Introduction
Ovarian cancer is the deadliest malignancy of the
female reproductive tract (1). The
5-year survival rate is >90% when the disease is limited to the
ovary; however, it decreases to only 30% when the disease has
extended (2). Lymphatic metastasis
is one of the prognostic parameters for ovarian cancer and may
account for a majority of deaths (3–5).
Patients with negative lymph nodes (LNs) survived significantly
longer than those who had positive lymph nodes (6). Despite advances in surgical
management and chemotherapy, lymphatic metastasis remains an
obstacle in cancer therapy. Accordingly, control of lymph node
metastasis is an important strategy for the treatment of ovarian
cancer.
The lymph-angiogenic growth factor, vascular
endothelial growth factor-D (VEGF-D) is involved in the growth of
lymphatic vessels and promotion of lymph node metastasis (7,8).
VEGF-D stimulates angiogenesis and lymphangiogenesis as well as
accelerates tumor growth. It is essential for the development of
lymphatic vessels within tumors, and therefore promotes metastatic
spread of tumor cells via the lymphatic route (9,10).
In this study, we established a feasible high lymphogenous
metastatic model using SKOV3 cells transfected with pcDNA3.1-VEGF-D
plasmid and then introduced it to experimental cancer therapy.
Vesicular stomatitis virus (VSV), a
negative-stranded RNA rhabdovirus with a single genome that encodes
five proteins (nucleoprotein N, phosphoprotein P, matrixprotein M,
glycoprotein G, and polymerase L), can preferentially replicate in
many types of tumor cells. Recent studies have demonstrated that
matrix protein (MP) of VSV is capable of inducing apoptosis in the
absence of other viral components in vitro mainly through
inhibiting host gene expression at the level of transcription
(11–14) and nucleocytoplasmic transport of
host RNA and proteins (15,16).
In addition, MP could cause inactivation of Akt, resulting in
dominant inhibition of Akt/protein kinase B signaling (17). Therefore, MP possesses potent
antitumor and anti-angiogenesis properties in various tumors
(18–20). It has also been observed abolishing
ascites formation via inhibiting VEGF production (21). However, it is still unclear whether
MP could effectively inhibit lymphangiogenesis and node
metastasis.
In this study, we tested the antitumor and
antimetastasis efficacy of a recombinant plasmid DNA carrying
MP-cDNA in the lymphogenous metastatic model. Cationic liposomes
were used as the gene delivery system. Our data demonstrated that
VEGF-D-enhanced lymphatic metastasis and lymphangiogenesis were
reversed by MP. The expression of VEGF-D and matrix
metalloproteinase-2 (MMP-2) were inhibited when treated with
pVAX-MP liposome complex. Suppression of tumor growth and increase
of apoptosis were also detected. MP may become a promising
therapeutic strategy against lymph node metastasis of ovarian
cancer without systemic toxic effects.
Materials and methods
Cell lines
Human epithelial serous cystadenocarcinoma cell line
SKOV3 cells were obtained from the American Type Culture Collection
(ATCC; Rockville, MD, USA), and cultured in RPMI-1640 medium
(Gibco) supplemented with 10% fetal calf serum, 100 U/ml
penicillin, and 100 mg/ml streptomycin. Cells were maintained in a
37°C humidified incubator with 5% CO2 atmosphere.
Establishment of VEGF-D overexpressing
clonal line
VEGF-D cDNA was cloned from mouse ovarian cDNA
library (Stratagene, La Jolla, CA, USA). The pcDNA3.1/VEGF-D was a
gift from Dr Xinjiang Xie. The SKOV3 cells were transfected with
either the recombinant pcDNA3.1/VEGF-D or pcDNA3.1 (empty vector)
as control. The transfected cells were cultured in G418 selection
medium (400 μg/ml) for screening. G418-resistant colonies which
carried VEGF-D plasmid stably were re-useable for additional
studies. Wild-type SKOV3 cells, SKOV3 cells transfected with
pcDNA3.1 plasmid and SKOV3 cells transfected with recombinant
pcDNA3.1/VEGF-D plasmid were named as WT-SK, EV-SK, and VEGFD-SK
cells, respectively.
RT-PCR
The total RNA was extracted by TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer's
instructions. Expression of MP in vitro was confirmed with
RT-PCR. The upstream and downstream primers for MP (GenBank
accession no. EU917223.1) were: 5′-CGAAC GACCTACACCGAAC-3′, and
5′-CCCTTCAGACCGAGA ATCTT-3′, respectively. Expression of VEGF-D
in vitro and in vivo were detected by RT-PCR. The
primer sequences of VEGF-D were as follow: 5′-GCAAGCTTATGTATGGAGAA
TGGGGAATG-3′, and 5′-CGTCTAGATCAAGGGTTCTC CTGGCTG-3′ (amplifies
nucleotides 1,077 bp of mouse VEGF-D, Genbank accession no.
GI6753873) (22). GAPDH was used
as an internal control.
Preparations of plasmid liposome
complexes
pVAX plasmid (Invitrogen, San Diego, CA, USA)
encoded MP was constructed as previously described (23). pVAX without MP was used as control.
The large-scale plasmid DNA was prepared using Endofree Plasmid
Giga kit (Qiagen, Chatsworth, CA, USA). Cationic liposomes were
gifts from Dr Chen and Professor Chen (State Key Laboratory of
Biotherapy) (24). Plasmids and
cationic liposomes were diluted in RPMI-1640 medium without serum
or D5W (dextrose 5% in water), and left at room temperature for 30
min. The pVAX liposome complexes were named pVAX:lip. The pVAX-MP
liposome complexes were named pVAX-MP:lip.
Cell transfection and flow analysis
VEGFD-SK cells (2×105/well) were seeded
in 6-well dishes and incubated to 80% confluence. pVAX:lip
(containing 2 μg/ml pVA plasmid) or pVAX-MP:lip (containing 2 μg/ml
pVAX-MP plasmid) was prepared in 2 ml RPMI-1640 medium without
serum. VEGFD-SK cells were incubated with the complexes for 6 h.
Then, VEGFD-SK cells were supplemented with medium containing serum
for further 48 h. Untreated VEGFD-SK cells were needed as control.
Quantitative evaluation of apoptotic VEGFD-SK cells was performed
by flow cytometric analysis after Annexin V binding and propidium
iodide staining (Invitrogen).
Transwell assay
Invasion inhibition was quantified with a Transwell
invasions assay. The cells were harvested, seeded and transfected
with plasmid complex for 48 h according to the above method.
Matrigel invasion chambers (Millipore, 8 μm) were hydrated with
serum-free media. The ovarian cancer cells were stained and counted
by trypan blue. The cells (4×104) were resuspended with
serum-free medium in the top of the chamber, and 72 h later fixed
by with 4% neutral formalin. The chambers were stained with 0.1%
crystal violet. The cells at the top of the Matrigel membrane were
removed through washing in dH2O. The number of the
invading cells on the lower surface was assessed by an inverted
microscope (x100).
Wound healing assay
Monolayer wound healing assay was used to assess the
role of pVAX-MP:lip in cell migration. The cells were transfected
in 24-well culture plates for 48 h. Then, the cells were detached,
resuspended and counted. The equal number of cells were cultured in
each well of a 6-well culture dish. Twenty-four hours later, the
monolayer was confluent. Parallel scratch wounds with similar
widths were created on the monolayer with 100 μm micropipette tip.
Migration of cells into the wound was observed using phase contrast
microscopy (x100). Cells that migrated into the wounded area or
cells with extended protrusions from the border of the wound were
photographed every 6 h. The status of wound closure was evaluated
by inverted microscope.
