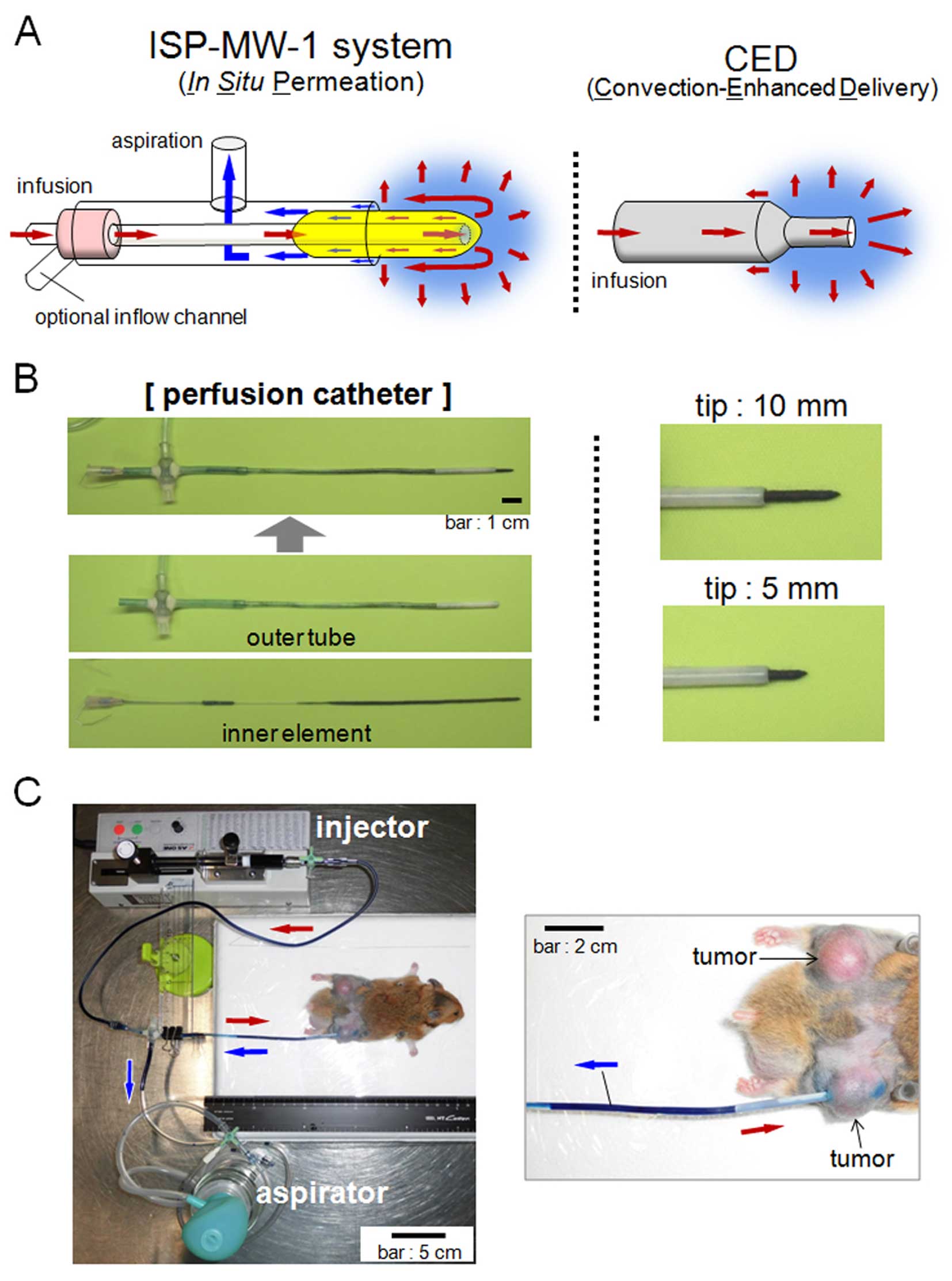
The principle of the ISP-MW-1 system-based
intratissue drug delivery is shown in Fig. 1A, the system used herein to achieve
the local diffusion and distribution of the liquid agents. In the
ISP-MW-1 system, another inflow channel is optionally added for the
air inflow (explained in Fig. 3C)
or infusion of a second agent. The perfusion catheter is inserted
and placed in the target tissue and used for in situ
permeation. The catheter consists of an outer tube and inner
element, the latter of which is inserted into and invaginated to
the outer tube (Fig. 1B). The
working tip of the perfusion catheter is scalable in length, so
that the tip at the cavity can be adjusted to the preferable length
based on the size of target tumor or tissue. Fig. 1B shows the appearance of the tip at
the 10- and 5-mm lengths. The thickness of the tip of the perfusion
catheter was approximately 1.5 mm in this study. The tip of the
inner element is covered with cotton, and the cotton cover is
proximally prolonged into the outer aspiration tube, through which
the infused agent can be efficiently absorbed and smoothly moved
into the outer tube spontaneously or via aspiration. In addition,
the placement of the prolonged cotton cover in the outer aspiration
tube prevents the entry of blood and separated small tissues into
the tube and clogging during aspiration.
