Cancer remains among the leading causes of mortality
word-wide with an increase of 7.5 million of deaths in 2008 to 8.2
million in 2012. Furthermore, 32.6 million people are living with
cancer according to the latest GLOBOCAN estimated cancer incidence,
mortality and prevalence (http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx).
Despite the advances in the diagnosis and treatment of human
malignancy, it is necessary to find new improved strategies to
treat cancer. The human gonadotropin-releasing hormone (hGnRH) is a
key hormone in the regulation of reproduction. However, an
increasing number of reports have been shown the participation of
GnRH and its receptor, not only in the reproduction but also in the
regulation of tumor cell behavior. The hGnRH has been shown
effective in controlling cell growth and invasiveness in certain
type of tumors, both in vivo and in vitro.
Furthermore, in some cases it has been reported to possess
anti-oncogenic activity. These characteristics make the hGnRH/hGnRH
receptor (hGnRHR) an ideal model in the study of new approaches for
cancer treatment. The present review will focus on the signal
transduction pathways activated when the hormone binds its
receptor, in gonadotrope cells and in several types of cancer and
how the relatively new concept of hGnRHR overexpression by
pharmacoperons could be a new therapeutic strategy for cancer
treatment.
The tissue distribution pattern of the hGnRH and its
receptor is wide and diversified. Therefore, it is not surprising
that the cell signaling pathways produced from the interaction of
the receptor with its hormone largely depend on the cellular
context in which this occurs (1).
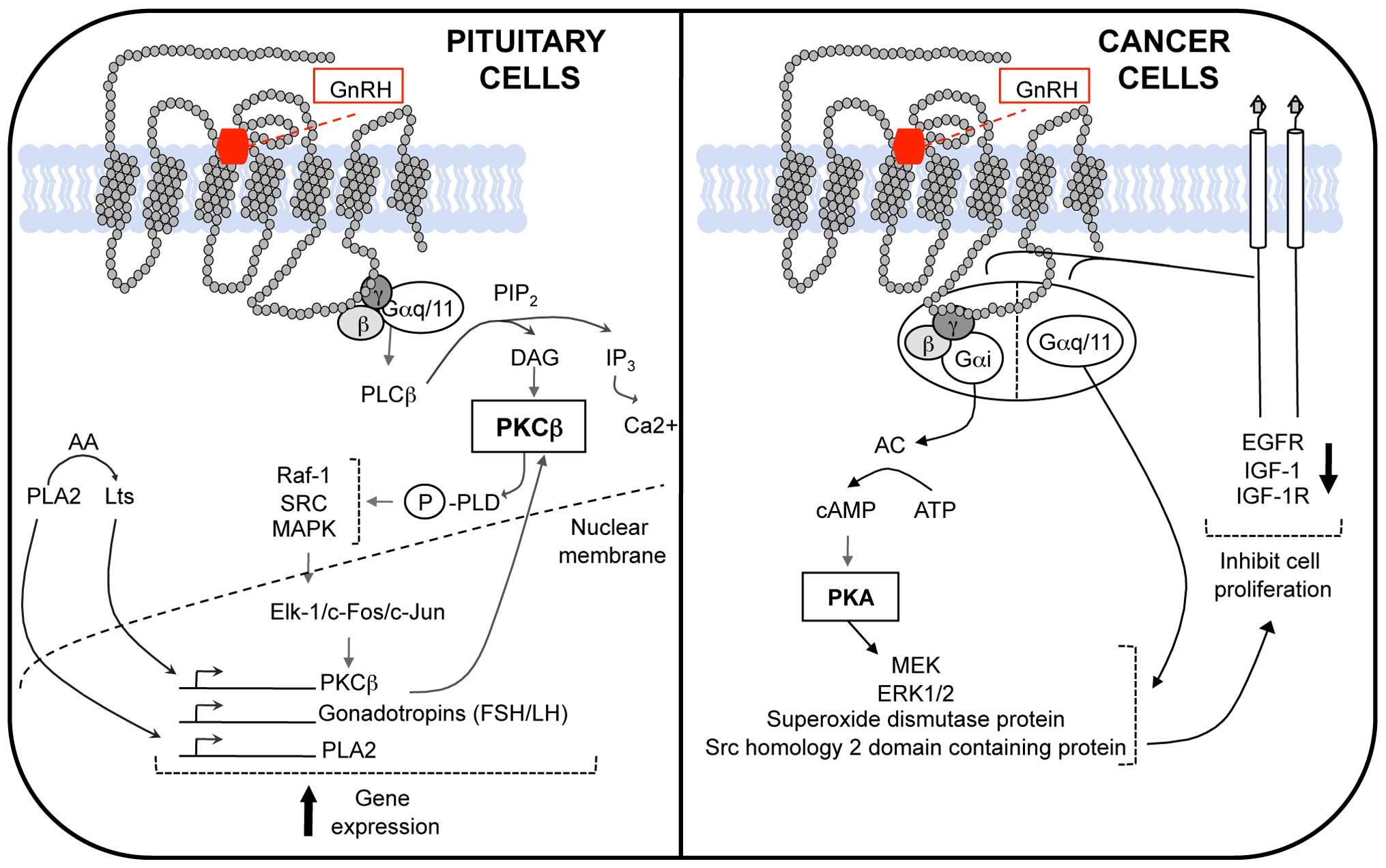
In pituitary gonadotropes, the interaction between hGnRHR with its
ligand induces conformational changes in both, the receptor itself
and the coupled G-protein. The G protein family belongs to the
heterotrimeric GTPases that are integrated by three subunits: Gα,
Gβ and Gγ. There is a wide range of G proteins, which have been
subclassified based on their structural differences in the Gα
subunit. However, in gonadotrope cells the hGnRHR can be coupled to
Gαq/11, and according to some reports, to Gαs subunit (2). The structural change of the G protein
modifies the affinity for GDP, and the guanine nucleotide exchange
factor facilitates the passive substitution of GDP for GTP. This
nucleotide exchange causes the Gα subunit separation of the
heterotrimer without affecting the association between the Gβ and
Gγ subunits, which remain as one (3). Early studies on hGnRH signaling
pathway in gonadotrophs, showed that only the Gαq/11 subunit could
initiate an intracellular signaling cascade, through its main
effector, the phospholipase Cβ (PLCβ) (4). However, further functional studies
have shown that Gβγ dimer is also able to activate PLCβ; in fact it
has been observed that in response to a sustained stimulation of
hGnRH other effectors such as phospholipase A2 (PLA2) and D (PLD)
can also be activated consecutively through both, Gα or Gβγ
(Fig. 1) (5).
Phospholipase C uses phosphatidylinositol 4,5
bisphosphate (PIP2) as substrate and the enzymatic hydrolysis of
this phospholipid produces the first wave of the second messengers,
diacylglycerol (DAG) and inositol 1,4,5 triphosphate (IP3)
(2). The endoplasmic reticulum
membrane contains IP3 receptors that can function as
Ca2+ channels when activated by their specific ligand.
