The treatment of ovarian cancer (OC) has improved
over the past 30 years, following the introduction of platinum- and
paclitaxel-based chemotherapy. However, most patients with OC will
suffer disease relapse despite having achieved a complete clinical
response. In many of these patients, the disease is incurable
mainly owing to the development of drug resistance (1). Treatment failure and death from OC
have been attributed to drug resistance in >90% of patients with
metastatic disease. Thus, a better understanding of the mechanisms
of drug resistance in OC will lead to improved treatment strategies
and perhaps, better survival (2).
In the present study, we provide evidence of a
marked decrease in TRPC1 mRNA levels in human OC vs. normal
specimens/cells and of their downregulation in drug-resistant OC
vs. sensitive cells. Comprehensive bioinformatics analyses
suggested the interaction of TRPC1 with numerous proteins/genes,
chemicals, biological processes and microRNAs, all of which are
involved in the regulation of OC drug resistance. In addition,
lower TRPC1 expression was shown to correlate with the high
histological grade of tumors in OC patients.
The human epithelial OC cell lines SKOV3 and A2780
were maintained in our laboratory and propagated in vitro by
serial passage in RPMI-1640 medium supplemented with 10% fetal
bovine serum (FBS). The cisplatin-resistant cell line SKOV3-DDP and
the carboplatin-resistant cell line A2780-CBP were established by
sequential exposure of cells to increasing concentrations of
cisplatin and carboplatin, respectively. The resistance index of
the SKOV3-DDP and A2780-CBP OC cells is 2.4 and 2.0,
respectively.
Total RNA was isolated from the cell lines SKOV3,
SKOV3-DDP, A2780 and A2780-CBP, using TRIzol reagent (Life
Technologies, Grand Island, NY, USA). The quantity and quality of
the RNA were measured using a Thermo Scientific NanoDrop 2000
spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA).
cDNA was synthesized from 2 μg of RNA using the SuperScript III
First-Strand Synthesis system (Life Technologies). TRPC1 mRNA
levels were measured using real-time quantitative polymerase chain
reaction (RT-qPCR) and the Power SYBR-Green PCR Master Mix (Applied
Biosystems, Life Technologies, Waltham, MA, USA). Data were
collected with the Applied Biosystems 7300 RT-PCR system in
accordance with the manufacturer's instructions. The RT-qPCR
gene-specific primers for TRPC1 were: (forward primer)
5′-ACGTCTAGTGACGAGCCTCT-3′ and (reverse primer)
5′-CCCGACATCTGTCCAAACCA-3′. For GAPDH, used as the control, the
forward primer was 5′-GAAGGTGAAGGTCGGAGT-3′ and the reverse primer
5′-GAAGATGGTGATGGGATTT-3′.
The data were analyzed using the SPSS 20.0 software.
The mRNA expression level of a particular gene is shown as the mean
± SD. The homogeneity of the variance was analyzed using the
t-test. The probability of survival and significance were
calculated using the Kaplan-Meier method and a log-rank test. The
correlation between microRNAs and the gene was analyzed using
bivariate correlations. The correlation between gene expression and
the clinicopathological characteristics was evaluated by Pearson's
χ2 test (2-sided). Expression values of a gene were
dichotomized into high and low expression using the median as a
cut-off in a Kaplan-Meier analysis, in accordance with previous
studies (31,32). A P-value <0.05 was considered to
indicate statistical significance.
TRPC1 mRNA expression was significantly and
consistently downregulated in OC tissues and cells compared with
the expressions in normal controls, as determined using data
retrieved from microarrays deposited in Oncomine and GEO Profiles.
