1. Introduction to collagen remodeling in
health and disease
One of the hallmarks of cancer is tumor invasion and
metastasis. A central process in these events is the degradation of
the tumor-surrounding extracellular matrix which is not only a
crucial rate-limiting step for tumors to invade adjacent tissue but
is also an important modification of the tumor microenvironment,
leading to changes in its regulatory function. When tumors expand,
the surrounding stroma undergoes constant remodeling and since
collagen is the major component of the extracellular matrix,
collagen synthesis and degradation is a key determinant for cancer
invasion. However, collagen turnover is not only central for tumor
invasion but is also a normal process in the healthy organism,
occurring continuously at sites with tissue remodeling. This
process is widespread in the body but occurs at highly different
rates. For example, cartilage and tendons are characterized by a
very slow rate of collagen turnover with a suggested half-life of
over 100 years for cartilage (1,2),
compared to the considerably accelerated rate in periodontal
ligaments with a half-life of 3–23 days (3,4).
The most thoroughly characterized pathway of
collagen turnover is the proteolytic breakdown process occurring in
the extracellular space. The distinctive triple-helix structure and
the ability to form large insoluble fibers makes collagen resistant
to most proteases (5).
Nevertheless, a few specialized collagenases in the matrix
metalloprotease family, including MMPs -1, -2, -8, -13–16, and the
cysteine protease cathepsin K, are able to perform a proteolytic
attack on the rigid structure (6–8). The
degradation process is initiated by the unwinding of collagen
fibers which exposes cleavage sites targeted by these specialized
proteases. Their activity produces well-defined ¼ and ¾ collagen
fragments, which are subsequently cleared by other proteases.
However, in addition to this well established extracellular
breakdown pathway, a less characterized intracellular process in
which collagen is internalized through binding to collagen-specific
receptors on the cell surface and delivered for lysosomal
degradation has also been identified. In 2000, two studies reported
the cloning of uPARAP/Endo180, a type-1 membrane protein belonging
to the mannose receptor family (9,10)
which later turned out to be responsible for this process. This
receptor, also referred to as CD280 (11), is the product of the MRC2 gene,
situated on chromosome 17q23 in humans (link to gene information:
http://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=9902;
human gene).
In this review, we summarize the features and
function of uPARAP/Endo180 and discuss the putative role of this
collagen receptor in various cancers and other diseases
characterized by dysregulation of collagen remodeling.
2. uPARAP/Endo180: an endocytic receptor for
collagen
The collagen binding function of uPARAP/Endo180 was
initially demonstrated in ligand competition experiments during the
characterization of the receptor on cultured cells (9). Studies with wild-type cells and cells
from uPARAP/Endo180-deficient mice, as well as cells transfected
with uPARAP/Endo180 cDNA in vitro, revealed that this
binding is associated with an active endocytosis machinery and that
the receptor functions in collagen internalization (12–14).
Thus, the bound collagen is routed through clathrin coated pits to
the endosomal/lysosomal compartment (13) where collagen degradation occurs in
a process dependent on cysteine proteases (15). This routing of the bound ligand is
governed by a short sequence motif in the small cytoplasmic domain
of the receptor (Fig. 1) (16). The receptor is recycled back to the
cell surface from the early endosomal compartment (16).
A complete survey of collagen subtypes has not been
undertaken. However, the evidence obtained so far makes it likely
that the binding and internalization capacity of uPARAP/Endo180
comprises most or all structural collagens. Positive binding and/or
internalization data have been obtained for at least fibrillary
collagens I, II and V and for sheet-like (basement membrane-type)
collagen IV (12,13). Although it should be noted that
additional roles of this receptor have also been studied in
vitro, particularly in relation to cell migration (17,18),
the discussion in this review will be focused on the function in
collagen turnover.
Important preferences exist regarding the physical
form of the internalized collagen. In the majority of studies on
uPARAP/Endo180 and collagen, internalization has been demonstrated
with solubilized collagens subjected to various means of labeling,
meaning that the conformational state of the material is not known
in detail. Furthermore, it is not completely clear what would be
the physical size limit for collagen units or fragment to allow
cell entry through internalization by this receptor. Studies in a
mouse mammary tumor model showed the uptake of collagen to occur
exclusively in uPARAP/Endo180-positive, fibroblast-like cells, with
at least some of the internalized material having a fibril-like
appearance as judged by electron microscopy (19). On the other hand, most studies so
far have counted against a function of uPARAP/Endo180 in regular
phagocytosis (16), thus pointing
to stringent size constraints for material to be internalized by
this receptor.
Whereas the internalization of strictly native
collagen fibrils is not easily quantified under controlled
conditions, a stringent pattern can be achieved after manipulation
with the collagen structure. Thus, a deliberate (heat-induced)
denaturation of collagen leads to increased binding to
uPARAP/Endo180 and increased uptake by cells expressing the
receptor (20). This observation
is important because a similar denaturation process occurs as a
consequence of initial (single-site) proteolytic cleavage of triple
helical collagen and indeed, the same study showed that pre-cleaved
collagen displays increased binding and uptake, just like the
heat-denatured material. In accordance with these findings, studies
with fibroblasts on a native (insoluble) collagen matrix showed
that the collagenase MT1-MMP on the cell surface is responsible for
a pre-cleavage of collagen into defined soluble fragments that are
then endocytosed by uPARAP/Endo180 (20). Experiments with cells seeded on a
pre-assembled matrix with polymerized collagen I and fibronectin
showed that uPARAP/Endo180 was indeed important for efficient
cellular uptake of matrix collagen (21). However, these studies also pointed
to a complicated interplay between collagen, the fibronectin
component, integrins and proteolytic (MMP-catalyzed) processes, in
addition to the role uPARAP/Endo180. Since these experiments did
not allow a direct evaluation of the single events involved, the
molecular form of collagen actually taken up by uPARAP/Endo180
could not be established with certainty.
Obviously, the role of this receptor must be
understood in the context of other collagen-binding proteins.
Several cell surface proteins are known to bind to structural
collagens, acting in diverse processes of adhesion, migration and
signaling (reviewed in ref. 22).
However, the majority of these proteins do not take part in
endocytic processes such as those described above. It should be
noted that a phagocytic collagen uptake mechanism in fibroblasts
was noted at an early point (23).
However, phagocytic uptake of collagen particles seems to be
governed mainly by collagen-binding integrins such as α2β1
(24), unlike the
clathrin-associated endocytosis mediated by uPARAP/Endo180 which is
integrin-independent (12,25). The role of the mannose receptor
(MR) in collagen endocytosis is described in the following
section.
3. Functional properties of uPARAP/Endo180
and related receptors
Cloning of the uPARAP/Endo180 cDNA (9,10,26)
revealed that the protein is a member of the mannose receptor
protein family, a well-characterized group of lectin-like endocytic
receptors. This family includes four members in mammals. In
addition to uPARAP/Endo180 itself, these are the (macrophage)
mannose receptor (MR), the M-type phospholipase A2 receptor (PLA2R)
and the dendritic cell receptor DEC-205. The avian immunoglobulin Y
receptor is a bird homologue of PLA2R (27,28).
All of these receptors share a characteristic domain
composition (29) (Fig. 1). They are type-1 membrane proteins
which, from the N-terminus, are composed of a cysteine-rich
(Cys-rich, Ricin B-like) domain, a fibronectin type-II (Fn-II)
domain, a series of 8–10 C-type lectin-like domains (CTLDs), a
transmembrane segment and a small cytoplasmic domain.