The interaction of IP3 changes the structural conformation of its
receptor, causing an increase in Ca2+ channel
sensitivity with the consequent release of the cation into the
cytosol. Subsequently, an increase in the concentration of
Ca2+ activates the L-type voltage-sensitive calcium
channels located in the cell membrane (6,7).
Several studies have demonstrated the functional association
between the accumulation of intracellular Ca2+ induced
by hGnRH and the exocytosis of gonadotropins (8). In gonadotrope cells, the production
of DAG and Ca2+ release into the cytosol are the main
events in the synthesis and release of gonadotropins in response to
hGnRH through the activation of protein kinase C (PKC) (9).
Due to the pleiotropic activity of the PKC, its
activation is considered to be the central event in the activation
of the hGnRH system. Studies performed in different cell systems
have reported that two PKC isoforms α and βII have the ability to
directly phosphorylate several residues of PLD and therefore
activate the kinase (10,11). PLD hydrolyzes the
phosphatidylcholine (PC) producing fosfatidilethanol (PET) and
phosphatidic acid (PA), and both can act as second messengers
increasing furthermore the signaling pathways initiated by hGnRH.
PLD also rapidly activates fibrosarcoma protein kinase 1 (Raf-1),
protein tyrosine kinase SRC and some mitogen-activated protein
kinases (MAPKs) that are the immediate effectors in gonadotropes
(10,12–14).
The direct or indirect activation of PKC upon via
the Raf-1/MAPKs ends with the phosphorylation of transcription
factors, such as Elk-1, c-Fos and c-Jun, which positively regulate
the transcription of the gonadotropin α subunit gene and the
phospholipase A2 (PLA2) gene (2,15,16).
In the gonadotrope cell line αT3-1, the PLA2 generates arachidonic
acid (AA), which in turn is substrate for the lipoxygenase that
converts AA into leukotrienes. These leukotrienes are also involved
in the induction of gene expression of the gonadotropins α subunit
as well as PKCβ (17,18). In turn all the signal transduction
pathways induced by hGnRH and transduced by the axis
hGnRHR/Gαq/11/PLC/PKC, culminate in the event of synthesis and
release of gonadotropins, luteinizing hormone (LH) and
follicle-stimulating hormone (FSH) (Fig. 1) (19). The activation of a second G protein
by the hGnRH stimulation in the gonadotrope remains controversial,
some reports have indicated the activation of Gαs with the
consequent activation of adenylate cyclase (AC) and the formation
of the second messenger, cyclic adenosine monophosphate (cAMP),
which in turn is the cofactor of the protein kinase A (PKA);
however, further experiments are necessary to confirm these results
(20).
Another component of these complex networks of
signal transduction pathways is regulated by both frequency and
amplitude of pulses of hypothalamic hGnRH secretion into the
bloodstream depending on the physiological needs of the organism
(21). It has been reported that
when gonadotrope cells are exposed to a high frequency pulsation of
hGnRH, the synthesis and release of gonadotropin α subunit and the
β subunit of LH is induced. While hGnRH-low frequency pulsatility
induces the synthesis and release of the β subunit of FSH (22).
Prostate tumors and cell lines derived from these
cancers express hGnRH, as well as the hGnRHR; in fact, almost 80%
malignant prostate tumors present binding sites for hGnRH (23,24).
Assays carried out in human prostate-cancer biopsy, shown that
these binding sites were mediated by specific hGnRH receptors
(25). Several studies have shown
that the hGnRH/hGnRHR system promotes a decrease in cellular
proliferation of malignant prostate tumors (26,27).
Keeping this in mind, several research groups have been employing
hGnRH as antineoplastic drugs for many years (28–33).
In tumor cell lines such as LNCaP
(androgen-sensitive prostatic-cancer cell line), DU145 cells (a
human androgen-independent cancer-cell line) and in PC-82 cells (an
androgen-dependent cell line) specific binding sites by hGnRH have
been shown (26,27,30).
The complete mechanism by which hGnRHR is activated in prostate
cancer is unknown; however, the identification of mRNA for hGnRH in
LNCaP cells suggests a local paracrine/autocrine system in this
tumor cells (34).
Many research groups have investigated the signaling
pathway activated by the hGnRH/hGnRHR system to inhibit cell
proliferation and ultimate demonstrate cross-talk between growth
factor-receptors and hGnRHR. This interaction was observed in LNCaP
and DU145 cells, where an association between hGnRHR and epidermal
growth factor receptor (EGFR) was shown. In both cell lines the
hGnRH stimulation abrogates epidermal growth factor (EGF)-induced
transcription factor c-fos expression and reduces the number of
EGF-binding sites in membrane. Furthermore, in DU145 cells,
inhibition of the EGF receptor phosphorylation and reduction of
EGF-binding sites in cell membrane after hGnRH treatment were
demonstrated (35). Collectively,
these results demonstrated that hGnRHR activation abrogates cell
proliferation via EGFR signaling. On the other hand, an important
inhibition of the mitogenic action of the insulin-like growth
factor 1 (IGF-1) was also observed in DU145 cells after
hGnRH-stimulation (36). The
inhibitory effects of hGnRH in the proliferation of prostate cancer
cells, appears to be mediated via coupling and activating the G
protein Gαi, and not by Gαq/11 as observed in gonadotropes
(37). In addition to the
antiproliferative effect exhibited by the hGnRH/hGnRHR system in
prostate cancer it also comprises direct induction of apoptotic
signaling (38,39). hGnRH induces apoptosis in DU145
cells involving the activation of c-Jun N-terminal kinase (JNK) by
a decrease of protein kinase B (PKB) activity. The activation of
JNK could also be mediated by inhibition of the upstream activator
of JNK, the mixed-lineage kinase 3 (MLK3) (38). However, details in the mechanisms
by which hGnRH induced apoptosis remain to be determined.
Temporal and specific expression of hGnRH/hGnRHR has
been shown in human ovary cells (40). Radioligand assay carried out in
different cell lines and tumor biopsy specimens demonstrated
high-affinity binding sites for 125I-labeled hGnRH
agonist ([D-Trp6] LHRH) in 70% of primary ovarian
cancers as well as in 83% of primary endometrial cancers (41,42).
The elucidation of the cellular function of hGnRHR system in
extra-pituitary tumor cells has been the goal of many researches.
These reports have shown that the expression of both hormone and
receptor are able to cause growth inhibition in malignant cells
(42–45). At the same time, clinical data show
that the expression of hGnRH/hGnRHR in epithelial malignant ovary
tumors could be considered as a favorable prognostic factor
(46).
Although the signaling pathways by which hGnRHR
affects cell proliferation in ovarian cancer are still
undetermined, it is clear that they are distinct from that in the
anterior pituitary (Fig. 1). The
specific intracellular signaling cascades that could be coupled to
hGnRH in human ovary cancer are the activation of the PKC system.