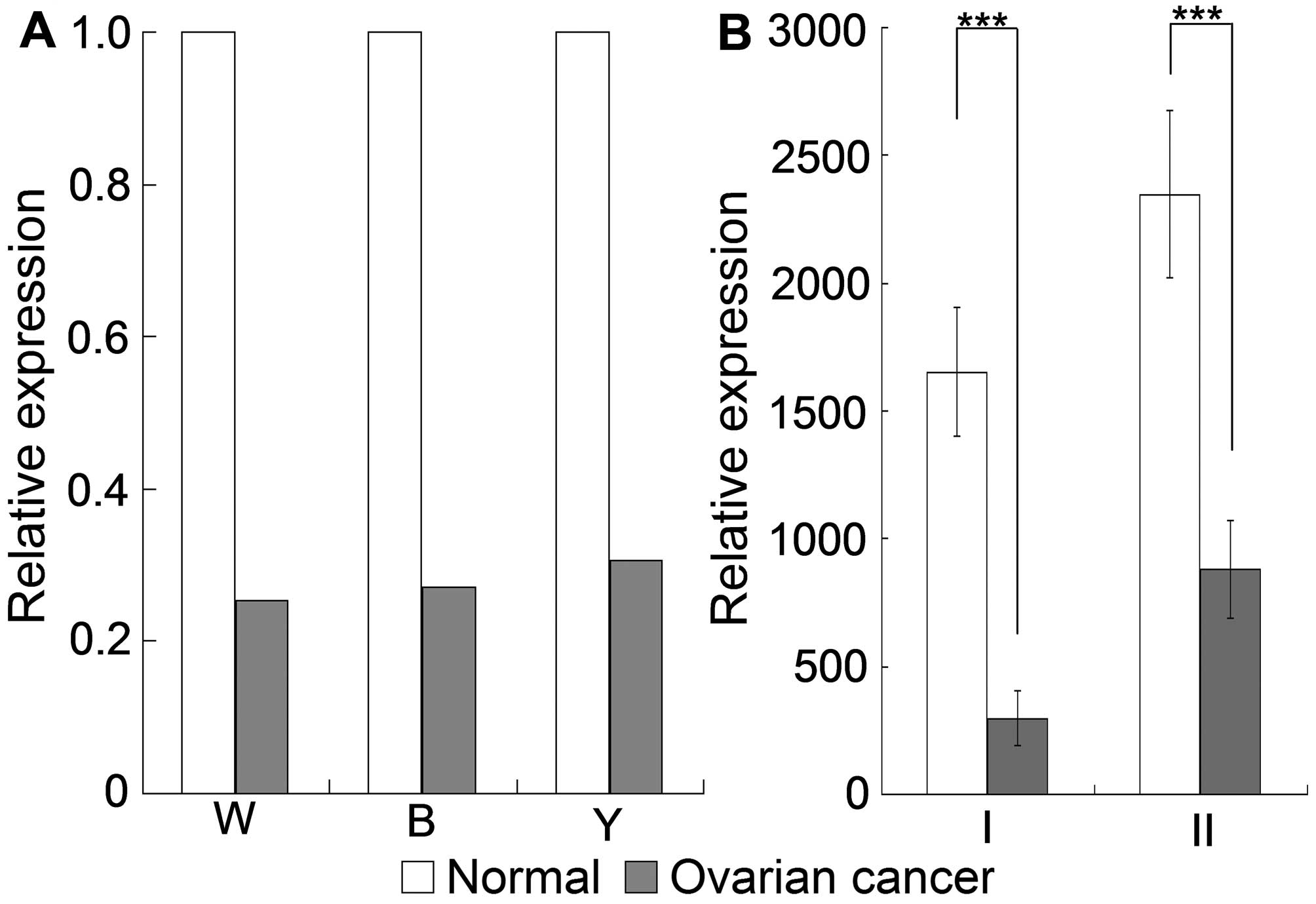
As indicated in Fig. 1A, TRPC1
mRNA expression was downregulated by 3.955-, 3.681- and 3.260-fold
in OC specimens according to the Oncomine Welsh ovarian microarray,
covering 28 ovarian serous surface papillary carcinomas and four
normal ovarian tissues; the Bonome ovarian microarray, covering 185
ovarian carcinomas and 10 ovarian surface epithelia; and the
Yoshihara ovarian array, covering 38 ovarian serous adenocarcinomas
and 10 peritoneal tissues. Consistent with the expression in OC
specimens, TRPC1 expression was downregulated by at least 3-fold in
OC cells compared with the expression in normal ovarian surface
epithelial cells, in accordance with GEO Profiles analyses
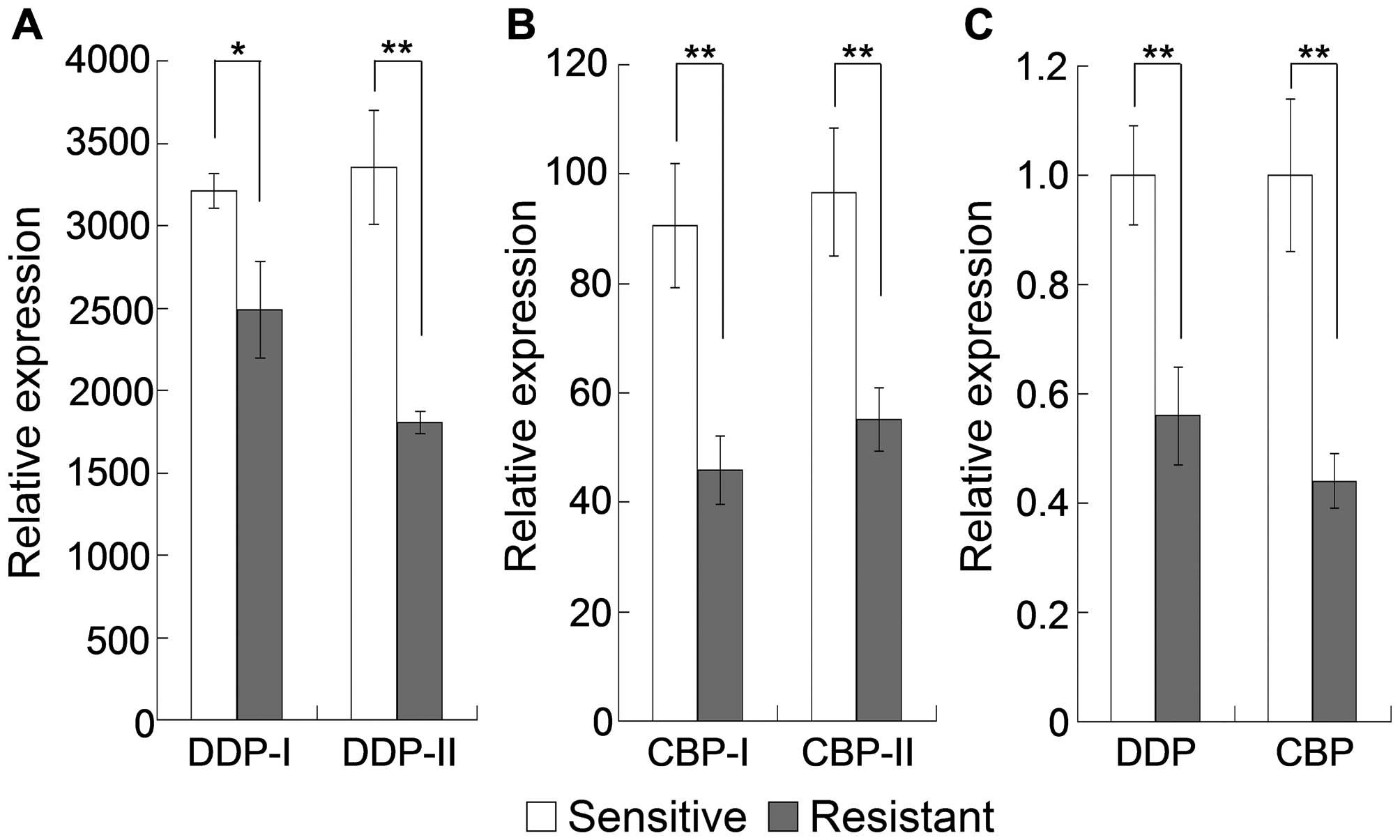
(Fig. 1B). There was also a
significant downregulation of TRPC1 mRNA expression in A2780
epithelial OC cells with acquired platinum resistance and in
carboplatin-resistant OC cells compared with expression in the
sensitive counterparts of both (Fig.
2A and B). This result was confirmed by the RT-qPCR analysis,
in which TRPC1 mRNA expression was significantly downregulated in
both cisplatin-resistant SKOV3 cells and carboplatin-resistant
A2780 cells compared with expression in the corresponding sensitive
cells (Fig. 2C). These results
suggested that the stable and significant downregulation of TRPC1
in specimens/cells from OC and drug-resistant OC plays a critical
role in the development drug resistance of these tumors.
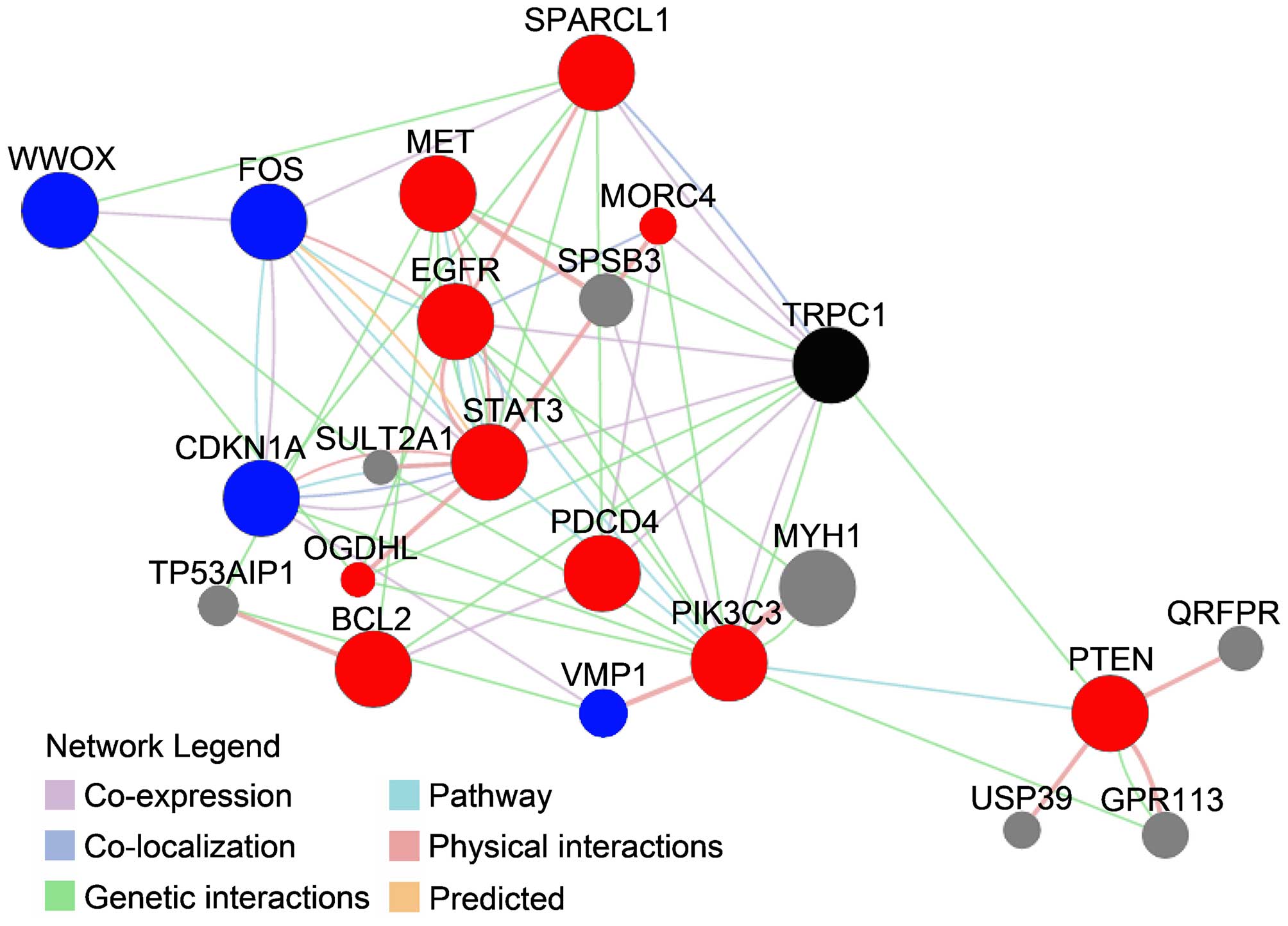
The protein/gene interactions of TRPC1 with other
proteins/genes were analyzed using the GeneMANIA tool. As shown in
Fig. 3, TRPC1 interacted directly
with 10 genes/proteins: MORC family CW-type zinc finger 4 (MORC4),
epidermal growth factor receptor (EGFR), signal transducer and
activator of transcription 3 (STAT3), programmed cell death 4
(PDCD4), MET proto-oncogene, receptor tyrosine kinase (MET),
oxoglutarate dehydrogenase-like (OGDHL), B-cell CLL/lymphoma 2
(BCL2), phosphatase and tensin homolog (PTEN), SPARC-like 1 (hevin)
(SPARCL1) and phosphatidylinositol 3-kinase, catalytic subunit type
3 (PIK3C3). Except for MORC4, all of these genes/proteins have been
implicated in the regulation of drug resistance in OC. For example,
PTEN is a TSG involved in the regulation of drug resistance via the
PI3K/AKT pathway and the p53-mediated apoptotic cascade. A
reduction in PTEN expression confers resistance to cisplatin in
OVCAR-3 cells through the activation of PI3K/Akt (36), and the overexpression of PTEN
reverses chemoresistance to cisplatin in human OC cells by
inactivating the PI3K/AKT cell survival pathway (37). However, in another study, the
overexpression of PTEN upregulated p53 and increased the
sensitivity of chemoresistant cells to cisplatin-induced apoptosis
without detectable changes in the levels of phosphorylated Akt,
suggesting that PTEN regulates drug resistance through a
p53-mediated apoptotic cascade independent of the PI3K/Akt pathway
(38). In a recent study, the
overexpression of PTEN improved the cisplatin-resistance of human
OC cells by upregulating KRT10 expression. Thus, the exogenously
induced overexpression of KRT10 may be a therapeutic strategy for
overcoming multi-drug resistance in OC (39). BCL2, STAT3, EGFR and MET are
drug-resistance-related oncogenes (10), and PDCD4 (40) and SPARCL1 (41) are drug-resistance-related TSGs. All
of these genes are expressed in OC. OGDHL is thought to modify
AKT-dependent signaling and NFKB1 function (42), both of which play crucial roles in
the regulation of drug resistance in OC (10).