The high degree of conservation of the domain
structure, including the presence of a fibronectin type-II domain
in all of the receptor family members (29), has prompted the investigation of
any collagen-binding function in the other members of the family.
It was soon realized that MR itself also has the capacity to bind
and internalize collagen (30,31),
most likely in a manner very similar to that of uPARAP/Endo180,
although these receptors are mostly expressed on different cell
types. Indeed, the expression of MR on liver sinusoidal endothelial
cells is responsible for hepatic clearance of injected, solubilized
collagen (32) whereas MR on
M2-like macrophages was shown to be active in the endocytosis of
fluorescence-labeled collagen that was polymerized after injection
under the skin of mice in vivo (33). A recent systematic study with
transfected cells confirmed that uPARAP/Endo180 and MR both possess
the capacity for collagen endocytosis but also showed that PLA2R
and DEC-205 have no such capacity (34). It should be noted that this notion
contradicts an early study, pointing to some degree of collagen
binding in PLA2R (35) and to a
recent study which led to the same conclusion although the
difference between mock-transfected and PLA2R-transfected cells was
rather small (36). The background
for this discrepancy remains to be determined. It is possible that
some species-specific difference exists in this interaction as
suggested in the last-mentioned study, or that PLA2R possesses an
activity which is just much smaller than that of uPARAP/Endo180 and
MR. Altogether, based on the direct comparison presented in the
report (34), it is our
interpretation that uPARAP/Endo180 and MR are the two dominant
collagen receptors in this family.
As would be expected from the evidence obtained from
several other collagen-binding proteins, the Fn-II domain of
uPARAP/Endo180 plays a decisive role in collagen binding. Although
the Fn-II domain does not appear to obtain the correct native
protein folding when expressed alone, stable truncated versions of
uPARAP/Endo180 can indeed be obtained which comprise the Fn-II
domain with its two flanking domains (i.e., constructs representing
the first three N-terminal domains of the receptor; Fig. 1). These protein constructs retain
collagen binding (13,37). Even more clearly, the importance of
the Fn-II domain has been shown by mutagenesis studies, using cells
transfected to express full-length receptor variants (34). These studies have shown, firstly,
that the Fn-II domain of uPARAP/Endo180 can be interchanged with
that from MR to still allow collagen internalization, whereas an
interchange with Fn-II domains from PLA2R or DEC-205 leads to loss
of this capability. Secondly, a 10-amino acid loop within the Fn-II
domain of uPARAP was found to be crucial for collagen binding and
uptake.
An additional domain in uPARAP/Endo180 with
functional importance is the second lectin-like domain (CTLD-2).
Although the receptor structure includes eight domains classified
as CTLDs on the basis of sequence homology, it appears that only
CTLD-2 has an active lectin function (38). This capacity to bind carbohydrate
plays a role in the binding of glycosylated collagen types to the
receptor. Whereas the major fibrillar collagen, collagen I, is
largely devoid of glycosylation, the sheet-like collagen IV of the
basement membrane is more heavily glycosylated (39). It turns out that the binding of
collagen IV to uPARAP/Endo180, while still dependent on the Fn-II
domain, is modulated by the lectin function of CTLD-2. Conversely,
in accordance with the collagen glycosylation difference, CTLD-2
does not seem to be involved in the binding of collagen I (37).
In addition to the direct binding contributions of
individual domains, it has been suggested that dynamic properties
of the inter-domain organization could be important for the
functional properties of this receptor. Thus, studies by
single-particle electron microscopy have pointed to pH-dependent
changes in the steric arrangement of the domains. At neutral pH,
the protein seems to adopt a ‘bent’ conformation with the Fn-II
domain contacting CTLD-2 (40)
whereas, at low pH, it takes up a more open structure (41). Since the receptor binds its ligand
at the neutral pH conditions in the extracellular environment while
dissociation occurs in endosomal compartments at low-pH, this
conformational change may be important in relation to ligand
binding and release (42).
4. Role of uPARAP/Endo180 in the healthy
organism
Gene knock-out studies (12,14)
have shown that uPARAP-deficient mice are born in the expected
Mendelian ratio, are viable and fertile, have normal survival and
that they phenotypically appear relatively normal, although they
display an effect on bone growth as detailed in the following
section. Even though the similar molecular function of
uPARAP/Endo180 and MR may suggest a redundant function of these two
receptors, mice with double deficiency for these two receptors
likewise show no obvious phenotypic abnormality (43).
uPARAP/Endo180 is highly expressed in osteogenic
tissue, and a number of reports have demonstrated a role for this
collagen receptor in bone development and homeostasis. Perhaps the
most striking evidence in this connection came from an independent
study investigating a hereditary cattle disease known as the
crooked tail syndrome (CTS). Animals suffering from CTS display
severe abnormalities in bone development. Following the genetics in
a particular strain of cattle and fine mapping of a locus
associated with the disease, a 2-bp deletion in the open reading
frame of the MRC2 gene coding for uPARAP/Endo180 was
identified as the causative mutation (44). A second uPARAP/Endo180 mutation,
likewise associated with this disease, was found in a later study
(45). In mice, the consequence of
uPARAP/Endo180 deficiency is less pronounced than that noted in
cattle. However, uPARAP/Endo180 knock-out mice show a small but
significant reduction in the length of the long bones, both in
newborn and adult mice (46,47)
and a reduced bone mineral density in adult mice. Interestingly,
the bone defect is strongly augmented when uPARAP/Endo180
deficiency is combined with deficiency for the collagenolytic
protease, MT1-MMP (46). An
increased effect on the growth retardation of the long bones is
also noted in uPARAP/Endo180 - MMP-2 double deficient mice
(47). However, in this case the
augmentation is less pronounced than that seen in mice with
combined uPARAP/Endo180 and MT1-MMP deficiency and an additional
difference is noted when comparing different bone compartments.
Thus, opposite to MT1-MMP-deficient mice, the lack of MMP-2 leads
to an increase in the thickness of the calvarium (48,49).
This effect is counteracted after combination with uPARAP/Endo180
deficiency, demonstrating that uPARAP/Endo180 and MMP-2 do not in
all cases support the same degradative processes (47). Studies in vitro have also
contributed to understanding the role of uPARAP/Endo180 in bone
homeostasis particularly in the context of the coupling between
bone formation and resorption. Throughout life, bone is maintained
by a remodeling system consisting of continuous bone resorption by
osteoclasts followed by bone formation by the osteoblasts.
Recently, it was suggested that uPARAP/Endo180 contributes to this
process since migration of osteoblast progenitors into resorption
pits created by osteoclasts appeared to be driven to some extent by
interactions between residual collagen fragments, left behind by
the osteoclasts, and the uPARAP/Endo180 receptor on
osteoprogenitors (50).
So far, deficiency for uPARAP/Endo180 has not been
reported in humans. However, a human polymorphism in the MRC2 gene,
in the form of a synonymous SNP in exon 30, has been associated
with the rate of recurrence after initially curative treatment of
patients with early-stage head and neck squamous cell carcinoma
(51). Furthermore, an SNP in an
MRC2 intron, suggested to be situated in a regulatory region, was
found to be associated with degenerative bony changes of the
temporomandibular joint (52).