In the human endometrial cancer cell line HHUA, hGnRHR activation
was able to downregulate the cellular proliferation via PKC and in
human ovarian mucinous cystadenocarcinoma samples, hGnRH agonists
also activate PKC protein (47,48).
The PKC activation by hGnRHR, could be the link between receptor
activation and MAPK kinase cascades to inhibit cell proliferation.
In ovarian cancer cells SKOV-3 and OVCAR-3, a pronounced cell
proliferation inhibition and activation of extracellular
signal-regulated kinase (ERK)1/2 via Gαq/PKC, was demonstrated
after hGnRH-stimulation (49).
On the other hand, the antiproliferative action of
hGnRHR via ERK1/2 also has been reported as a PKC-independent
process suggesting that hGnRHR signaling may vary by cell type
(50). In ovarian carcinoma and
endometrial carcinoma samples, the activation of the signaling
pathways by hGnRHR could be associated with the coupling between
the receptor and the Gi protein (51,52).
The link between hGnRHR and Gi could explain the differences in the
signal transduction pathways activated by hGnRH receptors in
malignant tumors and the anterior pituitary. Caov-3 cells shown
growth inhibition in response to hGnRH, and also are able to
activate several proteins such as adapter protein Src homology 2
domain containing (SHC), superoxide dismutase protein (SOD), MAP
kinase kinase protein (MEK) and activation of ERK, via Gi coupling
(50).
As mentioned above for prostate cancer, the effects
of hGnRHR in mitogenic signaling pathways have also shown
cross-talk between this receptor and growth factor receptors in
ovary cancer cells. In endometrial (HEC-1A) and ovarian (EFO-21 and
EFO-27) cancer cells, hGnRH receptor activation suppresses the
phosphorylation of EGFR and inhibits the activation of
MAP-kinase/ERK1/2 (53). Similar
results were observed in DU-145 cells where hGnRH receptor
activation abrogates EGFR-induced c-fos expression and
reduces the concentration of EGF-binding sites, resulting in
downregulation of cellular proliferation (35). In EFO-21 and EFO-27 cells the
inhibition of the EGF receptor is mediated through the coupling
between hGnRHR and Gi protein (54). The molecular mechanism for hGnRHR
to inhibit the EGFR action may be mediated by direct interaction
with intracellular mechanisms activated by EGF as well as by the
decrease in the number of EGF receptors present on these cells. For
example, in xenografts of OV-1063 cells, hGnRH agonists were able
to significantly decrease tumor growth as well as the levels in
membrane of EGFR and also its mRNA (55). Furthermore, chronic treatment with
hGnRH, in these cells, was able to decrease the levels of
insulin-like growth factor I receptor (IGF-1R) (44). A completely new mechanism of GnRH
action in endometrial cancer cells has been recently described by
Cho-Clark and colleagues (56)
demonstrating the participation of a hGnRH metabolite, the
GnRH(1–5), as an active component in the
transactivation of EGFR via an orphan GPCR, the GPR101, in Ishikawa
cells. Although cross-talk between hGnRHR and growth factor
receptors has been clearly demonstrated, it seems to vary in
different cellular contexts. Taken together these results show
different layers in the complexity of the hGnRH/hGnRHR system
regulation in cancer cells. Furthermore, a compound develop by
Schally's group, the Dox-14-O-hemiglutarate conjugated to [D-Lys6]
GnRH-I (AN-152, AEZS-108; Æterna Zentaris Inc., Quebec, QC, Canada)
is currently in phase III clinical trial on ovarian and endometrial
cancer due to its promising results in cancer treatment (57).
Breast cancer is the most common diagnosed cancer
and the main global cause of death in women. Approximately, from 75
to 80% of breast cancers are hormone-dependent expressing both
estrogen and progesterone receptors (58,59).
Approximately 15–20% of breast cancers overexpress the human
epidermal growth factor receptor-2 (HER2), and about half of these
tumors also express steroid hormone receptors. Unfortunately,
10–15% of breast cancers do not express estrogen or progesterone
receptors, nor HER2. These so-called triple-negative breast cancers
do not benefit from specific therapies that target these receptors
and, therefore, have the worst outcome (59). hGnRH stimulates gonadotropin
secretion from the hypothalamus and thereby controls gametogenesis
and steroidogenesis in the gonads (60). hGnRH-stimulated gonadotropin
secretion can be blocked with antagonists or with a sustained
stimulation with agonists, causing the so-called ‘medical
castration’ underlying the use of hGnRH analogs to treat
hormone-dependent neoplasms (59,60).
In addition to expressing the hGnRHR, the tumors also revealed the
presence of hGnRH, indicating the existence of an
autocrine/paracrine hGnRH/hGnRHR system that might regulate tumor
growth and invasiveness (61).
Approximately, 50–60% of human breast cancer expresses hGnRHR and
it has been highly speculated that activation or inhibition of
hGnRHR signaling may directly affect cell growth (62–66).
Morgan and colleagues (66)
demonstrated that hGnRH receptor was expressed in a wide range in
298 primary breast cancers, but most importantly, its expression
was significantly higher in patients with triple-negative
phenotype.
Initially, it was described that in tumor cells the
hGnRHR was exclusively coupled to Gi and consequently inhibit the
cAMP accumulation, this action presumably mediates the
anti-proliferative effect in these cells (61,67,68).
Some reports, however, indicated that for tumor cells the hGnRHR is
able to activate several G-proteins in a specific cell background
(69). The evidence so far
indicates that the anti-proliferative response induced by hGnRHR
activation results in apoptosis and G2/M arrest in the cell cycle.
This process is mediated via a coordinated dynamic pattern of MAPK,
cell cycle, apoptotic and cytoskeletal-related signaling (70).
As mentioned above, a broad variety of human
cancers, but not normal tissue, express the hGnRH/hGnRHR system,
thus, chronic administration of hGnRH agonists are widely and
successfully used for the treatment of hormone-dependent tumors.
However, study of the hGnRH system in non-hormone-dependent cancers
has recently been initiated. For instance, the cutaneous melanoma
is still the leading cause of skin cancer deaths in developed
countries (71). Moretti and
colleagues (72) demonstrated that
hGnRHR were expressed in melanoma cells, and the stimulation with a
hGnRH agonist had a significant inhibitory effect on tumor
progression and neoangiogenesis, by interfering with the activity
of growth factors. In multiple myeloma cells RPMI 8226, the use of
an hGnRH agonist induces apoptosis and inhibits cell growth by
increasing the expression of anti-oncogenes p21 and p53 (73). hGnRH as treatment in colon cancer
has been initiated, and preliminary reports suggest an
anti-angiogenic property of the hormone (74). As an innovative colon cancer
treatment, Schreier and colleagues (75) designed a derivate bioconjugate
between a chemotherapeutic agent and a hGnRH analog to target colon
cancer cells. Their results demonstrated that the treatment with
the bioconjugate changed the protein expression profile of multiple
intracellular processes. The use of hGnRH analogs in endometrial
cancer had variable responses, some patients respond to therapy but
the overall response was suboptimal (76). In some cases, such as in human
bladder cancer cells hGnRHR showed a tendency to form nanodomains;
however, the effect of any hGnRH analog has not been used in this
type of cancer (77).
