Introduction
Ribosome-inactivating proteins (RIPs) comprise a
large family of toxic proteins, which are widely distributed among
plants, fungi, algae and bacteria (1–5).
Based on their structural properties, RIPs have been divided into
three types (I, II and III). Types I and II are the most prevalent
forms of RIPs, where type I has a single polypeptide chain, while
type II consists of two polypeptide chains (A and B-chains) linked
together by a disulfide bond. RIPs inactivate the cellular ribosome
machinery by inhibiting the protein translation irreversibly that
leads to the death of the respective cells. Toxicity of RIPs is
greatly attributed to their RNA N-glycosidase activity, which is
responsible for depurination of 28S rRNA of eukaryotic ribosomes
(6–9). An additional mechanism of action for
type II RIP activity has also been identified, which is based on
the cellular endoplasmic reticulum (ER) stress (10). Since their discovery and especially
in the last ten years, RIPs have drawn a considerable amount of
attention as therapeutic agents for the treatment of cancer. In
this regard, toxic domains of RIPs are either linked to especially
designed molecules (immunotoxins) or delivered directly as cancer
gene therapy (11–14).
Ximenia americana, also known as sea lemon or
yellow plum, is a small sprawling tree which is widely distributed
over the tropical and subtropical areas. Powdered material of this
plant has been used in African regions as traditional medicine for
the treatment of certain maligant tumors. The active component of
X. americana, which is a 58–62-kDa protein, was identified
and purified almost a decade ago and named ‘Riproximin' (Rpx).
Based on its amino acid sequence and protein modeling structures,
it was placed along with the toxic group of type II RIPs. Closest
relatives of Rpx are ricin, viscum lectin I, ebulin and nigrin b,
which are classical examples of type II RIPs (15,16).
Initial in vitro toxicity data revealed the significant
antineoplastic potential of Rpx against different cell lines
belonging to leukemia and solid tumors. However, this cytotoxicity
of Rpx against different cancer cell lines varied over a broad
range (maximum by a factor of 100), which is possibly due to
differential expression of receptors required for Rpx binding,
specific molecular routes and negative feedback mechanisms
developed by the cancer cells over time. Nevertheless, Rpx
illustrated specific toxicity towards tumor cell lines, whereas
non-tumor cell lines showed either no or only a marginal
sensitivity (16–19). Further investigations on the
antineoplastic potential of Rpx were accomplished in colorectal and
pancreatic cancer liver metastasis rat animal models. Treatment of
both kind of animal models with Rpx (peroral or intraperitoneal)
showed a significant reduction in tumor burden as compared to
untreated control animals (20,21).
For illuminating the reasons, why gastrointestinal
cancer liver metastasis turned out to be a primary target of Rpx
activity, we expanded the experiments on metastasis-related aspects
of Rpx. For this purpose, we started investigating the effects of
Rpx exposure on properties of human and rat colorectal cancer (CRC)
cell lines akin to metastasis. While selecting appropriate cell
lines, we preferred two human cell lines with primary (SW480) and
metastatic (SW620) tumor origin and a primary rat cell line (CC531)
to have a comparison of the outcomes in two species for possible
extrapolation of experimental results. With regard to biological
properties of the cells, we studied the proliferation, colony
formation and wound healing assays, which link the steps from tumor
growth to metastasis. In addition, we performed the nuclear
staining, Annexin V-FITC and cell cycle analysis to assess the
possible nuclear/DNA fragmentation and/or cell cycle modulation,
respectively. We further aimed at investigating the changes in
expressional profiles of genes relevant for apoptosis and
mitochondrial/ER-stress in the selected cell lines, to gain insight
into the Rpx effects at molecular and mechanistic levels.
Materials and methods
Cell lines
Two human (SW480 and SW620) and one rat (CC531)
colon adenocarcinoma cell lines were cultured and maintained in
RPMI-1640 medium (Invitrogen, Darmstadt, Germany) supplemented with
L-glutamine (2 mM) and 10% fetal bovine serum (FBS). The cell
lines, free of pathogenic contaminations, were maintained under
standard incubation conditions with a humidified atmosphere (5%
CO2, at 37°C) and passaged routinely to maintain a
logarithmically growing cell population.
Cell proliferation assay
Proliferation of the selected cell lines was
assessed by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5
diphenyltetrazolium bromide) dye reduction assay. In brief, the
cells were seeded (5×103/well) in 96-well plates and
treated with increasing concentrations of Rpx (1.25–40 ng/ml) for
three time periods (24, 48 and 72 h). Surviving cell fractions from
treated and control groups (8 replicates/sample) were determined by
the addition of 10 μl/well MTT solution (10 mg/ml in PBS). After an
incubation period of 3 h under standard conditions, formazan
crystals formed by the viable cells were dissolved with 100 μl of
acidic 2-propanol (0.04 N HCl). Optical density was measured by an
ELISA plate reader (Anthos Mikrosysteme GmbH, Krefeld, Germany) at
540 nm wavelength (690-nm reference filter). Inhibitory
concentrations (IC) were determined by GraphPad Prism 6 software
and cell survival rates were calculated as the percentage of
untreated controls.
Colony formation assay
Following the Rpx (IC20) exposure for 48
h, 2×103 cells in a semi-liquid medium (0.4%
methyl-cellulose and 30% FBS in RPMI-1640 medium) were seeded into
6-well plates. Following the standard incubation conditions for 6–8
days, clusters of cells (>10 cells) were counted by an inverted
microscope (Leitz Fluovert FU Microscope, Wetzlar, Germany) and
grouped into small (<30 cells) or large (≥30 cells) colonies.
Furthermore, the colonies were fixed with a solution of methanol
and acetic acid (3:1 ratio respectively) and stained with 0.5%
crystal violet (in methanol). Data were represented as percentage
of the colonies formed by the untreated control cells.
Wound healing assay
The cells were seeded at optimized cell density
(2×105 SW480 and SW620, 1.25×105 CC531 cells)
in 12-well plates and were allowed to grow for 24 h under standard
conditions. Monolayers of the confluent cells were scratched in a
straight line by a 200-μl sterile yellow tip and free-floating
cells were removed. Opti-MEM®I serum reduced medium
(Invitrogen) was added and then the cells were exposed to the Rpx
(IC20). Effect on ‘wound closing' by the cells was
observed and photographed at zero and 24 h by an Axio Observer Z1
microscope (Carl Zeiss, Oberkochen, Germany). Three random
photographs were taken per sample for each time-point.
Cell cycle assay
Effects of Rpx treatment on cell cycle were
determined by propidium iodide (PI) fluorescent staining and flow
cytometry analysis (FACS). Briefly, the cells were exposed to Rpx
(IC25, IC50 or IC75) for 48 h,
harvested and washed with PBS followed by the addition of ice cold
ethanol (70%) to fix and permeabilize the cells. After an
incubation period of 2 h at 4°C, the cells were washed again and
re-suspended in PBS having RNaseA (1 mg/ml) and incubated for 30
min at 37°C. PI (50 μg/ml) was added to the cells and analysis was
done immediately (≤30 min) in a FACSCanto (BD Biosciences, San
Jose, CA, USA). Ten thousand cells (events) from each sample were
analyzed and distribution of the cells in G0/G1, S and G2/M phases
of cell cycle was calculated by ModFit LT software. In addition,
apoptotic cell fractions were determined by sub-G1 peak from the
DNA histogram using FACSDiva software (BD Biosciences).
Nuclear staining
The cells were seeded in 6-well plates having
sterilized cover slips inside and treated with Rpx
(IC25, IC50 or IC75) for 48 h.
Later on, the cells were washed with PBS, fixed with 4%
formaldehyde and permeablized with 0.3% Tritron X-100 (Sigma,
Munich, Germany). The cells were stained with 1.6 mM Hoechst 33342
dye (Invitrogen, Karlsruhe, Germany), spun on glass slides and were
systematically photographed at random areas with an Axiophot
microscope (Carl Zeiss).