5. Tissue expression and regulation
In the healthy organism, uPARAP/Endo180 is primarily
expressed by mesenchymal cells such as fibroblasts and osteogenic
cells and it is present in sites showing active tissue remodeling,
notably including developing bone (46,47,
53). This is in line with a
comparative study performed on a number of cultured cell lines
which also pointed to a clear preference for mesenchymal expression
(25). uPARAP/Endo180 is localized
in membrane-associated clathrin-coated pits or intracellular
endosomes and is a constitutively rapidly recycling receptor. In
fact, at any given time the largest pool (70–90%) of uPARAP/Endo180
can be found in intracellular cell compartments (16,54).
Indeed, when staining for uPARAP/Endo180 by immunohistochemistry,
the characteristic punctuated staining pattern is a reflection of
its localization in membrane-associated pits and endosomal
compartments (Figs. 2 and 5).
In the murine embryo, uPARAP/Endo180 is expressed in
all tissues undergoing primary ossification, both through the
intramembranous and the endochondral pathway, and the cells
expressing uPARAP/Endo180 in these tissues are of osteoblast linage
(26,53). The same expression pattern can be
seen in young mice where uPARAP/Endo180 is found on
osteoblasts/osteocytes but also on chondrocytes (46,55),
although it has been suggested that the expression by chondrocytes
declines with age (55).
In accordance with the strong prevalence of
uPARAP/Endo180 in bone tissue of murine embryos and young mice, the
calvaria and tibia are among the organs with the highest level of
uPARAP/Endo180 expression also in adult mice (47). When investigating the expression
pattern in adult bone tissue in more detail by
immunohistochemistry, it was demonstrated that the
uPARAP/Endo180-positive cells are the same as in young mice, i.e.,
osteoblastic lineage cells (47).
A strong expression of the receptor in humans and mice was seen on
the so-called bone lining cells which form a sheet covering the
bone surface (47,50) (Fig.
3). These cells are thought to be important for bone remodeling
(56) and therefore it seems that
uPARAP/Endo180 is important not only for bone generation, but also
for bone homeostasis.
In addition to the bone compartment, uPARAP/Endo180
is expressed in several organs and tissues. In adult mice, the
receptor has been demonstrated in a wide range of organs with
particularly high levels in the uterus and lung (47,57)
but with very low levels in liver, muscle and brain (47). In human tissue, one of the first
screenings performed by northern blotting revealed high levels of
uPARAP/Endo180 expression in the heart, prostate, testis, ovary,
and intestine, with lower levels in brain, placenta, lung, kidney,
pancreas, spleen, thymus and colon (26). Additional studies have reported
uPARAP/Endo180 to be present on monocyte-derived skin macrophages
(10), although the marker used
(CD14) might make it difficult to distinguish between different
cell types belonging to the monocyte lineage. Furthermore, the
receptor has been observed on human placental cells although there
is some discrepancy regarding their lineage, where one report
suggested endothelial cells in a subset of microvascular vessels
(10) and another mesenchymal
cells in the villous stroma (58).
A complicated expression pattern, including several cell types, has
been reported in human gingival epithelium (59); see further details below.
uPARAP/Endo180 has been shown to be expressed by a
large panel of cultured cell lines, primarily of mesenchymal
origin, and the level of expression has been shown to correlate
with the ability to internalize collagen (25) (Fig.
4). Studies in vitro also suggest that the regulation of
this receptor is correlated with the cellular activity in collagen
metabolism. Thus, in isolated rat hepatic stellate cells,
uPARAP/Endo180 was upregulated as a result of activation and
transdifferentiation into a more myofibroblast-like morphology.
This correlated with an increased ability to internalize collagen
(60,61). A similar pattern in acquiring
uPARAP/Endo180 expression and collagen internalization was seen
using in vitro differentiated pancreatic stellate cell lines
(62). The same study showed that
addition of TGF-β to induce an EMT-like process in the pancreatic
cancer cell lines enhanced uPARAP/Endo180 expression and collagen
internalization (62).
In other cell types, in vitro studies on the
regulation of uPARAP/Endo180 expression have likewise been focused
primarily on the role of TGF-β. For example, treatment of the
breast cancer cell line MCF-7, the U87MG glioma cell line and two
prostate cancer cell lines with TGF-β resulted in upregulation of
the collagen receptor (63–65)
and the same was observed when using human gingival fibroblasts
where both uPARAP/Endo180 protein expression and mRNA levels
increased in the presence of TGF-β (59). However, one report has shown the
opposite effect where uPARAP/Endo180 RNA expression actually
decreased in lung fibroblasts following TGF-β treatment (57). This difference between fibroblast
reaction to TGF-β may reflect a difference in tissue remodeling
potential depending on their origin and tissue surroundings.
6. uPARAP/Endo180 in breast and prostate
cancer
Early studies on the expression of uPARAP/Endo180 in
breast cancer and premalignant conditions showed the receptor to be
absent on tumor cells but strongly expressed by the
tumor-associated fibroblast-like cells in invasive breast
carcinomas and to a limited extent by myoepithelial cells in benign
lesions and ductal carcinoma in situ (66). Later studies showed a similar,
restrictive expression pattern in the polyomavirus middle T-induced
mammary tumor model in mice where uPARAP/Endo180 was found to be
present on periductal fibroblast-like mesenchymal cells whereas the
mammary epithelial cells and tumor cells were negative (19) (Fig.
5). Moreover, by combining uPARAP/Endo180 gene deficiency with
this model, it was found that mice developed less tumor burden and
increased collagen accumulation in the tumors as a result of
defective collagen clearance by tumor-associated stromal cells
(19). These studies pinpointed
uPARAP/Endo180 as a stromal contributor to mammary tumor
progression. However, studies on a large number of human breast
cancers revealed a small subset (3–6%) of breast tumors in which
the tumor cells were uPARAP/Endo180-positive (63). These samples belonged to a hormone
triple-negative subtype of breast carcinomas with basal-
(myoepithelium-) like characteristics. It was hypothesized that, in
some tumors, tumor cells might acquire uPARAP/Endo180 expression,
e.g., after stimulation with TGF-β, and that this might play a role
in invasive tumor growth. When human MCF-7 breast tumor cells,
which are initially uPARAP/Endo180 negative, were transfected to
express this receptor and grown in nude mice, they displayed
increased tumor growth and enhanced collagen turn-over, relative to
mock-transfected cells (63). A
recent study has proposed uPARAP/Endo180 as a potential marker for
metastatic breast cancer. In this report, uPARAP/Endo180
immunoreactivity was observed in patient plasma samples, ascribed
to protein shedding. Quantification of immunoreactivity by western
blotting indicated higher levels of uPARAP/Endo180 in recurrent
(metastatic) breast cancer patients as compared with patients with
early (localized) breast cancer (67).
The expression and the possible prognostic
significance of uPARAP/Endo180 has also been studied in prostate
cancer (68–70). It was found that uPARAP/Endo180 was
increasingly expressed in clinical high-risk group tumors and that
the expression was negatively correlated with survival. The
receptor was reported to be expressed in stromal as well as tumor
cells, although the use of tissue microarray (TMA) material in the
major part of these studies makes it difficult to quantitatively
evaluate the cellular composition of these samples.