The hGnRHR in its natural form exists as a misfolded
protein indicating that the cell intentionally synthesizes
conformational misrouted receptors. This characteristic made us
hypothesize that the decrease in protein membrane expression is the
result of a complex evolutionary reproductive process (78). This hGnRHR that is normally
functional become misrouted and then degraded by the cell quality
control, therefore ceasing its activity. Among the G-protein
coupled receptors, hGnRHR has several specific characteristics
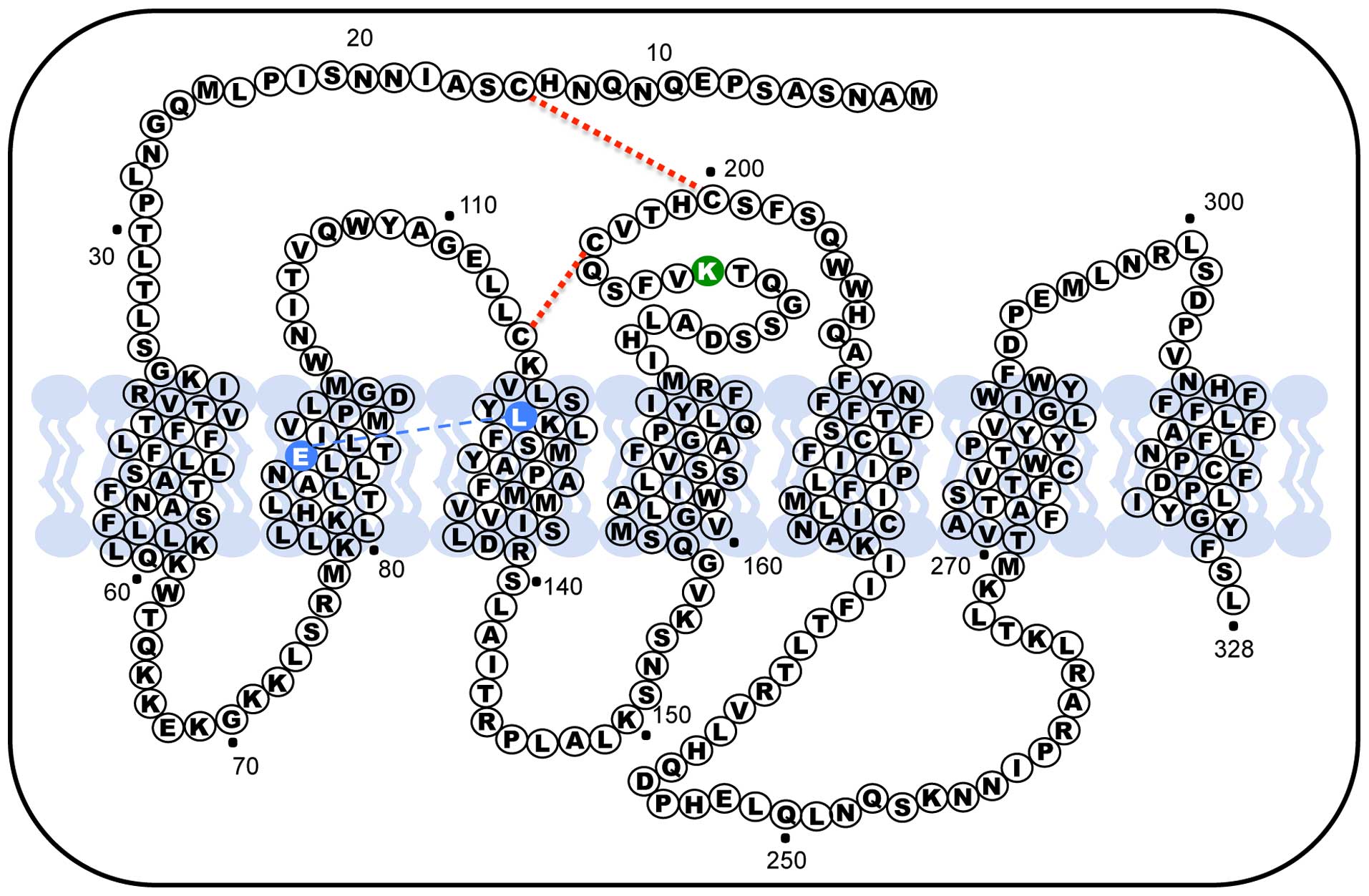
including the absence of a terminal carboxy-tail. Another
peculiarity of the hGnRHR is the presence of a Lys residue in
position 191 of the extracellular loop (EL) 2, in rodents GnRH
receptors this orthologous amino acid is absent, making the last
protein one residue smaller (327 amino acids). In rodent the lack
of this residue confers the GnRH receptor an increased plasma
membrane expression (79);
therefore, the presence of Lys191 in humans limits the number of
receptors exported from the endoplasmic reticulum to the membrane
by interfering, primarily, with formation of the Cys14-Cys200
disulphide bridge (Fig. 2)
(80). When the disulphide bridge
forms, the hGnRHR is recognized by the cell as correctly folded and
allows it to be exported to the plasma membrane. As a result, the
presence of the Lys191 interferes with the probability of bridge
formation and could explain the decrease in trafficking to the
plasma membrane. In fact, the deletion of Lys191 from the hGnRHR
increases the plasma membrane expression of the protein (81,82).
Another structural feature that modulate trafficking of the
receptor to the plasma membrane is the formation of a Glu90-Lys121
salt bridge that is essential for the transit through the quality
control system of the cell, in fact the Glu90Lys mutant, which led
to hypogonadotropic hypogonadism, was retained in the endoplasmic
reticulum and represents a loss of function receptor (83,84).
Further studies led to the demonstration that this particular
mutation was fully rescue by pharmacoperone drugs by the formation
of a surrogate bridge from residues Asp98 and Lys121 that can
substitute for the original salt bridge that is broken in Glu90Lys
mutant (84). Moreover, this human
receptor mutation was able to be rescued by four different chemical
classes of pharmacoperones that interact identically by creating
the same surrogate bridge (85).
In fact, pharmacoperones are able to rescue most of the hGnRH
receptor mutants, even though mutations appear through the whole
receptor sequence, further analysis indicated that these drugs
might stabilize the relation between TMD2 and TMD3 domains on the
hGnRHR allowing them to pass the quality control of the cell, and
thus reaching the membrane. Therefore, it is clear that in human
Lys191 is part of a complex motif that results in decreased
efficiency in expression (80,82,86).