Tumor models
Athymic nude mice (BALB/c, 6–8-week-old,
non-fertile, 18–20 g) were purchased from Sichuan University Animal
Center, and were maintained in pathogen-free conditions with
sterile chow. The nude mice were randomly assigned to three groups
(six mice/group). WT-SK, EV-SK, VEGFD-SK cells were injected
subcutaneously with 2×106 cells into the left hind paws
of mice respectively (25). The
tumor volume was determined by the following formula: tumor volume
(mm3) = 0.52 × length (mm) × width (mm) × height (mm).
All the animal experiments were approved by the Institute's Animal
Care and Use Committee.
Therapy for VEGF-D-enhanced
metastasis
Seven days following VEGFD-SK cells inoculation, the
tumors in the left hind paws of mice were visible. Systemic therapy
was initiated. The tumor-bearing mice were injected i.v. with the
corresponding reagent at 5-day intervals for a total of five times
(six mice/group): i) pVAX:lip group: mice were treated with 50 μg
pVAX/150 μg liposome complexes (volume = 200 μl); ii) pVAX-MP:lip
group: mice received 50 μg pVAX-MP/150 μg liposome complexes.
Evan's blue visualization of lymph
vessels
When tumors in foot grew to ~1 cm in diameter,
Evan's blue (5 mg/ml) was infused subcutaneously into the tumor
tissue areas until lymph vessel (LV) draining to the popliteal
lymph nodes were marked, and lymphatic network images were
obtained.
Metastasis rates
The volume of local tumors, number and the location
of metastasis in the lymph nodes were assessed. Specimens were
fixed in 10% formalin for examination of hematoxylin and eosin
(H&E). Metastasis rates in the three barrier LNs, including
popliteal space, parailiac, and renal hilum LNs in the drainage
routes from footpad tumors were assessed, and metastasis rate was
expressed as a ratio of metastatic lymph nodes counts out of the
total number of lymph nodes in each barrier.
Immunohistochemistry
Deparaffinized sections were immersed in 0.01 M
citrate buffer and heated in an autoclave. Endogenous peroxidase
activity was quenched in 3% hydrogen peroxide. Non-specific binding
sites were blocked with homeotypic non-immunoglobulin of the
secondary antibody at 37°C. VEGF-D antibody (Boster Biomart, CN,
USA) was used at a dilution of 1:100. MMP-2 antibody (Santa Cruz
Biotechnology, CA, USA) was used as primary antibody at a dilution
of 1:100. Sections were incubated with the primary antibody at 4°C
overnight, biotinylated secondary antibody at 37°C, and
streptavidin-biotin-peroxidase complex (SABC) sequentially. The
immunoreactions were visualized with diaminobenzidine (DAB)
solution and the crimson yellow precipitates were identified as
positive staining. Counterstaining was performed with
hematoxylin.
Estimation of VEGF-D and MMP-2
staining
The levels of VEGF-D and MMP-2 staining were
classified into four grades: ± (<25% of cells), meaning absent
or very weak staining; + (25–50% of cells), weak staining; ++
(50–75% of cells), moderate staining; and +++ (>75% of cells),
strong staining. Faint or equivocal immunoreactions were scored as
negative.
Computer-assisted morphometric
analysis
Human lymphatic vessel endothelial hyaluronan
receptor-1 (LYVE-1) antibody (Santa Cruz) was performed to identify
lymphatic microvessels at local tumor tissues. Lymphatic
microvessel counting was performed at high-power field (HPF)
(x400), and measured using computer assisted morphometric analysis.
Five high-power fields with abundant lymphatic sprout and highest
lymphatic vessel density (LVD) were identified and captured by Spot
digital camera. Parameters were evaluated using IP-Lab computer
aided image analysis software.
Apoptosis analysis
Qualitative apoptosis was determined by
Hoechst-33258 compounds (Sigma) according to the manufacturer's
instructions. Condensed chromatin or fragmented nuclei emitting
intense blue fluorescence were classified as apoptotic cells.
Toxicology analysis
The relevant indexes such as weight loss, skin,
behavior and feeding were evaluated. The weight of mice was
measured every four days. When the mice were sacrificed, tissues
and organs (including lung, brain, liver, kidney, heart, pancreas
and spleen) were fixed. The sections were evaluated with
H&E.
Statistical analysis
Statistical calculations were carried out using
StatView statistical software version 8.2 (SAS Institute, Cary,
N.C., USA). The χ2 test was used to analyze the
lymphatic metastasis rates. Differences in tumor volumes, LVD and
apoptosis index were assessed with analysis of variance (ANOVA).
All results were expressed as means ± standard deviation (SD).
P<0.05 was considered statistically significant.
Results
Expression of MP in vitro
RT-PCR analysis was employed to verify whether
transfection of pVAX-MP:lip could result in an evidently improved
expression level of MP in ovarian cancer cells. High expression of
MP was detected in cells transfected with pVAX-MP:lip. GAPDH served
as the internal positive control (Fig.
1A).
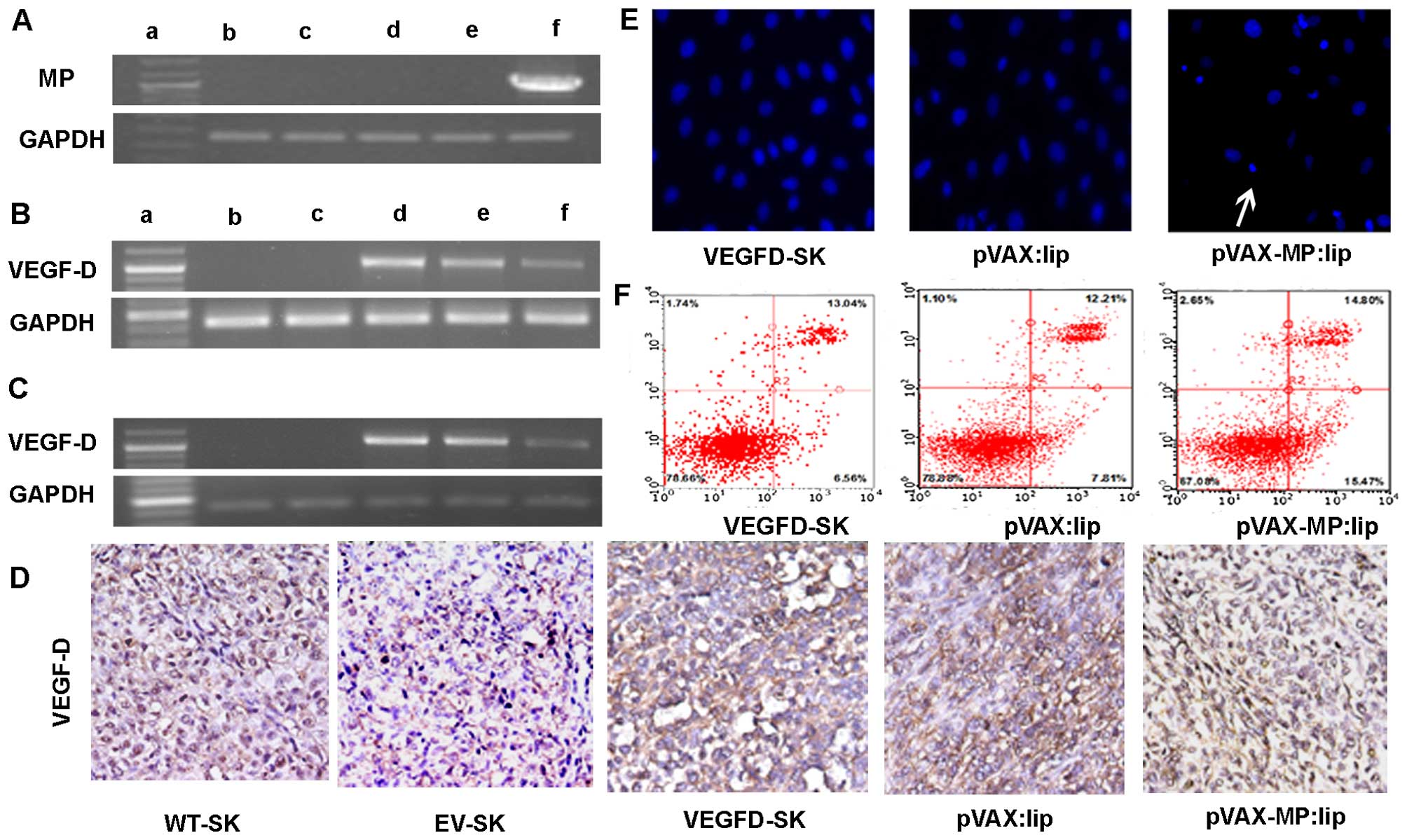 | Figure 1RT-PCR and apoptotic effect on
VEGFD-SK cells. (A) RT-PCR analysis of expression of MP in
vitro. High expression of MP in VEGFD-SK cells transfected with
pVAX-MP:lip was detected (1,036 bp). GAPDH was used for the
internal standard. The displaying panel of electrophoretic image
presents the following: DNA ladder marker (a), WT-SK (b), EV-SK
(c), VEGFD-SK (d), pVAX:lip (e), and pVAX-MP:lip (f), respectively.