7. uPARAP/Endo180 in glioma
Glioma originates from the glial cells in the brain
and accounts for about a third of all primary brain cancers. It is
divided into four main types where glioblastoma multiforme (GBM)
has the worst prognosis with a median survival of 15 months despite
aggressive treatment (71). The
tumors are highly invasive, probably being caused in part by the
increased interaction and degradation of the ECM which promotes
migration of the tumor cells (72). The collagen content in the brain
mainly consists of collagen IV, which is found on basement membrane
surrounding vascular endothelial cells. Although there are limited
amounts of collagen in the normal brain, this type of collagen,
together with other ECM components such as laminin and fibronectin,
is upregulated in gliomas due to increased microvasculature
(73). Additionally, it has been
shown that fibrillary collagen type I is deposited within GBM
tumors (65). Interestingly, in an
in silico analysis of available gene expression data,
uPARAP/Endo180 was shown to be highly upregulated in glioblastomas
(grade IV) compared to lower grade gliomas (grade II) (65). Upregulation of uPARAP/Endo180 was
also seen in a different study where the results were based on a
QT-PCR analysis on a cDNA panel from normal and GBM brain tissue
(74). These findings have been
confirmed at the protein level by immunohistochemistry in both
whole tissue and tissue micro arrays where 80–100% of GBM samples
analysed were positive for uPARAP/Endo180 (65). Also, in agreement with previous
studies, suggesting a preference for uPARAP/Endo180 expression on
cells of mesenchymal origin, uPARAP/Endo180 was found to be more
associated with the mesenchymal subclass of high-grade gliomas
compared to proneural and proliferative subgroups (65).
The expression and function of uPARAP/Endo180 has
also been investigated in glioma cell lines. The receptor is
expressed by several glioma-derived cell lines and down-regulation
of uPARAP/Endo180 expression has been shown to reduce their
migratory and collagen-invasive capacity in vitro,
suggesting an active participation of uPARAP/Endo180 in glioma cell
invasion (65,74). Therefore, considering the low
expression level of uPARAP/Endo180 in normal brain tissue, the fact
that it is highly upregulated in GBM and appears to have a role in
tumor cell migration makes it a promising therapeutic target.
8. uPARAP/Endo180 in bone cancer
The most common bone cancers occur as the result of
metastatic spread from tumors in other organs, such as breast, lung
or prostate carcinomas. When metastatic tumor cells spread to the
bones and form secondary tumors, the result on the bone matrix is
often a shift in bone homeostasis favoring bone degradation. This
is hypothesized to be due to a secondary effect of tumor cell
growth through increased osteoclast activity. The model is termed
the ‘vicious cycle’ whereby tumor cells, through release of
cytokines and growth factors activate osteoblasts which in turn
secrete an osteoclast activating factor known as RANKL. Increased
activity of the osteoclasts result in release of calcium and growth
factors from the bone matrix which in turn stimulate tumor growth,
thus augmenting the cycle (75).
The role of uPARAP/Endo180 in this setting is not clear but one
study has reported that upon co-culture with osteoblasts,
uPARAP/Endo180 was upregulated on prostate cancer cell lines while
simultaneously expression on osteoblasts was found to decrease
(64). In this cell culture
system, uPARAP/Endo180 expression on osteoblasts facilitated
deposition of collagen type I rather than degradation but the
co-culture with tumor cells suppressed this function. Further
studies on the co-culture system pointed to a dysregulated TGFβ1
signaling being responsible for these regulatory events (64). Another link between uPARAP/Endo180
and metastatic bone cancer was found in the above-mentioned study
on uPARAP/Endo180 in the plasma of breast cancer patients, where
the metastatic group included patients with bone metastases
(67).
9. uPARAP/Endo180 in other cancers
An early report, focused on endothelial gene
products preferentially expressed in tumors, employed differential
gene expression analysis with isolated endothelial cell material
from malignant versus normal colorectal tissue. This study
identified uPARAP/Endo180 among the 25 mostly elevated transcripts
in the tumor vessels (76).
However, although considerable care was taken to obtain pure cell
populations for comparison in this study, it is difficult to judge
whether this is actually a proof of endothelial upregulation
because most subsequent studies on cancer-associated expression of
this receptor have led to receptor identification on cell types
other than the endothelium (see above). An upregulation of
uPARAP/Endo180 has been shown in hepatocellular carcinoma where a
higher expression of the receptor was correlated with a poor
prognosis (77). In addition, an
upregulation of the receptor has been observed in the stromal
compartment of several types of head-and-neck cancers, relative to
the surrounding tissue (78).
Furthermore, in this study expression was found to be most
prominent in poorly differentiated tumors.
10. Expression and role of uPARAP/Endo180 in
other pathological conditions
Since uPARAP/Endo180 is involved in events related
to collagen remodeling, there has been a clear rationale to
investigate its influence in disorders characterized by
dysfunctional collagen turnover. Fibrosis can occur in several
organs in the body and includes a buildup of connective tissue
matrix, primarily collagen, in response to tissue damage or due to
an underlying genetic defect. In human liver cirrhosis, i.e., the
end-stage of liver fibrosis, a strong, localized upregulation of
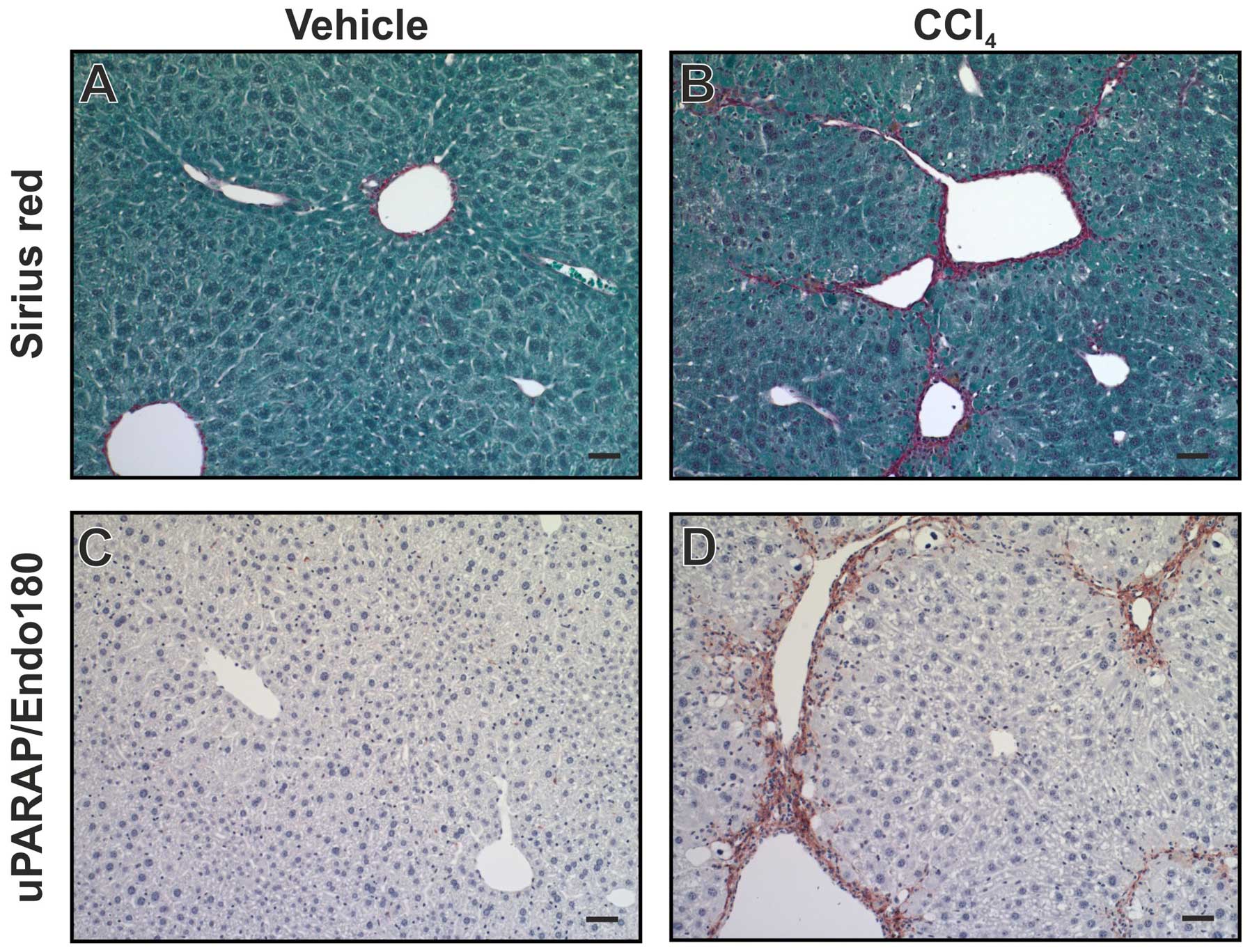
uPARAP/Endo180 was observed in fibroblast-like cells (79). Using a mouse model for liver
fibrosis, induced by injection of CCl4, a similar
upregulation was observed (Fig.