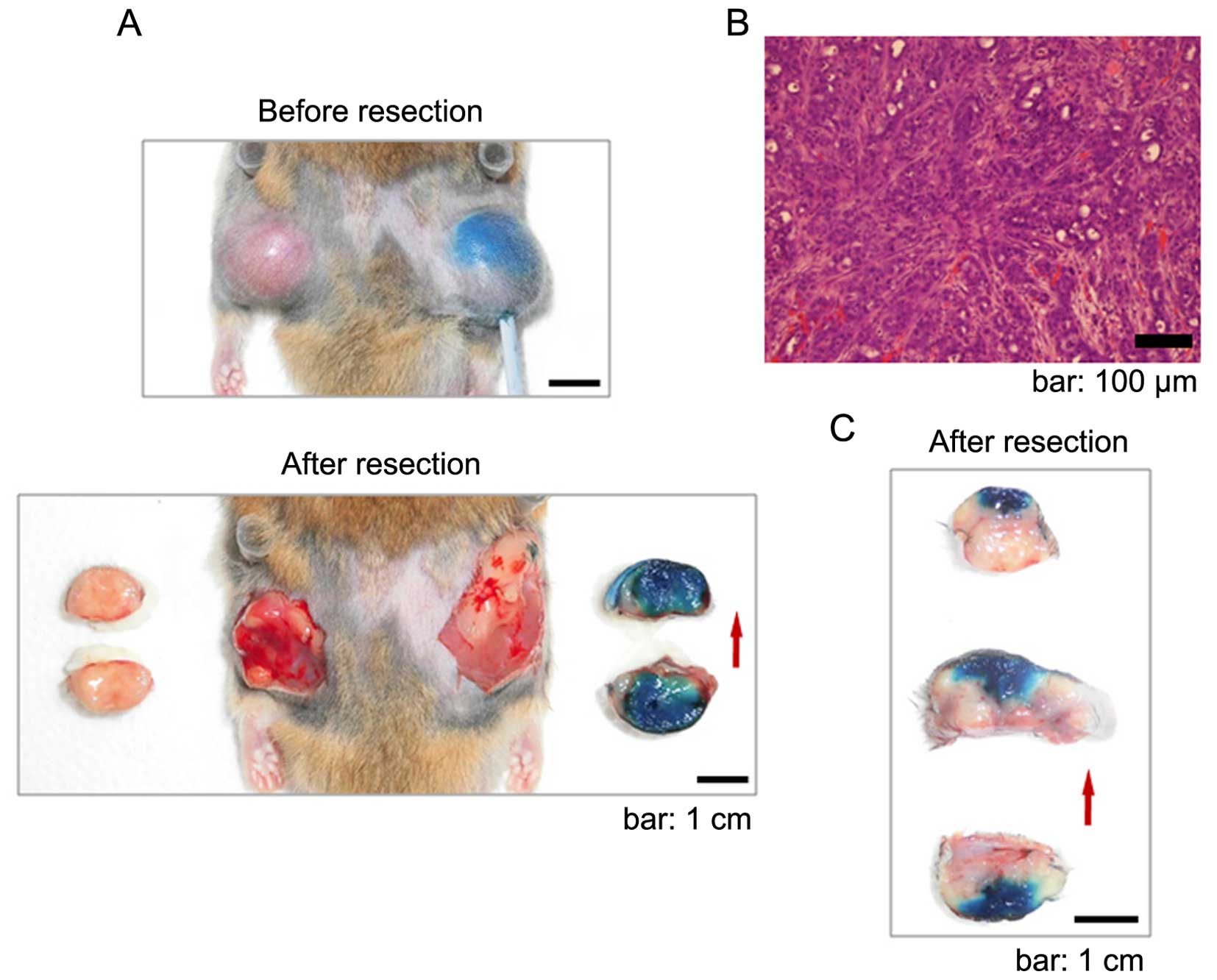
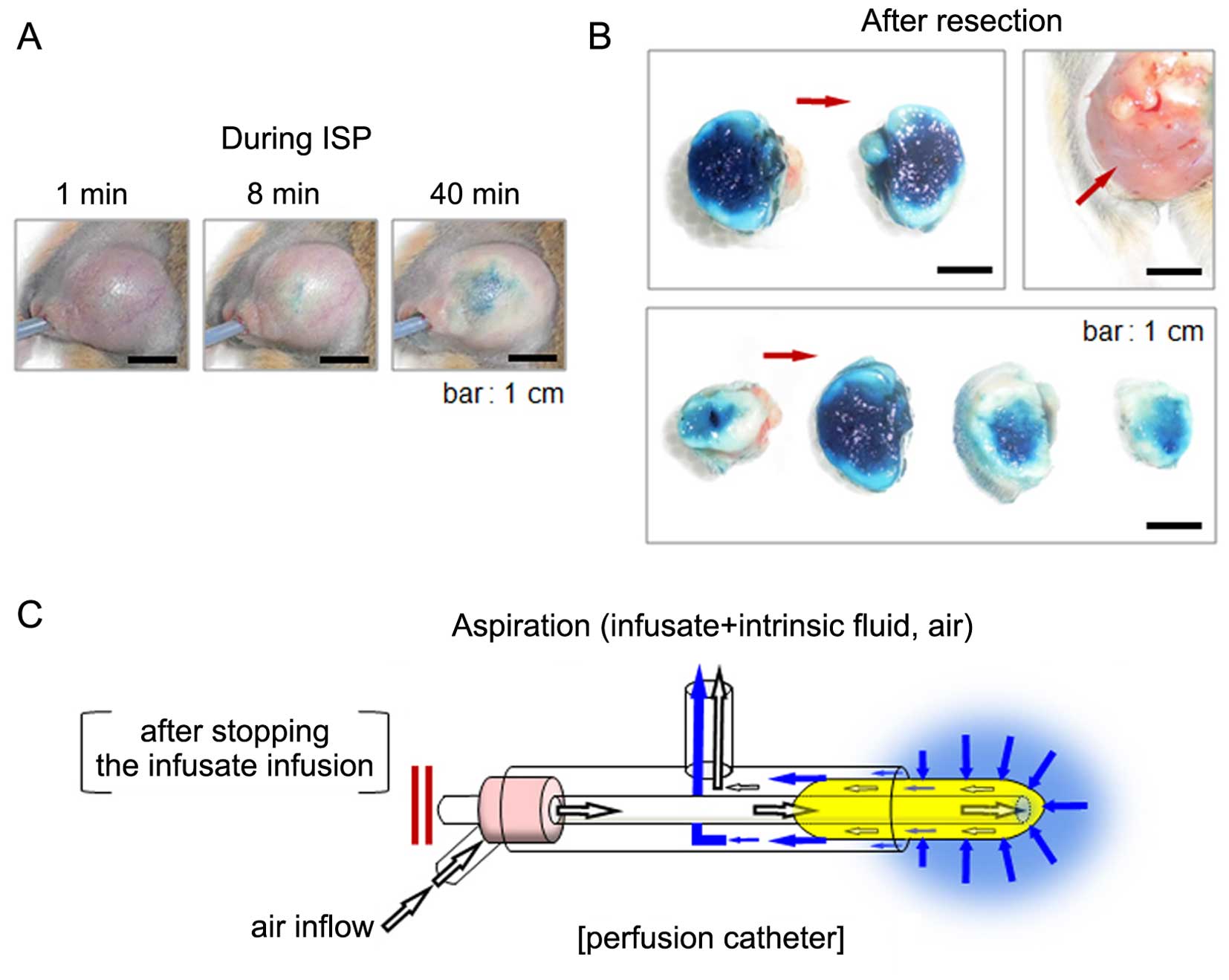 | Figure 3(A) The image of the subcutaneous
tumor during treatment with 50% acetic acid (methylene blue was
dissolved as a dye) using the ISP-MW-1 system. Before taking each
photograph at 8 and 40 min of ISP treatment, the infusate and
intrinsic fluid were intermittently removed via the
aspiration-enhanced procedure explained in (C). The tumor size is
almost the same in the three images, indicating that swelling of
the tumor tissue induced by diffusion of the agent was avoided with
the aspiration-enhanced procedure. (B) The subcutaneous tumor in
(A) was further treated with 50% acetic acid for a total 60 min
perfusion, and then the tumor was resected. Images of tumor
sections in two pieces (upper left panel) and four pieces (lower
panel) are shown. In both panels, full diffusion of the agent was
observed. The appearance of the subcutaneous area after resection
is shown in the upper right panel. The red arrow indicates the
direction of insertion of the perfusion catheter. (C) Schematic
figure of the aspiration-enhanced phase in the ISP-MW-1 system. For
removal of the infusate and tissue-intrinsic fluid from the tip of
perfusion catheter and intratissue cavity, the infusion of the
infusate is temporarily stopped and another inflow channel is
opened for the air inflow. In this phase, the aspiration induces a
continuous air flow and circulation through the tip-covered cotton,
and most of the infusate and intrinsic fluid around the tip can be
efficiently aspirated to the outer tube. The procedure with the air
inflow enables the prompt removal of the agent and intrinsic fluid
from and around the cavity. After this phase, the status of tissue
swelling and high intratissue pressure is improved, after which
flesh agents are resupplied and refilled in the cavity, resulting
in more efficient in situ permeation in the target
tissue. |
In the ISP-MW-1 system, a perfusion catheter is
connected to tubes attached to an injector and hand-operated
aspirator (Muranaka Medical Instruments Co., Osaka, Japan), which
enable the intratissue perfusion of the agents in the tip-inserted
cavity (Fig. 1C). The injector
consists of a syringe (1005TLL, 5 ml SYR; Hamilton Co., Reno, NV,
USA) and microsyringe pump (MSPE-1; AS One Co., Osaka, Japan), and
the flow rate of the infusate is controlled with the micropump. The
aspirator supplies a vacuum pressure of more than 150 mmHg by
compressing the bulb made of silicone. We herein used an outer tube
with a polypropylene component (outer diameter: 2.6 mm, inner
diameter: 2.2 mm, length: 3.5 cm) at the distal head. The proximal