(B) Overexpression of VEGF-D using RT-PCR could be detected in
VEGFD-SK cells, but not in WT-SK and EV-SK cells (1,077 bp).
However, the VEGF-D expression was suppressed when VEGFD-SK cells
suffered from pVAX-MP:lip. The overexpression of VEGF-D did not
decreased obviously when VEGFD-SK cells were treated with pVA. (C)
VEGF-D expression in vivo was analysis by RT-PCR. (D) The
expression of VEGF-D in vivo was determined with
immunohistochemistry using VEGF-D antibody: VEGFD-SK tumors were
detected with much stronger staining. Whereas, the VEGFD-SK tumors
in pVAX-MP:lip group present nearly negative staining. Tumors in
pVAX:lip group also exhibited strong staining. Very weak VEGF-D
protein was observed in WT-SK and EV-SK tumors. (E) Hoechst-33258
stained fluorescence microscopy (x200) in vitro: apoptosis
cells were observed apparently when the VEGFD-SK cells were treated
with pVAX-MP:lip (arrow). (F) A quantitative comparison of
apoptotic cells was carried out by flow analysis stained with
Annexin V-FITC and propidium iodide: pVAX-MP significantly
increased VEGFD-SK cells apoptosis compared with the controls. |
Expression of VEGF-D in vitro and in
vivo
VEGF-D expression in vitro was evaluated
using RT-PCR (Fig. 1B).
Overexpression of VEGF-D mRNA was recognized in VEGFD-SK cells, but
not in WT-SK and EV-SK cells. However, the overexpression of VEGF-D
was inhibited in pVAX-MP:lip-treated VEGFD-SK cells. This
expression of VEGF-D did not decrease obviously when VEGFD-SK cells
were treated with pVAX:lip. VEGF-D expression in vivo was
detected by RT-PCR and immunohistochemistry (Fig. 1C and D). As expected,
overexpression of VEGF-D mRNA was confirmed in VEGFD-SK group. The
expression of VEGF-D was suppressed when mice were treated with
pVAX-MP:lip. However, the expression of VEGF-D was still obvious in
the pVAX:lip group. VEGF-D protein in vivo was also
evaluated with immunohistochemistry. Our data showed only very weak
staining (±) for VEGF-D protein in WT-SK and EV-SK tumor tissues
(Fig. 1D); however, tumors in
VEGFD-SK group showed much stronger staining (+++) compared with
tumors in the WT-SK or EV-ST groups, which indicated the
overexpression of VEGF-D in VEGFD-SK tumors. Whereas, nearly
negative staining was observed in tumors of pVAX-MP:lip group.
Local tumors also exhibited VEGF-D staining obviously in pVAX:lip
group.
Apoptosis of VEGFD-SK cells in vitro
pVAX-MP:lip-treated VEGFD-SK cells resulted in
morphological changes characterized as apoptosis using
Hoechst-33258 staining (Fig. 1E):
a brightly fluorescent condensed nuclei and apoptotic bodies.
However, these changes rarely occurred in pVAX:lip-treated or
untreated VEGFD-SK cells. A quantitative comparison of apoptotic
VEGFD-SK cells in vitro was carried out to evaluate the
action of pVAX-MP using flow-cytometric analysis (Fig. 1F). Treatment with pVAX-MP
significantly increased the populations of Annexin V-positive cells
(apoptotic cells) in the right quadrants of flow cytometry graphs
compared with empty vector and untreated group.
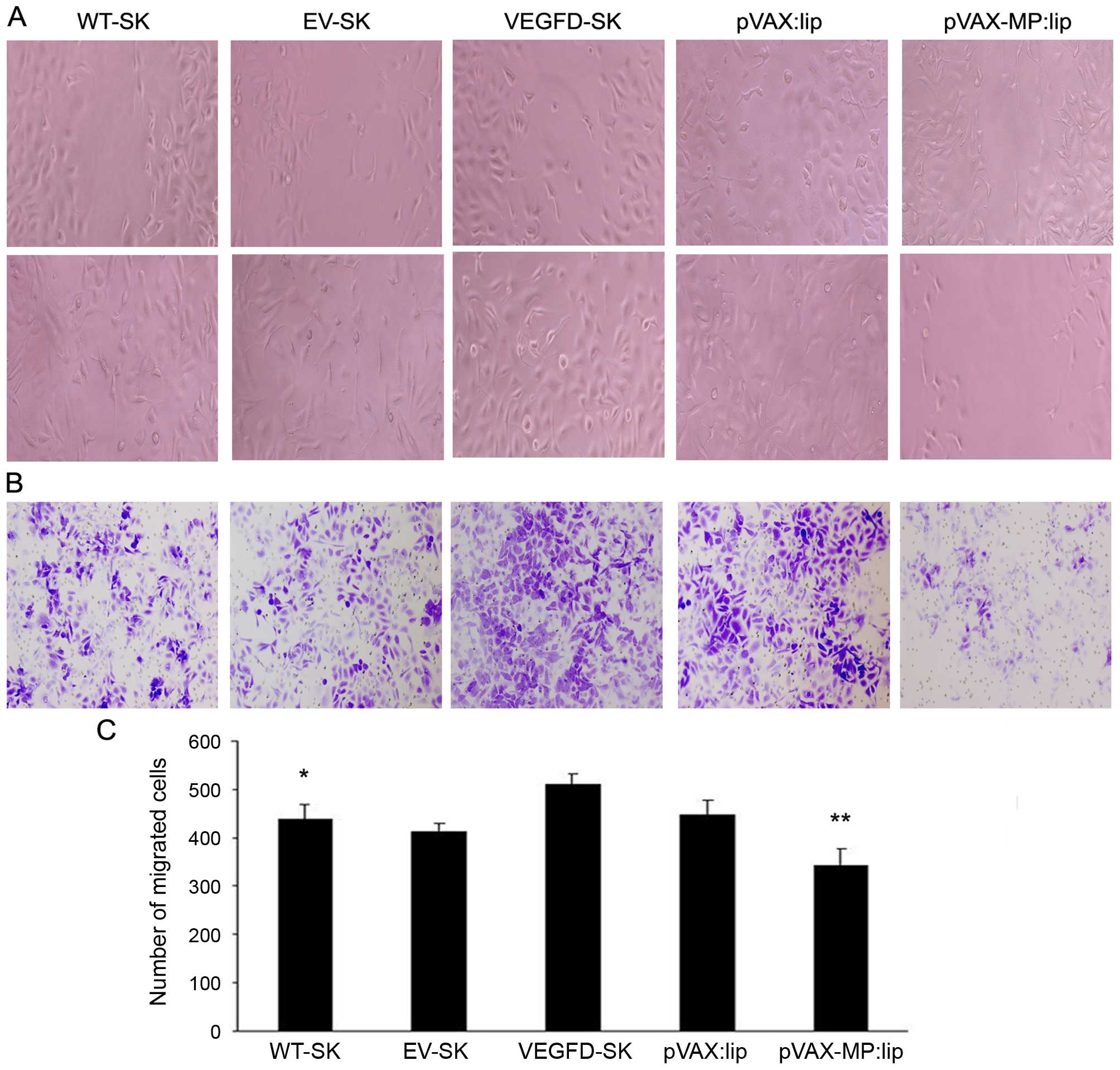
Inhibition of cell invasion and migration
induced by pVAX-MP:lip in vitro
Wound healing assay was used to analyze the
migration of the cells. The cells in the VEGFD-SK group were nearly
closed, demonstrating increased migration in VEGFD-SK group.
