Introduction
Prostate cancer (PCa) is one of the most common
malignancies for men (1). Most PCa
depends on androgens for growth and survival, and androgen ablation
therapy represents the most effective initial treatment (2). Unfortunately, most PCa will progress
to castration-resistant prostate cancer (CRPC) with higher
potential of invasion and metastasis in 2–3 years (3). Though efforts have been made for
treating CRPC for years, an efficient therapeutic treatment is
still not available (4).
Therefore, identification of mechanisms involved in CRPC
carcinogenesis and metastasis may be the key to novel
treatments.
Tumor metastasis is a crucial hallmark of cancer
progression. It involves numerous factors including defects in
programmed cell death, degradation of the extracellular matrix
(ECM) and tumor angiogenesis (5).
ECM provides structural support to cells and tissues by
heterogeneous macromolecules and is a rich source of angiogenesis
promoters (6). Matrix
metalloproteases 9 and 2 (MMP-9 and MMP-2) catalyze the degradation
of essentially the majority of ECM and components of the basement
membrane and proteolytically cleave and activate precursors of
angiogenesis promoters, which play an important role for tumor
invasion and metastasis (7–10).
The degradation activities of MMP-9 and MMP-2 are regulated by
NF-κB activity (11,12).
Matrine
(C15H24N2O), an alkaloid derived
from Sophora flavescens, a traditional Chinese herb
medicine, has been identified to exhibit bioactive and
pharmacological effects such as anti-inflammatory (13) and antiviral activities (14). It also exhibits an anticancer
effect on malignancies such as lung cancer, breast cancer and
castration-sensitive prostate cancer (15–17).
In our previous study, we have identified the inhibitory abilities
of matrine for growth of CRPC cells through NF-κB signaling pathway
in vitro (18). However,
the anti-metastatic effects of matrine on CRPC in vivo and
the underlying mechanisms regulating NF-κB by matrine are still
unknown.
In this study, we proved that matrine reduced the
growth and invasion abilities of CRPC cell lines both in
vitro and in vivo by reducing the expression levels of
MMP-2 and MMP-9 via NF-κB signaling pathway. Our results imply that
matrine may be developed as a novel anti-metastasis therapeutic
drug for CRPC in future.
Materials and methods
Reagents
Matrine [chemical formula:
C15H24N2O; molecular weight:
248.36] was purchased from Dalian Mellon (Dalian, China).
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum
(FBS), penicillin, streptomycin, phosphate-buffered saline, and
RPMI-1640 media were purchased from Hyclone (Logan, UT, USA).
Cell lines and culture
Human castration-resistant prostate cancer cell
lines DU145 and PC-3 were obtained from the cell bank of the Center
for Experiment Animals of Sun Yat-sen University (Guangzhou,
China). Cells were maintained in RPMI-1640 supplemented with 10%
(vol/vol) FBS, 1% penicillin and streptomycin at 37°C in an
incubator containing 5% CO2. Different concentrations of
matrine were added into culture medium to treat cells for different
times.
Cell viability assay
DU145 and PC-3 cells were seeded in 96-well plates
(100 µl/well) at a density of 1×104 cells/well
for 24 h. Then the cells were treated with various concentrations
of matrine (0, 0.025, 0.05, 0.1, 0.25, 0.5, 0.75, 1.0, 1.5, 2.0,
3.0, 4.0 and 6.0 g/l). After incubation for 0, 24, 48 and 72 h, the
viability of the cells was evaluated using the MTS kit
(cellTiter96AQ, Promega Corp., Madison, WI, USA) according to the
manufacturer's instructions, and the absorption was read at 490
nm.
Migration and invasion assay
The migration and invasion assays were performed
using Transwell® Inserts (BD Biosciences, Bedford, MA,
USA). Approximately 1×105 cells in 100 µl
serum-free RMPI-1640 medium were placed in the upper chamber, and
600 µl RMPI-1640 medium containing 10% (vol/vol) FBS were
placed in the lower chamber. For the invasion assay,
Transwell® membranes were pre-coated with 25% Matrigel
(BD Biosciences). The cells were then incubated for 48 h at 37°C in
5% CO2 with 0.5 g/l matrine, 10 µM Bay11-7082
(Bay), 0.5 g/l matrine plus 10 µM Bay or fresh median (the
untreated control). Cells were fixed in 4% paraformaldehyde for 20
min and stained with 0.05% crystal violet in PBS for 10 min. The
cells on the upper side of the filter were removed with
cotton-tipped swabs, and the filter was washed with 1X PBS. The
cells on the underside of the filter were examined and counted
under a microscope with a power of ×200 magnification.
