Introduction
Epithelial-mesenchymal transition (EMT), a crucial
phenotypic conversion during embryonic development, wound healing
and tissue remodeling, plays an essential role in tumor invasion
and metastasis (1–3). As a reversible process, EMT often
occurs at the invasive front of many metastatic cancers (4). Different signals received from tumor
microenvironment can trigger EMT conversion, such as TGFβ, TNFα,
EGF and Notch (5,6). During EMT conversion, epithelial
cells lose intercellular adhesion, acquire mesenchymal
characteristics and increase migratory and invasive capabilities
(7). The hallmark of EMT is loss
of E-cadherin expression (1). A
set of transcription factors have been implicated in the regulation
of EMT, including Twist, Snail, Slug and ZEB1 (8). Twist, a member of zinc-finger
transcription factor, has been proved as a central EMT regulator.
Studies showed that Twist induces EMT by binding to the E-box site
in the promoter of E-cadherin and repressing E-cadherin
transcription (9). Twist plays an
important role in the pathogenesis of various malignant tumors,
predominantly by promoting invasiveness and metastatic behavior.
Twist is overexpressed in a variety of cancers, including breast
cancer, hepatocellular carcinoma, prostate cancer, head and neck
squamous cell carcinoma and ovarian cancer (9). In breast cancer and lung cancer,
Twist expression is associated with a more aggressive phenotype and
poorer clinical outcome (10).
Furthermore, several studies revealed a correlation between Twist
expression and metastatic disease. Twist expression levels were
positively correlated with high grade and metastasis of prostate
cancer and bladder cancer (11,12).
Recently, increasing studies suggested that EMT
phenotype also led to the acquisition of other properties involved
in tumor progression, such as increased resistance to chemotherapy
drug and acquisition of cancer stem cell (CSC)-like features
(13). The CSCs are a group of
cells within a tumor that possess the capacity to self-renew and
differentiate into the heterogeneous lineages of cancer cells which
compose the whole tumor (14).
Mounting studies showed that somatic cells can be reprogramed into
pluripotent stem-like cells via co-expression of pluripotent makers
such as Bmi-1, Nanog and CD44 (15). A recent study confirmed that
drug-resistant lung cancer cells display a cancer stem-like trait
and EMT phenotype (16). Taken
together, these observations suggested that EMT and CSC-like
properties are mechanistically associated to chemoresistance.
Colorectal cancer (CRC) is a major worldwide health
concern. Most deaths from CRC are due to metastases that are
resistant to conventional therapies. Analysis of Twist in CRC
specimens in a recent study suggested that Twist can be an
important marker of poor prognosis (17). However, the specific role of Twist
in aggressive phenotype of CRC is has not been fully elucidated. In
this study we showed that upregulation of Twist confers aggressive
phenotype of CRC cells by inducing EMT and CSC properties.
Moreover, we demonstrated that high levels of Twist expression in
CRC cells are linked to increased chemoresistance.
Materials and methods
Chemicals and reagents
Primary antibody against E-cadherin was obtained
from Cell Signaling Technology (Danvers, MA, USA). Primary
antibodies against N-cadherin, vimentin, Twist, P-gp and α-tubulin
were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Primary antibodies against Bmi-1 and Nanog were obtained from
Epitomics. Alexa Fluor 488/594 conjugated secondary antibody,
Horseradish peroxidase (HRP)-conjugated secondary antibody, DAPI
and Lipofectamine 2000 were from Invitrogen (Carlsbad, CA, USA).
PrimeScript® RT reagent kit and SYBR® Premix
Ex Taq™ were from Takara. E.Z.N.A® HP Total RNA kit was
obtained from Omega Bio-Tek (Doraville, GA, USA).
Cell culture
The colorectal carcinoma cell lines HCT116 and SW480
were obtained from the Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). SW480 cells were cultured in
DMEM culture medium (Gibco BRL) supplemented with 10% fetal bovine
serum, and HCT116 cells were maintained in McCoy'5a culture medium
(Gibco BRL) supplemented with 10% fetal bovine serum under a
humidified 5% CO2 atmosphere at 37°C in an
incubator.
Transwell migration and invasion
assay
Invasion and migration assays were performed in
Boyden chambers. The polycarbonate filters, pre-coated with
Matrigel matrix, were used for invasion assay, and uncoated filters
were used for migration assay. Cells (1×105) in 300 μl
medium (containing 0.1% FBS) transfected with empty vector plasmid
or pcDNA-Twist were seeded in the upper chamber. Then 600 ml medium
with 10% FBS, served as a chemotactic agent, and was added to the
lower chamber. For migration, after 24 h incubation, the cells
migrated onto the lower chamber were fixed with 4% paraformaldehyde
for 30 min, stained with hematoxylin and counted under microscope.
For invasion, the cells in the upper chamber were fixed in 4%
paraformaldehyde for 30 min. Then the Matrigel was removed from the
filter with a cotton swab. The cells adhered to the underside of
the filter were stained with hematoxylin and counted under a
microscope. Each assay was repeated in three independent
experiments.
Gene overexpression
Cells/well (2×105) were seeded on a
6-well plate and left in culture until the next day. They were then
transfected with 2 μg empty vector plasmid or pcDNA-Twist mixed
with Lipofectamine 2000 reagent in serum-free medium according to
the manufacturer's instructions. After 6 h, medium was changed to
complete culture medium and the cells were incubated for another
24–48 h before harvest.