However, the wound widths in the pVAX-MP:lip group was wider than
that in VEGFD-SK or pVAX:lip group, indicationg the significant
effect of MP on cell migration (Fig.
2A). VEGFD-SK cells had much high migration ability, but
pVAX-MP:lip group had the lowest migration tendency in wound
healing assay (Fig. 2C) (P<0.05
WT-SK group versus VEGFD-SK group; P<0.05 pVAX-lip group versus
pVAX:lip group).
The monolayer Transwell assays were performed to
explore the role of MP in VEGFD-SK cell invasion. Consistent with
the results of the wound healing assay, the number of invading
cells in the VEGFD-SK group was increased compared with WT-SK or
EV-SK group. While the number of invading cells in the pVAX-MP:lip
group was clearly lower than that in the pVAX:lip group (Fig. 2B).
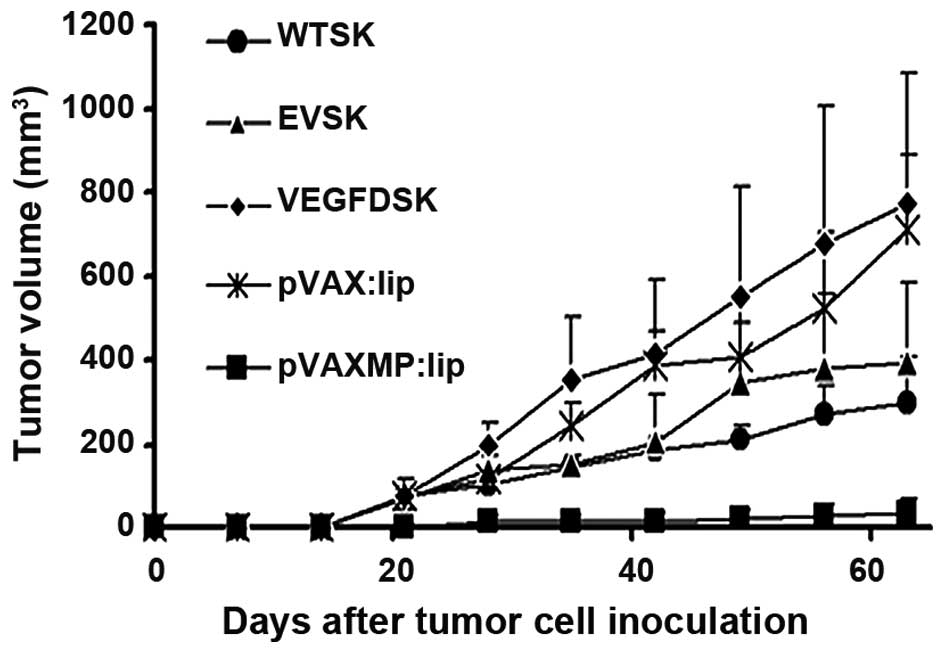
Tumor growth
Tumor volume was measured consecutively. As shown in
Fig. 3, VEGFD-SK group has a
faster growth rate of tumor compared with WT-SK, EV-SK groups. The
tumor in VEGFD-SK group exhibited fast tumor formation and growth,
similar to the tumor progression in pVAX:lip group. However,
tumorigenesis and growth in pVAX-MP:lip group was extremely
suppressed after treatment with pVAX-MP:lip (P<0.05 versus any
other group).
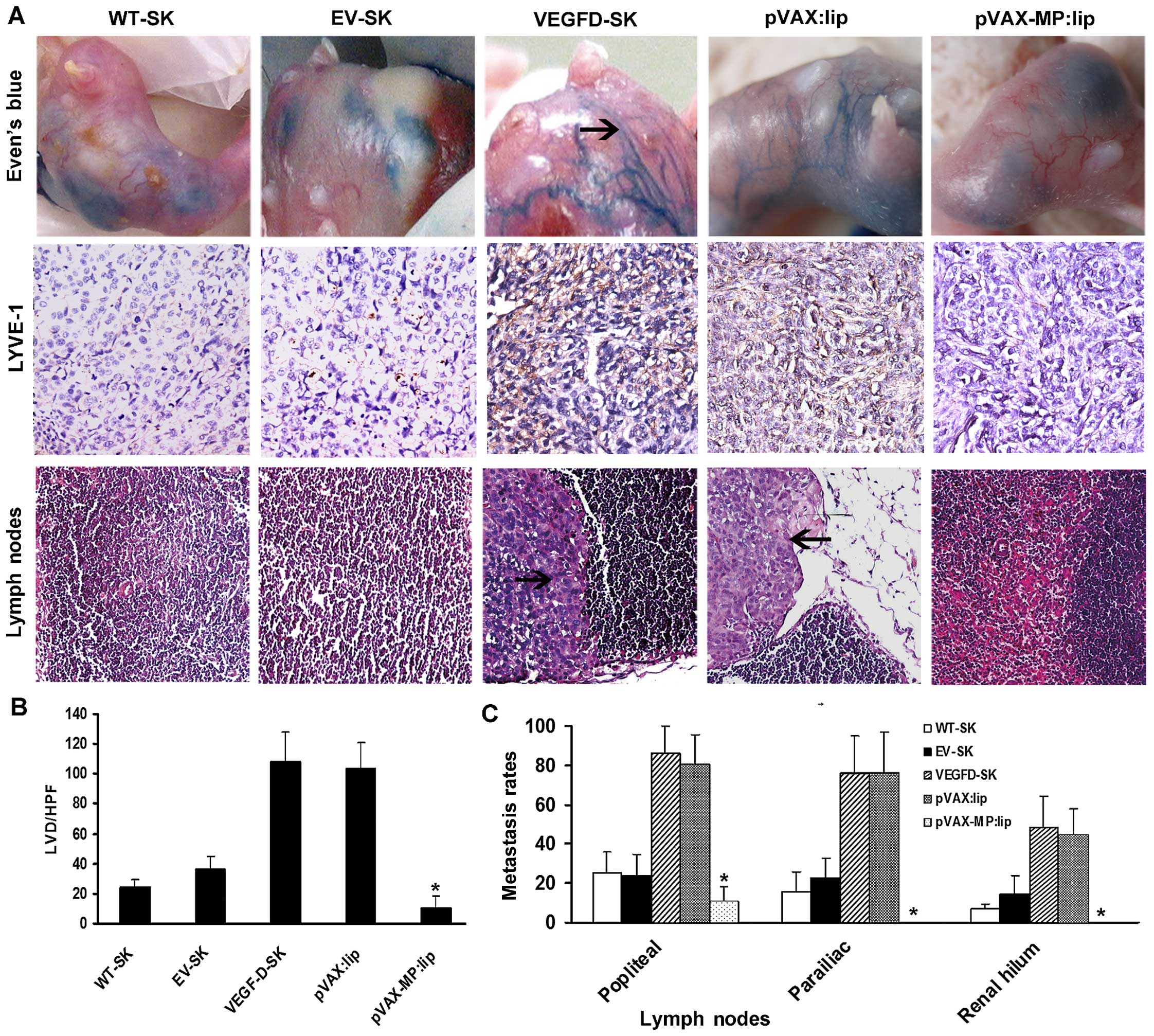
Evan's blue in vivo visualization and
microscopic lymphatic vessel density
Lymphangiogenesis were evaluated with lymphangiogram
stained by Evan's blue (Fig. 4A,
top panel) as well as LVD stained by LYVE-1 (Fig. 4A, middle panel). The staining
revealed few LVs draining Evan's blue detected in the WT-SK and
EV-SK groups. Twisted peritumoral blue LVs with dilated sprout
clumps emerged around tumors in VEGFD-SK and pVAX:lip groups
(arrow, blue lymphatic vessels), but no visible LVs were seen in
pVAX-MP:lip group. Anti-LYVE-1 immunostaining showed that there
were some LVs in WT-SK and EV-SK groups, especially sparse LVs in
pVAX-MP:lip group. However, rich nascent lymphatic microvessels
with dilated lumina were present in VEGFD-SK and pVAX:lip group.