end of the outer tube is attached to the inner element tubing to
the infusion micropump. The aspiration tube of the outer tube is
directly connected to the hand-operated aspirator. In order to
adjust the location of the perfusion catheter, a stand that allows
the catheter to be held and moved is utilized.
Subcutaneous tumor model in hamsters
Male Syrian hamsters were used for the animal
experiments in this study. The experiments employing the tumor
model in hamsters were approved by the Animal Care and Use
Committee, Okayama University. The animals were kept in a specific
pathogen-free housing facility at Okayama University. A hamster
pancreatic cancer cell line (HaP-T1) was provided by the RIKEN
BioResource Center (Ibaraki, Japan) through the National
Bio-Resource Project of the MEXT in Japan, and the cells were
cultivated as previously described (15). The HaP-T1 cells were inoculated
into the left and right femurs, and a hamster model bearing
bilateral subcutaneous tumors was developed.
Animal experiments of infusate diffusion
with the ISP-MW-1 system
Prior to inserting the perfusion catheter into the
tumor tissue, the animals were deeply anesthetized with
pentobarbital solution via intraperitoneal injection. A small skin
incision was made in the appropriate position for concentric
diffusion of the agent, and a needle was placed along the direction
of insertion. The tract was dilated using larger sheaths up to the
outer diameter, so that the perfusion catheter may be inserted into
the tract. Depending on the tumor size, either a 10- or 5-mm-long
tip of the perfusion catheter was selected for in situ
permeation in this study (Fig.
1B). The perfusion catheter was carefully inserted into the
tract so that the tip was located as centrally as possible within
the tumor tissue. Dehydrated ethanol, saline and 50% acetic acid
(diluted with H2O) were evaluated as the vehicle, and
methylene blue (Wako Pure Chemical Industries, Osaka, Japan) was
added to the vehicle as a dye at a concentration of 2 mg/ml. Before
applying the agents, the insoluble material was removed as
precipitation. We used the dye to evaluate the degree of
intratissue diffusion and distribution of the agent achieved with
the ISP-MW-1 system, and the dye diffused edge was interpreted as
indicating the extent of permeation of the agent in the in
vivo experiments. The agent was drawn into a Hamilton syringe
and the attached tube, so that no dead space was apparent. The tube
was subsequently connected to the perfusion catheter, and the
catheter was then flushed until the solution dripped from the
tip.