Quantitative real-time PCR
Total mRNA of the cells was extracted after
transfected with plasmids for 24 h. First step cDNA synthesis was
generated from 500 ng total mRNA. Quantification of target and
reference genes were detected in triplicate on
LightCycler® 480 II. The primers used in each reaction
are shown in Table I. Expression
levels for each target gene were normalized to GAPDH gene. Results
were calculated using the comparative threshold cycle (ΔΔCT)
method. Data are presented as the mean ± standard deviation (SD)
from three independent experiments.
 | Table IPrimers used in this study. |
Table I
Primers used in this study.
| Gene | Forward primer
5′-3′ | Reverse primer
5′-3′ |
|---|
| Twist |
GGAGTCCGCAGTCTTACGAG |
TCTGGAGGACCTGGTAGAGG |
| E-cadherin |
TACACTGCCCAGGAGCCAGA |
TGGCACCAGTGTCCGGATTA |
| Vimentin |
TGAGTACCGGAGACAGGTGCAG |
TAGCAGCTTCAACGGCAAAGTTC |
| Bmi-1 |
TGGAGAAGGAATGGTCCACTTC |
GTGAGGAAACTGTGGATGAGGA |
| Nanog |
CAAAGGCAAACAACCCACTT |
TCTGCTGGAGGCTGAGGTAT |
| CD44 |
GACACATATTGTTTCAATGCTTCAGC |
GATGCCAAGATGATCAGCCATTCTGGAAT |
| ABCB1 |
TGCTCAGACAGGATGTGAGTTG |
AATTACAGCAAGCCTGGAACC |
| ABCC1 |
GCCAAGAAGGAGGAGACC |
AGGAAGATGCTGAGGAAGG |
| ABCC2 |
TGGTGGCAACCTGAGCATAGG |
ACTCGTTTTGGATGGTCGTCTG |
| ABCC3 |
CTTAAGACTTCCCCTCAACATGC |
GGTCAAGTTCCTCTTGGCTC |
| ABCG2 |
TATAGCTCAGATCATTGTCACAGTC |
GTTGGTCGTCAGGAAGAAGAG |
| ABCC4 |
GGTTCCCCTTGGAATCATTT |
AATCCTGGTGTGCATCAAACAG |
| ABCC5 |
ACCCGTTGTTGCCATCTTAG |
GCTTTGACCCAGGCATACAT |
| ERCC1 |
CTCAAGGAGCTGGCTAAGATGT |
CATAGGCCTTGTAGGTCTCCAG |
| XRCC1 |
CATCGTGCGTAAGGAGTGGGTG |
AGTGGGCTTGGTTTTGGTCTGG |
| MSH2 |
GCTTCTCCTGGCAATCTCTCTC |
TACCCAACTCCAACCTGTCTCT |
| TUBB3 |
TCAGCAAGGTGCGTGAGGAGTAT |
CGGAAGCAGATGTCGTAGAGCG |
| POLH |
CGTGGGAGCAGTGATTGTGGAGGA |
GGTTTGGCGGTTGGGCTTGTTTAG |
| GSTπ |
ACCTCCGCTGCAAATACATC |
CTCAAAAGGCTTCAGTTGCC |
| γGT1 |
GCCTGGATTCTCCCAGAGAT |
GGAGAGCACCTCTTCCTCAG |
| GAPDH |
GCACCGTCAAGGCTGAGAAC |
TGGTGAAGACGCCAGTGGA |
Western blot analysis
The cells were washed three times with ice-cold PBS
and then lysed in lysis buffer. Lysates were cleared by
centrifugation and denatured by boiling for 5 min in Laemmli
buffer. Equal quantity of protein samples were loaded per well and
separated on SDS-polyacrylamide gels, and then transferred
electrophoretically onto PVDF membranes. After blocked with 5%
non-fat milk at room temperature for 2 h, membranes were incubated
with primary antibodies at 4°C overnight and then incubated with
HRP-conjugated secondary antibodies for 1.5 h at room temperature.
Immune complexes were detected using Western Blotting Plus
chemiluminescence reagent.
Immunofluorescence
The cells transfected with plasmids were cultured on
chamber slides for 24 h, then washed three times with PBS, fixed
with 4% paraformaldehyde for 30 min and permeabilized with 0.3%
Triton X-100 for 10 min. After blocked with goat serum for 2 h at
room temperature, cells were incubated with antibodies against
E-cadherin, vimentin (1:100 dilution) at 4°C overnight. Slides were
washed three times with PBS and incubated with Alexa Fluor 488 or
Alexa Fluor 594-conjugated secondary antibodies (1:1,000 dilution)
for 1 h at room temperature. Nuclei were stained with DAPI (10
μg/ml) for 10 min. Samples were examined with Confocal Laser
Scanning Microscopy (Zeiss) to analyze expression of E-cadherin,
vimentin.
Mammosphere formation assay
Stem cells are enriched in mammospheres of cancer
cells and assay is based on the ability of stem cells to grow and
form spheres in serum-free medium. Mammosphere culture was done in
a serum-free DMEM/F12 supplemented with 20 ng/ml EGF, B27, 20 ng/ml
basic fibroblast growth factor (bFGF), 1 g/ml hydrocortisone, 5
g/ml insulin and 1% antibiotic-antimycotic. Single cells prepared
from mechanical dissociation were plated in 12-well ultralow
attachment plates at a density of 1,000 cells/ml in culture. Single
cell status was confirmed under a microscope. Fresh mammosphere
culture was added every 3–4 days. After 7 days of culture, the
number of mammospheres (>20 μm) was counted under an upright
microscope and the images were acquired with the upright
microscope. Each assay was carried out in triplicate and repeated
in three independent experiments.
Cell viability assay
Cell viability was performed using the MTT assay.
Briefly, cells (1×104) transfected with plasmids were
seeded into each well of a 96-well plate and treated with
oxaliplatin at concentrations from 5 to 20 μM for 24 h. After
treatment, the cells were washed twice with PBS, and 100 ml of 0.25
mg/ml MTT inculture medium was added to each well. The plate was
incubated at 37°C for 4 h. Then, the culture medium was removed,
and 100 ml DMSO was added to each well to dissolve the dark blue
crystal. The absorbance was measured at 570 nm using a microplate
reader.
Immunohistochemistry
Sections cut from the human colorectal tumor
paraffin blocks were subjected to deparaffinization/rehydration,
and antigen retrieval by boiling in 0.01 M sodium citrate buffer
for 30 min. The sections were blocked with 10% goat serum, then
incubated with the primary antibodies against Twist and P-gp at a
dilution 1:200 at 4°C overnight in a humidified chamber. After
washing with PBS three times, slides were incubated with
HRP-conjugated antibody, and DAB was used as substrate. Mayer's
hematoxylin was applied as a counter stain. Throughout the above
analyses, controls were prepared by omitting the primary
antibodies.
Statistical analysis
Results are expressed as mean ± SD of three
independent experiments unless otherwise specified. Data were
analyzed by two-tailed unpaired Student's t-test between any two
groups. One-way ANOVA analysis of variance was used to assess the
difference of means among groups. These analyses were performed
using GraphPad Prism Software Version 5.0 (GraphPad Software Inc.,
La Jolla, CA, USA). A P-value of <0.05 was considered
statistically significant.