The tumor tissues in VEGFD-SK (107.6±20.23) and pVAX:lip
(103.4±17.86) groups displayed overt enhancement of LVD compared
with the LVD of WT-SK (23.9±7.75) and EV-SK (36.7±7.87) groups,
whereas pVAX-MP:lip group showed a tendency of reduction of LVD
(10.1±7.77). Data of the microvessels with five high-power fields
(HPF) are represented as the mean ± SD (Fig. 4B) (*P<0.05 versus any
other group).
pVAX-MP:lip reversed high metastasis
induced by VEGF-D
Histopathological assay showed that clumpy deposits
of metastatic growth had occupied almost entire lymph nodes in
VEGFD-SK and pVAX:lip groups (arrow, tumor cells in lymph node),
but very few or no metastasis was established in pVAX-MP:lip group
(Fig. 4A, bottom panel). The
metastasis comparison for tumor-draining lymph nodes showed that
VEGFD-SK and pVAX:lip groups had a much higher metastasis tendency
than other groups. The enhanced metastasis of multilevel lymph
nodes induced by VEGF-D were evidently reversed after the
pVAX-MP:lip administration, with zero metastasis in renal hilum LNs
of pVAX-MP:lip group. Rate of metastasis comparisons were expressed
as the number of metastatic lymph node counts out of the total
lymph nodes of each barrier among the five groups, according to the
three-step barrier LNs (Fig. 4C),
namely, barrier one popliteal LNs (WT-SK, 25.2±10.7%; EV-SK,
23.7±10.9%; VEGFD-SK, 86.1±13.7%; pVAX:lip, 80.28±15.29%;
pVAX-MP:lip, 7.5±10.7%); barrier two parailiac LNs (WT-SK,
15.6±10.1%; EV-SK, 22.5±10.3%; VEGFD-SK, 76.4±18.3%; pVAX:lip,
75.83±21.3%; pVAX-MP:lip, 0); and barier three renal hilum LNs
(WT-SK, 6.7±2.5%; EV-SK, 14.2±9.42%; VEGFD-SK, 48.7±15.6%;
pVAX:lip, 44.72±13.14%; pVAX-MP:lip, 0). VEGFD-SK group had much
higher metastasis tendency in each barrier, but pVAX-MP:lip group
had the lowest or zero metastasis tendency (P<0.05).
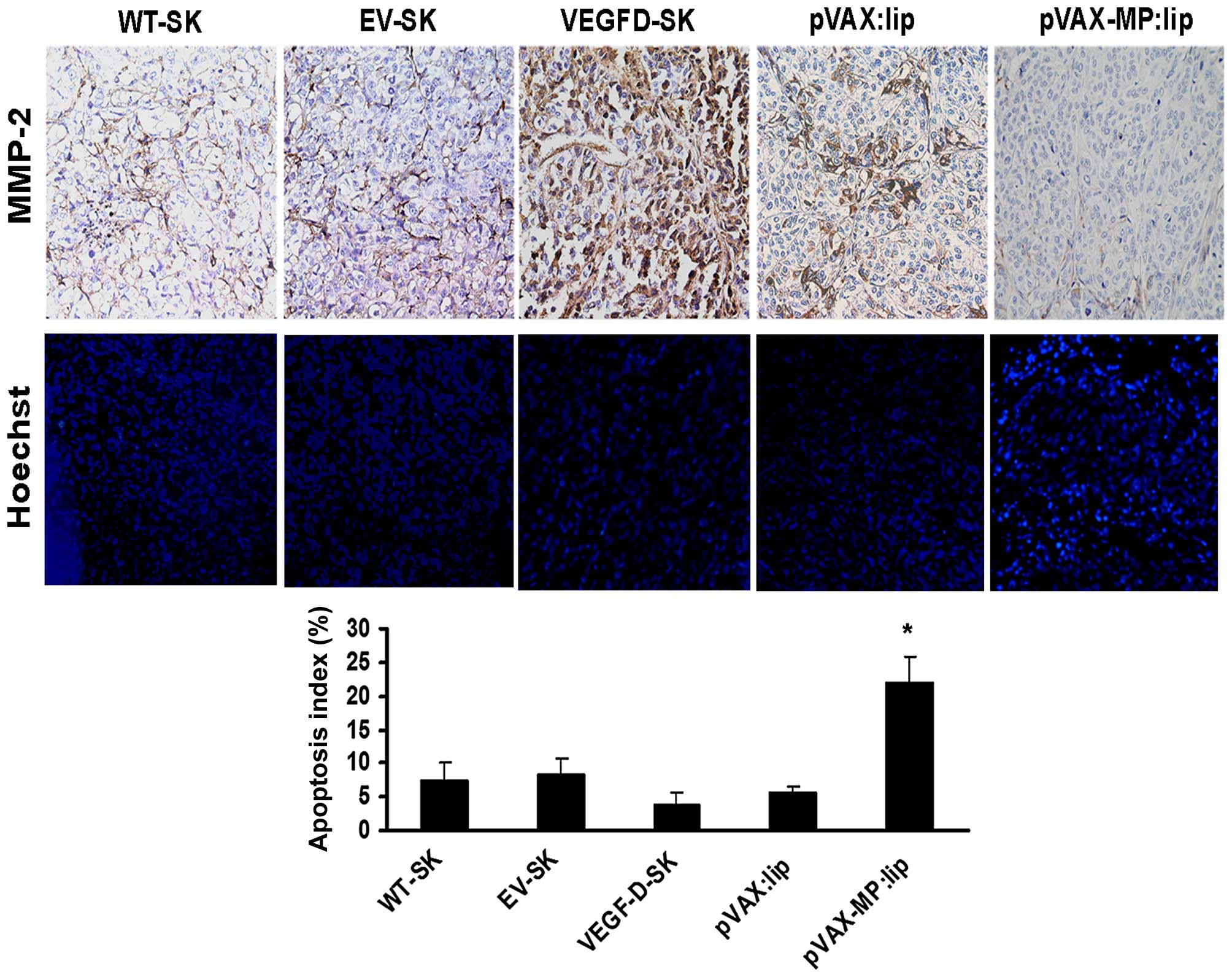
Expression of MMP-2
The microscopic column showed expression of MMP-2 of
local tumor in different groups (Fig.