After inserting the perfusion catheter into the
tumor, the microsyringe pump was placed and the intratissue
perfusion of the agent was started at the indicated flow rate for
each treatment. The total time of ISP-MW-1 therapy was also
indicated for each treatment. During the ISP procedure, periodical
aspiration using the hand-operated aspirator was performed in order
to vacuum the cavity and keep it at negative pressure. As explained
in Fig. 3C, the
aspiration-enhanced procedure was further added to remove the agent
and tissue-intrinsic fluid efficiently from and around the tip
cavity. Aspiration was conducted at a frequency required to avoid
leakage of the infusate from the point of catheter insertion. An
image of the entire device during the ISP-MW-1 procedure with
dehydrated ethanol in the hamster model bearing a subcutaneous
tumor is shown in Fig. 1C. When
the perfusion of the infusate was complete, the perfusion catheter
was slowly withdrawn over a period of 10 sec and the tumors were
resected. The tumor was cut into 8- to 10-mm-thick sections,
allowing for observation of the distribution of the dye within the
tumor.
Animal experiments for cancer chemical
ablation using the ISP-MW-1 system
Three hamsters bearing bilateral subcutaneous tumors
were used for the cancer ablation study. Before treatment, the size
of all tumors was measured and the tumor volume was calculated
using the previously described formula (16). On day 0, the side of treatment was
randomly selected, and the tumor was treated with 50% acetic acid
using the ISP-MW-1 system. Sham treatment was performed on the
untreated tumor side simply by inserting another perfusion
catheter. All hamsters were examined to determine the bilateral
tumor volume on day 7 after treatment and then sacrificed.
Histological procedure
For the histological observation of the
HaP-T1-derived tumors, the subcutaneous tumors were dissected at
the time of sacrifice of the animals. The tumor tissue was fixed in
formalin and embedded in paraffin to obtain sections. The sections
(5 μm) were stained with hematoxylin and eosin and photographed for
histopathology.
Results
Whole intratissue diffusion of the
chemical agents was achieved with the ISP-MW-1 system
In order to investigate the utility of the ISP-MW-1
system for achieving the in vivo focal distribution of a
liquid drug, a subcutaneous tumor model in hamsters was used. The
histopathology of the tumors indicated solid and malignant
proliferation of cancer cells (Fig.
2B). Dehydrated ethanol, saline and 50% acetic acid were used
as the vehicle, and the findings of intratissue diffusion are shown
in Figs. 2A and C, and 3B, respectively. The perfusion parameters
of the perfusion catheter and ISP-MW-1 treatment were as follows:
[dehydrated ethanol; tip length: 10 mm, flow rate: 50 μl/min, total
perfusion time: 60 min], [saline; tip length: 10 mm, flow rate: 100
μl/min, total perfusion time: 3 h] and [50% acetic acid; tip
length: 10 mm, flow rate: 100 μl/min (first 45 min) and 250 μl/min
(latter 15 mins, total perfusion time: 60 min]. Within 10 min of
perfusion with the ISP-MW-1 system, noticeable dye staining was
typically observed through the tumor skin (Figs. 1C and 3A). Regarding the results for the
diffusion experiments with dehydrated ethanol and 50% acetic acid,
the agent was distributed throughout the tumor tissue in each
section and confined within the area of the tumor, with no
significant leakage into the peritumoral space (Figs. 2A and 3B). When saline was used as the vehicle,
the intratissue diffusion into the tumor tissue was relatively
partial in comparison to that observed with the other two vehicles
(Fig. 2C). When 50% acetic acid
used as the vehicle, the edge of intratissue diffusion usually
presented as a white band (Fig.
3B), indicating that only acetic acid had reached that area. In
the ISP-MW-1 system, the flow rate (injection speed) can be
controlled with the microsyringe pump, which may have influenced
the extent of agent diffusion into the tumor tissue. No obvious
leakage of the infused agents was observed from the catheter/tissue
interface of the point of insertion during the ISP-MW-1 procedure.
In addition, upon withdrawal of the perfusion catheter after the
completion of perfusion, no leakage of the agents was observed at
the point of insertion of the perfusion catheter. No apparent side
effects were observed during the ISP-MW-1 procedure in the hamsters
treated with the three types of vehicle.
An aspiration-enhanced phase was added as
a step to the ISP-MW-1 system
In order to control the rate of diffusion and
distribute a greater volume of agent, we herein attempted to remove
the infused agent and target tissue-intrinsic fluid surrounding the
tip of the catheter. As shown in Fig.
3C, an aspiration-enhanced procedure was intermittently added
as a step to the ISP-MW-1 system in the experiments conducted with
50% acetic acid as the vehicle. In order to obtain the prompt and
efficient removal of the fluid from and around the tip cavity, the
air inflow was induced to the tip and the air was subsequently
aspirated with the agent and intrinsic fluid through the outer
tube. In the aspiration-enhanced phase, the air was taken through
the optional inflow channel after stopping the infusate infusion
(Fig. 3C). Consequently, the tumor
size remained almost the same during the ISP-MW-1 procedure
(Fig. 3A), indicating that
swelling of the tumor tissue as a result of diffusion of the agent
was avoided with the aspiration-enhanced procedure. These results
also suggest that both the infusate and intrinsic fluid at or
around the tip were smoothly eliminated in the ISP-MW-1 step, which
increased the intratissue standby capacity and enhanced the
consequent agent flow and diffusion within the tissue.
