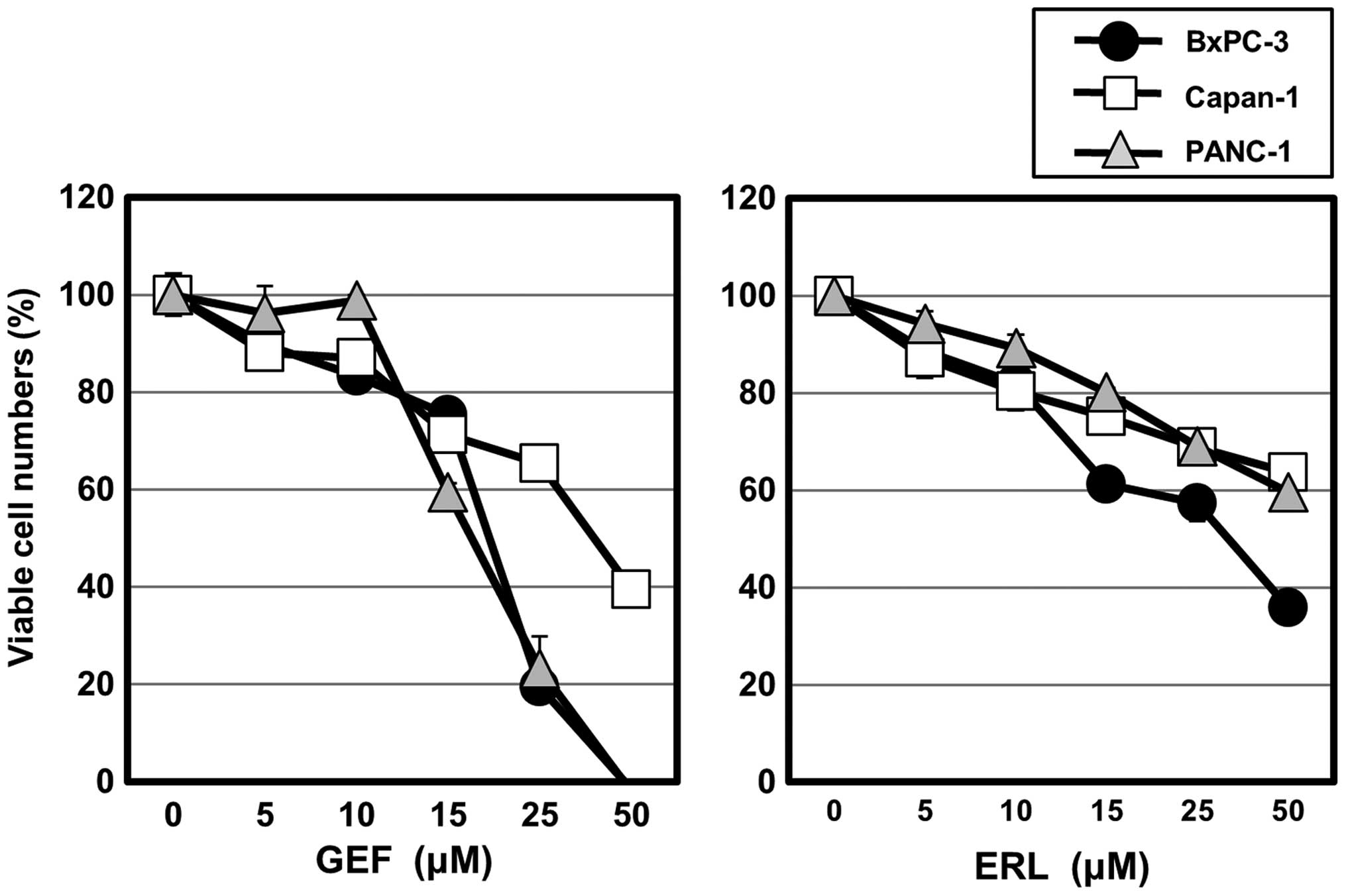
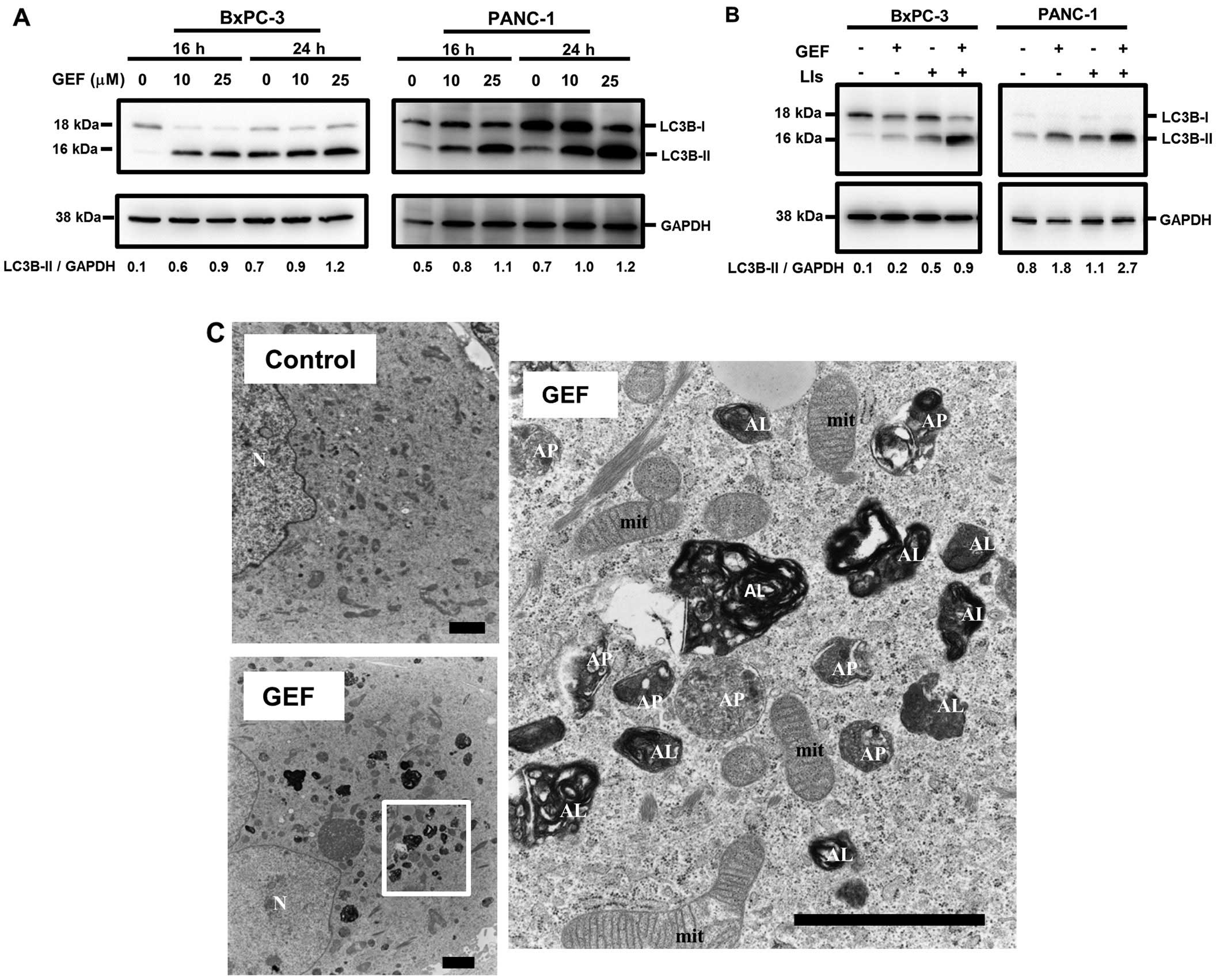
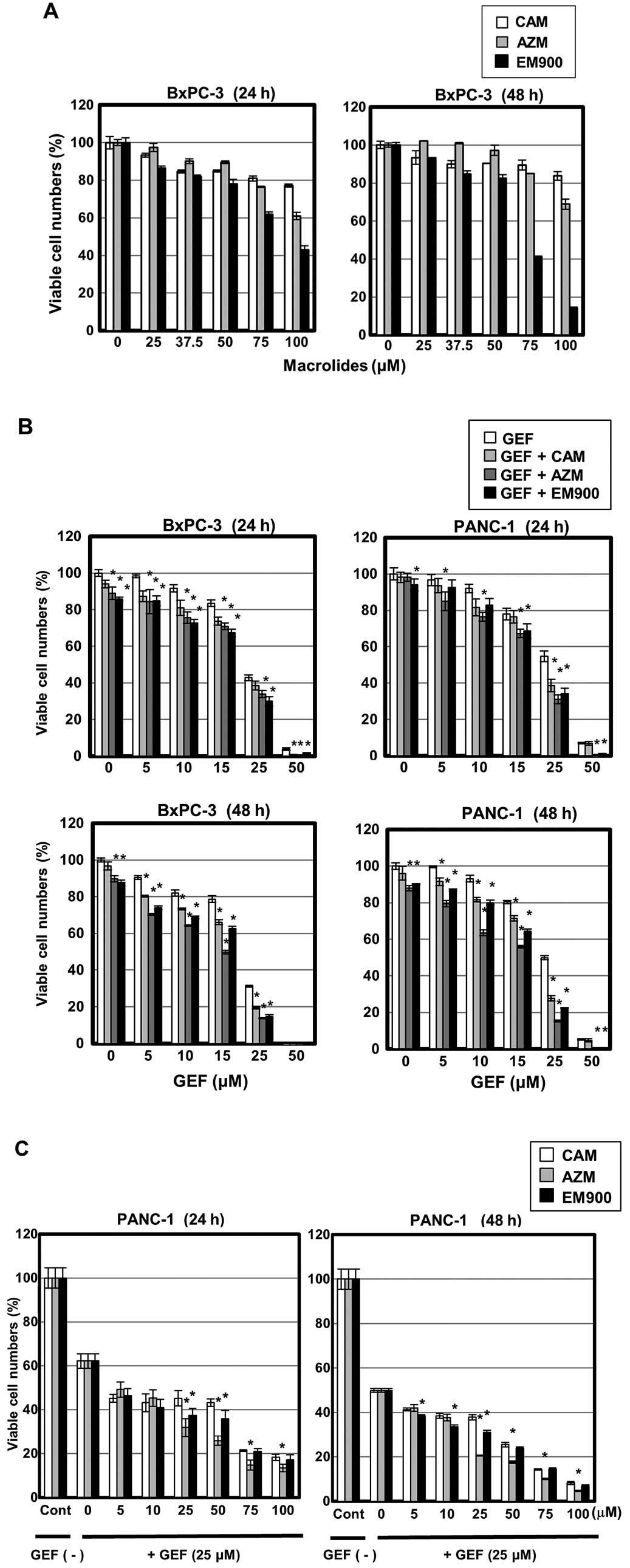
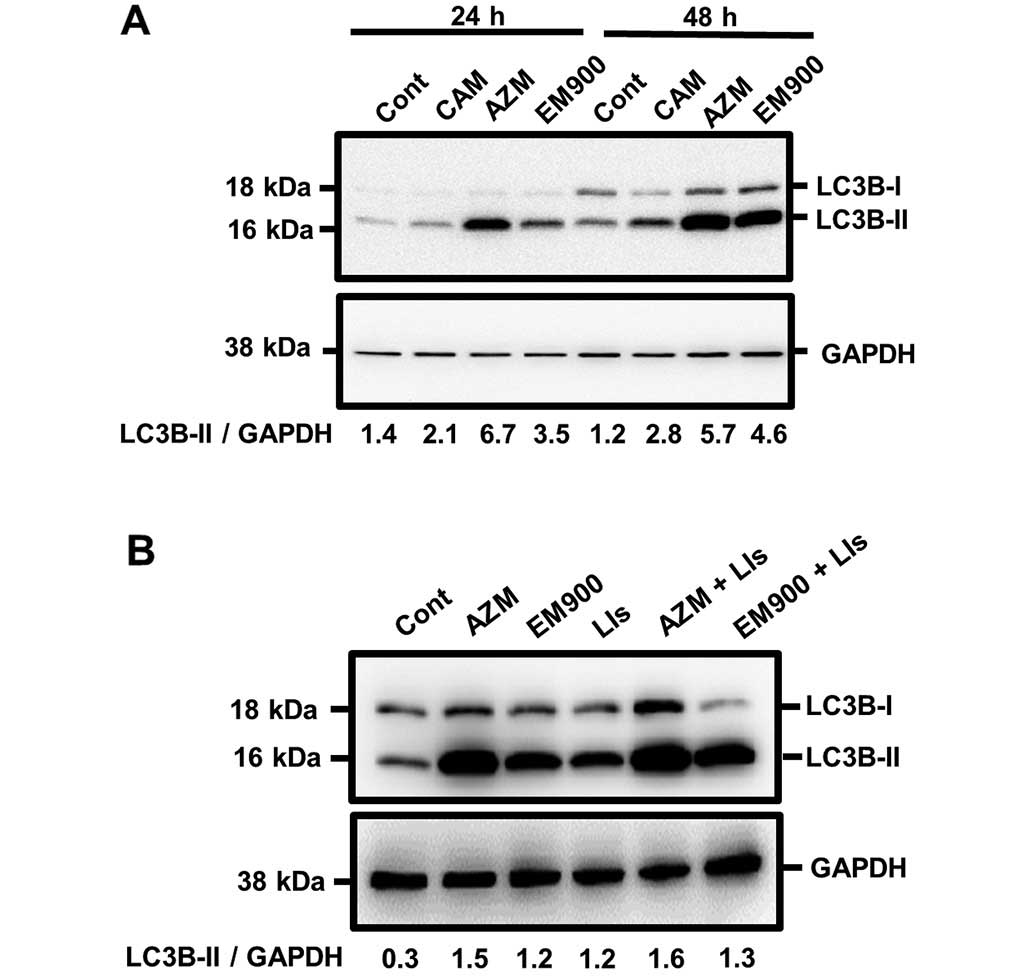
|
1
|
Gresham GK, Wells GA, Gill S, Cameron C
and Jonker DJ: Chemotherapy regimens for advanced pancreatic
cancer: A systematic review and network meta-analysis. BMC Cancer.
14:4712014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ueda S, Ogata S, Tsuda H, Kawarabayashi N,
Kimura M, Sugiura Y, Tamai S, Matsubara O, Hatsuse K and Mochizuki
H: The correlation between cytoplasmic overexpression of epidermal
growth factor receptor and tumor aggressiveness: Poor prognosis in
patients with pancreatic ductal adenocarcinoma. Pancreas. 29:e1–e8.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moore MJ, Goldstein D, Hamm J, Figer A,
Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al;
National Cancer Institute of Canada Clinical Trials Group.
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: A phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang ZY, Yuan JQ, Di MY, Zheng DY, Chen
JZ, Ding H, Wu XY, Huang YF, Mao C and Tang JL: Gemcitabine plus
erlotinib for advanced pancreatic cancer: A systematic review with
meta-analysis. PLoS One. 8:e575282013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Diaz Beveridge R, Alcolea V, Aparicio J,
Segura Á, García J, Corbellas M, Fonfría M, Giménez A and Montalar
J: Management of advanced pancreatic cancer with gemcitabine plus
erlotinib: Efficacy and safety results in clinical practice. JOP.