Results
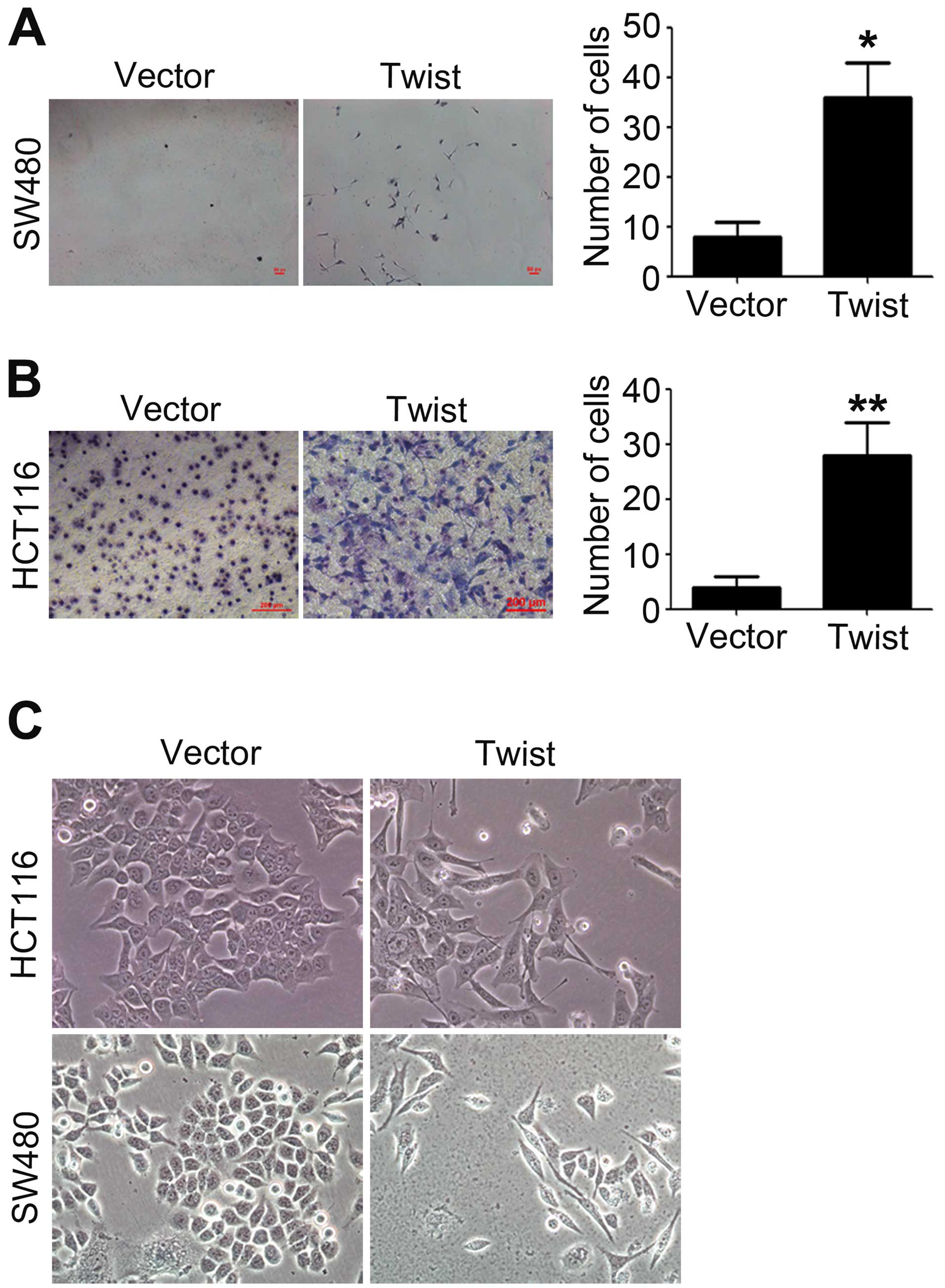
Overexpression of Twist promotes
migration and invasion of CRC cells
Tumor cells with an aggressive phenotype acquire
invasive and migratory capabilities, which will promote the
dissemination of tumors cells to distant organs (1). The migratory and invasive
capabilities of HCT116 and SW480 cells transfected with vector
plasmid or pcDNA-Twist were measured by using Transwell migration
and invasion assays. As shown in Fig.
1A and B, overexpression of Twist led to an obvious increase in
cell migration and invasion. Compared with control, the number of
migrated and invasive cells increased about 5-fold (migration) and
15-fold (invasion) after overexpression of Twist.
The increased migratory and invasive capabilities of
tumor cells are reminiscent of the molecular events at EMT. The
morphology of HCT116 and SW480 cells was observed after
transfection with pcDNA-Twist, we found that overexpression of
Twist resulted in a significant change in cell morphology, from
cobblestone phenotype to spindle-like and fusiform features
(Fig. 1C).