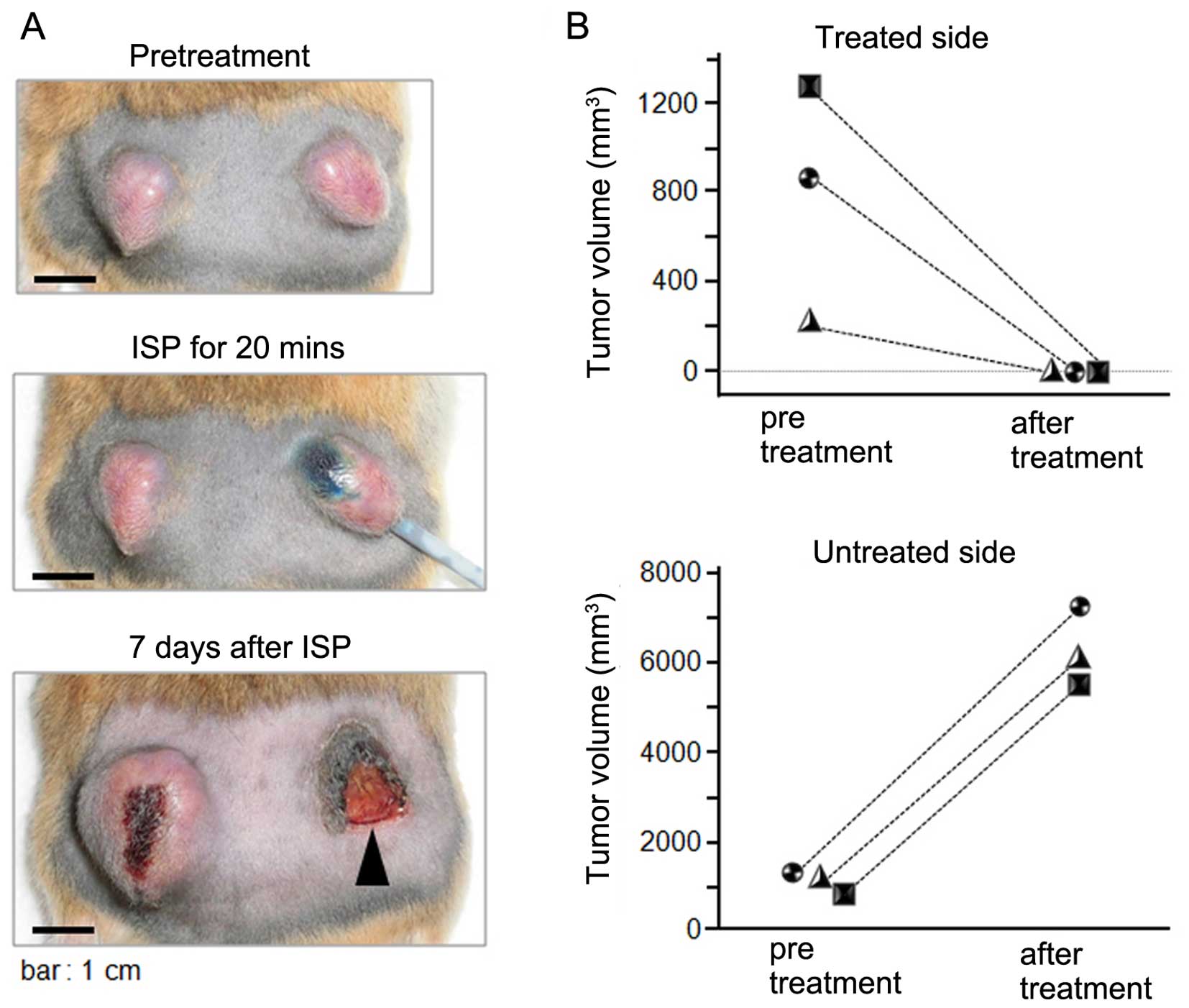
Complete tumor ablation was achieved with
the perfusion of 50% acetic acid using the ISP-MW-1 system
Based on the promising results obtained in the dye
diffusion studies, we next explored the utility of the ISP-MW-1
system for achieving therapeutic tumor ablation. The anti-tumor
effects of the local therapy with 50% acetic acid were evaluated
using three tumor-bearing hamsters (Fig. 4). In the tumors treated with acetic
acid, the length of the tip of the perfusion catheter and the total
time for perfusion was altered in reference to the tumor size prior
to treatment. The perfusion parameters of the perfusion catheter
and ISP-MW-1 treatment (Fig. 4B)
were as follows: [hamster 1 (square symbol); tip length: 10 mm,
flow rate: 100 μl/minute, total perfusion time: 20 min], [hamster 2
(circle symbol); tip length: 10 mm, flow rate: 100 μl/min, total
perfusion time: 10 min] and [hamster 3 (triangle symbol); tip
length: 5 mm, flow rate: 100 μl/min, total perfusion time: 5 min].
In the untreated tumors, the perfusion catheter was temporarily
inserted as a sham operation, and the tumor growth was monitored as
a negative control. As shown in Fig.
4B, all treated tumors completely disappeared following
chemical ablation on day 7 after the treatment. An image of the
treatment course in one of the hamsters is shown in Fig. 4A and only scar, without residual
cancer tissue, was observed on the treated side. On the other hand,
the tumor on the untreated side had rapidly grown. No apparent
complications were observed in the hamsters during the cancer
ablative therapy. Therefore, significant therapeutic effects were
obtained using the chemical ablation method with the ISP-MW-1
system.
Discussion
We evaluated the utility of the ISP-MW-1 system for
achieving the in situ permeation of liquid agents in a
subcutaneous tumor model in hamsters. Dehydrated ethanol, saline
and 50% acetic acid were employed as the vehicle, and methylene
blue was used as a dissolved substance to assess the extent of
agent diffusion. As a result, almost all of the tumor tissue within
the capsule (tumor size: ~3 cm) was permeated with dehydrated
ethanol and 50% acetic acid and partially with saline. We further
demonstrated that ISP treatment with 50% acetic acid completely
ablated the subcutaneous tumors in all of the treated hamsters.
Therefore, the ISP-MW-1 system is a promising approach for
achieving an optimal intratissue drug/vehicle distribution and
providing local ablation therapy for cancer lesions.