15:19–24. 2014.PubMed/NCBI
|
|
7
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galluzzi L, Pietrocola F, Levine B and
Kroemer G: Metabolic control of autophagy. Cell. 159:1263–1276.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
White E: Deconvoluting the
context-dependent role for autophagy in cancer. Nat Rev Cancer.
12:401–410. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ciardiello F and Tortora G: EGFR
antagonists in cancer treatment. N Engl J Med. 358:1160–1174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han W, Pan H, Chen Y, Sun J, Wang Y, Li J,
Ge W, Feng L, Lin X, Wang X, et al: EGFR tyrosine kinase inhibitors
activate autophagy as a cytoprotective response in human lung
cancer cells. PLoS One. 6:e186912011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jutten B and Rouschop KM: EGFR signaling
and autophagy dependence for growth, survival, and therapy
resistance. Cell Cycle. 13:42–51. 2014. View Article : Google Scholar :
|
|
13
|
Fung C, Chen X, Grandis JR and Duvvuri U:
EGFR tyrosine kinase inhibition induces autophagy in cancer cells.
Cancer Biol Ther. 13:1417–1424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim YC and Guan KL: mTOR: A pharmacologic
target for autophagy regulation. J Clin Invest. 125:25–32. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei Y, Zou Z, Becker N, Anderson M,
Sumpter R, Xiao G, Kinch L, Koduru P, Christudass CS, Veltri RW, et
al: EGFR-mediated Beclin 1 phosphorylation in autophagy
suppression, tumor progression, and tumor chemoresistance. Cell.
154:1269–1284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan X, Thapa N, Sun Y and Anderson RA: A
kinase-independent role for EGF receptor in autophagy initiation.
Cell. 160:145–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakamura M, Kikukawa Y, Takeya M, Mitsuya
H and Hata H: Clarithromycin attenuates autophagy in myeloma cells.
Int J Oncol. 37:815–820. 2010.PubMed/NCBI
|
|
18
|
Renna M, Schaffner C, Brown K, Shang S,
Tamayo MH, Hegyi K, Grimsey NJ, Cusens D, Coulter S, Cooper J, et
al: Azithromycin blocks autophagy and may predispose cystic
fibrosis patients to mycobacterial infection. J Clin Invest.
121:3554–3563. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moriya S, Che XF, Komatsu S, Abe A,
Kawaguchi T, Gotoh A, Inazu M, Tomoda A and Miyazawa K: Macrolide
antibiotics block autophagy flux and sensitize to bortezomib via
endoplasmic reticulum stress-mediated CHOP induction in myeloma
cells. Int J Oncol. 42:1541–1550. 2013.PubMed/NCBI
|
|
20
|
Moriya S, Komatsu S, Yamasaki K, Kawai Y,
Kokuba H, Hirota A, Che XF, Inazu M, Gotoh A, Hiramoto M, et al:
Targeting the integrated networks of aggresome formation,
proteasome, and autophagy potentiates ER stress-mediated cell death
in multiple myeloma cells. Int J Oncol. 46:474–486. 2015.
|
|
21
|
Verfaillie T, Salazar M, Velasco G and
Agostinis P: Linking ER stress to autophagy: Potential implications
for cancer therapy. Int J Cell Biol. 2010:9305092010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang M and Kaufman RJ: The impact of the
endoplasmic reticulum protein-folding environment on cancer
development. Nat Rev Cancer. 14:581–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sugita S, Ito K, Yamashiro Y, Moriya S,
Che XF, Yokoyama T, Hiramoto M and Miyazawa K: EGFR-independent
autophagy induction with gefitinib and enhancement of its cytotoxic
effect by targeting autophagy with clarithromycin in non-small cell
lung cancer cells. Biochem Biophys Res Commun. 461:28–34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sugawara A, Sueki A, Hirose T, Nagai K,
Gouda H, Hirono S, Shima H, Akagawa KS, Omura S and Sunazuka T:
Novel 12-membered non-antibiotic macrolides from erythromycin A;
EM900 series as novel leads for anti-inflammatory and/or
immunomodulatory agents. Bioorg Med Chem Lett. 21:3373–3376. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dragowska WH, Weppler SA, Wang JC, Wong
LY, Kapanen AI, Rawji JS, Warburton C, Qadir MA, Donohue E, Roberge
M, et al: Induction of autophagy is an early response to gefitinib
and a potential therapeutic target in breast cancer. PLoS One.