In addition to proteins/genes that directly interact
with TRPC1, there were others in the network whose interaction with
TRPC1 was indirect (Fig. 3). Among
those, CDKN1A (43,44), FOS (45,46),
WWOX (47) and VMP1 (48) are associated with drug resistance
in OC. Collectively, among the 21 proteins/genes found to interact
with TRPC1, 14 were associated with drug resistance in OC. These
results strongly suggest an association between TRPC1 and drug
resistance in OC.
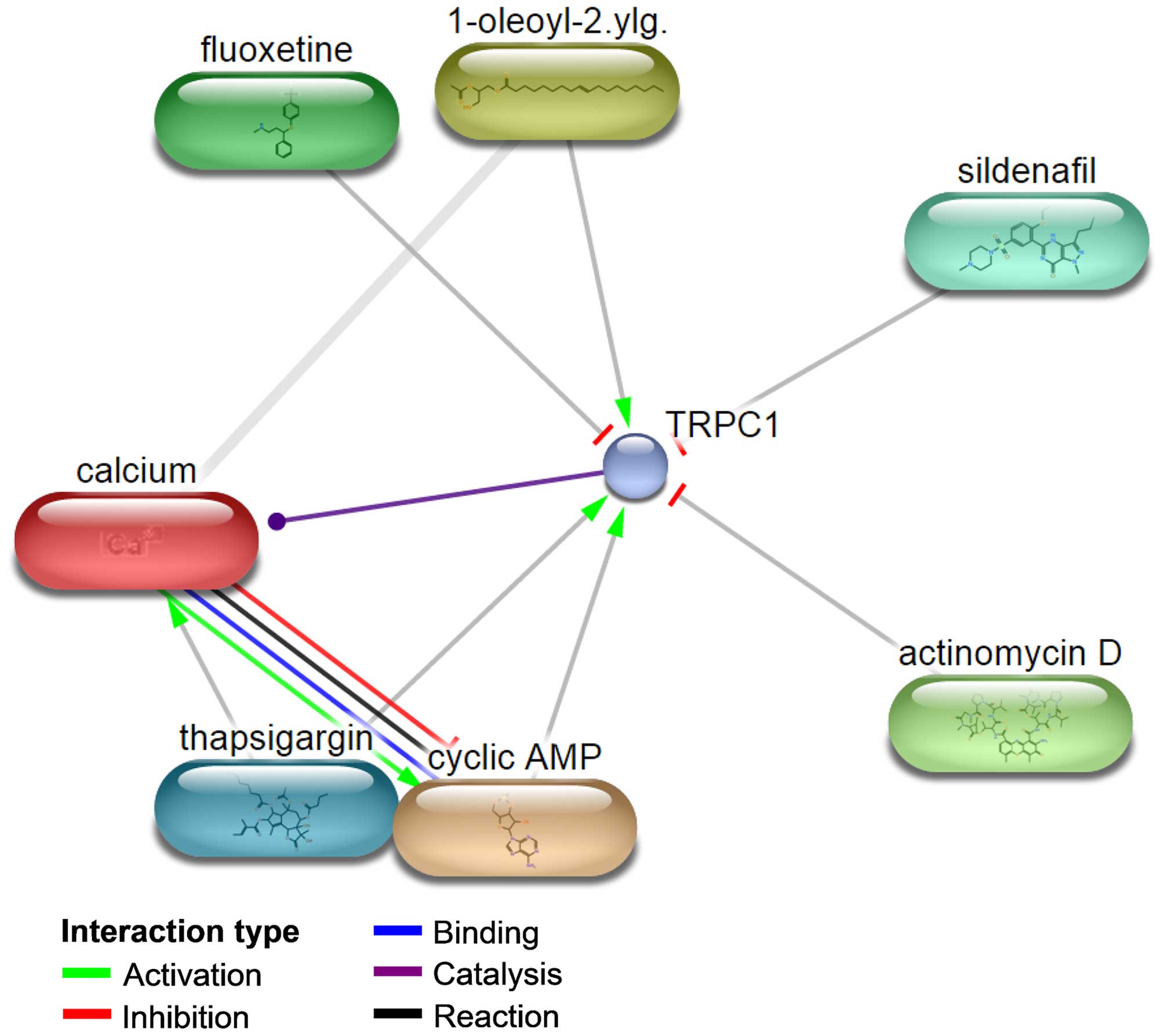
The protein and small molecule/chemical interaction
was assessed in an attempt to further explain the
drug-resistance-related functions of TRPC1 in OC. As shown in
Fig. 4, TRPC1 interacts with
1-oleoyl-2-acetylglycerol, cyclic AMP, thapsigargin, calcium,
actinomycin D, fluoxetine and sildenafil. The first three compounds
activate TRPC1 and the last three inhibit TRPC1. Except for
sildenafil, all of these compounds are associated with drug
resistance or are otherwise involved in chemotherapy in ovarian and
other cancers. For example, 1-oleoyl-2-acetylglycerol enhances the
phosphorylation of P-glycoprotein (49), which contributes to the development
of drug resistance in OC by regulating several pathways, such as
nuclear factor of kappa light polypeptide gene enhancer in B-cells
1 (NFKB1), mitogen-activated protein kinase (MAPK) and
phosphatidylinositol-4,5-bisphosphate 3-kinase, and catalytic
subunit alpha (PI3K) signaling (50). Cyclic AMP reduces the induction of
AP-1 binding, required for the activation of interleukin 8 (IL8) by
paclitaxel (51). The presence of
IL8 in paclitaxel-treated OC cells contributes to the development
of paclitaxel resistance (52). In
a recent study, thapsigargin exhibited pharmacological activity
against OC stem cells (53).
Fluoxetine is an antidepressant that greatly enhances the
cytotoxicity of cisplatin in HCT116 wild-type (wt), HCT116
(p53−/−), HT-29, SKOV3 and A2780 cells (54) and induces apoptosis through the
reactive oxygen species-dependent activation of NFKB1, frequently
implicated in the regulation of drug resistance (55). Actinomycin D is an antitumor agent
used in the chemotherapy of OC (56). Thus, among the identified small
molecules, 1-oleoyl-2-acetylglycerol, cyclic AMP, thapsigargin,
actinomycin D and fluoxetine are drug-resistance- related or are
involved in the chemotherapy of OC. Their interaction with TRPC1
implicates this protein in the drug resistance of these tumors.
There is also evidence for a relationship between calcium and drug
resistance in OC. A calcium channel blocker verapamil was reported
to reverse adriamycin resistance through inhibition of adriamycin
efflux in OC resistant cells (57). Similarly, doxorubicin resistance is
reversed by verapamil (58). Since
TRPC1 is an activator of calcium, these results also suggest its
involvement in drug resistance.
MicroRNA-mediated post-transcriptional gene
regulation is an important regulator of many cellular processes,
both physiological and pathological (63,64).