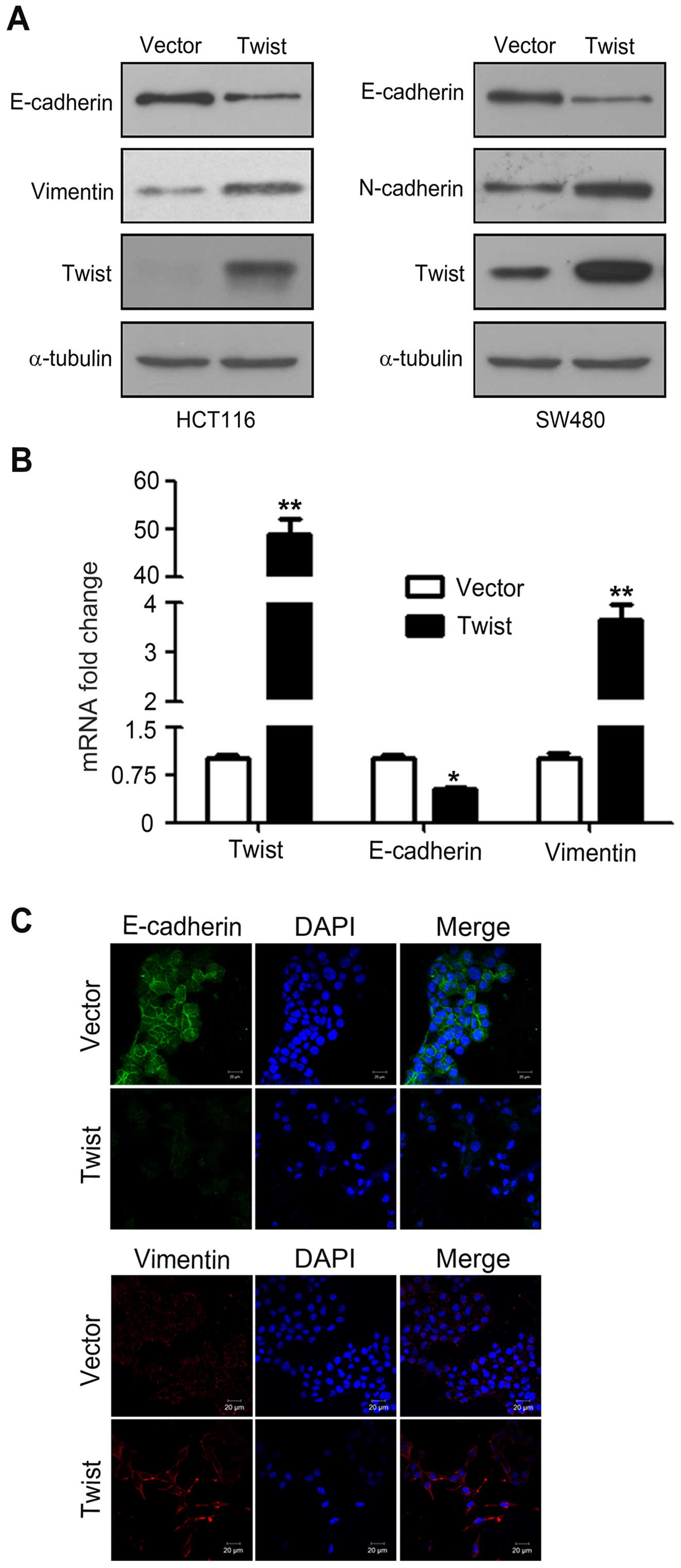
Overexpression of Twist induces EMT of
CRC cells
During EMT, the epithelial maker E-cadhein is
downregulated, whereas the mesenchymal markers vimentin and
N-cadherin are upregulated (18).
To confirm whether HCT116 and SW480 cells transfected with
pcDNA-Twist truly acquire EMT features, western blotting was used
to examine the expression of EMT makers. As shown in Fig. 2A, overexpression of Twist decreased
E-cadherin expression, which is consistent with increased vimentin
and N-cadherin. Similarly, real-time PCR analysis further confirmed
the increasing expression of vimentin and N-cadherin, and the
decreased expression of E-cadherin at mRNA levels (Fig. 2B). Furthermore, immunofluorescence
analysis showed that overexpression of Twist downregulated
E-cadherin and upregulated vimentin (Fig. 2C). Collectively, these observations
suggested that HCT116 and SW480 cells had undergone EMT following
overexpression of Twist.
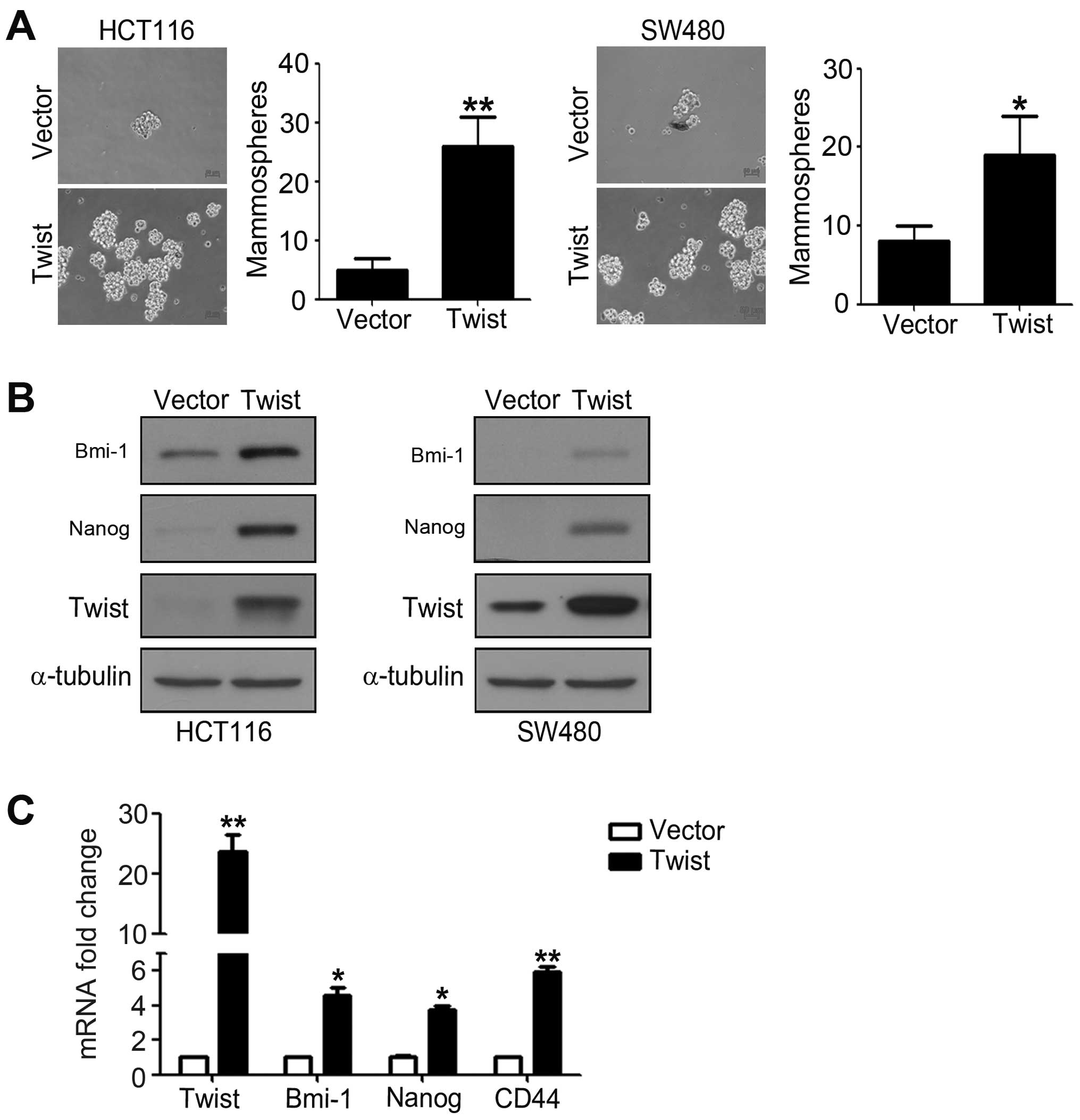
Overexpression of Twist induces a
CSC-like phenotype of CRC cells
To investigate whether cells with EMT phenotype
could display CSC-like features, we detected mammosphere forming
ability of HCT116 and SW480 cells transfected with pcDNA-Twist. We
found that cells overexpressing Twist showed obviously enhanced
ability to form mammosphere compared to control cells. As shown in
Fig. 3A, overexpression of Twist
increased the number of mammospheres about 3–7-fold of HCT116 and
SW480 cells. To further investigate the genes maintaining and
regulating the CSC properties, we performed western blotting and
real-time PCR analysis. The results from western blotting showed
that overexpression of Twist significantly increased pluripotent
makers Nanog and Bmi-1 expression levels (Fig. 3B). Real-time PCR analysis also
demonstrated that the mRNA expression levels of Nanog, Bmi-1 and
CD44 are obviously enhanced in cells overexpressing Twist compared
to control cells (Fig. 3C). These
findings demonstrated that overexpression of Twist induces a
CSC-like phenotype of CRC cells.