5, top panel). In comparison with the weak stromal staining (+)
in WT-SK group and EV-SK group, >90% tumor cells and stroma of
VEGFD-SK group showed much stronger staining for MMP-2 (+++). The
pVAX:lip group exhibited deep MMP-2 staining, especially in part of
tumor cells (++); whereas the pVAX-MP:lip group showed slight MMP-2
staining.
Apoptosis of tumor cells in vivo
Microscopic fluorescence images showed apoptotic
cells of tumor tissues in different groups by Hoechst-33258
staining (Fig. 5, middle panel).
Apoptosis index of VEGFD-SK tumors (3.9±1.7) was much lower than
that of any other group (Fig. 5,
bottom panel). No appreciable differences were observed between
WT-SK group (7.3±2.68) and EV-SK group (8.28±2.31). Lower apoptosis
index was also observed in pVAX:lip group (5.571±0.903). Whereas,
higher apoptosis index was only detected after the pVAX-MP:lip
administration (21.875±4.01, P<0.05 versus any other group).
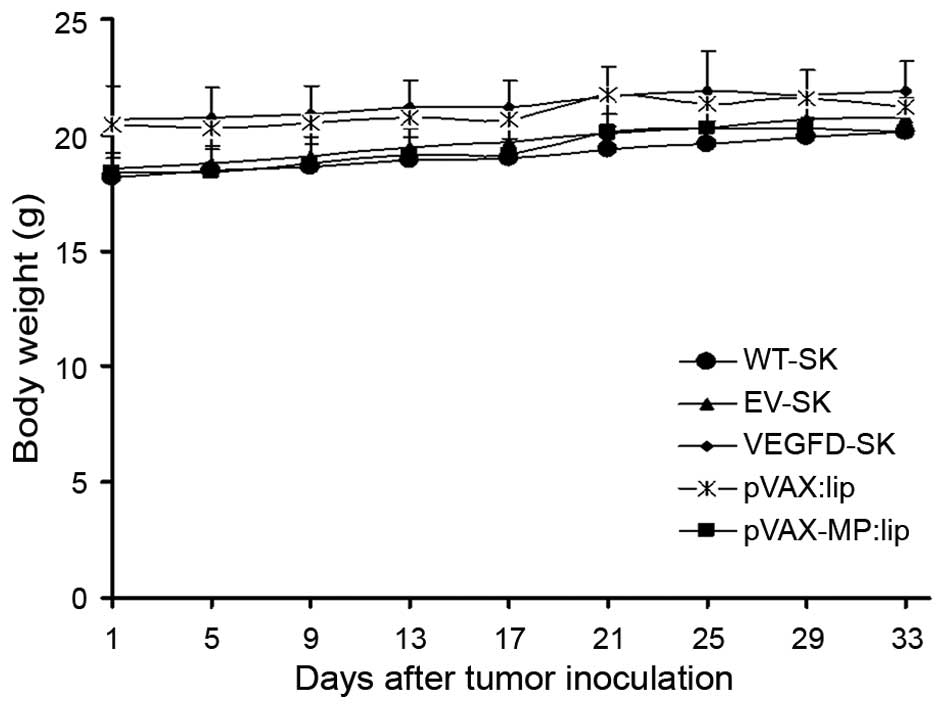
Safety
No significant differences in the weight of mice
were found among the five groups (P>0.05) (Fig. 6). No gross abnormalities were
detected in this study. Additionally, toxic reaction was confirmed
by H&E staining. The results indicated that there were no
significant pathologic alterations in major organs among the five
groups (data not shown).
Discussion
Ovarian cancer has the highest mortality rate of the
malignancies of the female reproductive tract. Lymph node
metastasis is a major prognostic factor in ovarian cancer. However,
there is no feasible therapeutic strategy against lymphatic
metastasis of ovarian cancer at present.
Metastasis to lymph nodes via lymphatic vessels is a
common step in the dissemination of ovarian cancer. Although the
molecular mechanisms of how lymphangiogenesis facilitates
metastasis remains unclear, research has suggested that VEGF-D is
associated with lymphangiogenesis-induced tumor metastasis to lymph
node by stimulating LV proliferation and increasing the diameter
and number of LVs (26).
Lymphangiogenesis can exist in the tumor mass and proliferate
rapidly in the presence of VEGF-D. With increasing numbers of LVs,
as passage for tumor cells, the overexpression of VEGF-D further
encourages lymphatic metastasis. It has been reported that high
expression of VEGF-D results in accelerated tumor growth and
develops metastasis via the lymphatic vessels (9,10).
In this study, we prepared a lymph node metastatic mouse model
using SKOV3 transfected with recombinant pcDNA3.1/VEGF-D plasmid.
Strong positive expression of VEGF-D in SKOV3 tumor cells were
detected both in vitro and in vivo. In addition, our
study showed high expression of MMP-2 in agreement with the
overexpression of VEGF-D, which might be associated with easy and
high metastasis of tumor cells via LVs (27,28).
At present, therapeutic intervention by
neutralization of VEGF-D has become a promising approach for the
treatment of malignant diseases (29). Recent studies have indicated
soluble VEGFR-3 protein or monoclonal antibodies which could block
the VEGF-D pathways have been used as a potentially biotherapeutic
agent for inhibition of metastatic spread and collapse of tumor
vessels (9,30,31).
High-level expression of VEGF-D could induce invasive and
metastatic behaviors, while lymphangiogenesis and lymphatic
metastasis were inhibited by knock-down of VEGF-D (32).
MP is capable of inducing apoptosis via inhibiting
host gene expression (11–14), nucleocytoplasmic transport of host
RNA and proteins (15,16) as well as inactivation of Akt
(17).
It has been reported to possess potent antitumor and
anti-angiogenesis properties (18–20).
However, whether MP could play a suppressive role in lymphatic
metastasis remains unclear. In this study, the antitumor and
antimetastasis efficacy of a recombinant plasmid DNA carrying
MP-cDNA in the lymphogenous metastatic model was tested. MP showed
anti-lymphangiogenic and anti-lymphatic metastasic properties
efficiently by analysis of lymphography, LVD, and pelvic lymph node
metastasis rates in mice. VEGF-D-enhanced metastasis to multilevel
lymph nodes in ovarian carcinoma were evidently reversed after
administration with pVAX-MP:lip. It is especially worth noting that
the nodal metastasis in pVAX-MP:lip group was evidently blockaded
and there is zero metastasis in parailiac and renal hilum LNs.
Moreover, MP exhibited potent antitumor efficiency, and resulted in
more than 80% inhibition in tumor volume compared with empty
vector.
Tumor progression and metastasis is a complex
process involving multiple cellular steps and mechanisms. To
elucidate the possible mechanism underlying the antitumor and
antimetastatic property of MP, expression of VEGF-D was evaluated.
Overexpression of VEGF-D was observed in the VEGFD-SK cells.