The target genes of microRNAs are a focus of interest based on
their diagnostic, prognostic and therapeutic relevance (65), and the function of a gene can be
predicted based on functionality of the microRNAs that target it
(41). To identify microRNAs that
target TRPC1, a microRNA-mRNA interaction analysis was performed
with miRWalk, which resulted in the identification of 481 microRNAs
predicted to transcriptionally target TRPC1 and suggested the
regulation of TRPC1 by microRNA. Those microRNAs predicted by at
least 6 of the 8 prediction tools were submitted to pathway
enrichment using DIANA miRPath (30). Pathways involved in the regulation
of cancer development and progression are also likely to be
involved in the regulation of drug resistance in ovarian and other
cancers. As shown in Table I, 11
pathways were enriched from the top 38 microRNAs that potentially
target TRPC1. Among the 11 pathways, at least 8 are involved in the
regulation of drug resistance in OC, including the PI3K-Akt, MAPK
and Wnt pathways. PI3K/AKT signaling is a major cell survival
pathway (66). It is involved in
the onset of drug resistance in OC (34) by promoting cell survival and
blocking apoptosis (36).
To confirm the potential regulation roles of the
microRNAs on TRPC1, the correlation between top 10 microRNAs
(Table II) and TRPC1 was
performed, on the basis of their expressions in ovarian cancer
tissues. The expression data of the microRNAs and TRPC1 were
retrieved from TCGA cohort covering 489 cases of ovarian cancer
tissues. Among the 8 microRNAs (the expression data of miR-603 and
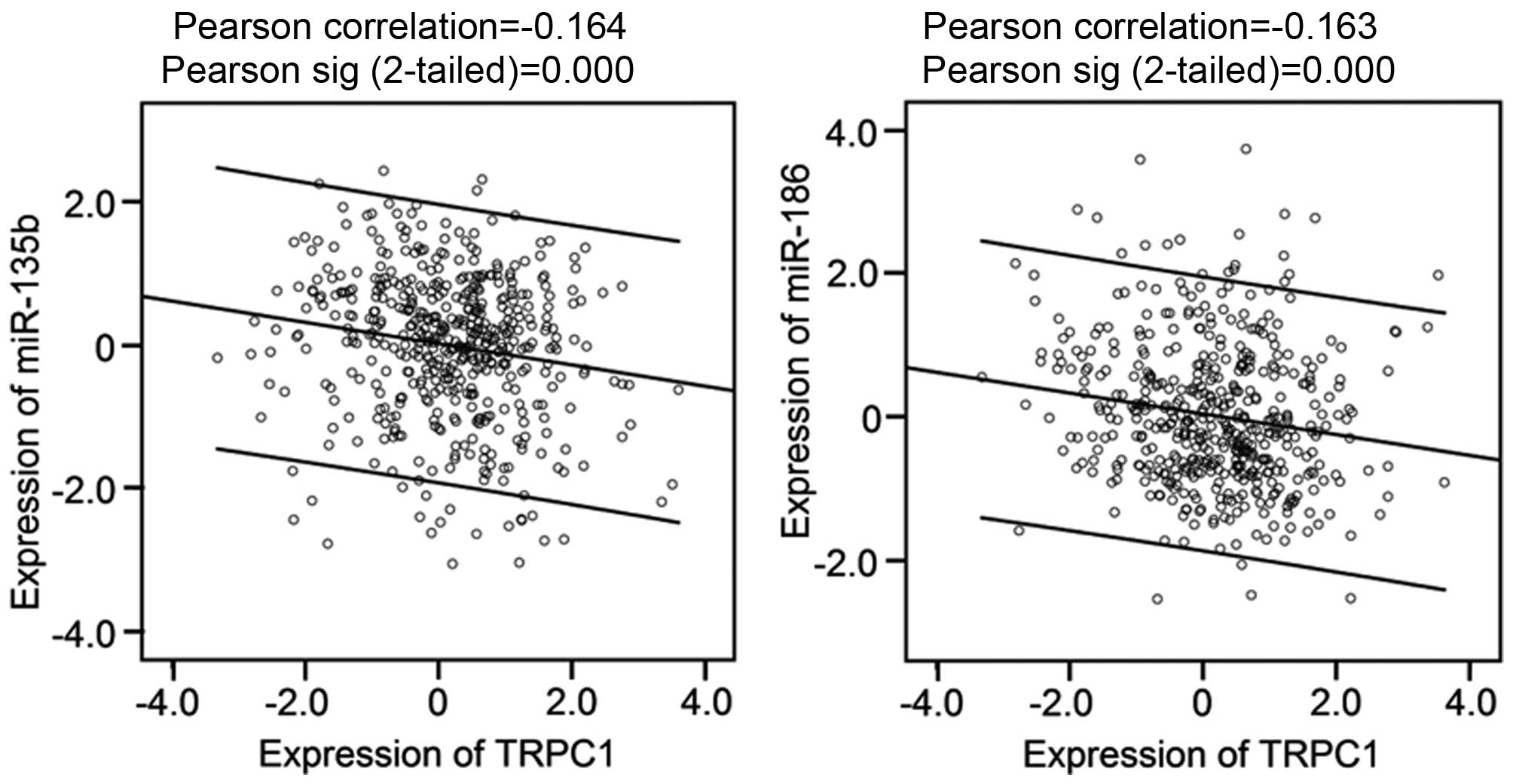
miR-103 are not available), 5 of them including miR-135b, miR-186,
miR-26a, miR-497 and miR-548b-3p are significantly correlated with
TRPC1, in particular the former 2, their high expression is
significantly correlated with the low expression of TRPC1 (Fig. 6).
Eight of the top 10 microRNAs that targeted TRPC1
are associated with drug resistance in ovarian and other cancers
(Table II). For example, miR-135a
is a tumor suppressor in epithelial OC and regulates HOXA10
expression (67), which correlates
with platinum resistance in OC (68). Upregulation of miR-497 in
Taxol-resistant OC is closely associated with Taxol resistance and
is therefore of interest in the development of microRNA therapies
for OC (69).
Taken together, these results provide further
support for the involvement of TRPC1 in drug resistance in OC.
We next examined the expression profile and clinical
significance of TRPC1 in a TCGA cohort. The clinical data of 489
patients with OC for whom TRPC1 mRNA expression data were available
was retrieved from cBioPortal for Cancer Genomics (http://cbio-portal.org) (23,24).
Data from the 341 patients with complete characteristics, including
disease-free survival and status, overall survival and status,
histological grade of the neoplasm, primary therapy outcome and
tumor stage, were evaluated for an association of drug resistance
in OC and TRPC1 expression. Gene expression was categorized high or
low by the median value in accordance with a previous study
(31). As shown in Table III, TRPC1 expression differed
significantly between patients with grade 2
(moderately-differentiated) OC and those with grade 3
(poorly-differentiated) OC (P=0.006). Low-level expression
correlated with higher tumor grade. A comparison of the
disease-free and overall survival of the 341 OC patients with TRPC1
expression above or below the median level showed a disadvantage in
terms of overall survival for patients with low vs. high expression
(48.700±2.307 vs. 51.300±2.843), although the difference was not
statistically significant.
Tumorigenesis and tumor progression are driven by
the aberrant function of genes that regulate genome stability, cell
proliferation, apoptosis, angiogenesis, cell cycle, invasion,
metastasis and drug resistance (89). With the rapid development of
high-throughput technologies, databases such as GEO Profiles
(22) and TCGA (90) have provided high-resolution
molecular profiles of gene expression, microRNA expression, copy
number and methylation in cancer tissues. In the present study,
microarray data retrieved from GEO and TCGA were used to analyze
TRPC1 expression in OC and its clinical relevance.
Bioinformatics analysis based on the various
proteomic and genomic datasets can exploit the context of a
protein/gene in cellular networks, provide insights into the
functional role of a protein/gene, facilitate the rapid annotation
of protein/gene function, and thereby guide laboratory experiments
(91–94). For example, a comprehensive
bioinformatics approach, including microarrays, motif analysis,
protein/gene interaction, protein-chemical interaction, biological
process annotation, pathway enrichment and mRNA-microRNA
interactions, has been used to identify the association of
dysregulated SPARCL1 and chemokine (C-C motif) ligand 21 (CCL21)
(41), NIMA-related kinase 11
(NEK11) (95) and NIMA-related
kinase 2 (NEK2) (96), and
ribonuclease T2 (RNASET2) and gametogenetin binding protein 2
(GGNBP2) (97) with drug
resistance in OC. In hepatocellular and gastric carcinomas,
bioinformatics analysis revealed the association of E2F
transcription factor 3 (E2F3) (98) and spalt-like transcription factor 4
(SALL4) (99), respectively, with
prognosis. In this study, widely used bioinformatics tools and
networks, including GeneMania (25), STITCH (version 4.0) (26,27),
Coremine Medical (28), and DIANA
miRPath (30), and six
mRNA-microRNA prediction tools, including TargetScan, were used to
analyze the associations of TRPC1 with drug resistance in OC.