6). Furthermore, when uPARAP/Endo180-deficient mice were
studied using the same model, fibrosis was worsened with increased
collagen accumulation, relative to littermate wild-type mice
(79). This points to a protective
role of uPARAP/Endo180 in this condition, completely in line with
the function in collagen clearance. Chronic kidney disease (CKD) is
caused in part by defective tissue remodeling of the tubules
resulting in accumulation of interstitial collagens. In a mouse
model for CKD, induced by unilateral ureteral obstruction,
uPARAP/Endo180 deficiency was shown to give rise to aggravated
renal fibrosis with increased collagen content and reduced collagen
turnover (80), suggesting a
function of the receptor similar to that found in liver fibrosis.
The role of uPARAP/Endo180 in lung fibrosis has also been studied.
Using a mouse model for lung fibrosis induced by bleomycin, a
decrease in uPARAP/Endo180 expression following bleomycin
administration was demonstrated. When challenging
uPARAP/Endo180-deficient mice with bleomycin, the collagen
accumulation was larger and the increase in lung permeability was
smaller compared to wild-type mice, indicating a role for
uPARAP/Endo180 also in matrix remodeling following lung injury
(57).
Wound healing and the associated scar formation and
subsequent resolution are complicated processes that require an
intricate balance between collagen synthesis and degradation. Some
tissues, like the mucosal gingiva, have the ability of faster wound
healing with less scarring, i.e., less collagen deposition,
compared to other tissues, which makes it relevant to study the
molecular components involved in collagen turnover in this
situation. Thus, the expression of uPARAP/Endo180 has been studied
in biopsies collected from normal and wounded human gingiva
(59). In contrast to localization
studies in most other tissues, this report pointed to expression of
the receptor in a very broad range of cell types, including
epithelial cells in addition to several cell populations in the
connective tissue, and with an upregulation of expression on the
migrating keratinocytes after the wound was made. Although it was
suggested that various particular properties of the gingival
epithelium may lead to a unique ability to express uPARAP/Endo180
(59), the use of
immunofluorescence for detection made it difficult to evaluate the
strength of the specific signal obtained in these tissues. During
skin wound healing in mice, uPARAP/Endo180 expression was also seen
to be upregulated and a delayed response in re-epithelialization
was noted in mice deficient for uPARAP/Endo180 (81). However, deficiency for
uPARAP/Endo180 did not affect the time course of the macroscopic
process of wound closure and there was no difference in collagen
content in wounds from wild-type and uPARAP/Endo180-deficient mice.
The authors suggested the latter observation to reflect that other,
compensatory mechanisms are in play in the skin (81).
Collagen is also abundant in human skin and during
UV-induced aging of the skin, also known as photoaging, collagen
integrity is lost. In more severe cases, photoaging can lead to
development of actinic keratoses and ultimately squamous cell
carcinoma (82). By comparing
sun-exposed and sun-protected areas of the skin from patients
diagnosed with early photoaging, it was demonstrated that
uPARAP/Endo180 was downregulated in the sun-damaged skin areas and
that collagen fragments were accumulated in the same locations
(83). Although this result was
based on a comparison of skin surfaces from different parts of the
body, the same study also demonstrated an acute downregulation of
uPARAP/Endo180 after experimental, short-term UV-irradiation of
human skin. Furthermore, using an in vitro approach it was
demonstrated that UV-exposed human dermal fibroblasts had a reduced
expression of uPARAP/Endo180, as well as a reduced capacity for
collagen internalization (83). A
downregulation of uPARAP/Endo180 by UV-irradiation of human dermal
fibroblasts was also seen in another study which, furthermore,
demonstrated that the drug all trans retinoic acid (ATRA) could
serve to reverse the effect on uPARAP/Endo180 expression (84). The authors suggested that, through
this effect, ATRA might be useful in stimulating endocytic collagen
internalization which might in turn contribute to reduce the
symptoms associated with photoaging.
11. Conclusions and perspectives
The discovery of uPARAP/Endo180 as an endocytic
collagen receptor has led to the delineation of a novel
intracellular pathway of matrix breakdown. This pathway both
complements and acts in concert with the well-established function
of matrix-degrading extracellular proteases. In spite of the
requirement for certain proteolytic events in conjunction with the
endocytic mechanism, the action of uPARAP/Endo180 is in some cases
rate limiting with respect to extracellular matrix turnover in
vivo. This is reflected by phenotypic abnormalities in bone
growth upon uPARAP/Endo180 deficiency, by protective functions in
conjunction with the excessive build-up of extracellular matrix in
fibrosis and by active pathological functions in the matrix
breakdown processes associated with cancer invasion. Since
inactivation of uPARAP/Endo180 would probably be well tolerated in
the adult organism, the degradative function of this receptor makes
it an interesting target for anti-invasive cancer therapy and
treatment of other degenerative diseases. To pursue this
possibility, important questions to be answered will include the
details of the receptor's expression pattern in various cancers, an
improved understanding of its functional redundancy and interplay
with other degradation mechanisms and the result of experimental
targeting in mouse models. Such studies may open the way for a
whole new concept of treatment in matrix degenerative disease.
Acknowledgements
This study was supported by the Danish Cancer
Society, the Danish Medical Research Council, the Danish Cancer
Research Foundation, the Lundbeck Foundation, the Novo Nordisk
Foundation, the Danish National Research Foundation (Danish-Chinese
Center for Proteases and Cancer) and the European Community's
Seventh Framework Programme FP7/2007–2011 under grant agreement no.
201279.
Abbreviations:
|
MMP
|
matrix metalloprotease
|
|
uPARAP
|
urokinase plasminogen activator
receptor associated protein
|
|
MR
|
mannose receptor
|
|
PLA2R
|
M-type phospholipase A2 receptor
|
|
Fn-II
|
fibronectin type-II
|
|
CTLD
|
C-type lectin-like domain
|
|
CTS
|
crooked tail syndrome
|
|
EMT
|
epithelial mesenchymal transition
|
|
TGF-β
|
transforming growth factor-β
|
|
GBM
|
glioblastoma multiforme
|
|
RANKL
|
receptor activator of nuclear
factor-κB ligand
|
|
CKD
|
chronic kidney disease
|
|
ATRA
|
all trans retinoic acid
|
References
|
1
|
Maroudas A, Bayliss MT and Venn MF:
Further studies on the composition of human femoral head cartilage.