This overview shows that the hGnRH/hGnRHR system
activates different signal transduction pathways in gonadotrope and
tumor cells, coupling to different G-proteins depending on the cell
context. Most interesting is the finding that this hormone/receptor
system is present not only in reproductive tissue but also in tumor
cells with various degrees of the expression. In some cases the
expression of hGnRHR is related to cancer progression; for example,
in ovarian carcinomas of early stages the presence of this receptor
is higher when compared with advanced stages of carcinoma. Also in
prostate cancer loss of hGnRHR expression with tumor progression
has been demonstrated (87,88).
Moreover, nanomolar concentrations of GnRH II antagonists induce
apoptotic cell death in human endometrial, ovarian, and breast
cancer in vitro and in vivo, via dose-dependent loss
of mitochondrial membrane potential and activation of caspase-3
(89). According to the
literature, in several tumor cell lines, the expression level of
GnRHR is significantly lower than in pituitary or the gonadotropes
(57). The demonstration that
hGnRHR exists as a misfolded protein has raised the speculation
that in tumor cells this compartmentalization and retention of the
receptor in the ER function as a protective mechanism to avoid cell
death induced by hGnRHR activation (88,90).
In prostate cancer cultures it was demonstrated that IN3 enhances
the GnRH agonist apoptotic effect by increasing the hGnRHR in the
plasma membrane (88). The use of
a careful pulsatile pharmacoperone therapy in a knock-in mouse
expressing the hGnRHR mutant E90K is able to restore the mutation
from ER retention to the plasma membrane. Also spermatogenetic
proteins associated with steroid transport and synthesis, and
androgen levels were restored with pharmacoperone administration
(91). The hGnRHR plasma membrane
increase by pharmacoperones, due to the trafficking of the receptor
from the ER, may represent an important research area to evaluate
the clinical use of IN3 in tumor cells. Given the clinical utility
of hGnRH, further studies of pharmacoperones are necessary to
characterize this compound as a step to a more effective and
perhaps new therapeutic strategy for cancer patients.
The present study was supported by grants
FIS/IMSS/PROT/559, FIS/IMSS/PROT/G11/936 (G.M-N) and
FIS/IMSS/PROT/G12/1147 (A.A-R).
|
1
|
Kakar SS and Jennes L: Expression of
gonadotropin-releasing hormone and gonadotropin-releasing hormone
receptor mRNAs in various non-reproductive human tissues. Cancer
Lett. 98:57–62. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu F, Usui I, Evans LG, Austin DA, Mellon
PL, Olefsky JM and Webster NJ: Involvement of both G(q/11) and G(s)
proteins in gonadotropin-releasing hormone receptor-mediated
signaling in L beta T2 cells. J Biol Chem. 277:32099–32108. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hamm HE: How activated receptors couple to
G proteins. Proc Natl Acad Sci USA. 98:4819–4821. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gilman AG: G proteins: Transducers of
receptor-generated signals. Annu Rev Biochem. 56:615–649. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ford CE, Skiba NP, Bae H, Daaka Y, Reuveny
E, Shekter LR, Rosal R, Weng G, Yang CS, Iyengar R, et al:
Molecular basis for interactions of G protein betagamma subunits
with effectors. Science. 280:1271–1274. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berridge MJ and Irvine RF: Inositol
trisphosphate, a novel second messenger in cellular signal
transduction. Nature. 312:315–321. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Limor R, Ayalon D, Capponi AM, Childs GV
and Naor Z: Cytosolic free calcium levels in cultured pituitary
cells separated by centrifugal elutriation: Effect of
gonadotropin-releasing hormone. Endocrinology. 120:497–503. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tse FW, Tse A, Hille B, Hille B, Horstmann
H and Almers W: Local Ca2+ release from internal stores
controls exocytosis in pituitary gonadotrophs. Neuron. 18:121–132.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Naor Z, Harris D and Shacham S: Mechanism
of GnRH receptor signaling: Combinatorial cross-talk of
Ca2+ and protein kinase C. Front Neuroendocrinol.
19:1–19. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim Y, Han JM, Park JB, Lee SD, Oh YS,
Chung C, Lee TG, Kim JH, Park SK, Yoo JS, et al: Phosphorylation
and activation of phospholipase D1 by protein kinase C in vivo:
Determination of multiple phosphorylation sites. Biochemistry.
38:10344–10351. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mitchell R, Robertson DN, Holland PJ,
Collins D, Lutz EM and Johnson MS: ADP-ribosylation
factor-dependent phospholipase D activation by the M3 muscarinic
receptor. J Biol Chem. 278:33818–33830. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kolch W, Heidecker G, Kochs G, Hummel R,
Vahidi H, Mischak H, Finkenzeller G, Marmé D and Rapp UR: Protein
kinase C alpha activates RAF-1 by direct phosphorylation. Nature.
364:249–252. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Levi NL, Hanoch T, Benard O, Rozenblat M,
Harris D, Reiss N, Naor Z and Seger R: Stimulation of Jun
N-terminal kinase (JNK) by gonadotropin-releasing hormone in
pituitary alpha T3-1 cell line is mediated by protein kinase C,
c-Src, and CDC42. Mol Endocrinol. 12:815–824. 1998.PubMed/NCBI
|
|
14
|
Mulvaney JM and Roberson MS: Divergent
signaling pathways requiring discrete calcium signals mediate
concurrent activation of two mitogen-activated protein kinases by
gonadotropin-releasing hormone. J Biol Chem. 275:14182–14189. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seger R and Krebs EG: The MAPK signaling
cascade. FASEB J. 9:726–735. 1995.PubMed/NCBI
|
|
16
|
Maurer RA, Kim KE, Schoderbek WE, Roberson
MS and Glenn DJ and Glenn DJ: Regulation of glycoprotein hormone
alpha-subunit gene expression. Recent Prog Horm Res. 54:455–484;
discussion 485. 1999.PubMed/NCBI
|
|
17
|
Ben-Menahem D, Shraga-Levine Z, Limor R
and Naor Z: Arachidonic acid and lipoxygenase products stimulate
gonadotropin alpha-subunit mRNA levels in pituitary alpha T3-1 cell
line: Role in gonadotropin releasing hormone action. Biochemistry.
33:12795–12799. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shraga-Levine Z, Ben-Menahem D and Naor Z:
Arachidonic acid and lipoxygenase products stimulate protein kinase
C beta mRNA levels in pituitary alpha T3-1 cell line: Role in
gonadotropin-releasing hormone action. Biochem J. 316:667–670.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bliss SP, Navratil AM, Xie J and Roberson
MS: GnRH signaling, the gonadotrope and endocrine control of
fertility. Front Neuroendocrinol. 31:322–340. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miller RS, Wolfe A, He L, Radovick S and
Wondisford FE: CREB binding protein (CBP) activation is required
for luteinizing hormone beta expression and normal fertility in
mice. Mol Cell Biol. 32:2349–2358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khadra A and Li YX: A model for the
pulsatile secretion of gonadotropin-releasing hormone from
synchronized hypothalamic neurons. Biophys J. 91:74–83. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haisenleder DJ, Dalkin AC, Ortolano GA,
Marshall JC and Shupnik MA: A pulsatile gonadotropin-releasing
hormone stimulus is required to increase transcription of the
gonadotropin subunit genes: Evidence for differential regulation of
transcription by pulse frequency in vivo. Endocrinology.