TRPC1 mRNA expression was significantly and
consistently downregulated by at least 3.260-fold in 251 OC samples
compared with 24 normal control samples, according to three
independent microarrays (Fig. 1A).
In a previous study TRPC1 mRNA expression was lower in 5 ovarian
serous papillary adenocarcinomas than in five normal samples
(20). TRPC1 mRNA was at least
3-fold lower in OC cells than in normal ovarian surface epithelial
cells (Fig. 1B). Significant
downregulation of TRPC1 mRNA was also detected in
cisplatin-resistant A2780 and SKOV3 cells and carboplatin-resistant
A2780 cells when compared with expression in their sensitive
counterparts (Fig. 2). These
results indicated that the stable and significant downregulation of
TRPC1 in OC and drug-resistant cells plays a critical role in the
development OC and in the regulation of drug resistance.
Further support for this conclusion was obtained in
a comprehensive bioinformatics analyses. Protein/gene-protein/gene
and protein-chemical interactions indicated the interaction of
TRPC1 with 14 proteins/genes and 6 chemicals, all of which are
associated with drug resistance in OC (Figs. 3 and 4). Annotation of TRPC1, OC and drug
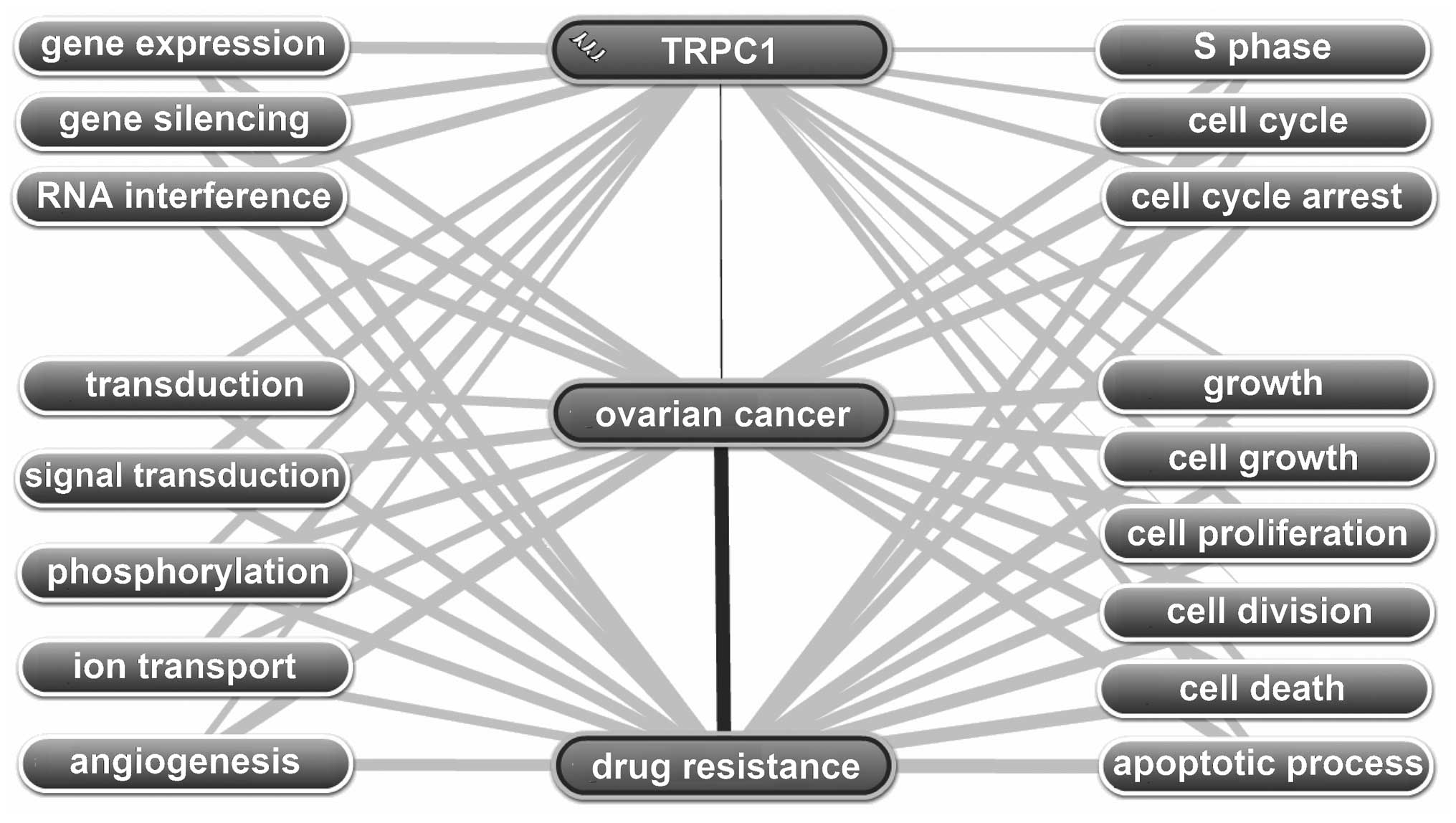
resistance supported a role for TRPC1 in drug-resistance-related
functions in OC through 17 biological processes related to the cell
cycle, gene expression and cell growth and cell death (Fig. 5). Among the top 11 pathways
enriched from the top 38 microRNAs targeting TRPC1, 8 were involved
in the regulation of drug resistance in OC (Table I). In addition, 8 of the top 10
microRNAs targeting TRPC1 were implicated in drug resistance in
ovarian and other cancers (Table
II).
A possible mechanism underlying the role of TRPC1 in
drug resistance in OC is the regulation of autophagy. As shown in
Fig. 3, co-expression and genetic
interactions between TRPC1 and PIK3C3 and co-expression and
co-localization with SPARCL1 were determined. PIK3C3 plays a
critical role in the regulation of autophagy in vitro and
in vivo (100). Autophagy,
which acts both in protecting against cancer as well as promoting
the growth of cancer, has attracted increased attention as an
important mechanism for cancer development (101). Autophagy also contributes to drug
resistance in ovarian and other cancers (101,102). For instance, it has been reported
that the induction of ERK-mediated autophagy conferred cisplatin
resistance to ovarian cancer cells (103). The relationship between PIK3C3
and cancer has been targeted in chemotherapy via the drug
paclitaxel, which has been used to block PIK3C3 activation
(104). SPARCL1 was shown to be
involved in the regulation of drug resistance via several pathways,
including autophagy (41). The
strong interactions of SPARCL1 with TRPC1 suggest the involvement
of the latter in the regulation of autophagy and in drug resistance
in OC.
Finally, our analysis of the relationship between
TRPC1 mRNA expression and the histological grade of OC in 341
patients of a TCGA cohort showed significant differences between
grade 2 (moderately-differentiated) and grade 3
(poorly-differentiated) tumors, with the low-level expression of
TRPC1 correlating with high tumor grade (Table III). This result provides
clinical support for a link between the down-regulation of TRPC1
and drug resistance in OC.
In summary, evidence obtained from RT-qPCR
measurement, microarray data retrieval, comprehensive
bioinformatics analyses, and clinical analysis of a TCGA cohort
together suggest that the downregulation of TRPC1 contributes to
drug resistance in OC and to the high histological grade of these
tumors. Our results provide the basis for further investigation of
the drug-resistance-related functions of TRPC1 in OC.
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81302283, 81560424
and 81460397), the China Postdoctoral Science Foundation (nos.
2014M552535XB and 2014M552291), the Natural Science Foundation of
Guangxi (nos. 2014GXNSFCA118010, 2015GXNSFBA139115,
2015GXNSFAA139151 and 2014GXNSFBA118155), and the Youth Science
Foundation of Guangxi Medical University (no. GXMUYSF201312).
|
1
|
Matsuo K, Eno ML, Im DD, Rosenshein NB and
Sood AK: Clinical relevance of extent of extreme drug resistance in
epithelial ovarian carcinoma. Gynecol Oncol. 116:61–65. 2010.