Annexin V-FITC binding assay
Apoptotic response of the cells to Rpx treatment was
investigated by Annexin V-FITC apoptosis detection kit
(eBioscience, Frankfurt, Germany). In brief, the cells were treated
with Rpx (IC25, IC50 or IC75) for
48 h and harvested with EDTA free trypsin. After the two washing
steps (PBS and 1X binding buffer), the cells (2×105)
were resuspended in 100 μl of 1X binding buffer (provided by the
kit) and 5 μl of fluorochrome-conjugated Annexin V-FITC was added,
followed by 15-min incubation at room temperature in the dark.
Cells were washed with 1X binding buffer to remove additional
unbound Annexin V-FITC and resuspended in 200 μl of 1X binding
buffer. PI (5 μl) was added to each sample and analyses were done
with a FACS Calibur flow cytometer (BD Biosciences).
RNA isolation and cDNA synthesis
The cells were seeded in 6-well plates and treated
with increasing concentrations of Rpx (IC25,
IC50 or IC75) the next day. After 48 h of
exposure time, the cells were harvested, washed and total RNA was
extracted from the cell pellets with RNeasy Mini kit (Qiagen,
Hilden, Germany) following the manufacturer's protocol.
Concentrations of the isolated RNA were measured by a GeneQuant pro
spectrophotometer (GE Healthcare, Munich, Germany) and
complementary DNA (cDNA) was synthesized by Maxima reverse
transcriptase enzyme (Thermo Scientific, Schwerte, Germany).
Real-time qPCR
Effects of Rpx exposure on expression profile of
apoptosis relevant genes (IL24/MDA-7 and GADD family) were studied
by qRT-PCR methodology. For this purpose, prepared cDNA (1 μg) from
the control and treated samples was subjected to PCR amplification
procedure by using 2X LC480 master mix along with an appropriate
probe from the Universal probe library (Roche, Mannheim, Germany).
Samples were processed in triplicate and the expression level of
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and gamma-tubulin
(G-tubulin) were used for normalization of the data. The
fold-changes in the expression levels were calculated by the
2−ΔΔCT method (22).
Primer sequences used for the amplifications of selected genes are
given in Table I.
 | Table IPrimers for selected genes. |
Table I
Primers for selected genes.
| Genes | Forward primer | Reverse primer | Probe no.a | Probe IDa |
|---|
| Human |
| IL24/MDA-7 |
cccagaaactgtgggaagc |
gggcactcgtgatgttatcc | 26 | 04687574001 |
| GADD45A |
ggagagcagaagaccgaaag |
agtgatcgtgcgctgactc | 37 | 04687957001 |
| GADD45B |
cattgtctcctggtcacgaa |
taggggacccactggttgt | 10 | 04685091001 |
| GADD45G |
cagccaaagtcttgaacgtg |
cctggatcagcgtaaaatgg | 71 | 04688945001 |
| GAPDH |
agccacatcgctcagacac |
gcccaatacgaccaaatcc | 60 | 04688945001 |
| Rat |
| IL24/MDA-7 |
gctgttgaaaccacaggttct |
ttgaatttgactattttgctgtgat | 40 | 04687990001 |
| GADD45A |
cagagcagaagatcgaaagga |
gactccgagccttgctga | 65 | 04688643001 |
| GADD45B |
ctgcctcctggtcacgaa |
ttgcctctgctctcttcaca | 10 | 04685091001 |
| GADD45G |
gtccgccaaagtcctgaat |
tcgccctcatcttcttcgt | 71 | 04688945001 |
| GADD34 |
tcctctgaagggtagaaaggtg |
cttcgatctcgtgcaaactg | 26 | 04687574001 |
| GADD153 |
accaccacacctgaaagca |
agctggacactgtctcaaagg | 13 | 04685121001 |
| G-tubulin |
tctacaacccagagaacatctacct |
tgatgtcaaaaatgtcctcgtg | 25 | 04686993001 |
Statistical analysis
GraphPad Prism 6 software was used for the
statistical analysis of the data. All the data are represented as
mean ± standard deviation (SD). A P<0.05 was selected as
significant to interpret statistical significance of the
results.
Results
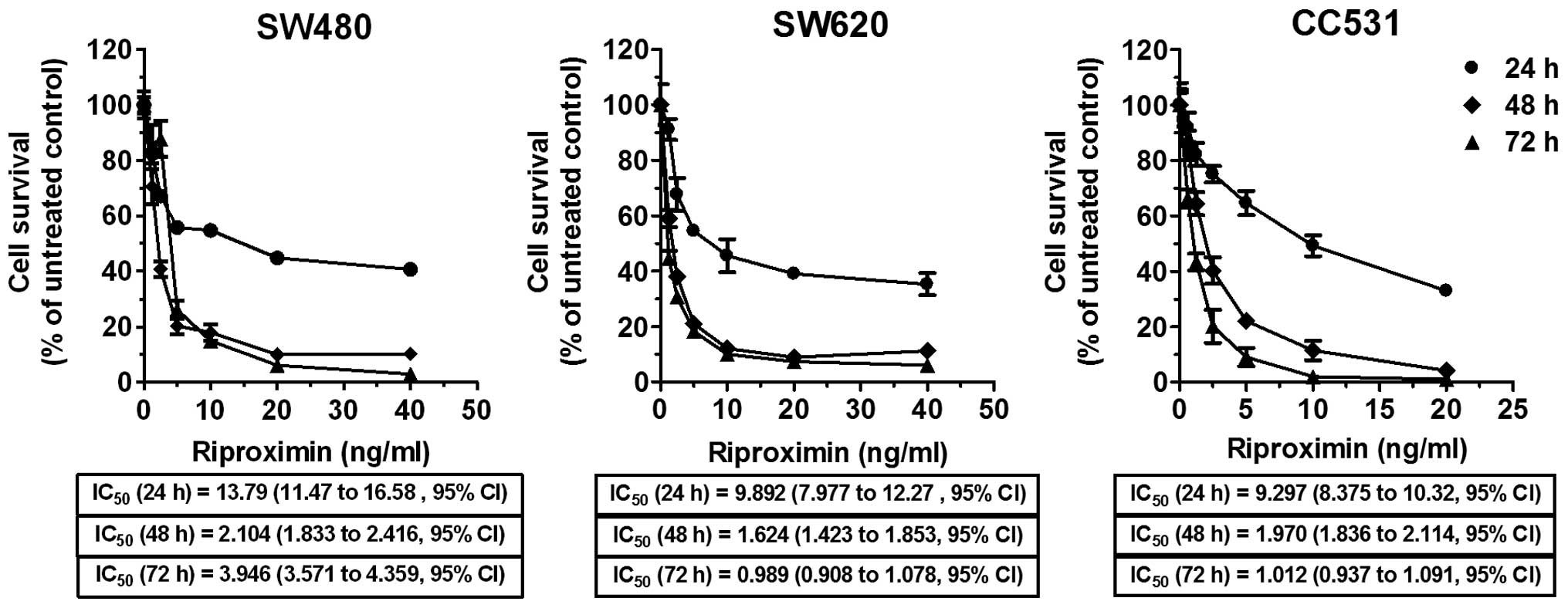
Rpx induces antiproliferative effects in
CRC cells
Cells from selected CRC cell lines were treated with
increasing concentrations of purified Rpx (1.25–40 ng/ml) for 24,
48 and 72 h, and antiproliferative effects were evaluated by MTT
dye-reduction assay. An effective antiproliferative activity was
observed as survival rates, following Rpx exposure, declined in a
concentration and time-dependent format in the exposed cells. At
low Rpx concentrations (≤5 ng/ml), a steep inhibition in survival
rate was found, especially following the 48 and 72 h exposure
periods in dose-response curves as shown in Fig. 1. The relevant concentrations
(IC20–IC75) were calculated based on this
viability assay and applied in all subsequent experimental
procedures.