Western blotting
Cells treated with different concentrations of
matrine, Bay and TNF-α (T6674 Sigma, USA) were lysed in a RIPA
lysis buffer system (50 mM Tris-HCl, 1 mM EDTA, 150 mM NaCl, 0.5%
sodium deoxycholate, 1% Triton X-100, 0.1% sodium dodecyl sulfate
(SDS) and 1 mmol/l PMSF, pH 7.4). Supernatants were then collected
via centrifugation at 14,000 × g for 15 min at 4°C. Proteins were
separated by electrophoresis in 10% SDS-polyacrylamide gel and
transferred onto polyvinylidene fluoride (PVDF) membranes
(Millipore Corp., Billerica, MA, USA). PVDF membranes were
incubated in blocking buffer (1X Tris-buffered saline, 0.1%
Tween-20 with 5% non-fat dry milk) for 1 h and probed overnight at
4°C with specific primary antibodies against p-P65, P65, MMP-2,
MMP-9 (1:1,000; Cell Signaling Technology, Beverly, MA, USA) or
GAPDH (1:1,000; Kangcheng Biology, Shanghai, China). After being
washed, membranes were incubated with horseradish peroxidase
(HRP)-conjugated secondary antibody (Dako) for 1 h at room
temperature. Membranes were subsequently visualized with the
Western Blotting Luminal Reagent (Millipore Corp.) according to the
manufacturer's protocol.
Animal xenograft model assays
Male Balb/c nude mice (5-weeks old) were purchased
from Experimental Animal Center of Sun Yat-sen University
(Certificate of compliance: 44008500010058; Guangzhou, China) and
raised under specific pathogen-free (SPF) conditions. All
experimental procedures were performed according to the
institutional ethical guidelines approved by the Institutional
Animal Care and Use Committee of Sun Yat-sen Memorial Hospital, Sun
Yat-sen University. Suspensions of DU145 and PC-3 cells (0.1 ml
5×107 viable cells/ml) were subcutaneously injected into
the right flank of mice. Nine days later, the tumor volumes were
~100 mm3 in all mice. The treatment and control groups
were subjected to intraperitoneal injection of 50 or 200 mg/kg
matrine and an equal volume of saline three times per week,
respectively (6 mice in each group). Tumor sizes were measured
twice weekly, and the volumes (cm3) were calculated
according to the formula: V = (length × width2) / 2.
Animals of both groups were sacrificed to measure tumor weights 3
weeks later.
Statistical analysis
All the experiments were repeated successfully at
least three times. Two-tailed Student's t-test was used to
determine the difference between groups by GraphPad Prism (San
Diego, CA, USA). A P-value ≤0.05 was considered statistically
significant.