Ann Rheum Dis. 39:514–523. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maroudas A, Palla G and Gilav E:
Racemization of aspartic acid in human articular cartilage. Connect
Tissue Res. 28:161–169. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ahuja TD, Dhakray V, Mittal M, Khanna P,
Yadav B and Jain M: Role of collagen in the periodontal ligament
(review). Internet J Microbiol. 10:2012.
|
|
4
|
Sodek J and McKee MD: Molecular and
cellular biology of alveolar bone. Periodontol 2000. 24:99–126, ,
2000.PubMed/NCBI
|
|
5
|
Ottani V, Martini D, Franchi M, Ruggeri A
and Raspanti M: Hierarchical structures in fibrillar collagens.
Micron. 33:587–596. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saftig P, Hunziker E, Wehmeyer O, Jones S,
Boyde A, Rommerskirch W, Moritz JD, Schu P and von Figura K:
Impaired osteoclastic bone resorption leads to osteopetrosis in
cathepsin-K-deficient mice. Proc Natl Acad Sci USA. 95:13453–13458.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hotary K, Li XY, Allen E, Stevens SL and
Weiss SJ: A cancer cell metalloprotease triad regulates the
basement membrane transmigration program. Genes Dev. 20:2673–2686.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi J, Son MY, Yamada S, Szabova L, Kahan
S, Chrysovergis K, Wolf L, Surmak A and Holmbeck K: Membrane-type
MMPs enable extracellular matrix permissiveness and mesenchymal
cell proliferation during embryogenesis. Dev Biol. 313:196–209.
2008. View Article : Google Scholar :
|
|
9
|
Behrendt N, Jensen ON, Engelholm LH, Mørtz
E, Mann M and Danø K: A urokinase receptor-associated protein with
specific collagen binding properties. J Biol Chem. 275:1993–2002.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sheikh H, Yarwood H, Ashworth A and Isacke
CM: Endo180, an endocytic recycling glycoprotein related to the
macrophage mannose receptor is expressed on fibroblasts,
endothelial cells and macrophages and functions as a lectin
receptor. J Cell Sci. 113:1021–1032. 2000.PubMed/NCBI
|
|
11
|
Thomas EK, Nakamura M, Wienke D, Isacke
CM, Pozzi A and Liang P: Endo180 binds to the C-terminal region of
type I collagen. J Biol Chem. 280:22596–22605. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Engelholm LH, List K, Netzel-Arnett S,
Cukierman E, Mitola DJ, Aaronson H, Kjøller L, Larsen JK, Yamada
KM, Strickland DK, et al: uPARAP/Endo180 is essential for cellular
uptake of collagen and promotes fibroblast collagen adhesion. J
Cell Biol. 160:1009–1015. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wienke D, MacFadyen JR and Isacke CM:
Identification and characterization of the endocytic transmembrane
glycoprotein Endo180 as a novel collagen receptor. Mol Biol Cell.
14:3592–3604. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
East L, McCarthy A, Wienke D, Sturge J,
Ashworth A and Isacke CM: A targeted deletion in the endocytic
receptor gene Endo180 results in a defect in collagen uptake. EMBO
Rep. 4:710–716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kjøller L, Engelholm LH, Høyer-Hansen M,
Danø K, Bugge TH and Behrendt N: uPARAP/endo180 directs lysosomal
delivery and degradation of collagen IV. Exp Cell Res. 293:106–116.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Howard MJ and Isacke CM: The C-type lectin
receptor Endo180 displays internalization and recycling properties
distinct from other members of the mannose receptor family. J Biol
Chem. 277:32320–32331. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sturge J, Wienke D, East L, Jones GE and
Isacke CM: GPI-anchored uPAR requires Endo180 for rapid directional
sensing during chemotaxis. J Cell Biol. 162:789–794. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sturge J, Wienke D and Isacke CM:
Endosomes generate localized Rho-ROCK-MLC2-based contractile
signals via Endo180 to promote adhesion disassembly. J Cell Biol.
175:337–347. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Curino AC, Engelholm LH, Yamada SS,
Holmbeck K, Lund LR, Molinolo AA, Behrendt N, Nielsen BS and Bugge
TH: Intracellular collagen degradation mediated by uPARAP/Endo180
is a major pathway of extracellular matrix turnover during
malignancy. J Cell Biol. 169:977–985. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Madsen DH, Engelholm LH, Ingvarsen S,
Hillig T, Wagenaar-Miller RA, Kjøller L, Gårdsvoll H, Høyer-Hansen
G, Holmbeck K, Bugge TH, et al: Extracellular collagenases and the
endocytic receptor, urokinase plasminogen activator
receptor-associated protein/Endo180, cooperate in
fibroblast-mediated collagen degradation. J Biol Chem.
282:27037–27045. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi F, Harman J, Fujiwara K and Sottile J:
Collagen I matrix turnover is regulated by fibronectin
polymerization. Am J Physiol Cell Physiol. 298:C1265–C1275. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leitinger B and Hohenester E: Mammalian
collagen receptors. Matrix Biol. 26:146–155. 2007. View Article : Google Scholar
|
|
23
|
Everts V, van der Zee E, Creemers L and
Beertsen W: Phagocytosis and intracellular digestion of collagen,
its role in turnover and remodelling. Histochem J. 28:229–245.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arora PD, Manolson MF, Downey GP, Sodek J
and McCulloch CA: A novel model system for characterization of
phagosomal maturation, acidification, and intracellular collagen
degradation in fibroblasts. J Biol Chem. 275:35432–35441. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Madsen DH, Ingvarsen S, Jürgensen HJ,
Melander MC, Kjøller L, Moyer A, Honoré C, Madsen CA, Garred P,
Burgdorf S, et al: The non-phagocytic route of collagen uptake: A
distinct degradation pathway. J Biol Chem. 286:26996–27010. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu K, Yuan J and Lasky LA:
Characterization of a novel member of the macrophage mannose
receptor type C lectin family. J Biol Chem. 271:21323–21330. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tesar DB, Cheung EJ and Bjorkman PJ: The
chicken yolk sac IgY receptor, a mammalian mannose receptor family
member, transcytoses IgY across polarized epithelial cells. Mol
Biol Cell. 19:1587–1593. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He Y and Bjorkman PJ: Structure of FcRY,
an avian immunoglobulin receptor related to mammalian mannose
receptors, and its complex with IgY. Proc Natl Acad Sci USA.
108:12431–12436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
East L and Isacke CM: The mannose receptor
family. Biochim Biophys Acta. 1572:364–386. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Napper CE, Drickamer K and Taylor ME:
Collagen binding by the mannose receptor mediated through the
fibronectin type II domain. Biochem J. 395:579–586. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martinez-Pomares L, Wienke D, Stillion R,
McKenzie EJ, Arnold JN, Harris J, McGreal E, Sim RB, Isacke CM and
Gordon S: Carbohydrate-independent recognition of collagens by the
macrophage mannose receptor. Eur J Immunol. 36:1074–1082. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Malovic I, Sørensen KK, Elvevold KH,
Nedredal GI, Paulsen S, Erofeev AV, Smedsrød BH and McCourt PA: The
mannose receptor on murine liver sinusoidal endothelial cells is
the main denatured collagen clearance receptor. Hepatology.