Inhibition of colony formation by Rpx
exposure
Colony formation assay was used to monitor the
effects of Rpx on colony forming ability of single tumor cells. For
this purpose, the cells were exposed to the respective
IC20 of Rpx and colony forming units (CFU >10 cells)
were counted 6–8 days later. In the untreated control cells, totals
of 421, 438 and 219 colonies (small and large) were identified,
which corresponded to ratios of 21, 22 and 11% CFU for SW480, SW620
and CC531 cells, respectively. Rpx exposure reduced this ability to
produce small colonies by 32 (P=0.0075), 27 (P=0.0032) and 23%
(P=0.0063) in SW480, SW620 and CC531 cells, respectively. Rpx
treatment was more effective in inhibiting the formation of large
colonies in CC531 cells (43%, P=0.0040), as compared to SW480 (18%,
P=0.0823) and SW620 (24%, P=0.0463) cells, respectively.
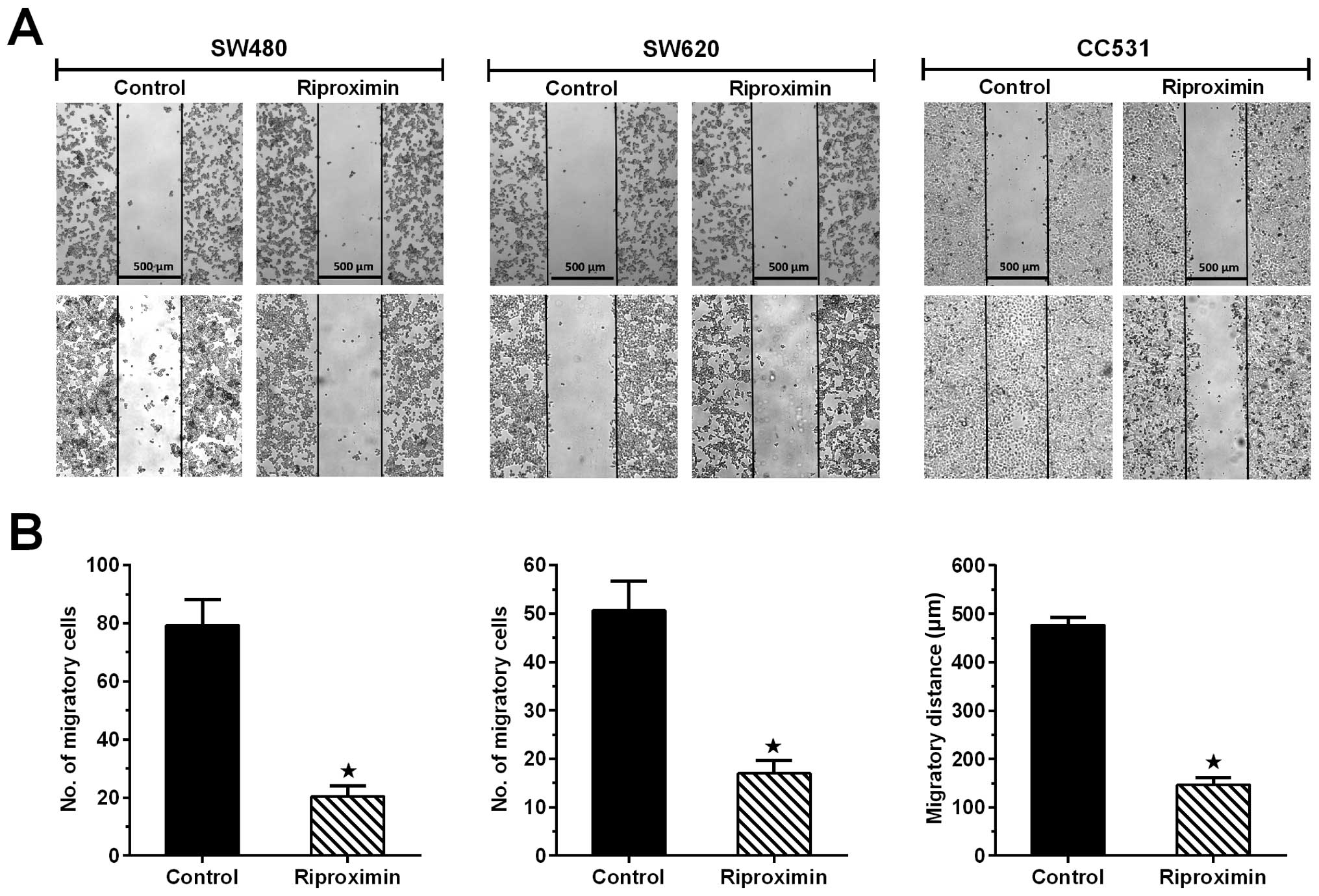
Rpx treatment interrupts the wound
healing process
The cells from the three CRC cell lines were
subjected to the ‘wound healing' assay and concomitantly exposed to
Rpx (IC20). The ability of cells to cover the scratched
area was followed for 24 h. The cell lines, following Rpx exposure,
showed significant reduction in their ability to fill the area
between the scratched edges. CC531 control cells covered almost the
complete area between the scratched edges (477±9 μm), whereas Rpx
exposure inhibited this potential by 69% (147±9 μm). For the human
CRC cell lines, wound healing response was recorded as number of
migratory cells between the scratched areas. Following Rpx
treatment, the effects were more profound in SW480 cells with 75%
inhibition of migratory potential of cells as compared to SW620
cells, where maximum reduction was 67%. The differences in the
control and treated groups were statistically significant
(P<0.05) for all cell lines as shown in Fig. 3.