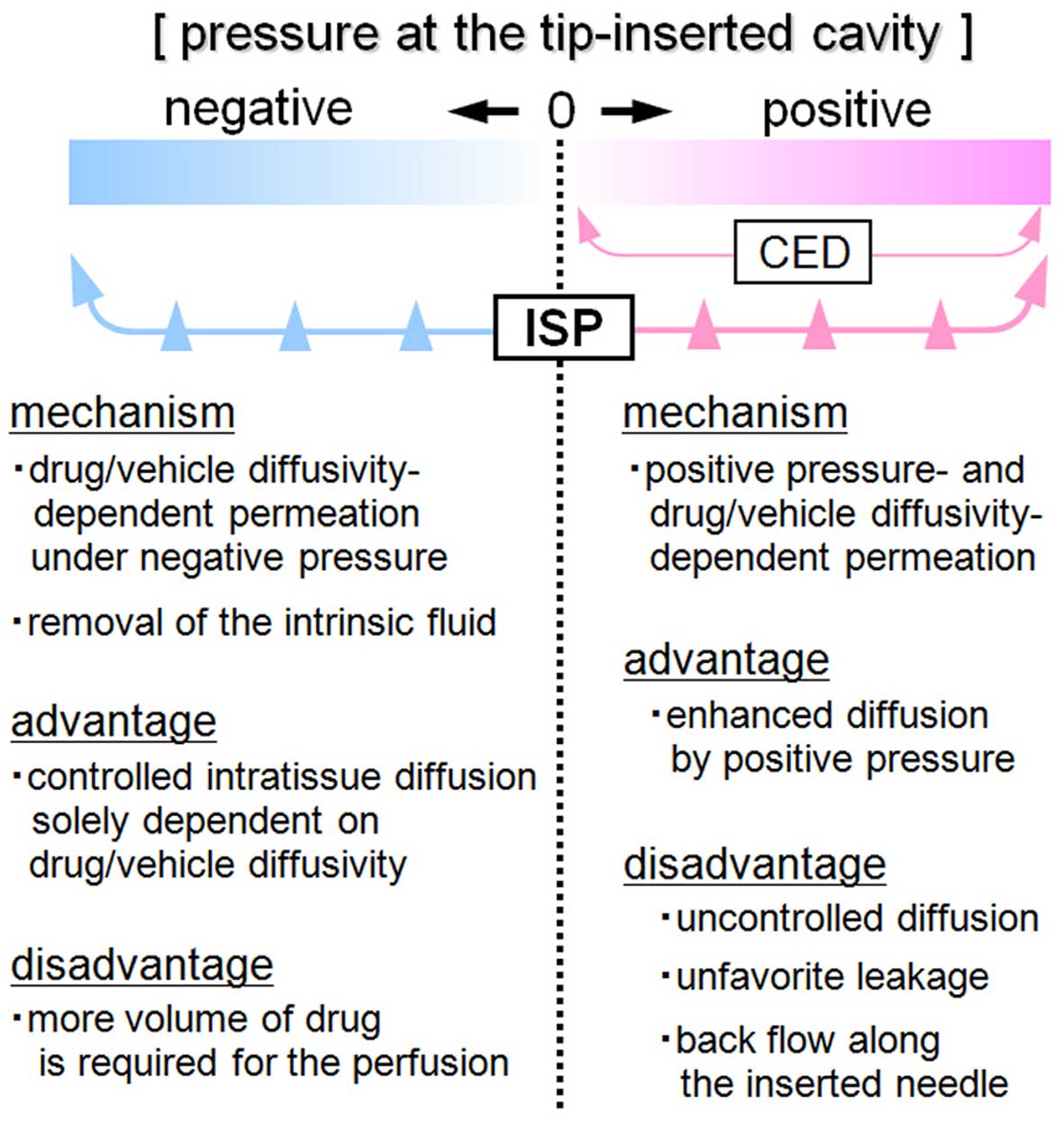
To our knowledge, ISP-MW-1 is the first infusion
device that enables the clinician to control the extent of in
situ permeation by changing the pressure in the tip-inserted
cavity from positive to negative (Fig.
5). When the cavity pressure is maintained positive by setting
the appropriate infusion speed and aspiration pressure, the
ISP-MW-1 works as a CED system and can be employed to acquire more
enhanced diffusion in comparison to that seen with negative
pressure. On the other hand, when the pressure is maintained to be
negative, more controllable diffusion is expected due to the
diffusivity of the agent itself. In this study, we validated the
ability of the ISP-MW-1 system and its perfusion catheter for
achieving controllable in situ perfusion of the liquid
agents. Intratissue perfusion was performed under conditions of
controlled infusion and aspiration, and there were no apparent
signs of leakage of the infusate from the point of catheter
insertion. The tip of the inner element is covered with cotton and
the cotton cover is prolonged into the outer aspiration tube,
through which the liquid agent smoothly moves into the outer tube
for aspiration. In addition, the placement of the prolonged cotton
cover in the outer aspiration tube prevents the entry of blood and
separated small tissues into the tube and clogging during
aspiration. It was important that the pressure in the tip-inserted
cavity be periodically kept negative in all experiments in this
study. The type of aspirator used in the current study was useful
for maintaining a negative cavity pressure, as confirmed in
additional experiments (data not shown). Due to the effects of
aspiration, intratissue perfusion can be promoted through the
distal to proximal part of the tip of the perfusion catheter. In
addition, perfusion of the solute and vehicle continuously supplies
fresh agents to the target tissue, which subsequently promotes the
concentration-dependent intratissue diffusion of the agent. When
the cavity pressure is kept negative using the ISP-MW-1 system and
there is no positive pressure-dependent fluid convection, the
diffusion depends solely on the drug permeation induced by the
concentration gradient and diffusivity of the agent in the target
tissue.
One obstacle to achieving optimal intratissue drug
diffusion is the presence of limited interstitial space and/or low
capacity of the target tissue to accept the fluid flow. In order to
overcome these issues, we developed the ISP-MW-1 system with the
goal of removing the tissue-intrinsic fluid and widening the
interstitial pathway. In the perfusion phase of the ISP-MW-1
system, the intrinsic fluid mixed with the infusate can be
aspirated and removed continuously. Furthermore, in the
aspiration-enhanced phase with the air inflow after stopping the
infusate infusion, both the infusate and intrinsic fluid at or
around the tip may be efficiently eliminated and replaced with air
(Fig. 3C). For this purpose, the
cotton-covered tip is important for absorbing the fluid in the
cavity and aspirating this fluid out to the outer tube. Hence,
using the ISP-MW-1 device, we established a procedure for achieving
intratissue fluid removal in order to increase the standby capacity
and thus enhance the agent flow and degree of diffusion within the
tissue. It is likely that tumor swelling and intratissue pressure
elevation due to the diffusion of the agent may be efficiently
improved using the aspiration-enhanced procedure, as the tumor size
remained almost same during the ISP-MW-1 procedure in this study
(Fig. 3A).