128:509–517. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qayum A, Gullick W, Clayton RC, Sikora K
and Waxman J and Waxman J: The effects of gonadotrophin releasing
hormone analogues in prostate cancer are mediated through specific
tumour receptors. Br J Cancer. 62:96–99. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Halmos G, Arencibia JM, Schally AV, Davis
R and Bostwick DG: High incidence of receptors for luteinizing
hormone-releasing hormone (LHRH) and LHRH receptor gene expression
in human prostate cancers. J Urol. 163:623–629. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fekete M, Redding TW, Comaru-Schally AM,
Pontes JE, Connelly RW, Srkalovic G and Schally AV: Receptors for
luteinizing hormone-releasing hormone, somatostatin, prolactin, and
epidermal growth factor in rat and human prostate cancers and in
benign prostate hyperplasia. Prostate. 14:191–208. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Limonta P, Dondi D, Moretti RM, Maggi R
and Motta M: Antiproliferative effects of luteinizing
hormone-releasing hormone agonists on the human prostatic cancer
cell line LNCaP. J Clin Endocrinol Metab. 75:207–212.
1992.PubMed/NCBI
|
|
27
|
Dondi D, Limonta P, Moretti RM, Marelli
MM, Garattini E and Motta M: Antiproliferative effects of
luteinizing hormone-releasing hormone (LHRH) agonists on human
androgen-independent prostate cancer cell line DU 145: Evidence for
an autocrine-inhibitory LHRH loop. Cancer Res. 54:4091–4095.
1994.PubMed/NCBI
|
|
28
|
Redding TW and Schally AV: Inhibition of
prostate tumor growth in two rat models by chronic administration
of D-Trp6 analogue of luteinizing hormone-releasing hormone. Proc
Natl Acad Sci USA. 78:6509–6512. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tolis G, Ackman D, Stellos A, Mehta A,
Labrie F, Fazekas AT, Comaru-Schally AM and Schally AV: Tumor
growth inhibition in patients with prostatic carcinoma treated with
luteinizing hormone-releasing hormone agonists. Proc Natl Acad Sci
USA. 79:1658–1662. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koppán M, Nagy A, Schally AV, Plonowski A,
Halmos G, Arencibia JM and Groot K: Targeted cytotoxic analog of
luteinizing hormone-releasing hormone AN-207 inhibits the growth of
PC-82 human prostate cancer in nude mice. Prostate. 38:151–158.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arencibia JM, Schally AV, Halmos G, Nagy
A, Kiaris H and Klaris H: In vitro targeting of a cytotoxic analog
of luteinizing hormone-releasing hormone AN-207 to ES-2 human
ovarian cancer cells as demonstrated by microsatellite analyses.
Anticancer Drugs. 12:71–78. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gnanapragasam VJ, Darby S, Khan MM, Lock
WG, Robson CN and Leung HY and Leung HY: Evidence that prostate
gonadotropin-releasing hormone receptors mediate an
anti-tumourigenic response to analogue therapy in hormone
refractory prostate cancer. J Pathol. 206:205–213. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gründker C, Huschmand Nia A and Emons G:
Gonadotropin-releasing hormone receptor-targeted gene therapy of
gynecologic cancers. Mol Cancer Ther. 4:225–231. 2005.PubMed/NCBI
|
|
34
|
Limonta P, Dondi D, Moretti RM, Fermo D,
Garattini E and Motta M: Expression of luteinizing
hormone-releasing hormone mRNA in the human prostatic cancer cell
line LNCaP. J Clin Endocrinol Metab. 76:797–800. 1993.PubMed/NCBI
|
|
35
|
Moretti RM, Marelli MM, Dondi D, Poletti
A, Martini L, Motta M and Limonta P: Luteinizing hormone-releasing
hormone agonists interfere with the stimulatory actions of
epidermal growth factor in human prostatic cancer cell lines, LNCaP
and DU 145. J Clin Endocrinol Metab. 81:3930–3937. 1996.PubMed/NCBI
|
|
36
|
Marelli MM, Moretti RM, Dondi D, Motta M
and Limonta P: Luteinizing hormone-releasing hormone agonists
interfere with the mitogenic activity of the insulin-like growth
factor system in androgen-independent prostate cancer cells.
Endocrinology. 140:329–334. 1999.PubMed/NCBI
|
|
37
|
Maudsley S, Davidson L, Pawson AJ, Chan R,
López de Maturana R and Millar RP: Gonadotropin-releasing hormone
(GnRH) antagonists promote proapoptotic signaling in peripheral
reproductive tumor cells by activating a Galphai-coupling state of
the type I GnRH receptor. Cancer Res. 64:7533–7544. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kraus S, Levy G, Hanoch T, Naor Z and
Seger R: Gonadotropin-releasing hormone induces apoptosis of
prostate cancer cells: Role of c-Jun NH2-terminal kinase, protein
kinase B, and extracellular signal-regulated kinase pathways.
Cancer Res. 64:5736–5744. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kraus S, Naor Z and Seger R:
Gonadotropin-releasing hormone in apoptosis of prostate cancer
cells. Cancer Lett. 234:109–123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Choi JH, Gilks CB, Auersperg N and Leung
PC: Immuno-localization of gonadotropin-releasing hormone (GnRH)-I,
GnRH-II, and type I GnRH receptor during follicular development in
the human ovary. J Clin Endocrinol Metab. 91:4562–4570. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Emons G, Ortmann O, Schulz KD and Schally
AV: Growth-inhibitory actions of analogues of Luteinizing hormone
releasing hormone on tumor cells. Trends Endocrinol Metab.
8:355–362. 1997. View Article : Google Scholar
|
|
42
|
Völker P, Gründker C, Schmidt O, Schulz KD
and Emons G: Expression of receptors for luteinizing
hormone-releasing hormone in human ovarian and endometrial cancers:
Frequency, autoregulation, and correlation with direct
antiproliferative activity of luteinizing hormone-releasing hormone
analogues. Am J Obstet Gynecol. 186:171–179. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Emons G, Ortmann O, Becker M, Irmer G,
Springer B, Laun R, Hölzel F, Schulz KD and Schally AV: High
affinity binding and direct antiproliferative effects of LHRH
analogues in human ovarian cancer cell lines. Cancer Res.