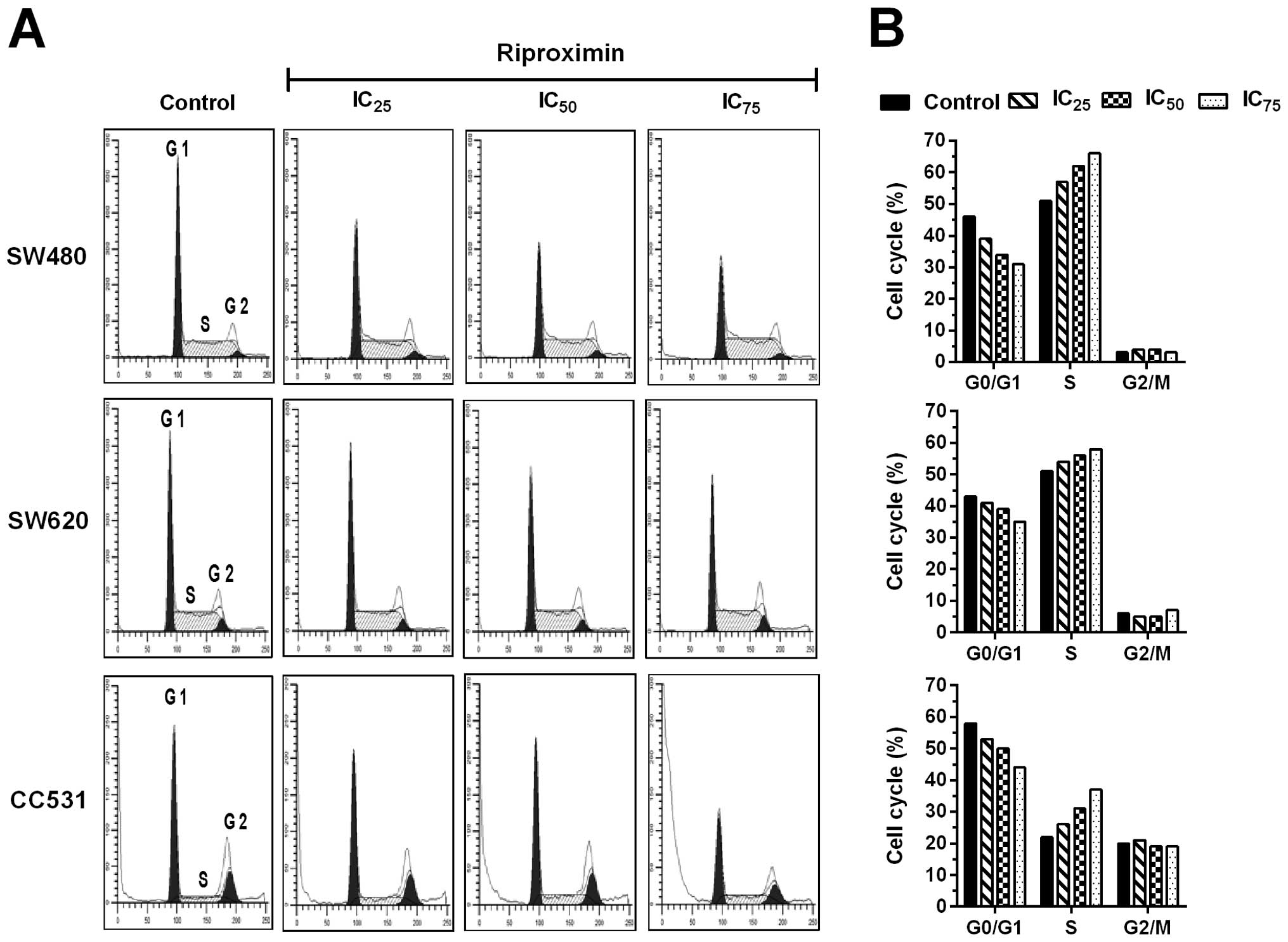
Rpx stimulated the arrest in S phase of
the cell cycle
Comparison of the two human CRC cell lines (SW480
and SW620) illustrated a similar cell cycle distribution with
relatively high and balanced ratios of G0/G1 (43–46%) and S phase
(51%) cells, while the respective proportions of cells in G2/M
phase were remarkably low (3–6%). In contrast, the rat cell line
(CC531) was characterized by preponderance of G0/G1 phase cells
(58%) and a relative equal distribution of S (20%) and G2/M phase
cells (22%). Following Rpx exposure (IC25,
IC50 or IC75) for 48 h, FACS analysis showed
significant alterations in cell cycle distributions of all three
cell lines. Rpx induced a noteworthy arrest in the S phase of the
cell cycle, as indicated by a steady increase in the cell
percentages in this phase. This phenomenon was persistent for all
cell lines in a concentration-dependent format, where G0/G1 cells
dropped by maximally 15, 8 and 14% for SW480, SW620 and CC531
cells, respectively. This loss was subsequently gained in S phase,
where the maximum increases were 15, 7 and 15% for SW480, SW620 and
CC531 cells, respectively. The G2/M phase remained almost unaltered
with the respective percentages reflecting the significant
differences between untreated cells (Fig. 4).
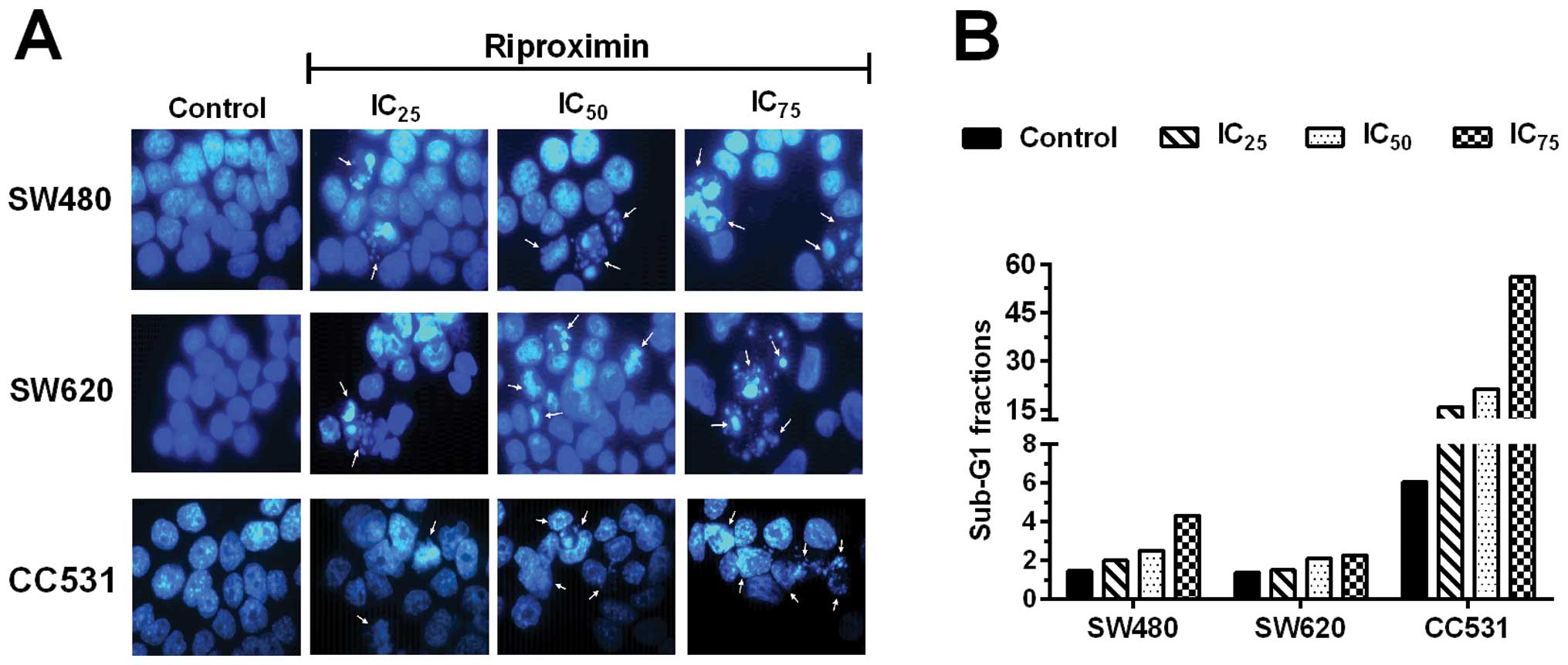
Induction of nuclear fragmentation
For determining the ability of Rpx to induce
apoptotic effects, we studied the nuclear morphology by Hoechst
33342 staining in the selected cell lines. For this purpose, the
cells were exposed to increasing concentrations of Rpx
(IC25, IC50 or IC75) and resulting
effects were monitored after 48 h of treatment. Rpx induced
destructive changes in the nuclei of all three cell lines
concentration-dependently. The effects were exerted even at lower
concentrations (IC25), where chromatin condensation and
nuclear shrinkage were observed. At higher Rpx concentrations
(IC50 or IC75), nuclear/DNA fragmentation was
also noted in the cell lines. These apoptotic effects are reflected
by the sub-G1 fractions of the three cell lines obtained by flow
cytometry (Fig. 5).