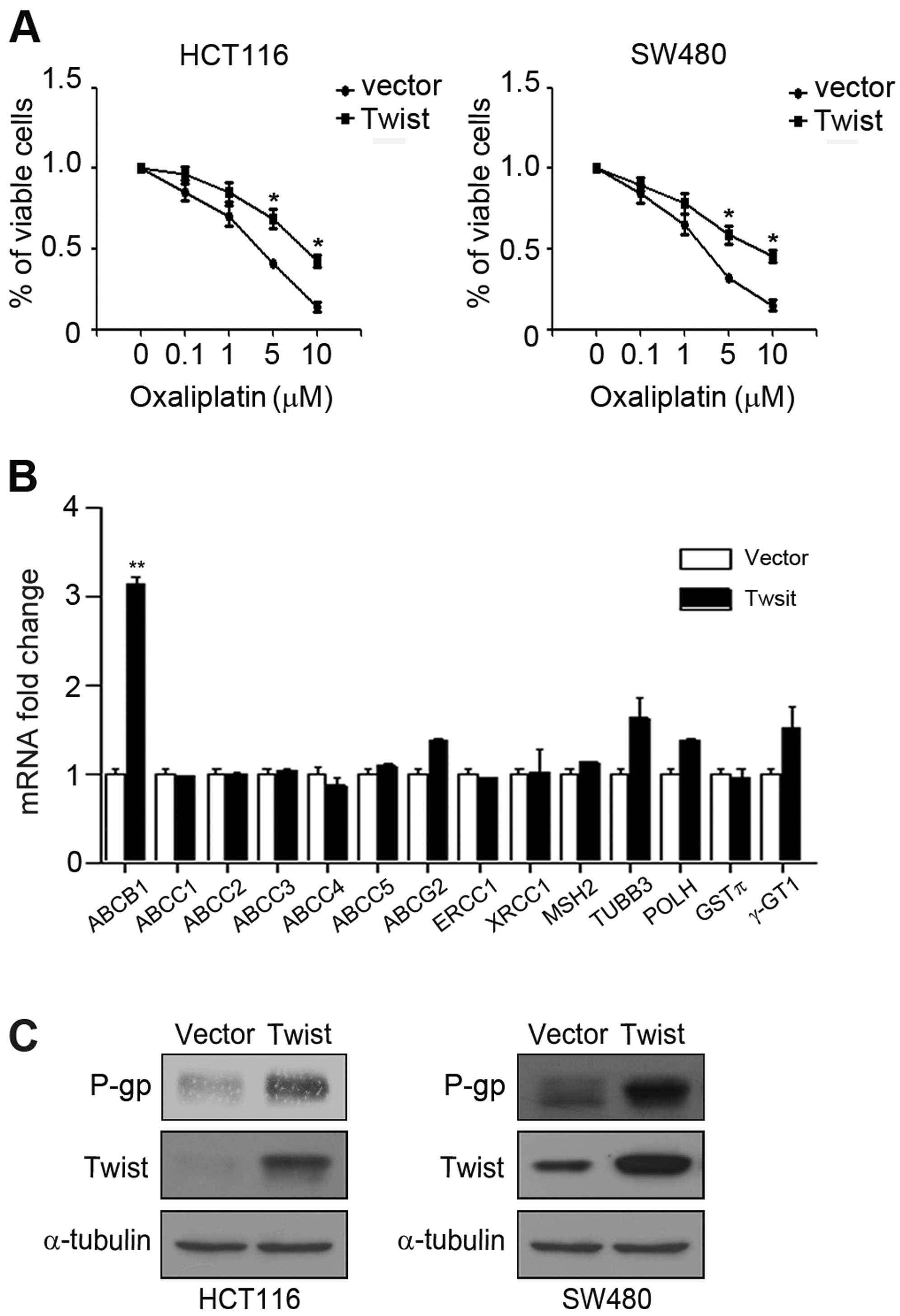
Overexpression of Twist mediates
chemoresistance of CRC cells
Recent studies showed that EMT phenotype and
CSC-like trait are closely related to chemoresistance of cancer
cells (13). We next observed
whether overexpression of Twist can affect sensitivity of HCT116
and SW480 cells to oxaliplatin. HCT116 and SW480 cells transfected
with control vector plasmid or pcDNA-Twist were treated with
different concentration of oxaliplatin and MTT assay was performed.
The results showed that overexpression of Twist led to a distinct
increase in cell viability compared with cells transfected with
control vector (Fig. 4A).