View Article : Google Scholar
|
|
2
|
Cannistra SA: Cancer of the ovary. N Engl
J Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Agarwal R and Kaye SB: Ovarian cancer:
Strategies for over-coming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parikh A, Lee C, Joseph P, Marchini S,
Baccarini A, Kolev V, Romualdi C, Fruscio R, Shah H, Wang F, et al:
microRNA-181a has a critical role in ovarian cancer progression
through the regulation of the epithelial-mesenchymal transition.
Nat Commun. 5:29772014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shah JS, Cole AJ, Dickson KA, Soon P and
Marsh DJ: Investigating the role of long non-coding RNAs in
cisplatin resistance in ovarian cancer. Asia Pac J Clin Oncol.
10:42. 2014.
|
|
6
|
Sorrentino A, Liu CG, Addario A, Peschle
C, Scambia G and Ferlini C: Role of microRNAs in drug-resistant
ovarian cancer cells. Gynecol Oncol. 111:478–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suh DH, Kim MK, No JH, Chung HH and Song
YS: Metabolic approaches to overcoming chemoresistance in ovarian
cancer. Ann NY Acad Sci. 1229:53–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yin F, Liu X, Li D, Wang Q, Zhang W and Li
L: Tumor suppressor genes associated with drug resistance in
ovarian cancer (Review). Oncol Rep. 30:3–10. 2013.PubMed/NCBI
|
|
9
|
Richardson A and Kaye SB: Drug resistance
in ovarian cancer: The emerging importance of gene transcription
and spatio-temporal regulation of resistance. Drug Resist Updat.
8:311–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu X, Gao Y, Lu Y, Zhang J, Li L and Yin
F: Oncogenes associated with drug resistance in ovarian cancer. J
Cancer Res Clin Oncol. 141:381–395. 2015. View Article : Google Scholar
|
|
11
|
Kahl CR and Means AR: Regulation of cell
cycle progression by calcium/calmodulin-dependent pathways. Endocr
Rev. 24:719–736. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roderick HL and Cook SJ: Ca2+
signalling checkpoints in cancer: Remodelling Ca2+ for
cancer cell proliferation and survival. Nat Rev Cancer. 8:361–375.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rajewskaya TA, Goncharova SA, Konovalova
NP, Kotelnikova RA and Tatyanenko LV: Effect of drug resistance
modulator, NO donor, on membrane structure and function of
membrane-bound Ca2+-activated Mg2+-dependent
ATPase. Bull Exp Biol Med. 146:200–202. 2008. View Article : Google Scholar
|
|
14
|
Clapham DE, Runnels LW and Strübing C: The
TRP ion channel family. Nat Rev Neurosci. 2:387–396. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nilius B and Szallasi A: Transient
receptor potential channels as drug targets: From the science of
basic research to the art of medicine. Pharmacol Rev. 66:676–814.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ong HL and Ambudkar IS: The dynamic
complexity of the TRPC1 channelosome. Channels (Austin). 5:424–431.
2011. View Article : Google Scholar
|
|
17
|
Selli C, Erac Y and Tosun M: Simultaneous
measurement of cytosolic and mitochondrial calcium levels:
Observations in TRPC1-silenced hepatocellular carcinoma cells. J
Pharmacol Toxicol Methods. 72:29–34. 2015. View Article : Google Scholar
|
|
18
|
He B, Liu F, Ruan J, Li A, Chen J, Li R,
Shen J, Zheng D and Luo R: Silencing TRPC1 expression inhibits
invasion of CNE2 nasopharyngeal tumor cells. Oncol Rep.
27:1548–1554. 2012.PubMed/NCBI
|
|
19
|
Tajeddine N and Gailly P: TRPC1 protein
channel is major regulator of epidermal growth factor receptor
signaling. J Biol Chem. 287:16146–16157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng B, Yuan C, Yang X, Atkin SL and Xu
SZ: TRPC channels and their splice variants are essential for
promoting human ovarian cancer cell proliferation and
tumorigenesis. Curr Cancer Drug Targets. 13:103–116. 2013.
View Article : Google Scholar
|
|
21
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: A cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edgar R, Domrachev M and Lash AE: Gene
Expression Omnibus: NCBI gene expression and hybridization array
data repository. Nucleic Acids Res. 30:207–210. 2002. View Article : Google Scholar :
|
|
23
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multi-dimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBio-Portal. Sci Signal. 6:pl12013. View Article : Google Scholar
|
|
25
|
Zuberi K, Franz M, Rodriguez H, Montojo J,
Lopes CT, Bader GD and Morris Q: GeneMANIA prediction server 2013
update. Nucleic Acids Res. 41(W1): W115–W122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuhn M, von Mering C, Campillos M, Jensen
LJ and Bork P: STITCH: Interaction networks of chemicals and
proteins. Nucleic Acids Res. 36(Database): D684–D688. 2008.
View Article : Google Scholar :
|
|
27
|
Kuhn M, Szklarczyk D, Pletscher-Frankild
S, Blicher TH, von Mering C, Jensen LJ and Bork P: STITCH 4:
Integration of protein-chemical interactions with user data.
Nucleic Acids Res. 42(D1): D401–D407. 2014. View Article : Google Scholar
|
|
28
|
de Leeuw N, Dijkhuizen T, Hehir-Kwa JY,
Carter NP, Feuk L, Firth HV, Kuhn RM, Ledbetter DH, Martin CL, van
Ravenswaaij-Arts CM, et al: Diagnostic interpretation of array data
using public databases and internet sources. Hum Mutat. 33:930–940.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dweep H, Sticht C, Pandey P and Gretz N:
miRWalk-database: Prediction of possible miRNA binding sites by
‘walking’ the genes of three genomes. J Biomed Inform. 44:839–847.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vlachos IS, Kostoulas N, Vergoulis T,
Georgakilas G, Reczko M, Maragkakis M, Paraskevopoulou MD,
Prionidis K, Dalamagas T and Hatzigeorgiou AG: DIANA miRPath v.20:
Investigating the combinatorial effect of microRNAs in pathways.
Nucleic Acids Res. 40(W1): W498–W504. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hedditch EL, Gao B, Russell AJ, Lu Y,
Emmanuel C, Beesley J, Johnatty SE, Chen X, Harnett P, George J, et
al; Australian Ovarian Cancer Study Group. ABCA transporter gene
expression and poor outcome in epithelial ovarian cancer. J Natl
Cancer Inst. 106:1062014. View Article : Google Scholar
|
|
32
|
Meng D, Chen Y, Zhao Y, Wang J, Yun D,
Yang S, Chen J, Chen H and Lu D: Expression and prognostic
significance of TCTN1 in human glioblastoma. J Transl Med.
12:2882014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bowen NJ, Walker LD, Matyunina LV, Logani
S, Totten KA, Benigno BB and McDonald JF: Gene expression profiling
supports the hypothesis that human ovarian surface epithelia are
multipotent and capable of serving as ovarian cancer initiating
cells. BMC Med Genomics. 2:712009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li M, Balch C, Montgomery JS, Jeong M,
Chung JH, Yan P, Huang TH, Kim S and Nephew KP: Integrated analysis
of DNA methylation and gene expression reveals specific signaling
pathways associated with platinum resistance in ovarian cancer. BMC
Med Genomics. 2:342009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peters D, Freund J and Ochs RL:
Genome-wide transcriptional analysis of carboplatin response in
chemosensitive and chemoresistant ovarian cancer cells. Mol Cancer
Ther. 4:1605–1616. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee S, Choi EJ, Jin C and Kim DH:
Activation of PI3K/Akt pathway by PTEN reduction and PIK3CA mRNA
amplification contributes to cisplatin resistance in an ovarian
cancer cell line. Gynecol Oncol. 97:26–34. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu H, Cao Y, Weng D, Xing H, Song X, Zhou
J, Xu G, Lu Y, Wang S and Ma D: Effect of tumor suppressor gene
PTEN on the resistance to cisplatin in human ovarian cancer cell
lines and related mechanisms. Cancer Lett. 271:260–271. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yan X, Fraser M, Qiu Q and Tsang BK:
Over-expression of PTEN sensitizes human ovarian cancer cells to
cisplatin-induced apoptosis in a p53-dependent manner. Gynecol
Oncol. 102:348–355. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu H, Wang K, Liu W and Hao Q: PTEN
overexpression improves cisplatin-resistance of human ovarian
cancer cells through upregulating KRT10 expression. Biochem Biophys
Res Commun. 444:141–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang X, Wang X, Song X, Liu C, Shi Y,
Wang Y, Afonja O, Ma C, Chen YH and Zhang L: Programmed cell death
4 enhances chemosensitivity of ovarian cancer cells by activating
death receptor pathway in vitro and in vivo. Cancer Sci.