53:5439–5446. 1993.PubMed/NCBI
|
|
44
|
Yano T, Pinski J, Halmos G, Szepeshazi K,
Groot K and Schally AV: Inhibition of growth of OV-1063 human
epithelial ovarian cancer xenografts in nude mice by treatment with
luteinizing hormone-releasing hormone antagonist SB-75. Proc Natl
Acad Sci USA. 91:7090–7094. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
So WK, Cheng JC, Poon SL and Leung PC:
Gonadotropin-releasing hormone and ovarian cancer: A functional and
mechanistic overview. FEBS J. 275:5496–5511. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wilkinson SJ, Kucukmetin A, Cross P, Darby
S, Gnanapragasam VJ, Calvert AH, Robson CN and Edmondson RJ:
Expression of gonadotrophin releasing hormone receptor I is a
favorable prognostic factor in epithelial ovarian cancer. Hum
Pathol. 39:1197–1204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Imai A, Ohno T, Furui T, Takahashi K,
Matsuda T and Tamaya T: Gonadotropin-releasing hormone stimulates
phospholipase C but not protein phosphorylation/dephosphorylation
in plasma membrane from human epithelial ovarian cancer. Int J
Gynecol Cancer. 3:311–317. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shibata S, Sato H, Ota H, Karube A,
Takahashi O and Tanaka T: Involvement of annexin V in
antiproliferative effects of gonadotropin-releasing hormone
agonists on human endometrial cancer cell line. Gynecol Oncol.
66:217–221. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim KY, Choi KC, Auersperg N and Leung PC:
Mechanism of gonadotropin-releasing hormone (GnRH)-I and
-II-induced cell growth inhibition in ovarian cancer cells: Role of
the GnRH-I receptor and protein kinase C pathway. Endocr Relat
Cancer. 13:211–220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kimura A, Ohmichi M, Kurachi H, Ikegami H,
Hayakawa J, Tasaka K, Kanda Y, Nishio Y, Jikihara H, Matsuura N, et
al: Role of mitogen-activated protein kinase/extracellular
signal-regulated kinase cascade in gonadotropin-releasing
hormone-induced growth inhibition of a human ovarian cancer cell
line. Cancer Res. 59:5133–5142. 1999.PubMed/NCBI
|
|
51
|
Imai A, Takagi H, Horibe S, Fuseya T and
Tamaya T and Tamaya T: Coupling of gonadotropin-releasing hormone
receptor to Gi protein in human reproductive tract tumors. J Clin
Endocrinol Metab. 81:3249–3253. 1996.PubMed/NCBI
|
|
52
|
Imai A, Horibe S, Takagi A and Tamaya T:
Gi protein activation of gonadotropin-releasing hormone-mediated
protein dephosphorylation in human endometrial carcinoma. Am J
Obstet Gynecol. 176:371–376. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Emons G, Muller V, Ortmann O, Grossmann G,
Trautner U, Stuckrad B, Schulz K and Schally A: Luteinizing
hormone-releasing hormone agonist triptorelin antagonizes signal
transduction and mitogenic activity of epidermal growth factor in
human ovarian and endometrial cancer cell lines. Int J Oncol.
9:1129–1137. 1996.PubMed/NCBI
|
|
54
|
Gründker C, Völker P and Emons G:
Antiproliferative signaling of luteinizing hormone-releasing
hormone in human endometrial and ovarian cancer cells through G
protein alpha(I)-mediated activation of phosphotyrosine
phosphatase. Endocrinology. 142:2369–2380. 2001.PubMed/NCBI
|
|
55
|
Shirahige Y, Cook C, Pinski J, Halmos G,
Nair R and Schally A: Treatment with luteinizing-hormone-releasing
hormone antagonist sb-75 decreases levels of epidermal
growth-factor receptor and its messenger-RNA in ov-1063 human
epithelial ovarian-cancer xenografts in nude-mice. Int J Oncol.
5:1031–1035. 1994.PubMed/NCBI
|
|
56
|
Cho-Clark M, Larco DO, Semsarzadeh NN,
Vasta F, Mani SK and Wu TJ: GnRH-(1–5) transactivates EGFR in
Ishikawa human endometrial cells via an orphan G protein-coupled
receptor. Mol Endocrinol. 28:80–98. 2014. View Article : Google Scholar
|
|
57
|
Szabó I, Bősze S, Orbán E, Sipos É, Halmos
G, Kovács M and Mező G: Comparative in vitro biological evaluation
of daunorubicin containing GnRH-I and GnRH-II conjugates developed
for tumor targeting. J Pept Sci. 21:426–435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cheang MC, Voduc D, Bajdik C, Leung S,
McKinney S, Chia SK, Perou CM and Nielsen TO: Basal-like breast
cancer defined by five biomarkers has superior prognostic value
than triple-negative phenotype. Clin Cancer Res. 14:1368–1376.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gründker C, Föst C, Fister S, Nolte N,
Günthert AR and Emons G: Gonadotropin-releasing hormone type II
antagonist induces apoptosis in MCF-7 and triple-negative
MDA-MB-231 human breast cancer cells in vitro and in vivo. Breast
Cancer Res. 12:R492010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sedgley KR, Finch AR, Caunt CJ and McArdle
CA: Intracellular gonadotropin-releasing hormone receptors in
breast cancer and gonadotrope lineage cells. J Endocrinol.
191:625–636. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Limonta P, Montagnani Marelli M, Mai S,
Motta M, Martini L and Moretti RM: GnRH receptors in cancer: From
cell biology to novel targeted therapeutic strategies. Endocr Rev.
33:784–811. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fekete M, Wittliff JL and Schally AV:
Characteristics and distribution of receptors for
[D-TRP6]-luteinizing hormone-releasing hormone, somatostatin,
epidermal growth factor, and sex steroids in 500 biopsy samples of
human breast cancer. J Clin Lab Anal. 3:137–147. 1989. View Article : Google Scholar
|
|
63
|
Baumann KH, Kiesel L, Kaufmann M, Bastert
G and Runnebaum B: Characterization of binding sites for a
GnRH-agonist (buserelin) in human breast cancer biopsies and their
distribution in relation to tumor parameters. Breast Cancer Res
Treat. 25:37–46. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Moriya T, Suzuki T, Pilichowska M, Ariga
N, Kimura N, Ouchi N, Nagura H and Sasano H: Immunohistochemical
expression of gonadotropin releasing hormone receptor in human
breast carcinoma. Pathol Int. 51:333–337. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mangia A, Tommasi S, Reshkin SJ, Simone G,
Stea B, Schittulli F and Paradiso A: Gonadotropin releasing hormone
receptor expression in primary breast cancer: Comparison of
immunohistochemical, radioligand and Western blot analyses. Oncol
Rep. 9:1127–1132. 2002.PubMed/NCBI
|
|
66
|
Morgan K, Meyer C, Miller N, Sims AH,
Cagnan I, Faratian D, Harrison DJ, Millar RP and Langdon SP: GnRH
receptor activation competes at a low level with growth signaling
in stably transfected human breast cell lines. BMC Cancer.