45:1454–1461. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Madsen DH, Leonard D, Masedunskas A, Moyer
A, Jürgensen HJ, Peters DE, Amornphimoltham P, Selvaraj A, Yamada
SS, Brenner DA, et al: M2-like macrophages are responsible for
collagen degradation through a mannose receptor-mediated pathway. J
Cell Biol. 202:951–966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jürgensen HJ, Johansson K, Madsen DH,
Porse A, Melander MC, Sørensen KR, Nielsen C, Bugge TH, Behrendt N
and Engelholm LH: Complex determinants in specific members of the
mannose receptor family govern collagen endocytosis. J Biol Chem.
289:7935–7947. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ancian P, Lambeau G and Lazdunski M:
Multifunctional activity of the extracellular domain of the M-type
(180 kDa) membrane receptor for secretory phospholipases A2.
Biochemistry. 34:13146–13151. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takahashi S, Watanabe K, Watanabe Y,
Fujioka D, Nakamura T, Nakamura K, Obata JE and Kugiyama K: C-type
lectin-like domain and fibronectin-like type II domain of
phospholipase A(2) receptor 1 modulate binding and migratory
responses to collagen. FEBS Lett. 589:829–835. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jürgensen HJ, Madsen DH, Ingvarsen S,
Melander MC, Gårdsvoll H, Patthy L, Engelholm LH and Behrendt N: A
novel functional role of collagen glycosylation: Interaction with
the endocytic collagen receptor uparap/ENDO180. J Biol Chem.
286:32736–32748. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
East L, Rushton S, Taylor ME and Isacke
CM: Characterization of sugar binding by the mannose receptor
family member, Endo180. J Biol Chem. 277:50469–50475. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sage H, Woodbury RG and Bornstein P:
Structural studies on human type IV collagen. J Biol Chem.
254:9893–9900. 1979.PubMed/NCBI
|
|
40
|
Rivera-Calzada A, Robertson D, MacFadyen
JR, Boskovic J, Isacke CM and Llorca O: Three-dimensional interplay
among the ligand-binding domains of the
urokinase-plasminogen-activator-receptor-associated protein,
Endo180. EMBO Rep. 4:807–812. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Boskovic J, Arnold JN, Stilion R, Gordon
S, Sim RB, Rivera-Calzada A, Wienke D, Isacke CM, Martinez-Pomares
L and Llorca O: Structural model for the mannose receptor family
uncovered by electron microscopy of Endo180 and the mannose
receptor. J Biol Chem. 281:8780–8787. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Llorca O: Extended and bent conformations
of the mannose receptor family. Cell Mol Life Sci. 65:1302–1310.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sturge J, Todd SK, Kogianni G, McCarthy A
and Isacke CM: Mannose receptor regulation of macrophage cell
migration. J Leukoc Biol. 82:585–593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fasquelle C, Sartelet A, Li W, Dive M,
Tamma N, Michaux C, Druet T, Huijbers IJ, Isacke CM, Coppieters W,
et al: Balancing selection of a frame-shift mutation in the MRC2
gene accounts for the outbreak of the Crooked Tail Syndrome in
Belgian Blue Cattle. PLoS Genet. 5:e10006662009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sartelet A, Klingbeil P, Franklin CK,
Fasquelle C, Géron S, Isacke CM, Georges M and Charlier C: Allelic
heterogeneity of Crooked Tail Syndrome: Result of balancing
selection? Anim Genet. 43:604–607. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wagenaar-Miller RA, Engelholm LH, Gavard
J, Yamada SS, Gutkind JS, Behrendt N, Bugge TH and Holmbeck K:
Complementary roles of intracellular and pericellular collagen
degradation pathways in vivo. Mol Cell Biol. 27:6309–6322. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Madsen DH, Jürgensen HJ, Ingvarsen S,
Melander MC, Albrechtsen R, Hald A, Holmbeck K, Bugge TH, Behrendt
N and Engelholm LH: Differential actions of the endocytic collagen
receptor uPARAP/Endo180 and the collagenase MMP-2 in bone
homeostasis. PLoS One. 8:e712612013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Inoue K, Mikuni-Takagaki Y, Oikawa K, Itoh
T, Inada M, Noguchi T, Park JS, Onodera T, Krane SM, Noda M, et al:
A crucial role for matrix metalloproteinase 2 in osteocytic
canalicular formation and bone metabolism. J Biol Chem.
281:33814–33824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mosig RA, Dowling O, DiFeo A, Ramirez MC,
Parker IC, Abe E, Diouri J, Aqeel AA, Wylie JD, Oblander SA, et al:
Loss of MMP-2 disrupts skeletal and craniofacial development and
results in decreased bone mineralization, joint erosion and defects
in osteoblast and osteoclast growth. Hum Mol Genet. 16:1113–1123.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Abdelgawad ME, Søe K, Andersen TL, Merrild
DM, Christiansen P, Kjærsgaard-Andersen P and Delaisse JM: Does
collagen trigger the recruitment of osteoblasts into vacated bone
resorption lacunae during bone remodeling? Bone. 67:181–188. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wu X, Spitz MR, Lee JJ, Lippman SM, Ye Y,
Yang H, Khuri FR, Kim E, Gu J, Lotan R, et al: Novel susceptibility
loci for second primary tumors/recurrence in head and neck cancer
patients: Large-scale evaluation of genetic variants. Cancer Prev
Res (Phila). 2:617–624. 2009. View Article : Google Scholar
|
|
52
|
Yamaguchi T, Nakaoka H, Yamamoto K,
Fujikawa T, Kim YI, Yano K, Haga S, Katayama K, Shibusawa T, Park
SB, et al: Genome-wide association study of degenerative bony
changes of the temporomandibular joint. Oral Dis. 20:409–415. 2014.
View Article : Google Scholar
|
|
53
|
Engelholm LH, Nielsen BS, Netzel-Arnett S,
Solberg H, Chen XD, Lopez Garcia JM, Lopez-Otin C, Young MF,
Birkedal-Hansen H, Danø K, et al: The urokinase plasminogen
activator receptor-associated protein/endo180 is coexpressed with
its interaction partners urokinase plasminogen activator receptor
and matrix metalloprotease-13 during osteogenesis. Lab Invest.
81:1403–1414. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Isacke CM, van der Geer P, Hunter T and
Trowbridge IS: p180, a novel recycling transmembrane glycoprotein
with restricted cell type expression. Mol Cell Biol. 10:2606–2618.
1990.PubMed/NCBI
|
|
55
|
Howard MJ, Chambers MG, Mason RM and
Isacke CM: Distribution of Endo180 receptor and ligand in
developing articular cartilage. Osteoarthritis Cartilage. 12:74–82.
2004. View Article : Google Scholar
|
|
56
|
Everts V, Delaissé JM, Korper W, Jansen
DC, Tigchelaar-Gutter W, Saftig P and Beertsen W: The bone lining
cell: Its role in cleaning Howship's lacunae and initiating bone
formation. J Bone Miner Res. 17:77–90. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bundesmann MM, Wagner TE, Chow YH,
Altemeier WA, Steinbach T and Schnapp LM: Role of urokinase
plasminogen activator receptor-associated protein in mouse lung. Am
J Respir Cell Mol Biol. 46:233–239. 2012. View Article : Google Scholar :
|
|
58
|
Engelholm LH, Nielsen BS, Danø K and
Behrendt N: The urokinase receptor associated protein
(uPARAP/endo180): A novel internalization receptor connected to the
plasminogen activation system. Trends Cardiovasc Med. 11:7–13.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Honardoust HA, Jiang G, Koivisto L, Wienke
D, Isacke CM, Larjava H and Häkkinen L: Expression of Endo180 is
spatially and temporally regulated during wound healing.