Underlying molecular mechanisms of chemoresistance are extremely
complicated, involving ATP binding cassette (ABC) membrane
transport proteins and DNA repair proteins (19). We overexpressed Twist and detected
drug resistance-associated genes, including ABCB1, ABCC1, ABCC2,
ABCC3, ABCC4, ABCC5, ABCG2, ERCC1, XRCC1, MSH2, TUBB3, POLH, GSTπ
and γ-GT1. Noteworthy, overexpression of Twist distinctly increased
the mRNA expression of ABCB1, but not the others (Fig. 4B). P-gp, a transmembrane
glycoprotein characterized based on its ability to confer a
multidrug-resistance phenotype to cancer cells is an ABCB1 encoded
protein (20). Further, we
demonstrated that overexpression of Twist also enhanced the
expression of P-gp in HCT116 and SW480 cells (Fig. 4C). Taken together, these
observations suggested that P-gp may be essential for drug
resistant phenotype induced by Twist.
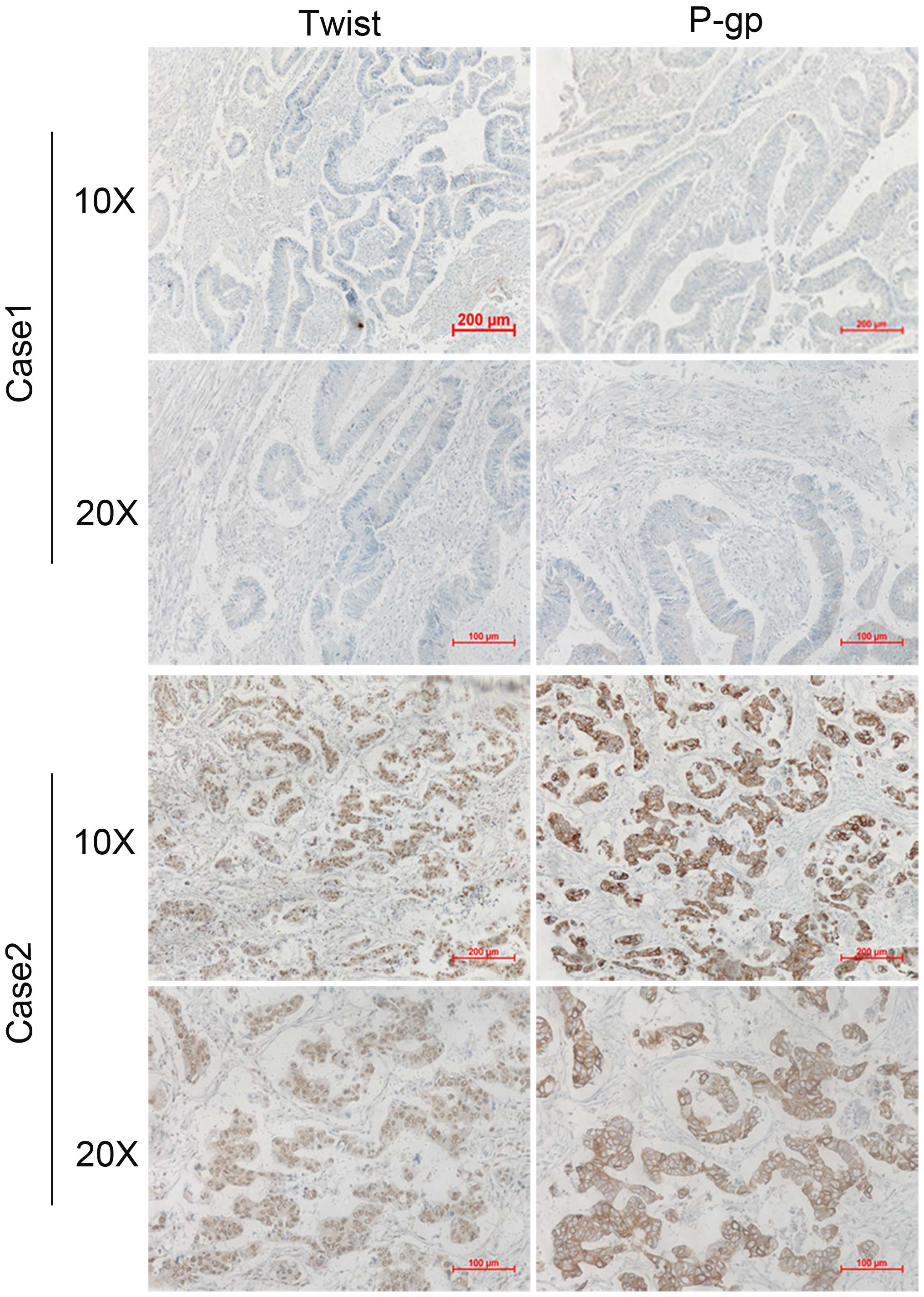
Clinical corelation of Twist and P-gp
expression in human CRC specimens
To further extend our findings in vivo, we
determined whether there is a correlation between the expression of
Twist and P-gp in resected human colorectal cancer specimens.
Immunohistochemical analyses of Twist and P-gp were performed for
46 examples of tumor samples from colorectal cancer patients. A
representative immunohistochemistry result is shown in Fig. 5, Twist expression was on the cell
nuclei, while P-gp expressed on the cell membrane. Of note,
expression of P-gp occurred in 28 cases (60.8%) and expression of
Twist occurred in 26 cases (56.5%), indicating that there was an
obvious association between Twist and P-gp expression (P<0.01;
Table II).
 | Table IIImmunohistochemical staining of Twist
and P-gp was performed in 46 tumor samples from colorectal cancer
patients.a |
Table II
Immunohistochemical staining of Twist
and P-gp was performed in 46 tumor samples from colorectal cancer
patients.a
| P-gp | |
|---|
|
| |
|---|
| Positive | Negative | Total | P-value |
|---|
| Twist |
| Positive | 21 | 5 | 26 | |
| Negative | 7 | 13 | 20 | <0.01 |
| Total | 28 | 18 | 46 | |
Discussion
Metastasis involves many steps such as successful
invasion, intravasation, survival in the circulation, extravasation
and colonization by the cancer cells. Many cancer types depend on
the process of EMT for successful completion of these steps
(1). EMT is thought to be the
first step of tumor invasion and metastasis (4). It has been reported that the
expression profiles of EMT are associated with tumor grades and
metastasis of breast carcinoma (21,22).