8:e765032013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mizushima N and Yoshimori T: How to
interpret LC3 immunoblotting. Autophagy. 3:542–545. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang P and Mizushima N: LC3- and
p62-based biochemical methods for the analysis of autophagy
progression in mammalian cells. Methods. 75:13–18. 2015. View Article : Google Scholar
|
|
29
|
Degterev A, Hitomi J, Germscheid M, Ch'en
IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, et al:
Identification of RIP1 kinase as a specific cellular target of
necrostatins. Nat Chem Biol. 4:313–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sui X, Kong N, Zhu M, Wang X, Lou F, Han W
and Pan H: Cotargeting EGFR and autophagy signaling: A novel
therapeutic strategy for non-small-cell lung cancer. Mol Clin
Oncol. 2:8–12. 2014.PubMed/NCBI
|
|
31
|
Mindell JA: Lysosomal acidification
mechanisms. Annu Rev Physiol. 74:69–86. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Harada M, Sakisaka S, Yoshitake M, Kin M,
Ohishi M, Shakado S, Mimura Y, Noguchi K, Sata M and Tanikawa K:
Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPases,
inhibits the receptor-mediated endocytosis of asialoglycoproteins
in isolated rat hepatocytes. J Hepatol. 24:594–603. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nujić K, Smith M, Lee M, Belamarić D,
Tomašković L, Alihodžić S, Malnar I, Polančec D, Schneider K and
Eraković Haber V: Valosin containing protein (VCP) interacts with
macrolide antibiotics without mediating their anti-inflammatory
activities. Eur J Pharmacol. 677:163–172. 2012. View Article : Google Scholar
|
|
34
|
Watts GD, Wymer J, Kovach MJ, Mehta SG,
Mumm S, Darvish D, Pestronk A, Whyte MP and Kimonis VE: Inclusion
body myopathy associated with Paget disease of bone and
frontotemporal dementia is caused by mutant valosin-containing
protein. Nat Genet. 36:377–381. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meyer H and Weihl CC: The VCP/p97 system
at a glance: Connecting cellular function to disease pathogenesis.
J Cell Sci. 127:3877–3883. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meyer H, Bug M and Bremer S: Emerging
functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat
Cell Biol. 14:117–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gonzalez MA, Feely SM, Speziani F,
Strickland AV, Danzi M, Bacon C, Lee Y, Chou TF, Blanton SH, Weihl
CC, et al: A novel mutation in VCP causes Charcot-Marie-Tooth Type
2 disease. Brain. 137:2897–2902. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nikoletopoulou V, Markaki M, Palikaras K
and Tavernarakis N: Crosstalk between apoptosis, necrosis and
autophagy. Biochim Biophys Acta. 1833.3448–3459. 2013.
|
|
39
|
Vanden Berghe T, Linkermann A,
Jouan-Lanhouet S, Walczak H and Vandenabeele P: Regulated necrosis:
The expanding network of non-apoptotic cell death pathways. Nat Rev
Mol Cell Biol. 15:135–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Feoktistova M and Leverkus M: Programmed
necrosis and necroptosis signalling. FEBS J. 282:19–31. 2015.
View Article : Google Scholar
|
|
41
|
Bray K, Mathew R, Lau A, Kamphorst JJ, Fan
J, Chen J, Chen HY, Ghavami A, Stein M, DiPaola RS, et al:
Autophagy suppresses RIP kinase-dependent necrosis enabling
survival to mTOR inhibition. PLoS One. 7:e418312012. View Article : Google Scholar : PubMed/NCBI
|