Histopathology. 49:634–648. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mousavi SA, Sato M, Sporstøl M, Smedsrød
B, Berg T, Kojima N and Senoo H: Uptake of denatured collagen into
hepatic stellate cells: Evidence for the involvement of urokinase
plasminogen activator receptor-associated protein/Endo180. Biochem
J. 387:39–46. 2005. View Article : Google Scholar :
|
|
61
|
Mousavi SA, Fønhus MS and Berg T:
Up-regulation of uPARAP/Endo180 during culture activation of rat
hepatic stellate cells and its presence in hepatic stellate cell
lines from different species. BMC Cell Biol. 10:392009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ikenaga N, Ohuchida K, Mizumoto K, Akagawa
S, Fujiwara K, Eguchi D, Kozono S, Ohtsuka T, Takahata S and Tanaka
M: Pancreatic cancer cells enhance the ability of collagen
internalization during epithelial-mesenchymal transition. PLoS One.
7:e404342012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wienke D, Davies GC, Johnson DA, Sturge J,
Lambros MB, Savage K, Elsheikh SE, Green AR, Ellis IO, Robertson D,
et al: The collagen receptor Endo180 (CD280) Is expressed on
basal-like breast tumor cells and promotes tumor growth in vivo.
Cancer Res. 67:10230–10240. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Caley MP, Kogianni G, Adamarek A, Gronau
JH, Rodriguez-Teja M, Fonseca AV, Mauri F, Sandison A, Rhim JS,
Pchejetski D, et al: TGFβ1-Endo180-dependent collagen deposition is
dysregulated at the tumour-stromal interface in bone metastasis. J
Pathol. 226:775–783. 2012. View Article : Google Scholar
|
|
65
|
Huijbers IJ, Iravani M, Popov S, Robertson
D, Al-Sarraj S, Jones C and Isacke CM: A role for fibrillar
collagen deposition and the collagen internalization receptor
endo180 in glioma invasion. PLoS One. 5:e98082010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Schnack Nielsen B, Rank F, Engelholm LH,
Holm A, Danø K and Behrendt N: Urokinase receptor-associated
protein (uPARAP) is expressed in connection with malignant as well
as benign lesions of the human breast and occurs in specific
populations of stromal cells. Int J Cancer. 98:656–664. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Palmieri C, Caley MP, Purshouse K, Fonseca
AV, Rodriguez-Teja M, Kogianni G, Woodley L, Odendaal J, Elliott K,
Waxman J, et al: Endo180 modulation by bisphosphonates and
diagnostic accuracy in metastatic breast cancer. Br J Cancer.
108:163–169. 2013. View Article : Google Scholar :
|
|
68
|
Kogianni G, Walker MM, Waxman J and Sturge
J: Endo180 expression with cofunctional partners MT1-MMP and
uPAR-uPA is correlated with prostate cancer progression. Eur J
Cancer. 45:685–693. 2009. View Article : Google Scholar
|
|
69
|
Rodriguez-Teja M, Gronau JH, Minamidate A,
Darby S, Gaughan L, Robson C, Mauri F, Waxman J and Sturge J:
Survival outcome and EMT suppression mediated by a lectin domain
interaction of Endo180 and CD147. Mol Cancer Res. 13:538–547. 2015.
View Article : Google Scholar
|
|
70
|
Rodriguez-Teja M, Gronau JH, Breit C,
Zhang YZ, Minamidate A, Caley MP, McCarthy A, Cox TR, Erler JT,
Gaughan L, et al: AGE-modified basement membrane cooperates with
Endo180 to promote epithelial cell invasiveness and decrease
prostate cancer survival. J Pathol. 235:581–592. 2015. View Article : Google Scholar
|
|
71
|
Stupp R, van den Bent MJ and Hegi ME:
Optimal role of temo-zolomide in the treatment of malignant
gliomas. Curr Neurol Neurosci Rep. 5:198–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
D'Abaco GM and Kaye AH: Integrins:
Molecular determinants of glioma invasion. J Clin Neurosci.
14:1041–1048. 2007. View Article : Google Scholar
|
|
73
|
Payne LS and Huang PH: The pathobiology of
collagens in glioma. Mol Cancer Res. 11:1129–1140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Takahashi S, Yamada-Okabe H, Hamada K,
Ohta S, Kawase T, Yoshida K and Toda M: Downregulation of uPARAP
mediates cytoskeletal rearrangements and decreases invasion and
migration properties in glioma cells. J Neurooncol. 103:267–276.
2011. View Article : Google Scholar
|
|
75
|
Mundy GR: Mechanisms of bone metastasis.
Cancer. 80(Suppl): 1546–1556. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
St Croix B, Rago C, Velculescu V, Traverso
G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C,
Vogelstein B, et al: Genes expressed in human tumor endothelium.
Science. 289:1197–1202. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gai X, Tu K, Lu Z and Zheng X: MRC2
expression correlates with TGFβ1 and survival in hepatocellular
carcinoma. Int J Mol Sci. 15:15011–15025. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sulek J, Wagenaar-Miller RA, Shireman J,
Molinolo A, Madsen DH, Engelholm LH, Behrendt N and Bugge TH:
Increased expression of the collagen internalization receptor
uPARAP/Endo180 in the stroma of head and neck cancer. J Histochem
Cytochem. 55:347–353. 2007. View Article : Google Scholar
|
|
79
|
Madsen DH, Jürgensen HJ, Ingvarsen S,
Melander MC, Vainer B, Egerod KL, Hald A, Rønø B, Madsen CA, Bugge
TH, et al: Endocytic collagen degradation: A novel mechanism
involved in protection against liver fibrosis. J Pathol.
227:94–105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
López-Guisa JM, Cai X, Collins SJ,
Yamaguchi I, Okamura DM, Bugge TH, Isacke CM, Emson CL, Turner SM,
Shankland SJ, et al: Mannose receptor 2 attenuates renal fibrosis.
J Am Soc Nephrol. 23:236–251. 2012. View Article : Google Scholar :
|
|
81
|
Rohani MG, Chow YH, Razumova MV, Ash S,
Hung CF and Schnapp LM: uPARAP function in cutaneous wound repair.
PLoS One. 9:e926602014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Oppel T and Korting HC: Actinic keratosis:
The key event in the evolution from photoaged skin to squamous cell
carcinoma. Therapy based on pathogenetic and clinical aspects. Skin
Pharmacol Physiol. 17:67–76. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tang S, Lucius R, Wenck H, Gallinat S and
Weise JM: UV-mediated downregulation of the endocytic collagen
receptor, Endo180, contributes to accumulation of extracellular
collagen fragments in photoaged skin. J Dermatol Sci. 70:42–48.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Shim JH, Shin DW, Noh MS and Lee TR:
Reduced collagen internalization via down-regulation of MRC2
expression by UVA irradiation and its recovery by all-trans
retinoic acid. J Dermatol Sci. 73:163–166. 2014. View Article : Google Scholar
|
|
85
|
Taylor ME: Evolution of a family of
receptors containing multiple C-type carbohydrate-recognition
domains. Glycobiology. 7:v–viii. 1997.PubMed/NCBI
|