EMT also plays a crucial role in the metastasis of colorectal
cancer, which occurs at the invasive front of colon carcinoma
concomitant with a selective loss of basement membrane (23). Twist, a zinc-finger transcription
factor, has been considered as a critical regulator that promotes
EMT and induces invasiveness as well as metastasis in various types
of malignant tumors (24). In CRC
tissues, Twist was highly expressed and the inverse correlation
between Twist and E-cadherin was observed (25). In addition, aberrant Twist
expression associated significantly with lymph node metastasis of
CRC (25). Consistent with the
above investigations, our studies demonstrated that overexpression
of Twist in CRC cells led to decreased E-cadherin expression and
increased vimentin and N-cadherin expression. Furthermore,
acquisition of mesenchymal phenotype has been associated with
enhanced invasive and metastatic behavior.
Recently, it was proposed that EMT not only enables
cancer cells to disseminate but also to acquire the ability to
self-renew by inducing a CSC trait (26). The CSCs represent a small subset of
cells within a malignant tumor thought to be capable of initiating
the tumor and of driving its growth and recurrence after treatment
(14). The relationship between
EMT and CSC has been the focus of recent research. Mani et
al demonstrated that overexpression of EMT regulators Snail and
Twist in immortalized human mammary epithelial cells (HMLEs)
induced an EMT state and conferred to the cells an increased
ability to form mammospheres, which is characteristic of CSCs
(27). Comparably, McCoy et
al proved that the homeobox transcription factor Six1 induced
tumors that underwent EMT in mouse mammary gland epithelium by
increasing the population of cells displaying CSC markers (28). Similarly, recent research showed
that gene expression patterns of CSC-associated pathways were
involved in EMT (29). In line
with these studies, we found that Twist-induced EMT conferred a
CSC-like phenotype in CRC cells. Overexpression of Twist in two CRC
cell lines resulted in increased expression of the CSC markers
Bmi-1, Nanog and CD44 as well as enhanced ability to form
mammospheres.
Increasing evidence suggested that both EMT and CSC
phenotype are associated with chemoresistance (13). Cancer cells undergoing EMT become
resistant to chemotherapy, and cancer cells selected for
chemoresistance acquire EMT phenotype (1,16,30,31).
Induction of EMT was detected to promote the decreased efficacy of
chemotherapy in colorectal, breast and ovarian cancers (30,32,33).
It has been demonstrated that chemoresistance is due to the
existence of CSC features, which remain after treatment and result
in cancer relapse. In this study, we also showed that the CRC cells
overexpressing Twist exhibited EMT and CSC properties as well as
resistance to chemotherapy. The cancer cells resistant to
conventional therapies was primary due to altered expression of ATP
binding cassette (ABC) membrane transport proteins (34), overexpression of the DNA repair
enzymes (35), activation of DNA
damage checkpoint responses and inhibition of cell apoptosis
(19,36).
We next investigated the underlying mechanism of
chemoresistance induced by Twist overexpression and our results
showed that Twist significantly enhance ABCB1 mRNA level, but not
the other drug resistance genes, such as ABCC1, ABCC2, ABCC3,
ABCC4, ABCC5, ABCG2, ERCC1, XRCC1, MSH2, TUBB3, POLH, GSTπ and
γ-GT1. A recent study found that Twist can directly combine ABCC4
and ABCC5 promoters and increase their expression levels in breast
epithelial cells (37). However,
in our study, we did not find this phenomenon, which may be due to
the different cell lines. P-gp, a transmembrane glycoprotein
encoded by the ABCB1 gene, is the first and most important human
ABC transporter and confer a multidrug-resistance phenotype to
cancer cells (20). Clinically,
P-gp is involved in the process of local metastatic dissemination
and drug resistance of colorectal carcinoma cells (38). We further confirmed that high
expression of Twist increased P-gp expression in CRC cells.
Importantly, we found clinical correlation of Twist and P-gp
expression in human CRC specimens. Software analysis showed that
the promoter of ABCB1 have Twist binding sites (CANNTG). It is
worth further investigation to assess whether Twist can directly
combine the promoter of ABCB1 and promote P-gp expression.
In summary, our studies have demonstrated that Twist
expression in human CRC cells can mediate EMT and the development
of the CSC phenotype as well as chemoresistance. Therapeutic
targeting of Twist may be a novel method to enhance the efficacy of
chemotherapy and improve outcomes for patients with metastatic
CRC.
Acknowledgements
This work was funded by the Natural Science
Foundation of Hubei Province (no. 2015CFB206).
References
|
1
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu Y and Zhou BP: Snail: More than EMT.
Cell Adh Migr. 4:199–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Christofori G: New signals from the
invasive front. Nature. 441:444–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barrallo-Gimeno A and Nieto MA: The Snail
genes as inducers of cell movement and survival: implications in
development and cancer. Development. 132:3151–3161. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang H, Wang HS, Zhou BH, Li CL, Zhang F,
Wang XF, Zhang G, Bu XZ, Cai SH and Du J: Epithelial-mesenchymal
transition (EMT) induced by TNF-α requires AKT/GSK-3β-mediated
stabilization of snail in colorectal cancer. PLoS One.
8:e566642013. View Article : Google Scholar
|
|
7
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: an alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nuti SV, Mor G, Li P and Yin G: TWIST and
ovarian cancer stem cells: implications for chemoresistance and
metastasis. Oncotarget. 5:7260–7271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wushou A, Hou J, Zhao YJ and Shao ZM:
Twist-1 up-regulation in carcinoma correlates to poor survival. Int
J Mol Sci. 15:21621–21630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C,
Zhang X, Chua CW, Chan KW, Chan FL, Glackin C, et al: Up-regulation
of TWIST in prostate cancer and its implication as a therapeutic
target. Cancer Res. 65:5153–5162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Z, Xie D, Li X, Wong YC, Xin D, Guan
XY, Chua CW, Leung SC, Na Y and Wang X: Significance of TWIST
expression and its association with E-cadherin in bladder cancer.