It is notable that the diffusion of acetic acid
obtained with the ISP-MW-1 system reached the whole tumor and was
limited to within the tumor tissue. The advantages of controlled
intratissue diffusion have also been demonstrated in experiments
with ethanol. We consider that infusion with the ISP-MW-1 system
achieves a homogeneous distribution of infusate and improves the
extent of controllability of in situ permeation via the
following mechanisms (Fig. 5): i)
performing focal perfusion under negative cavity pressure enables
the diffusion to solely depend on the drug permeation induced by
the concentration gradient and diffusivity of the agent into the
target tissue, ii) removing the intratissue fluid, including the
tissue-intrinsic fluid, increases the standby capacity of the
extracellular space and makes it easy to control the subsequent
agent permeation within the tissue. As for other advantages of the
ISP-MW-1 system, the tip of the perfusion catheter is scalable in
both length and thickness. The tip length and thickness should be
altered for proper use based on the size of the target tumor or
tissue. In addition, the outer diameter (OD) of the outer tube of
the perfusion catheter can be also altered based on the target
situation in each case. We used 2.6-mm OD outer tubes in the
present study and are currently developing thinner tubes with an OD
of ~1.2 mm, thereby enabling more non-invasive access to the target
tissue. Preliminary tests of the thinner perfusion catheter are
already underway to assess the utility for intratissue diffusion.
On the other hand, it should be noted that there are disadvantages
with respect to in situ permeation with the ISP-MW-1 system.
Under negative cavity pressure, it is conceivable that the degree
of intratissue permeation may be limited due to the lack of
promoting power. Since the ISP-MW-1 procedure requires maintenance
of the perfusion of the infused drug, a greater volume or amount of
the drug is consumed in comparison with the CED system.
The application of ISP-MW-1 treatment with 50%
acetic acid eradicated the hamster tumors in this study, consistent
with the findings that whole intratissue diffusion of the tumor can
be successfully achieved using the ISP-MW-1 procedure. Many studies
have demonstrated the effectiveness of chemical tumor ablation
using a variety of chemical liquid agents-ethanol, carbolic acid,
acetic acid and glycerin (2,6–8,17).
Among these agents, acetic acid has been reported to be a very
strong ablative agent and is utilized in the clinical field
(2,7,18).
However, has also been reported that the direct injection of acetic
acid into hepatomas in a rat model results in a significantly high
rate of death, likely due to uncontrolled leakage of the agent into
other spaces (19). Since the
chemical ablative agents used in local therapy are usually
cytotoxic, the ability to control the extent of intratissue
diffusion and distribution is essential for preventing off-target
side effects. In this study, we demonstrated that the ISP-MW-1
system is a promising approach for obtaining controlled intratissue
diffusion of therapeutic agents and providing local ablation
therapy for cancer lesions.
To date, a variety of local therapies using drugs
have been developed for the treatment of cancer and non-cancer
diseases (20–25). We expect that the ISP-MW-1 system
may be used to enhance the effectiveness of local therapies by
achieving widespread intratissue diffusion of these drugs. It is
also expected that the improved degree of controllability of
intratissue drug delivery will expand the application of local
therapies for various diseases and promote the development of novel
drugs and vehicles for use in local therapy. In the protocol for
local therapy, the choice of vehicle for the drugs is particularly
important for each individual disease. As for cancer therapeutics,
cytotoxic chemical agents are available as a vehicle. If commonly
used chemotherapeutic drugs could be dissolved as solutes in
chemical vehicles, the compound agents would exert much greater
ablative effects for the target tumor. On the other hand, regarding
treatment for local lesions of non-cancer diseases, gene
therapeutic approaches, cellular transplantation and
anti-pathogenic drugs are available to locally reconstruct the
tissue functions and eliminate the pathogens. In any case, the
vehicle for the drug should not impede the intrinsic efficacy of
the drug itself. In order to utilize the ISP-MW-1 system in the
clinical setting, feasibility studies are needed, taking into
consideration the characteristics of each disease and the
investigated drug.
We herein demonstrated that the in situ
permeation method using the ISP-MW-1 system is useful for
controlling and increasing the intratissue distribution of locally
infused agents. Regarding the possible clinical application of
ISP-MW-1, intra-operative imaging guidance with computed tomography
(CT), ultrasonography or magnetic resonance imaging (MRI) may help
to yield a more accurate distribution of the infused agents. We
believe that this system enables improved efficacious infusion of
therapeutic agents and further elicits the effectiveness of local
therapies as a minimally invasive option. We finally note that the
ISP-MW-1 method may be applicable to a broad range of medicinal and
industrial fields, such as regenerative medicine, drug delivery
systems, biochemistry and materials technologies, as well as cancer
therapeutics.
Acknowledgements
This work was supported by scientific research
grants (KAKENHI: 24390368, 25462478, 25670683, 26293352) from the
Ministry of Education, Culture, Sports, Science and Technology of
Japan. The author thanks Ms. Shun-Ai Li, Ms. Fusaka Oonari and Mr.
Hideo Ueki (Okayama University) for their valuable assistance. Dr
M. Watanabe is the inventor of the ISP-MW-1 system. Okayama
University is applying for a patent for the ISP system.
References
|
1
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohnishi K, Yoshioka H, Ito S and Fujiwara
K: Prospective randomized controlled trial comparing percutaneous
acetic acid injection and percutaneous ethanol injection for small
hepatocellular carcinoma. Hepatology. 27:67–72. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shah SS, Jacobs DL, Krasinkas AM, Furth
EE, Itkin M and Clark TW: Percutaneous ablation of VX2
carcinoma-induced liver tumors with use of ethanol versus acetic
acid: Pilot study in a rabbit model. J Vasc Interv Radiol.