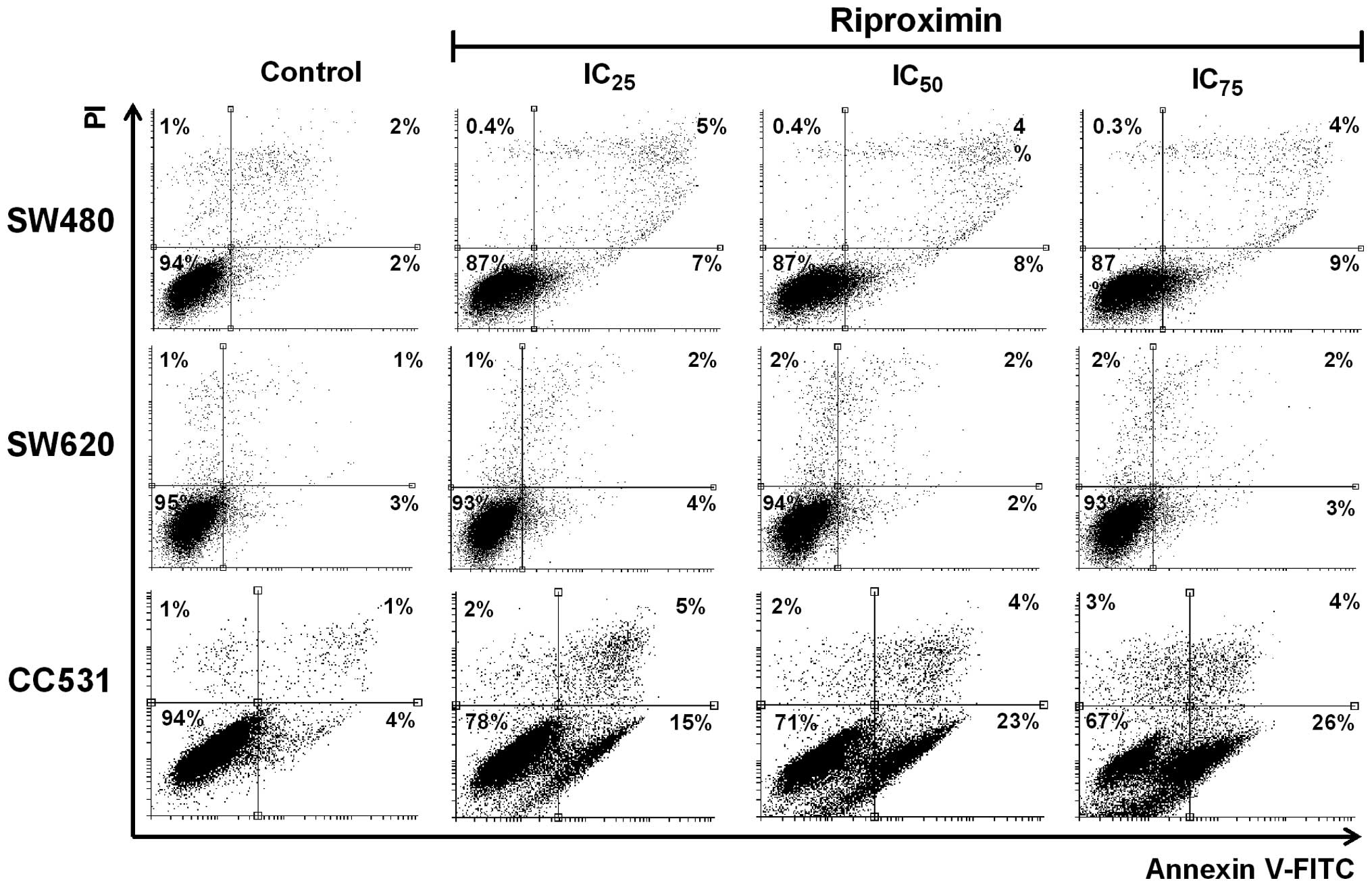
Rpx as a stimulus of apoptosis
To validate the ability of Rpx as an apoptosis
inducer, which we observed initially by Hoechst 33342 staining,
Annexin V-FITC based assay was performed in the selected cell
lines. The cells were exposed to Rpx (IC25,
IC50 or IC75) for 48 h, labeled with Annexin
V-FITC and examined by FACS analysis. A significant increase in
Annexin V-FITC-positive cells (7–22%) was observed in response to
the increasing concentrations of Rpx, as shown in Fig. 6. This steady increase in the
percentage of Annexin V-FITC bound cells concentration-dependently
corroborated our cytotoxicity and DNA/nuclear fragmentation data
from MTT and nuclear staining assays, respectively.
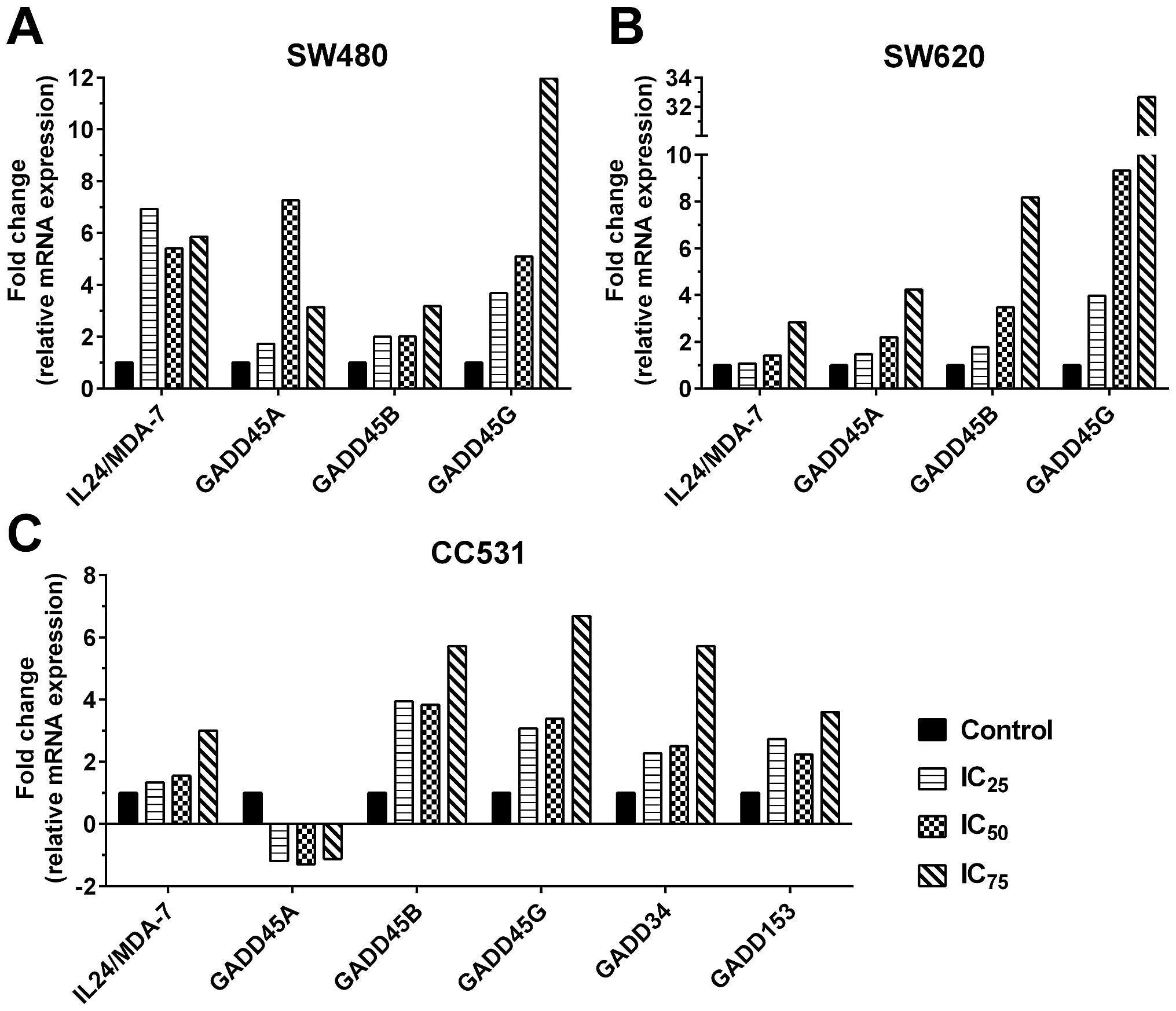
Rpx mediates induction of
apoptosis/ER-stress relevant genes
The observed apoptotic processes such as
fragmentation of nuclei/DNA and high percentages of Annexin
V-positive cells after Rpx treatment provoked us to study the
expression profiles of apoptosis/ER-stress-related genes. In this
regard, we selected a gene with anticancer potential (IL24/MDA-7)
and its downstream targets (GADD genes). The cells were treated
with Rpx (IC25, IC50 or IC75) for
48 h and the expression profiles of the selected genes were studied
by qRT-PCR technology. These experiments illustrated a significant
potential of Rpx to induce IL24/MDA-7 and GADD family genes in the
cell lines (Fig. 7). In SW480
cells, we observed a maximum induction of 6.9-, 7.3-, 3.2- and
12-fold for IL24/MDA-7, GADD45 A, B and G genes, respectively.