Results
Matrine reduces the viability of DU145
and PC-3 cells in vitro
DU145 and PC-3 cells were exposed to increasing
concentrations of matrine, and their viabilities were evaluated by
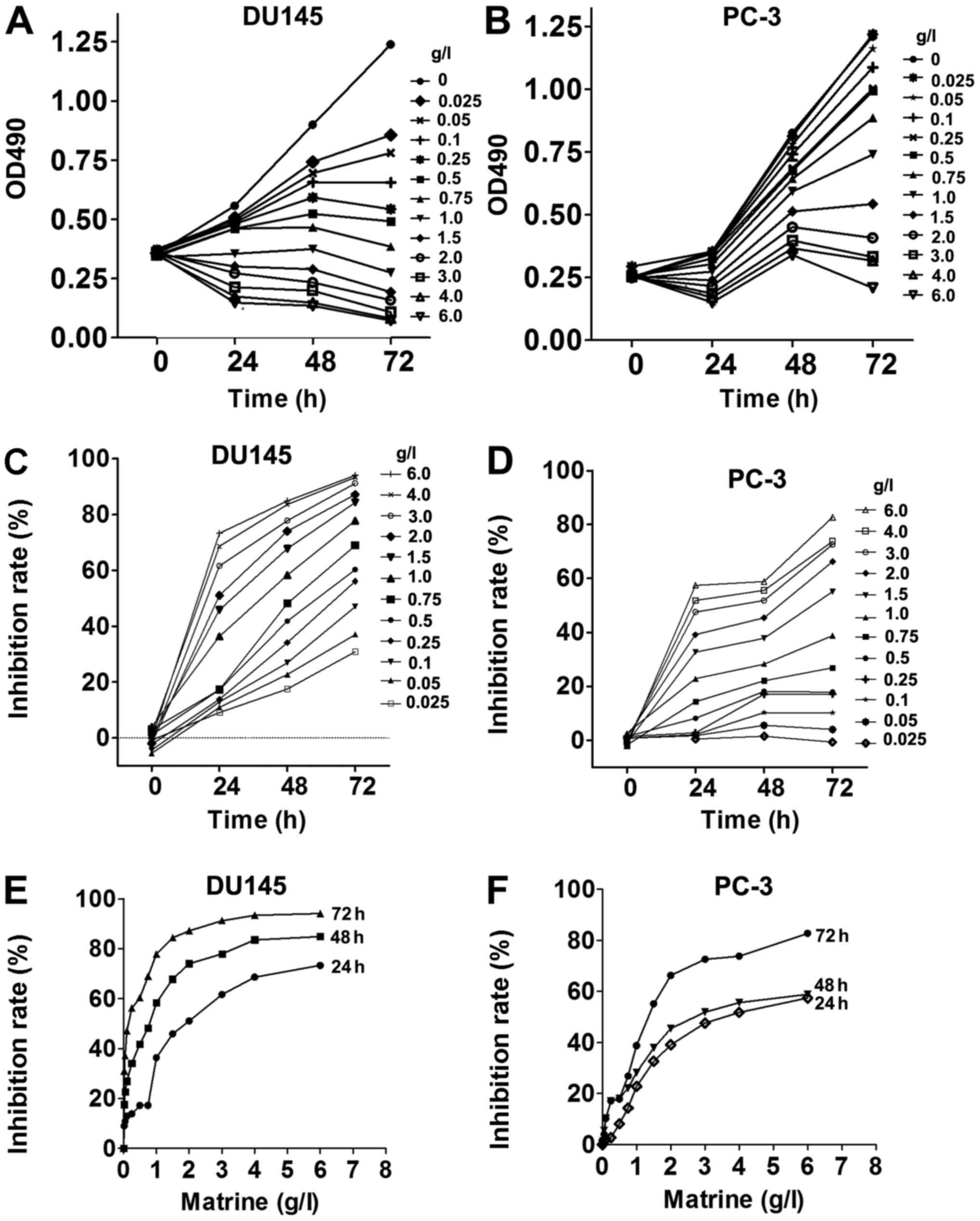
MTS assays at different time-points. Matrine inhibited the
proliferation of both CRPC cell lines in a dose- and time-dependent
manner (Fig. 1A and B). The
inhibition rates of both CRPC cell lines reached almost 90% when
cells were treated with 6.0 g/l matrine for 72 h (Fig. 1C and D). The potency of matrine to
suppress the growth of CRPC cells was increased with increasing
time (Fig. 1E and F). Thus,
matrine suppresses the viabilities of both CRPC cell lines DU145
and PC-3.