101:2163–2170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yin F, Liu X, Li D, Wang Q, Zhang W and Li
L: Bioinformatic analysis of chemokine (C-C motif) ligand 21 and
SPARC-like protein 1 revealing their associations with drug
resistance in ovarian cancer. Int J Oncol. 42:1305–1316.
2013.PubMed/NCBI
|
|
42
|
Sen T, Sen N, Noordhuis MG, Ravi R, Wu TC,
Ha PK, Sidransky D and Hoque MO: OGDHL is a modifier of
AKT-dependent signaling and NF-κB function. PLoS One. 7:e487702012.
View Article : Google Scholar
|
|
43
|
Xia X, Ma Q, Li X, Ji T, Chen P, Xu H, Li
K, Fang Y, Weng D, Weng Y, et al: Cytoplasmic p21 is a potential
predictor for cisplatin sensitivity in ovarian cancer. BMC Cancer.
11:3992011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Materna V, Surowiak P, Markwitz E,
Spaczynski M, Drag-Zalesinska M, Zabel M and Lage H: Expression of
factors involved in regulation of DNA mismatch repair- and
apoptosis pathways in ovarian cancer patients. Oncol Rep.
17:505–516. 2007.PubMed/NCBI
|
|
45
|
Moorehead RA and Singh G: Influence of the
proto-oncogene c-fos on cisplatin sensitivity. Biochem Pharmacol.
59:337–345. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mahner S, Baasch C, Schwarz J, Hein S,
Wölber L, Jänicke F and Milde-Langosch K: C-Fos expression is a
molecular predictor of progression and survival in epithelial
ovarian carcinoma. Br J Cancer. 99:1269–1275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu YY, Li L, Li DR, Zhang W and Wang Q:
Suppression of WWOX gene by RNA interference reverses platinum
resistance acquired in SKOV3/SB cells. Zhonghua Fu Chan Ke Za Zhi.
43:854–858. 2008.In Chinese. PubMed/NCBI
|
|
48
|
Liu T, Zhao L, Chen W, Li Z, Hou H, Ding L
and Li X: Inactivation of von Hippel-Lindau increases ovarian
cancer cell aggressiveness through the HIF1α/miR-210/VMP1 signaling
pathway. Int J Mol Med. 33:1236–1242. 2014.PubMed/NCBI
|
|
49
|
Hamada H, Hagiwara K, Nakajima T and
Tsuruo T: Phosphorylation of the Mr 170,000 to 180,000 glycoprotein
specific to multidrug-resistant tumor cells: Effects of verapamil,
trifluoperazine, and phorbol esters. Cancer Res. 47:2860–2865.
1987.PubMed/NCBI
|
|
50
|
Zhao BX, Sun YB, Wang SQ, Duan L, Huo QL,
Ren F and Li GF: Grape seed procyanidin reversal of P-glycoprotein
associated multi-drug resistance via down-regulation of NF-κB and
MAPK/ERK mediated YB-1 activity in A2780/T cells. PLoS One.
8:e710712013. View Article : Google Scholar
|
|
51
|
Lee LF, Haskill JS, Mukaida N, Matsushima
K and Ting JP: Identification of tumor-specific paclitaxel
(Taxol)-responsive regulatory elements in the interleukin-8
promoter. Mol Cell Biol. 17:5097–5105. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Duan Z, Feller AJ, Penson RT, Chabner BA
and Seiden MV: Discovery of differentially expressed genes
associated with paclitaxel resistance using cDNA array technology:
Analysis of interleukin (IL) 6, IL-8, and monocyte chemotactic
protein 1 in the paclitaxel-resistant phenotype. Clin Cancer Res.
5:3445–3453. 1999.PubMed/NCBI
|
|
53
|
Huang Y, Ju B, Tian J, Liu F, Yu H, Xiao
H, Liu X, Liu W, Yao Z and Hao Q: Ovarian cancer stem cell-specific
gene expression profiling and targeted drug prescreening. Oncol
Rep. 31:1235–1248. 2014.PubMed/NCBI
|
|
54
|
Engelmann BJ, Ryan JJ and Farrell NP:
Antidepressants and platinum drugs. Anticancer Res. 34:509–516.
2014.PubMed/NCBI
|
|
55
|
Lee CS, Kim YJ, Jang ER, Kim W and Myung
SC: Fluoxetine induces apoptosis in ovarian carcinoma cell line
OVCAR-3 through reactive oxygen species-dependent activation of
nuclear factor-kappaB. Basic Clin Pharmacol Toxicol. 106:446–453.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hiss DC, Gabriels GA and Folb PI:
Combination of tunicamycin with anticancer drugs synergistically
enhances their toxicity in multidrug-resistant human ovarian
cystadenocarcinoma cells. Cancer Cell Int. 7:52007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rogan AM, Hamilton TC, Young RC, Klecker
RW Jr and Ozols RF: Reversal of adriamycin resistance by verapamil
in human ovarian cancer. Science. 224:994–996. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ozols RF: Pharmacologic reversal of drug
resistance in ovarian cancer. Semin Oncol. 12(Suppl 4): 7–11.
1985.PubMed/NCBI
|
|
59
|
Gene Ontology consortium. http://www.geneontology.org.
|
|
60
|
Gamberoni G, Storari S and Volinia S:
Finding biological process modifications in cancer tissues by
mining gene expression correlations. BMC Bioinformatics. 7:62006.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lagreid A, Hvidsten TR, Midelfart H,
Komorowski J and Sandvik AK: Predicting gene ontology biological
process from temporal gene expression patterns. Genome Res.
13:965–979. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Medical COREMINE. http://www.coremine.com/medical/.
|
|
63
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Croce CM and Calin GA: miRNAs, cancer, and
stem cell division. Cell. 122:6–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tili E, Michaille JJ, Gandhi V, Plunkett
W, Sampath D and Calin GA: miRNAs and their potential for use
against cancer and other diseases. Future Oncol. 3:521–537. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Brazil DP, Park J and Hemmings BA: PKB
binding proteins. Getting in on the Akt. Cell. 111:293–303. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tang W, Jiang Y, Mu X, Xu L, Cheng W and
Wang X: MiR-135a functions as a tumor suppressor in epithelial
ovarian cancer and regulates HOXA10 expression. Cell Signal.
26:1420–1426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Matei D, Fang F, Shen C, Schilder J,
Arnold A, Zeng Y, Berry WA, Huang T and Nephew KP: Epigenetic
resensitization to platinum in ovarian cancer. Cancer Res.
72:2197–2205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kim YW, Kim EY, Jeon D, Liu JL, Kim HS,
Choi JW and Ahn WS: Differential microRNA expression signatures and
cell type-specific association with Taxol resistance in ovarian
cancer cells. Drug Des Devel Ther. 8:293–314. 2014.PubMed/NCBI
|
|
70
|
Arafa SA, Zhu Q, Barakat BM, Wani G, Zhao
Q, El-Mahdy MA and Wani AA: Tangeretin sensitizes
cisplatin-resistant human ovarian cancer cells through
downregulation of phosphoinositide 3-kinase/Akt signaling pathway.
Cancer Res. 69:8910–8917. 2009. View Article : Google Scholar
|
|
71
|
Lange TS, Stuckey AR, Robison K, Kim KK,
Singh RK, Raker CA and Brard L: Effect of a vitamin D3
derivative (B3CD) with postulated anti-cancer activity in an
ovarian cancer animal model. Invest New Drugs. 28:543–553. 2010.
View Article : Google Scholar :
|
|
72
|
Jiao JW and Wen F: Tanshinone IIA acts via
p38 MAPK to induce apoptosis and the down-regulation of ERCC1 and
lung-resistance protein in cisplatin-resistant ovarian cancer
cells. Oncol Rep. 25:781–788. 2011.
|
|
73
|
Kumar S, Kumar A, Shah PP, Rai SN,
Panguluri SK and Kakar SS: MicroRNA signature of cis-platin
resistant vs. cisplatin sensitive ovarian cancer cell lines. J
Ovarian Res. 4:172011. View Article : Google Scholar
|
|
74
|
Jin L, Huo Y, Zheng Z, Jiang X and Deng H,
Chen Y, Lian Q, Ge R and Deng H: Down-regulation of Ras-related
protein Rab 5C-dependent endocytosis and glycolysis in
cisplatin-resistant ovarian cancer cell lines. Mol Cell Proteomics.