These effects followed a concentration-dependent format for GADD45
B and G, whereas IL24/ MDA-7 and GADD45A genes were induced
maximally by IC25 and IC50 concentrations,
respectively. For SW620, the induction of all selected genes was
steady in response to Rpx concentrations, where maximum induction
was 2.8-, 4.2-, 8.2- and 32.7-fold for IL24/MDA-7, GADD45 A, B and
G genes, respectively. Interestingly, we did not find any
expression of GADD34 and 153 genes in our control or treated human
CRC cell lines. In CC531 cells, the response was mixed, where
IL24/MDA-7 followed a linear increase with a maximum induction of
3-fold, while GADD45A was downregulated to a moderate level
(maximum −1.3-fold). Significant induction was observed for the
other GADD genes in CC531 cells, where the maximum-fold change was
5.7-, 6.7-, 5.7- and 3.7-fold for GADD 45B, 45G, 34 and 153,
respectively.
Discussion
The high cytotoxic potential of Rpx in diverse types
of cancer cells and its ability to effectively reduce tumor burden
in colorectal and pancreatic liver metastasis rat models were
studied previously (20,21). With regard to CRC, rat cancer cells
(CC531) were found to be highly sensitive to Rpx exposure (16). In a subsequent report, HCT116 CRC
cells were also found sensitive to Rpx (10). Based on these promising results, we
incorporated another two human CRC cell lines (SW480 and SW620) and
determined the cytotoxic levels of Rpx by MTT dye reduction assay.
Considering the IC50 values (ng/ml) for 48 h, Rpx showed
comparable cytotoxicity in the three selected CRC cell lines and
indicated high anticancer potential in our previous studies in
vivo (17,21). All CRC cells demonstrated a steep
decline at low Rpx concentrations (≤5 ng/ml), while the effects
were not steady in proportion to further increasing Rpx
concentrations. In line with this, despite the similar
IC50 values at 48 h, the human CRC cells showed some
degree of resistance at higher Rpx concentrations (≥20 ng/ml).
Compared to the anti-proliferative effects, Rpx
caused similar in ratio anti-clonogenic effects, but a 3–4-fold
increased inhibition of migration. These profound anti-migratory
effects, observed by scratch assay, were significant regardless of
the primary or metastatic nature of CRC cells. A high potential of
Rpx, as an anti-migratory agent for CRC cells, illustrates its
ability to reduce the risk of tumor cell migration and its possible
metastasis. Different study reports have confirmed the potential of
RIPs to inhibit colony formation as well as migration of cancer
cells (23–29). To clarify the reasoning behind
anti-proliferative effects, observed by MTT assay, we investigated
the impact of Rpx exposure on cell cycle and apoptosis relevant
activities of CRC cells. Many studies have described the potential
of RIPs as cytostatic agents for cancer cells (29,30),
where they imposed arrest in different stages of the cell cycle
(G0/G1, S or G2/M). In our study, Rpx induced profound cytostatic
effects at the transition from S to G2 phases, thus high
proportions of these cells were arrested in a dose-dependent manner
in the S phase of the cell cycle. These cytostatic effects were
more prominent in primary CRC cells (SW480 and CC531) than in cells
of the metastatic line (SW620).
A vast majority of reports have illustrated the
potential of RIPs to exert apoptotic effects in cancer cells
(31–34). Keeping in view this fact, we aimed
at finding the possible apoptotic effects of Rpx in selected CRC
cells. Following Rpx exposure, we observed apoptotic effects at
morphological levels, which included blebbing, shrinkage and
detachment of CRC cells from the culture plates. Furthermore, we
also examined condensation and fragmentation of nuclear contents of
CRC cells concentration-dependently. Rpx mediated apoptotic effects
were further accomplished and confirmed by Annexin V-FITC assay.
Remarkably, these apoptotic effects, observed by nuclear staining
and Annexin V-FITC assays, were higher in rat than human CRC cells.
As these results paralleled the anti-proliferative effects, we
concentrated on factors which might have contributed to these
differences.
In our previous study (10), we confirmed that Rpx induced the
unfolded protein response (UPR), resulting in endoplasmic reticulum
(ER) stress of cancer cells and apoptosis. In the same study, we
also identified a marked upregulation of the IL24/MDA-7 expression
in MDA-MB-231 breast cancer cells. In this study, we investigated a
possible expressional modulation of the IL24/MDA-7 gene and its
downstream targets in CRC cells. Following Rpx exposure, we
observed significantly increased mRNA levels of the IL24/MDA-7 gene
(≥2-fold). The IL24/MDA-7 gene has been grouped with highly
effective anticancer genes (35)
and exerts tumor suppressor effects in a variety of solid and
non-solid cancers (36–39). With clinical perspective, low
levels of the IL24/MDA-7 have been observed in cancerous conditions
including CRC. Consequently, these low levels of the IL24/MDA-7
have been associated with poor prognosis, shorter disease-free
survival and metastasis (40–42).
In order to maintain high levels of this protein, recombinant
IL24/MDA-7 has been implicated in clinical trials for treatment of
various solid tumors. At the same time, efforts are being carried
out to find novel reagents, which can induce the expression of the
IL24/MDA-7 in cancerous conditions (43,44).
Taking into consideration the anticancer properties of the
IL24/MDA-7, the ability of Rpx to induce this gene in CRC cells
seems to be of paramount importance. With reference to the
downstream signaling factors of the IL24/MDA-7 gene, we focused on
growth arrest and DNA damage (GADD family) genes in this study. The
IL24/MDA-7 gene has been shown to induce the expression of GADD
genes by means of the p38 MAPK pathway (45). GADD genes are stress-related genes,
which play an important role in apoptosis and transcriptional
regulation of various pro- and/or anti-apoptosis genes (46). In this study, we found the
induction of three members of the GADD family genes (A, B and G) in
response to Rpx exposure in human CRC cells. In rat CRC cells, in
addition to GADD B and G, we also observed significant induction
(≥2-fold) of two additional GADD genes (GADD34 and 153). These
additional GADD genes have been reported to induce the production
of reactive oxygen species (ROS), high levels of ceramides and
inhibition of anti-apoptotic genes like BCL-2 (47,48).