Hum Pathol. 38:598–606. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: an emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kong D, Li Y, Wang Z and Sarkar FH: Cancer
stem cells and epithelial-to-mesenchymal transition
(EMT)-phenotypic cells: are they cousins or twins? Cancers (Basel).
3:716–729. 2011. View Article : Google Scholar
|
|
15
|
Kong D, Banerjee S, Ahmad A, Li Y, Wang Z,
Sethi S and Sarkar FH: Epithelial to mesenchymal transition is
mechanistically linked with stem cell signatures in prostate cancer
cells. PLoS One. 5:e124452010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Zhang G, Zhang H, Zhang F, Zhou B,
Ning F, Wang HS, Cai SH and Du J: Acquisition of
epithelial-mesenchymal transition phenotype and cancer stem
cell-like properties in cisplatin-resistant lung cancer cells
through AKT/β-catenin/Snail signaling pathway. Eur J Pharmacol.
723:156–166. 2014. View Article : Google Scholar
|
|
17
|
Kim YH, Kim G, Kwon CI, Kim JW, Park PW
and Hahm KB: TWIST1 and SNAI1 as markers of poor prognosis in human
colorectal cancer are associated with the expression of ALDH1 and
TGF-β 1. Oncol Rep. 31:1380–1388. 2014.PubMed/NCBI
|
|
18
|
Voulgari A and Pintzas A:
Epithelial-mesenchymal transition in cancer metastasis: mechanisms,
markers and strategies to overcome drug resistance in the clinic.
Biochim Biophys Acta. 1796:75–90. 2009.PubMed/NCBI
|
|
19
|
He H, Ni J and Huang J: Molecular
mechanisms of chemoresistance in osteosarcoma (Review). Oncol Lett.
7:1352–1362. 2014.PubMed/NCBI
|
|
20
|
Xue X and Liang XJ: Overcoming drug
efflux-based multidrug resistance in cancer with nanotechnology.
Chin J Cancer. 31:100–109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Logullo AF, Nonogaki S, Pasini FS, Osório
CA, Soares FA and Brentani MM: Concomitant expression of
epithelial-mesenchymal transition biomarkers in breast ductal
carcinoma: Association with progression. Oncol Rep. 23:313–320.
2010.PubMed/NCBI
|
|
22
|
Xue CS, Plieth D, Venkov C, Xu C and
Neilson EG: The gatekeeper effect of epithelial-mesenchymal
transition regulates the frequency of breast cancer metastasis.
Cancer Res. 63:3386–3394. 2003.PubMed/NCBI
|
|
23
|
Sheehan KM, Gulmann C, Eichler GS,
Weinstein JN, Barrett HL, Kay EW, Conroy RM, Liotta LA and
Petricoin EF III: Signal pathway profiling of epithelial and
stromal compartments of colonic carcinoma reveals
epithelial-mesenchymal transition. Oncogene. 27:323–331. 2008.
View Article : Google Scholar
|
|
24
|
Jung HY and Yang J: Unraveling the TWIST
between EMT and cancer stemness. Cell Stem Cell. 16:1–2. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Galván JA, Helbling M, Koelzer VH, Tschan
MP, Berger MD, Hädrich M, Schnüriger B, Karamitopoulou E, Dawson H,
Inderbitzin D, et al: TWIST1 and TWIST2 promoter methylation and
protein expression in tumor stroma influence the
epithelial-mesenchymal transition-like tumor budding phenotype in
colorectal cancer. Oncotarget. 6:874–885. 2015. View Article : Google Scholar :
|
|
26
|
Fan YL, Zheng M, Tang YL and Liang XH: A
new perspective of vasculogenic mimicry: EMT and cancer stem cells
(Review). Oncol Lett. 6:1174–1180. 2013.PubMed/NCBI
|
|
27
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McCoy EL, Iwanaga R, Jedlicka P, Abbey NS,
Chodosh LA, Heichman KA, Welm AL and Ford HL: Six1 expands the
mouse mammary epithelial stem/progenitor cell pool and induces
mammary tumors that undergo epithelial-mesenchymal transition. J
Clin Invest. 119:2663–2677. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang AD, Fan F, Camp ER, van Buren G, Liu
W, Somcio R, Gray MJ, Cheng H, Hoff PM and Ellis LM: Chronic
oxaliplatin resistance induces epithelial-to-mesenchymal transition
in colorectal cancer cell lines. Clin Cancer Res. 12:4147–4153.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arumugam T, Ramachandran V, Fournier KF,
Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey
DJ and Choi W: Epithelial to mesenchymal transition contributes to
drug resistance in pancreatic cancer. Cancer Res. 69:5820–5828.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD
and Wang LH: Twist transcriptionally up-regulates AKT2 in breast
cancer cells leading to increased migration, invasion, and
resistance to paclitaxel. Cancer Res. 67:1979–1987. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kajiyama H, Shibata K, Terauchi M,
Yamashita M, Ino K, Nawa A and Kikkawa F: Chemoresistance to
paclitaxel induces epithelial-mesenchymal transition and enhances
metastatic potential for epithelial ovarian carcinoma cells. Int J
Oncol. 31:277–283. 2007.PubMed/NCBI
|
|
34
|
Fukuda Y and Schuetz JD: ABC transporters
and their role in nucleoside and nucleotide drug resistance.
Biochem Pharmacol. 83:1073–1083. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hsu DS, Lan HY, Huang CH, Tai SK, Chang
SY, Tsai TL, Chang CC, Tzeng CH, Wu KJ, Kao JY, et al: Regulation
of excision repair cross-complementation group 1 by Snail
contributes to cisplatin resistance in head and neck cancer. Clin
Cancer Res. 16:4561–4571. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saxena M, Stephens MA, Pathak H and
Rangarajan A: Transcription factors that mediate
epithelial-mesenchymal transition lead to multidrug resistance by
upregulating ABC transporterss. Cell Death Dis. 2:e1792011.
View Article : Google Scholar
|
|
38
|
Zorzos HS, Lazaris AC, Korkolopoulou PA,
Kavantzas NG, Tseleni-Balafouta S, Patsouris ES, Tsavaris NV and
Davaris PS: Multidrug resistance proteins and topoisomerase IIalpha
expression in colon cancer: association with metastatic potential.
Pathology. 35:315–318. 2003. View Article : Google Scholar : PubMed/NCBI
|