11:4762011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Millar RP, Pawson AJ, Morgan K, Rissman EF
and Lu ZL: Diversity of actions of GnRHs mediated by ligand-induced
selective signaling. Front Neuroendocrinol. 29:17–35. 2008.
View Article : Google Scholar
|
|
68
|
Naor Z and Huhtaniemi I: Interactions of
the GnRH receptor with heterotrimeric G proteins. Front
Neuroendocrinol. 34:88–94. 2013. View Article : Google Scholar
|
|
69
|
Sviridonov L, Dobkin-Bekman M, Shterntal
B, Przedecki F, Formishell L, Kravchook S, Rahamim-Ben Navi L,
Bar-Lev TH, Kazanietz MG, Yao Z, et al: Differential signaling of
the GnRH receptor in pituitary gonadotrope cell lines and prostate
cancer cell lines. Mol Cell Endocrinol. 369:107–118. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Aguila r-Rojas A, Huer ta-Reyes M,
Maya-Núñez G, Arechavaleta-Velásco F, Conn PM, Ulloa-Aguirre A and
Valdés J: Gonadotropin-releasing hormone receptor activates GTPase
RhoA and inhibits cell invasion in the breast cancer cell line
MDA-MB-231. BMC Cancer. 12:5502012. View Article : Google Scholar
|
|
71
|
Goodson AG and Grossman D: Strategies for
early melanoma detection: Approaches to the patient with nevi. J Am
Acad Dermatol. 60:719–735; quiz 736–718. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Moretti RM, Mai S, Montagnani Marelli M,
Bani MR, Ghilardi C, Giavazzi R, Taylor DM, Martini PG and Limonta
P: Dual targeting of tumor and endothelial cells by
gonadotropin-releasing hormone agonists to reduce melanoma
angiogenesis. Endocrinology. 151:4643–4653. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wen J, Feng Y, Bjorklund CC, Wang M,
Orlowski RZ, Shi ZZ, Liao B, O'Hare J, Zu Y, Schally AV, et al:
Luteinizing Hormone-Releasing Hormone (LHRH)-I antagonist
cetrorelix inhibits myeloma cell growth in vitro and in vivo. Mol
Cancer Ther. 10:148–158. 2011. View Article : Google Scholar
|
|
74
|
Ghiringhelli F, Isambert N and Ladoire S:
Degarelix as a new antiangiogenic agent for metastatic colon
cancer? World J Gastroenterol. 19:769–772. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Schreier VN, Pethő L, Orbán E, Marquardt
A, Petre BA, Mező G and Manea M: Protein expression profile of
HT-29 human colon cancer cells after treatment with a cytotoxic
daunorubicin-GnRH-III derivative bioconjugate. PLoS One.
9:e940412014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Carlson MJ, Thiel KW and Leslie KK: Past,
present, and future of hormonal therapy in recurrent endometrial
cancer. Int J Womens Health. 6:429–435. 2014.PubMed/NCBI
|
|
77
|
Zhang J, Chtcheglova LA, Zhu R,
Hinterdorfer P, Zhang B and Tang J: Nanoscale organization of human
GnRH-R on human bladder cancer cells. Anal Chem. 86:2458–2464.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Conn PM, Janovick JA, Brothers SP and
Knollman PE: ‘Effective inefficiency’: Cellular control of protein
trafficking as a mechanism of post-translational regulation. J
Endocrinol. 190:13–16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Maya-Núñez G, Janovick JA and Conn PM:
Combined modification of intracellular and extracellular loci on
human gonadotropin-releasing hormone receptor provides a mechanism
for enhanced expression. Endocrine. 13:401–407. 2000. View Article : Google Scholar
|
|
80
|
Conn PM and Janovick JA: Drug development
and the cellular quality control system. Trends Pharmacol Sci.
30:228–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Janovick JA, Patny A, Mosley R, Goulet MT,
Altman MD, Rush TS III, Cornea A and Conn PM: Molecular mechanism
of action of pharmacoperone rescue of misrouted GPCR mutants: The
GnRH receptor. Mol Endocrinol. 23:157–168. 2009. View Article : Google Scholar :
|
|
82
|
Maya-Núñez G, Ulloa-Aguirre A, Janovick JA
and Conn PM: Pharmacological chaperones correct misfolded GPCRs and
rescue function: Protein trafficking as a therapeutic target.
Subcell Biochem. 63:263–289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Maya-Núñez G, Janovick JA, Ulloa-Aguirre
A, Söderlund D, Conn PM and Méndez JP: Molecular basis of
hypogonadotropic hypogonadism: Restoration of mutant (E(90)K) GnRH
receptor function by a deletion at a distant site. J Clin
Endocrinol Metab. 87:2144–2149. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Janovick JA, Maya-Nunez G and Conn PM:
Rescue of hypogonadotropic hypogonadism-causing and manufactured
GnRH receptor mutants by a specific protein-folding template:
Misrouted proteins as a novel disease etiology and therapeutic
target. J Clin Endocrinol Metab. 87:3255–3262. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Conn PM and Ulloa-Aguirre A:
Pharmacological chaperones for misfolded gonadotropin-releasing
hormone receptors. Adv Pharmacol. 62:109–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Janovick JA, Knollman PE, Brothers SP,
Ayala-Yáñez R, Aziz AS and Conn PM: Regulation of G protein-coupled
receptor trafficking by inefficient plasma membrane expression:
Molecular basis of an evolved strategy. J Biol Chem. 281:8417–8425.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Cheung LW and Wong AS:
Gonadotropin-releasing hormone: GnRH receptor signaling in
extrapituitary tissues. FEBS J. 275:5479–5495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Sánchez CA, Mercado AJ, Contreras HR,
Cabezas JC, Huidobro CC and Castellón EA: Pharmacoperone IN3
enhances the apoptotic effect of leuprolide in prostate cancer
cells by increasing the gonadotropin-releasing hormone receptor in
the cell membrane. Anticancer Drugs. 23:959–969. 2012.PubMed/NCBI
|
|
89
|
Fister S, Günthert AR, Aicher B, Paulini
KW, Emons G and Gründker C: GnRH-II antagonists induce apoptosis in
human endometrial, ovarian, and breast cancer cells via activation
of stress-induced MAPKs p38 and JNK and proapoptotic protein Bax.
Cancer Res. 69:6473–6481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Cheng CK and Leung PC: Molecular biology
of gonadotropin-releasing hormone (GnRH)-I, GnRH-II, and their
receptors in humans. Endocr Rev. 26:283–306. 2005. View Article : Google Scholar
|
|
91
|
Janovick JA, Stewart MD, Jacob D, Martin
LD, Deng JM, Stewart CA, Wang Y, Cornea A, Chavali L, Lopez S, et
al: Restoration of testis function in hypogonadotropic hypogonadal
mice harboring a misfolded GnRHR mutant by pharmacoperone drug
therapy. Proc Natl Acad Sci USA. 110:21030–21035. 2013. View Article : Google Scholar : PubMed/NCBI
|