Thus, induction of GADD34 and 153 was possibly responsible for the
more profound anti-proliferative and apoptotic effects in rat CRC
cells as observed by MTT assay, nuclear staining and Annexin V-FITC
based protocols in this study. This confirms Rpx induced,
previously reported UPR/ER-stress and also demonstrates the
possible involvement of the mitochondrial arm of apoptosis in
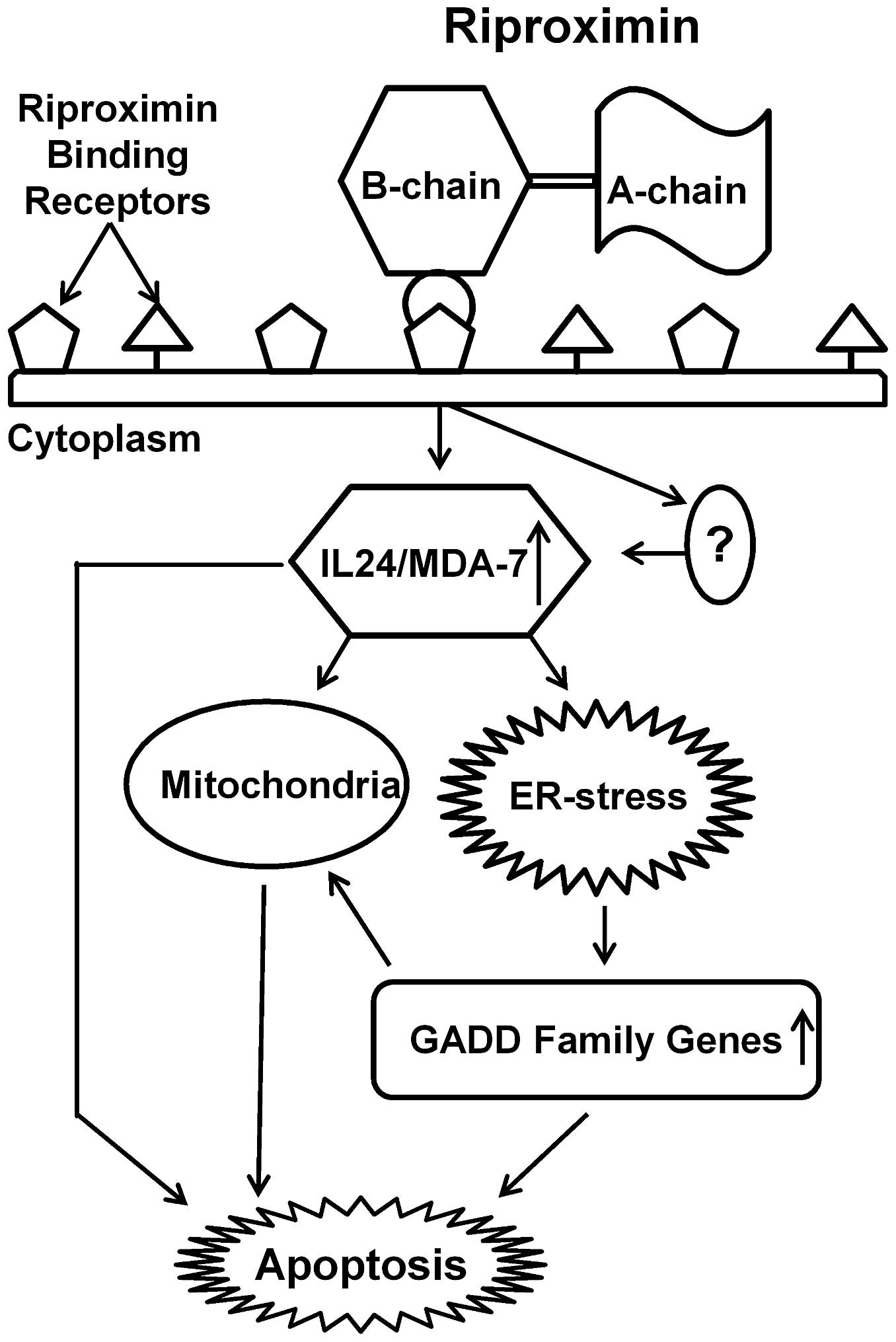
certain cancer cell lines. Based on our observations and the above
cited literature, we postulate a Rpx mediated pathway with the
involvement of IL24/MDA-7 and GADD genes as shown in Fig. 8, which has bearing for both, the ER
and mitochondrial pathways leading to apoptosis.
In conclusion, Rpx exerts significant
anti-migratory, cytostatic and apoptotic effects in CRC cells.
These anti-neoplastic effects were found in both, primary and
metastatic CRC cell lines. In addition, the ability of Rpx to
induce the anticancer gene IL24/MDA-7 and ER-stress via GADD
induction highlights the therapeutic potential of this naturally
existing compound.
References
|
1
|
Santana SS, Gennari-Cardoso ML, Carvalho
FC, Roque-Barreira MC, Santiago AS, Alvim FC and Pirovani CP:
Eutirucallin, a RIP-2 type lectin from the latex of Euphorbia
tirucalli L. presents proinflammatory properties. PLoS One.
9:e884222014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Girbes T, Ferreras JM, Arias FJ, Muñoz R,
Iglesias R, Jimenez P, Rojo MA, Arias Y, Perez Y, Benitez J, et al:
Non-toxic type 2 ribosome-inactivating proteins (RIPs) from
Sambucus: Occurrence, cellular and molecular activities and
potential uses. Cell Mol Biol (Noisy-le-grand). 49:537–545.
2003.
|
|
3
|
He WJ and Liu WY: Cinnamomin: A
multifunctional type II ribosome-inactivating protein. Int J
Biochem Cell Biol. 35:1021–1027. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stirpe F: Ribosome-inactivating proteins.
Toxicon. 44:371–383. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walsh MJ, Dodd JE and Hautbergue GM:
Ribosome-inactivating proteins: Potent poisons and molecular tools.
Virulence. 4:774–784. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hartley MR and Lord JM: Cytotoxic
ribosome-inactivating lectins from plants. Biochim Biophys Acta.
1701:1–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stirpe F and Battelli MG:
Ribosome-inactivating proteins: Progress and problems. Cell Mol
Life Sci. 63:1850–1866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peumans WJ, Hao Q and Van Damme EJ:
Ribosome-inactivating proteins from plants: more than RNA
N-glycosidases? FASEB J. 15:1493–1506. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fong WP, Mock WY and Ng TB: Intrinsic
ribonuclease activities in ribonuclease and ribosome-inactivating
proteins from the seeds of bitter gourd. Int J Biochem Cell Biol.
32:571–577. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horrix C, Raviv Z, Flescher E, Voss C and
Berger MR: Plant ribosome-inactivating proteins type II induce the
unfolded protein response in human cancer cells. Cell Mol Life Sci.
68:1269–1281. 2011. View Article : Google Scholar
|
|
11
|
de Virgilio M, Lombardi A, Caliandro R and
Fabbrini MS: Ribosome-inactivating proteins: From plant defense to
tumor attack. Toxins (Basel). 2:2699–2737. 2010. View Article : Google Scholar
|
|
12
|
Nielsen K and Boston RS:
Ribosome-inactivating proteins: A plant perspective. Annu Rev Plant
Physiol Plant Mol Biol. 52:785–816. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Massa S, Paolini F, Spanò L, Franconi R
and Venuti A: Mutants of plant genes for developing cancer
vaccines. Hum Vaccin. 7(Suppl): 147–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zarovni N, Vago R and Fabbrini MS: Saporin
suicide gene therapy. Methods Mol Biol. 542:261–283. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Preijers FW: Rationale for the clinical
use of immunotoxins: Monoclonal antibodies conjugated to
ribosome-inactivating proteins. Leuk Lymphoma. 9:293–304. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Voss C, Eyol E and Berger MR:
Identification of potent anti-cancer activity in Ximenia americana
aqueous extracts used by African traditional medicine. Toxicol Appl
Pharmacol. 211:177–187. 2006. View Article : Google Scholar
|
|
17
|
Voss C, Eyol E, Frank M, von der Lieth CW
and Berger MR: Identification and characterization of riproximin, a
new type II ribosome-inactivating protein with antineoplastic
activity from Ximenia americana. FASEB J. 20:1194–1196. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bayer H, Ey N, Wattenberg A, Voss C and
Berger MR: Purification and characterization of riproximin from
Ximenia americana fruit kernels. Protein Expr Purif. 82:97–105.