15:63–67. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lonser RR, Warren KE, Butman JA, Quezado
Z, Robison RA, Walbridge S, Schiffman R, Merrill M, Walker ML, Park
DM, et al: Real-time image-guided direct convective perfusion of
intrinsic brainstem lesions. Technical note. J Neurosurg.
107:190–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chittiboina P, Heiss JD, Warren KE and
Lonser RR: Magnetic resonance imaging properties of convective
delivery in diffuse intrinsic pontine gliomas. J Neurosurg Pediatr.
13:276–282. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fotiadis NI, Sabharwal T, Morales JP,
Hodgson DJ, O'Brien TS and Adam A: Combined percutaneous
radiofrequency ablation and ethanol injection of renal tumours:
Midterm results. Eur Urol. 52:777–784. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao YY, Tian JL, Li JK, Yang L and Zhang
JS: CT-guided percutaneous chemical ablation of adrenal neoplasms.
AJR Am J Roentgenol. 190:105–110. 2008. View Article : Google Scholar
|
|
8
|
Plante MK, Marks LS, Anderson R, Amling C,
Rukstalis D, Badlani G, Getlin L and Vang E: Phase I/II examination
of transurethral ethanol ablation of the prostate for the treatment
of symptomatic benign prostatic hyperplasia. J Urol. 177:1030–1035;
discussion 1035. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watanabe M, Nasu Y and Kumon H:
Adenovirus-mediated REIC/Dkk-3 gene therapy: Development of an
autologous cancer vaccination therapy (Review). Oncol Lett.
7:595–601. 2014.PubMed/NCBI
|
|
10
|
Bobo RH, Laske DW, Akbasak A, Morrison PF,
Dedrick RL and Oldfield EH: Convection-enhanced delivery of
macromolecules in the brain. Proc Natl Acad Sci USA. 91:2076–2080.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oh S, Odland R, Wilson SR, Kroeger KM, Liu
C, Lowenstein PR, Castro MG, Hall WA and Ohlfest JR: Improved
distribution of small molecules and viral vectors in the murine
brain using a hollow fiber catheter. J Neurosurg. 107:568–577.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barua NU, Gill SS and Love S:
Convection-enhanced drug delivery to the brain: Therapeutic
potential and neuropatho-logical considerations. Brain Pathol.
24:117–127. 2014. View Article : Google Scholar
|
|
13
|
Brady ML, Raghavan R, Singh D, Anand PJ,
Fleisher AS, Mata J, Broaddus WC and Olbricht WL: In vivo
performance of a micro-fabricated catheter for intraparenchymal
delivery. J Neurosci Methods. 229:76–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lonser RR, Sarntinoranont M, Morrison PF
and Oldfield EH: Convection-enhanced delivery to the central
nervous system. J Neurosurg. 122:697–706. 2015. View Article : Google Scholar
|
|
15
|
Watanabe M, Sakaguchi M, Kinoshita R, Kaku
H, Ariyoshi Y, Ueki H, Tanimoto R, Ebara S, Ochiai K, Futami J, et
al: A novel gene expression system strongly enhances the anticancer
effects of a REIC/Dkk-3-encoding adenoviral vector. Oncol Rep.
31:1089–1095. 2014.PubMed/NCBI
|
|
16
|
Huang P, Watanabe M, Kaku H, Ueki H,
Noguchi H, Sugimoto M, Hirata T, Yamada H, Takei K, Zheng S, et al:
Cancer stem cell-like characteristics of a CD133(+) subpopulation
in the J82 human bladder cancer cell line. Mol Clin Oncol.
1:180–184. 2013.PubMed/NCBI
|
|
17
|
Bhullar JS, Subhas G, Chaudhary S,
Silberberg B, Tilak J, Decker M and Mittal VK: Intratumoral acetic
acid injection eradicates human prostate cancer tumors in a murine
model. World J Urol. 31:331–337. 2013. View Article : Google Scholar
|
|
18
|
Germani G, Pleguezuelo M, Gurusamy K,
Meyer T, Isgrò G and Burroughs AK: Clinical outcomes of
radiofrequency ablation, percutaneous alcohol and acetic acid
injection for hepatocelullar carcinoma: A meta-analysis. J Hepatol.
52:380–388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zardi EM, Borzomati D, Cacciapaglia F,
Picardi A, Valeri S, Bianchi A, Galeotti T, Coppolino G, Coppola R
and Afeltra A: Percutaneous ultrasound-guided ablation of
BW7756-hepatoma using ethanol or acetic acid in a rat model. BMC
Gastroenterol. 7:452007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Muramatsu S, Fujimoto K, Kato S, Mizukami
H, Asari S, Ikeguchi K, Kawakami T, Urabe M, Kume A, Sato T, et al:
A phase I study of aromatic L-amino acid decarboxylase gene therapy
for Parkinson's disease. Mol Ther. 18:1731–1735. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
High KA and Aubourg P: rAAV human trial
experience. Methods Mol Biol. 807:429–457. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Juratli TA, Schackert G and Krex D:
Current status of local therapy in malignant gliomas - a clinical
review of three selected approaches. Pharmacol Ther. 139:341–358.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shien K, Tanaka N, Watanabe M, Soh J,
Sakaguchi M, Matsuo K, Yamamoto H, Furukawa M, Asano H, Tsukuda K,
et al: Anti-cancer effects of REIC/Dkk-3-encoding adenoviral vector
for the treatment of non-small cell lung cancer. PLoS One.
9:e879002014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaufman HL, Ruby CE, Hughes T and
Slingluff CL Jr: Current status of granulocyte-macrophage
colony-stimulating factor in the immunotherapy of melanoma. J
Immunother Cancer. 2:112014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimazu Y, Kurozumi K, Ichikawa T, Fujii
K, Onishi M, Ishida J, Oka T, Watanabe M, Nasu Y, Kumon H and Date
I: Integrin antagonist augments the therapeutic effect of
adenovirus-mediated REIC/Dkk-3 gene therapy for malignant glioma.
Gene Ther. 22:146–154. 2015. View Article : Google Scholar
|