However, the expression of the exogenous VEGF-D was decreased
obviously when interfered with MP. Some evidence has demonstrated
that lymphangiogenesis of sprout plays a significant role in cancer
progression and prognosis (33–36).
In this study, the high-LVD induced by VEGF-D was declined
significantly after MP administration.
Lymphangiogenesis is the result of the synergy of
many factors such as tumor and VEGF-D. MP not only reduces VEGF-D,
but also induce tumor cell apoptosis. Therefore, in our study, the
reduction of lymphangiogenesis is more obvious than the repression
of VEGF-D with the impact of MP.
Stimulation of motility and invasiveness represent
key steps in the process of metastasis by both vascular and
lymphatic routes. In vitro approaches including migration
and invasion assays for tumor cells revealed that anti-lymphatic
metastasis role of MP in ovarian cancer might be related to the
suppression of invasion and migration. Expression of MMP-2 is
associated with invasive and metastatic behavior (37–39).
MMP-2 has the ability to degrade type IV collagen in the basement
membrane, and thus has an important influence in tumor progression.
In this study, transfection of VEGF-D resulted in upregulation of
MMP-2. However, inhibition of MMP-2 expression by MP was observed
distinctly in VEGFD-SK ovarian cancer. A significant suppression of
apoptotic tumor cells was observed in VEGFD-SK ovarian carcinoma,
but MP could result in significant apoptotic tumor cells by
apoptosis assays in vitro and in vivo. Our data are
consistent with the previously reported mechanism that MP could
induce cytopathogenesis (18,19,23).
Tumor size has been regarded as a stimulating factor
of nodal invasion (40–42). It may be partly attributed to high
interstitial pressure as the results of few numbers of lymph
vessels, and of the worse microenvironment for tumor metastasis
resulting from inhibition of MMP-2 expression. We observed that
tumor growth was stimulated by the increased expression of VEGF-D.
The enhanced tumor growth was inhibited by MP therapy. Angiogenesis
is necessary in tumor growth and metastasis because the blood
vessels supply malignant cells with sufficient oxygen and
nutrients. In the previous study, anti-angiogenesis effect of MP
has been confirmed. MP can inhibit formation of neovascularization
and induce mouse pancreatic islet endothelial cell (MS1 cell line)
apoptosis (18). Similar effect
was observed in our research (data not show). It may be another
reason for the low lymphatic metastasis rate in pVAX-MP:lip
group.
The reversal of metastasis might be due to: i) MP
significantly suppressed the growth and metastasis potential of the
local tumor; ii) MP significantly suppressed VEGF-D-induced
lymphatic sprouting; iii) MP directly inhibited the growth of nodal
metastasis. Further studies are still necessary to elucidate the
precise mechanisms of anti-lymph node metastasis and antitumor
effect of MP.
In conclusion, VEGF-D promoted tumor metastasis via
lymph system in the mouse model. The overexpression of VEGF-D,
VEGF-D-enhanced cell invasion, migration, lymph node metastasis and
lymphangiogenesis of ovarian carcinoma could be reversed by MP.
Inhibition of tumor growth and suppression of MMP-2 expression were
detected. Our results may provide a potentially effective
therapeutic strategy against human lymphatic metastasis of advanced
ovarian cancer without systemic toxic effects.
Acknowledgements
We thank Dr Ping Chen for his expert technical
assistance. Dr Xinjiang Xie provided recombined pcDNA3.1/VEGF-D
plasmid. Steven Pan helped in polishing the English, and provided
with other useful suggestions. This study was supported by National
Key Basic Research Program of China (973) (Grant No.
2011CB910703).
Abbreviations:
|
VEGF-D
|
vascular endothelial growth factor
D
|
|
VSV
|
vesicular stomatitis virus
|
|
MP
|
matrix protein
|
|
LN
|
lymph node
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
MMP-2
|
matrix metalloproteinase-2
|
|
LYVE-1
|
human lymphatic vessel endothelial
hyaluronan receptor-1
|
References
|
1
|
Edwards BK, Brown ML, Wingo PA, Howe HL,
Ward E, Ries LA, Schrag D, Jamison PM, Jemal A, Wu XC, et al:
Annual report to the nation on the status of cancer, 1975–2002
featuring population-based trends in cancer treatment. J Natl
Cancer Inst. 97:1407–1427. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Concin N, Hefler L, van Bavel J,
Mueller-Holzner E, Zeimet A, Daxenbichler G, Speiser P, Hacker N
and Marth C: Biological markers in pT1 and pT2 ovarian cancer with
lymph node metastases. Gynecol Oncol. 89:9–15. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rao G, Crispens M and Rothenberg ML:
Intraperitoneal chemotherapy for ovarian cancer: Overview and
perspective. J Clin Oncol. 25:2867–2872. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ayhan A, Gultekin M, Taskiran C, Celik NY,
Usubutun A, Kucukali T and Yuce K: Lymphatic metastasis in
epithelial ovarian carcinoma with respect to clinicopathological
variables. Gynecol Oncol. 97:400–404. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
di Re F, Baiocchi G, Fontanelli R, Grosso
G, Cobellis L, Raspagliesi F and di Re E: Systematic pelvic and
paraaortic lymphadenectomy for advanced ovarian cancer: Prognostic
significance of node metastases. Gynecol Oncol. 62:360–365. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Millauer B, Wizigmann-Voos S, Schnürch H,
Martinez R, Møller NP, Risau W and Ullrich A: High affinity VEGF
binding and developmental expression suggest Flk-1 as a major
regulator of vasculogenesis and angiogenesis. Cell. 72:835–846.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaipainen A, Korhonen J, Mustonen T, van
Hinsbergh VW, Fang GH, Dumont D, Breitman M and Alitalo K:
Expression of the fms-like tyrosine kinase 4 gene becomes
restricted to lymphatic endothelium during development. Proc Natl
Acad Sci USA. 92:3566–3570. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stacker SA, Caesar C, Baldwin ME, Thornton
GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H and Achen
MG: VEGF-D promotes the metastatic spread of tumor cells via the
lymphatics. Nat Med. 7:186–191. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Guo Y, Wang B, Bi J, Li K, Liang
X, Chu H and Jiang H: Lymphatic microvessel density and vascular
endothelial growth factor-C and -D as prognostic factors in breast
cancer: A systematic review and meta-analysis of the literature.
Mol Biol Rep. 39:11153–11165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Black BL and Lyles DS: Vesicular
stomatitis virus matrix protein inhibits host cell-directed
transcription of target genes in vivo. J Virol. 66:4058–4064.
1992.PubMed/NCBI
|
|
12
|
Gerlier D and Lyles DS: Interplay between
innate immunity and negative-strand RNA viruses: Towards a rational
model. Microbiol Mol Biol Rev. 75:468–490. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ahmed M and Lyles DS: Effect of vesicular
stomatitis virus matrix protein on transcription directed by host
RNA polymerases I, II, and III. J Virol. 72:8413–8419.
1998.PubMed/NCBI
|
|
14
|
Yuan H, Puckett S and Lyles DS: Inhibition
of host transcription by vesicular stomatitis virus involves a
novel mechanism that is independent of phosphorylation of
TATA-binding protein (TBP) or association of TBP with
TBP-associated factor subunits. J Virol. 75:4453–4458. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahmed M, McKenzie MO, Puckett S, Hojnacki
M, Poliquin L and Lyles DS: Ability of the matrix protein of
vesicular stomatitis virus to suppress beta interferon gene
expression is genetically correlated with the inhibition of host
RNA and protein synthesis. J Virol. 77:4646–4657. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Petersen JM, Her LS, Varvel V, Lund E and
Dahlberg JE: The matrix protein of vesicular stomatitis virus
inhibits nucleocytoplasmic transport when it is in the nucleus and
associated with nuclear pore complexes. Mol Cell Biol.