2012. View Article : Google Scholar
|
|
19
|
Bayer H, Essig K, Stanzel S, Frank M,
Gildersleeve JC, Berger MR and Voss C: Evaluation of riproximin
binding properties reveals a novel mechanism for cellular
targeting. J Biol Chem. 287:35873–35886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adwan H, Bayer H, Pervaiz A, Sagini M and
Berger MR: Riproximin is a recently discovered type II ribosome
inactivating protein with potential for treating cancer. Biotechnol
Adv. 32:1077–1090. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adwan H, Murtaja A, Kadhim Al-Taee K,
Pervaiz A, Hielscher T and Berger MR: Riproximin's activity depends
on gene expression and sensitizes PDAC cells to TRAIL. Cancer Biol
Ther. 15:1185–1197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Kodama T, Doukas AG and Hamblin MR:
Delivery of ribosome-inactivating protein toxin into cancer cells
with shock waves. Cancer Lett. 189:69–75. 2003. View Article : Google Scholar
|
|
24
|
Gasperi-Campani A, Musa AR and Roncuzzi L:
Diverse activity of sc-RIP saporin 6 on primary and metastatic
melanoma cells in vitro. Melanoma Res. 3:363–367. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang C, Yang A, Zhang B, Yin Q, Huang H,
Chen M and Xie J: PANC-1 pancreatic cancer cell growth inhibited by
cucurmosin alone and in combination with an epidermal growth factor
receptor-targeted drug. Pancreas. 43:291–297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Podlech O, Harter PN, Mittelbronn M,
Poschel S and Naumann U: Fermented mistletoe extract as a
multimodal antitumoral agent in gliomas. eCAM.
2012:5017962012.PubMed/NCBI
|
|
27
|
Mohamed MS, Veeranarayanan S, Poulose AC,
Nagaoka Y, Minegishi H, Yoshida Y, Maekawa T and Kumar DS: Type 1
ribotoxin-curcin conjugated biogenic gold nanoparticles for a
multimodal therapeutic approach towards brain cancer. Biochim
Biophys Acta. 1840:1657–1669. 2014. View Article : Google Scholar
|
|
28
|
Wei F, Cao S, Ren X, Liu H, Yu J, Li H and
Hao X: Efficient antiproliferative and antiangiogenic effects on
human ovarian cancer growth by gene transfer of attenuated mutants
of Shiga-like toxin I. Int J Gynecol Cancer. 18:677–691. 2008.
View Article : Google Scholar
|
|
29
|
Fan X, He L and Meng Y, Li G, Li L and
Meng Y: A-MMC and MAP30, two ribosome-inactivating proteins
extracted from Momordica charantia, induce cell cycle arrest and
apoptosis in A549 human lung carcinoma cells. Mol Med Rep.
11:3553–3558. 2015.PubMed/NCBI
|
|
30
|
Fang EF, Zhang CZ, Ng TB, Wong JH, Pan WL,
Ye XJ, Chan YS and Fong WP: Momordica charantia lectin, a type II
ribosome inactivating protein, exhibits antitumor activity toward
human nasopharyngeal carcinoma cells in vitro and in vivo. Cancer
Prev Res (Phila). 5:109–121. 2012. View Article : Google Scholar
|
|
31
|
Narayanan S, Surendranath K, Bora N,
Surolia A and Karande AA: Ribosome inactivating proteins and
apoptosis. FEBS Lett. 579:1324–1331. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gan YH, Peng SQ and Liu HY: Molecular
mechanism of apoptosis induced by ricin in HeLa cells. Acta
Pharmacol Sin. 21:243–248. 2000.
|
|
33
|
Garrosa M, Jiménez P, Tejero J, Cabrero P,
Cordoba-Diaz D, Quinto EJ, Gayoso MJ and Girbés T: Toxicity of the
anti-ribosomal Lectin Ebulin f in lungs and intestines in elderly
mice. Toxins (Basel). 7:367–379. 2015. View Article : Google Scholar
|
|
34
|
Zhang D, Chen B, Zhou J, Zhou L, Li Q, Liu
F, Chou KY, Tao L and Lu LM: Low concentrations of trichosanthin
induce apoptosis and cell cycle arrest via c-Jun N-terminal protein
kinase/mitogen-activated protein kinase activation. Mol Med Rep.
11:349–356. 2015.
|
|
35
|
Grimm S and Noteborn M: Anticancer genes:
Inducers of tumour-specific cell death signalling. Trends Mol Med.
16:88–96. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tian H, Wang J, Zhang B, Di J, Chen F, Li
H, Li L, Pei D and Zheng J: MDA-7/IL-24 induces Bcl-2
denitrosylation and ubiq-uitin-degradation involved in cancer cell
apoptosis. PLoS One. 7:e372002012. View Article : Google Scholar
|
|
37
|
Dalloul A and Sainz-Perez A:
Interleukin-24: A molecule with potential anti-cancer activity and
a cytokine in search of a function. Endocr Metab Immune Disord Drug
Targets. 9:353–360. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Emdad L, Lebedeva IV, Su ZZ, Gupta P,
Sauane M, Dash R, Grant S, Dent P, Curiel DT, Sarkar D, et al:
Historical perspective and recent insights into our understanding
of the molecular and biochemical basis of the antitumor properties
of mda-7/IL-24. Cancer Biol Ther. 8:391–400. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gupta P, Su ZZ, Lebedeva IV, Sarkar D,
Sauane M, Emdad L, Bachelor MA, Grant S, Curiel DT and Dent P:
mda-7/IL-24: Multifunctional cancer-specific apoptosis-inducing
cytokine. Pharmacol Ther. 111:596–628. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Choi Y, Roh MS, Hong YS, Lee HS and Hur
WJ: Interleukin-24 is correlated with differentiation and lymph
node numbers in rectal cancer. World J Gastroenterol. 17:1167–1173.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ellerhorst JA, Prieto VG, Ekmekcioglu S,
Broemeling L, Yekell S, Chada S and Grimm EA: Loss of MDA-7
expression with progression of melanoma. J Clin Oncol.
20:1069–1074. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Patani N, Douglas-Jones A, Mansel R, Jiang
W and Mokbel K: Tumour suppressor function of MDA-7/IL-24 in human
breast cancer. Cancer Cell Int. 10:292010.PubMed/NCBI
|
|
43
|
Xu S, Oshima T, Imada T, Masuda M, Debnath
B, Grande F, Garofalo A and Neamati N: Stabilization of MDA-7/IL-24
for colon cancer therapy. Cancer Lett. 335:421–430. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Whitaker EL, Filippov VA and
Duerksen-Hughes PJ: Interleukin 24: Mechanisms and therapeutic
potential of an anti-cancer gene. Cytokine Growth Factor Rev.
23:323–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sarkar D, Su ZZ, Lebedeva IV, Sauane M,
Gopalkrishnan RV, Valerie K, Dent P and Fisher PB: mda-7 (IL-24)
mediates selective apoptosis in human melanoma cells by inducing
the coordinated overexpression of the GADD family of genes by means
of p38 MAPK. Proc Natl Acad Sci USA. 99:10054–10059. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cretu A, Sha X, Tront J, Hoffman B and
Liebermann DA: Stress sensor Gadd45 genes as therapeutic targets in
cancer. Cancer Ther. 7A:268–276. 2009.
|
|
47
|
McCullough KD, Martindale JL, Klotz LO, Aw
TY and Holbrook NJ: Gadd153 sensitizes cells to endoplasmic
reticulum stress by down-regulating Bcl2 and perturbing the
cellular redox state. Mol Cell Biol. 21:1249–1259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hollander MC, Sheikh MS, Yu K, Zhan Q,
Iglesias M, Woodworth C and Fornace AJ Jr: Activation of Gadd34 by
diverse apoptotic signals and suppression of its growth inhibitory
effects by apoptotic inhibitors. Int J Cancer. 96:22–31. 2001.
View Article : Google Scholar : PubMed/NCBI
|