13:3138–3151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Li J, Zhang Y, Gao Y, Cui Y, Liu H, Li M
and Tian Y: Downregulation of HNF1 homeobox B is associated with
drug resistance in ovarian cancer. Oncol Rep. 32:979–988.
2014.PubMed/NCBI
|
|
76
|
Rosanò L, Cianfrocca R, Tocci P, Spinella
F, Di Castro V, Caprara V, Semprucci E, Ferrandina G, Natali PG and
Bagnato A: Endothelin A receptor/β-arrestin signaling to the Wnt
pathway renders ovarian cancer cells resistant to chemotherapy.
Cancer Res. 74:7453–7464. 2014. View Article : Google Scholar
|
|
77
|
Ko MA, Zehong G, Virtanen C, Guindi M,
Waddell TK, Keshavjee S, et al: MicroRNA expression profiling of
esophageal cancer before and after induction chemoradiotherapy. Ann
Thorac Surg. 94:1094–1102; discussion 1102–1093. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wang FJ, Ding Y, Mao YY, Jing FY, Zhang
ZY, Jiang LF, Guo JF, Sun XJ, Jin MJ and Chen K: Associations
between hsa-miR-603 polymorphism, lifestyle-related factors and
colorectal cancer risk. Cancer Biomark. 14:225–231. 2014.PubMed/NCBI
|
|
79
|
Rogler A, Hoja S, Socher E, Nolte E, Wach
S, Wieland W, Hofstädter F, Goebell PJ, Wullich B, Hartmann A, et
al: Role of two single nucleotide polymorphisms in secreted
frizzled related protein 1 and bladder cancer risk. Int J Clin Exp
Pathol. 6:1984–1998. 2013.PubMed/NCBI
|
|
80
|
Zhang J, Zhang T, Ti X, Shi J, Wu C, Ren X
and Yin H: Curcumin promotes apoptosis in A549/DDP
multidrug-resistant human lung adenocarcinoma cells through an
miRNA signaling pathway. Biochem Biophys Res Commun. 399:1–6. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhu W, Zhu D, Lu S, Wang T, Wang J, Jiang
B, Shu Y and Liu P: miR-497 modulates multidrug resistance of human
cancer cell lines by targeting BCL2. Med Oncol. 29:384–391. 2012.
View Article : Google Scholar
|
|
82
|
Della Vittoria Scarpati G, Falcetta F,
Carlomagno C, Ubezio P, Marchini S, De Stefano A, Singh VK,
D'Incalci M, De Placido S and Pepe S: A specific miRNA signature
correlates with complete pathological response to neoadjuvant
chemoradiotherapy in locally advanced rectal cancer. Int J Radiat
Oncol Biol Phys. 83:1113–1119. 2012. View Article : Google Scholar
|
|
83
|
Tang J, Tao ZH, Wen D, Wan JL, Liu DL,
Zhang S, Cui JF, Sun HC, Wang L, Zhou J, et al: MiR-612 suppresses
the stemness of liver cancer via Wnt/β-catenin signaling. Biochem
Biophys Res Commun. 447:210–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yang Y, Li H, Hou S, Hu B, Liu J and Wang
J: The noncoding RNA expression profile and the effect of lncRNA
AK126698 on cisplatin resistance in non-small-cell lung cancer
cell. PLoS One. 8:e653092013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ichikawa T, Sato F, Terasawa K, Tsuchiya
S, Toi M, Tsujimoto G and Shimizu K: Trastuzumab produces
therapeutic actions by upregulating miR-26a and miR-30b in breast
cancer cells. PLoS One. 7:e314222012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gu YF, Zhang H, Su D, Mo ML, Song P, Zhang
F and Zhang SC: miR-30b and miR-30c expression predicted response
to tyrosine kinase inhibitors as first line treatment in non-small
cell lung cancer. Chin Med J (Engl). 126:4435–4439. 2013.
|
|
87
|
Pichiorri F, Palmieri D, De Luca L,
Consiglio J, You J, Rocci A, Talabere T, Piovan C, Lagana A,
Cascione L, et al: In vivo NCL targeting affects breast cancer
aggressiveness through miRNA regulation. J Exp Med. 210:951–968.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Huang JW, Wang Y, Dhillon KK, Calses P,
Villegas E, Mitchell PS, Tewari M, Kemp CJ and Taniguchi T:
Systematic screen identifies miRNAs that target RAD51 and RAD51D to
enhance chemosensitivity. Mol Cancer Res. 11:1564–1573. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Huang N, Shah PK and Li C: Lessons from a
decade of integrating cancer copy number alterations with gene
expression profiles. Brief Bioinform. 13:305–316. 2012. View Article : Google Scholar :
|
|
90
|
McLendon R, Friedman A, Bigner D, Van Meir
EG, Brat DJ, Mastrogianakis GM, Olson JJ, Mikkelsen T, Lehman N,
Aldape K, et al; Cancer Genome Atlas Research Network.
Comprehensive genomic characterization defines human glioblastoma
genes and core pathways. Nature. 455:1061–1068. 2008. View Article : Google Scholar
|
|
91
|
Sharan R, Ulitsky I and Shamir R:
Network-based prediction of protein function. Mol Syst Biol.
3:882007. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Phuong T and Nhung N: Predicting gene
function using similarity learning. BMC Genomics. 14(Suppl 4):
S42013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Janga SC, Díaz-Mejía JJ and
Moreno-Hagelsieb G: Network-based function prediction and
interactomics: The case for metabolic enzymes. Metab Eng. 13:1–10.
2011. View Article : Google Scholar
|
|
94
|
Yu G, Zhu H, Domeniconi C and Guo M:
Integrating multiple networks for protein function prediction. BMC
Syst Biol. 9(Suppl 1): S32015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Liu X, Gao Y, Lu Y, Zhang J, Li L and Yin
F: Downregulation of NEK11 is associated with drug resistance in
ovarian cancer. Int J Oncol. 45:1266–1274. 2014.PubMed/NCBI
|
|
96
|
Liu X, Gao Y, Lu Y, Zhang J, Li L and Yin
F: Upregulation of NEK2 is associated with drug resistance in
ovarian cancer. Oncol Rep. 31:745–754. 2014.
|
|
97
|
Yin F, Liu L, Liu X, Li G, Zheng L, Li D,
Wang Q, Zhang W and Li L: Downregulation of tumor suppressor gene
ribonuclease T2 and gametogenetin binding protein 2 is associated
with drug resistance in ovarian cancer. Oncol Rep. 32:362–372.
2014.PubMed/NCBI
|
|
98
|
Zeng X, Yin F, Liu X, Xu J, Xu Y, Huang J,
Nan Y and Qiu X: Upregulation of E2F transcription factor 3 is
associated with poor prognosis in hepatocellular carcinoma. Oncol
Rep. 31:1139–1146. 2014.PubMed/NCBI
|
|
99
|
Liu J, Wang LY, Yang AJ, Jiang PF and Wang
MC: Up-regulation of SALL4 associated with poor prognosis in
gastric cancer. Hepatogastroenterology. 61:1459–1464.
2014.PubMed/NCBI
|
|
100
|
Jaber N, Dou Z, Lin RZ, Zhang J and Zong
WX: Mammalian PIK3C3/VPS34: The key to autophagic processing in
liver and heart. Autophagy. 8:707–708. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yang ZJ, Chee CE, Huang S and Sinicrope
FA: The role of autophagy in cancer: Therapeutic implications. Mol
Cancer Ther. 10:1533–1541. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Peracchio C, Alabiso O, Valente G and
Isidoro C: Involvement of autophagy in ovarian cancer: A working
hypothesis. J Ovarian Res. 5:222012. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wang J and Wu GS: Role of autophagy in
cisplatin resistance in ovarian cancer cells. J Biol Chem.
289:17163–17173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Veldhoen RA, Banman SL, Hemmerling DR,
Odsen R, Simmen T, Simmonds AJ, Underhill DA and Goping IS: The
chemotherapeutic agent paclitaxel inhibits autophagy through two
distinct mechanisms that regulate apoptosis. Oncogene. 32:736–746.
2013. View Article : Google Scholar
|