20:8590–8601. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dunn EF and Connor JH: Dominant inhibition
of Akt/protein kinase B signaling by the matrix protein of a
negative-strand RNA virus. J Virol. 85:422–431. 2011. View Article : Google Scholar :
|
|
18
|
Zhong Q, Wen YJ, Yang HS, Luo H, Fu AF,
Yang F, Chen LJ, Chen X, Qi XR, Lin HG, et al: Efficient inhibition
of cisplatin-resistant human ovarian cancer growth and prolonged
survival by gene transferred vesicular stomatitis virus matrix
protein in nude mice. Ann Oncol. 19:1584–1591. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gou M, Men K, Zhang J, Li Y, Song J, Luo
S, Shi H, Wen Y, Guo G, Huang M, et al: Efficient inhibition of
C-26 colon carcinoma by VSVMP gene delivered by biodegradable
cationic nanogel derived from polyethyleneimine. ACS Nano.
4:5573–5584. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahmed M, Puckett S and Lyles DS:
Susceptibility of breast cancer cells to an oncolytic matrix (M)
protein mutant of vesicular stomatitis virus. Cancer Gene Ther.
17:883–892. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou Y, Wen F, Zhang P, Tang R and Li Q:
Matrix protein of vesicular stomatitis virus: A potent inhibitor of
vascular endothelial growth factor and malignant ascites formation.
Cancer Gene Ther. 20:178–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wen J, Fu AF, Chen LJ, Xie XJ, Yang GL,
Chen XC, Wang YS, Li J, Chen P, Tang MH, et al: Liposomal honokiol
inhibits VEGF-D-induced lymphangiogenesis and metastasis in
xenograft tumor model. Int J Cancer. 124:2709–2718. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin X, Chen X, Wei Y, Zhao J, Fan L, Wen
Y, Wu H and Zhao X: Efficient inhibition of intraperitoneal human
ovarian cancer growth and prolonged survival by gene transfer of
vesicular stomatitis virus matrix protein in nude mice. Gynecol
Oncol. 104:540–546. 2007. View Article : Google Scholar
|
|
24
|
Chen X, Wang X, Wang Y, Yang L, Hu J, Xiao
W, Fu A, Cai L, Li X and Ye X: Improved tumor-targeting drug
delivery and therapeutic efficacy by cationic liposome modified
with truncated bFGF peptide. J Control Release. 145:17–25. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou L, Du L, Chen X, Li X, Li Z, Wen Y,
Li Z, He X, Wei Y, Zhao X, et al: The antitumor and antimetastatic
effects of N-trimethyl chitosan-encapsulated camptothecin on
ovarian cancer with minimal side effects. Oncol Rep. 24:941–948.
2010.PubMed/NCBI
|
|
26
|
Jussila L and Alitalo K: Vascular growth
factors and lymphangiogenesis. Physiol Rev. 82:673–700. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kabashima A, Maehara Y, Kakeji Y, Baba H,
Koga T and Sugimachi K: Clinicopathological features and
overexpression of matrix metalloproteinases in intramucosal gastric
carcinoma with lymph node metastasis. Clin Cancer Res. 6:3581–3584.
2000.PubMed/NCBI
|
|
28
|
Mitra A, Chakrabarti J, Banerji A and
Chatterjee A: Cell membrane-associated MT1-MMP dependent activation
of MMP-2 in SiHa (human cervical cancer) cells. J Environ Pathol
Toxicol Oncol. 25:655–666. 2006. View Article : Google Scholar
|
|
29
|
Griffioen AW: Lymphangiogenesis factors: a
target for therapy? Blood. 113:4135–4136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kubo H, Fujiwara T, Jussila L, Hashi H,
Ogawa M, Shimizu K, Awane M, Sakai Y, Takabayashi A, Alitalo K, et
al: Involvement of vascular endothelial growth factor receptor-3 in
maintenance of integrity of endothelial cell lining during tumor
angiogenesis. Blood. 96:546–553. 2000.PubMed/NCBI
|
|
31
|
Pytowski B, Goldman J, Persaud K, Wu Y,
Witte L, Hicklin DJ, Skobe M, Boardman KC and Swartz MA: Complete
and specific inhibition of adult lymphatic regeneration by a novel
VEGFR-3 neutralizing antibody. J Natl Cancer Inst. 97:14–21. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Majumder M, Tutunea-Fatan E, Xin X,
Rodriguez-Torres M, Torres-Garcia J, Wiebe R, Timoshenko AV,
Bhattacharjee RN, Chambers AF and Lala PK: Co-expression of α9β1
integrin and VEGF-D confers lymphatic metastatic ability to a human
breast cancer cell line MDA-MB-468LN. PLoS One. 7:e350942012.
View Article : Google Scholar
|
|
33
|
Renyi-Vamos F, Tovari J, Fillinger J,
Timar J, Paku S, Kenessey I, Ostoros G, Agocs L, Soltesz I and Dome
B: Lymphangiogenesis correlates with lymph node metastasis,
prognosis, and angiogenic phenotype in human non-small cell lung
cancer. Clin Cancer Res. 11:7344–7353. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hall FT, Freeman JL, Asa SL, Jackson DG
and Beasley NJ: Intratumoral lymphatics and lymph node metastases
in papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg.
129:716–719. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dadras SS, Paul T, Bertoncini J, Brown LF,
Muzikansky A, Jackson DG, Ellwanger U, Garbe C, Mihm MC and Detmar
M: Tumor lymphangiogenesis: A novel prognostic indicator for
cutaneous melanoma metastasis and survival. Am J Pathol.
162:1951–1960. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bono P, Wasenius VM, Heikkilä P, Lundin J,
Jackson DG and Joensuu H: High LYVE-1-positive lymphatic vessel
numbers are associated with poor outcome in breast cancer. Clin
Cancer Res. 10:7144–7149. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
O'Grady A, Dunne C, O'Kelly P, Murphy GM,
Leader M and Kay E: Differential expression of matrix
metalloproteinase (MMP)-2, MMP-9 and tissue inhibitor of
metalloproteinase (TIMP)-1 and TIMP-2 in non-melanoma skin cancer:
Implications for tumour progression. Histopathology. 51:793–804.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Taniwaki K, Fukamachi H, Komori K, Ohtake
Y, Nonaka T, Sakamoto T, Shiomi T, Okada Y, Itoh T, Itohara S, et
al: Stroma-derived matrix metalloproteinase (MMP)-2 promotes
membrane type 1-MMP-dependent tumor growth in mice. Cancer Res.
67:4311–4319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nathanson SD, Anaya P, Avery M, Hetzel FW,
Sarantou T and Havstad S: Sentinel lymph node metastasis in
experimental melanoma: Relationships among primary tumor size,
lymphatic vessel diameter and 99mTc-labeled human serum albumin
clearance. Ann Surg Oncol. 4:161–168. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Carter CL, Allen C and Henson DE: Relation
of tumor size, lymph node status, and survival in 24,740 breast
cancer cases. Cancer. 63:181–187. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nagamoto N, Saito Y, Ohta S, Sato M, Kanma
K, Sagawa M, Takahashi S, Usuda K, Nakada T and Hashimoto K:
Relationship of lymph node metastasis to primary tumor size and
microscopic appearance of roentgenographically occult lung cancer.
Am J Surg Pathol. 13:1009–1013. 1989. View Article : Google Scholar : PubMed/NCBI
|