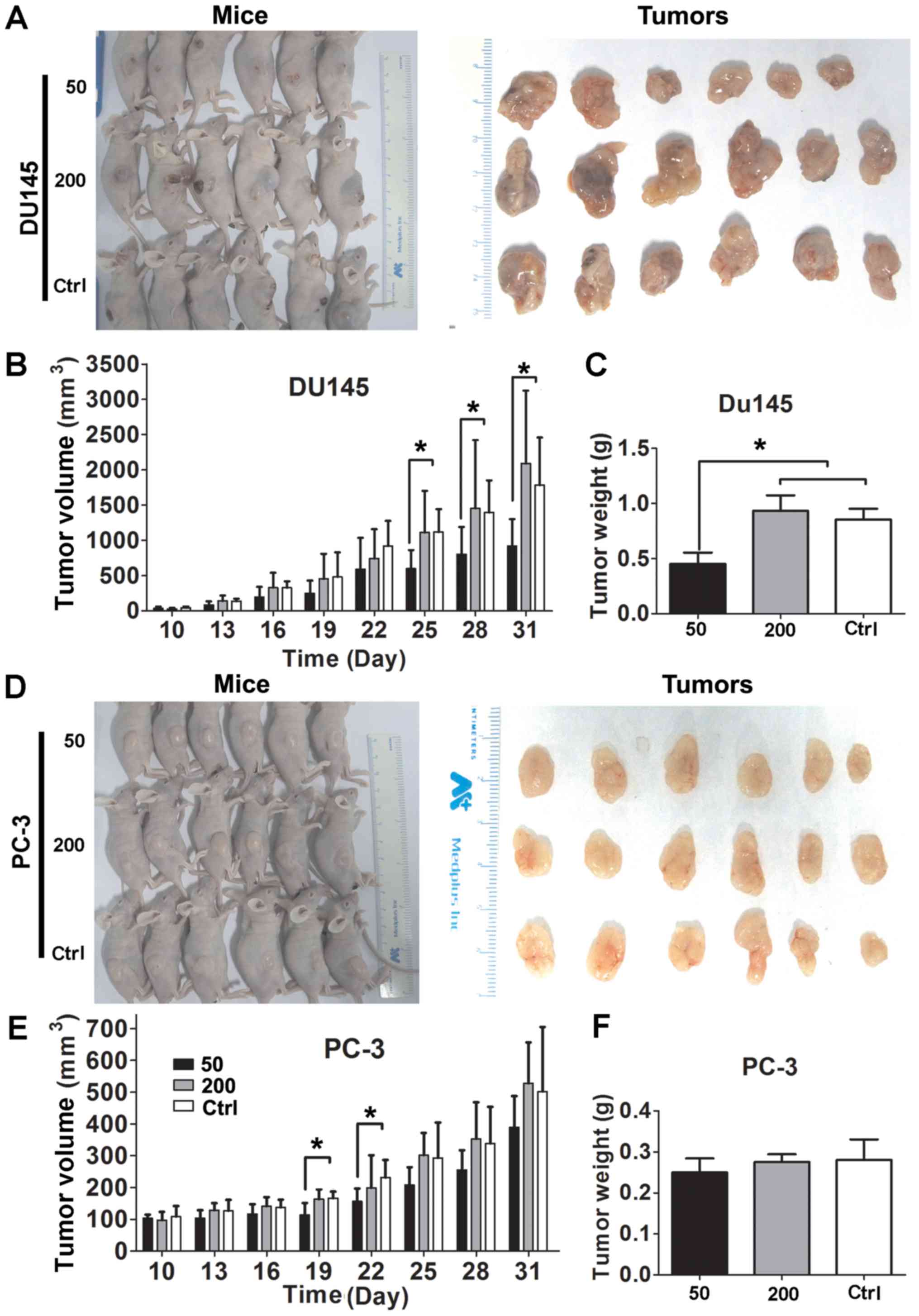
Matrine suppresses tumor growth in nude
mice inoculated with CRPC cells
DU145 and PC-3 cells were inoculated into male
Balb/c nude mice. The time courses of CRPC xenograft growth with
and without matrine treatment are shown in Fig. 2. Inhibitory impacts of matrine on
tumor volume and weight were observed in DU145-inoculated mice
treated with 50 mg/kg/d matrine (Fig.
2A–C). Such impact of matrine on tumor growth was only observed
on the 19th and 22nd days in PC-3-inoculated mice treated with 50
mg/kg/d matrine (Fig. 2D–F).
Interestingly, matrine at dosage of 200 mg/kg/d did not show any
impact on the tumor size and weight of either DU145- or
PC-3-inoculated mice, we did notice that tumors in the sacrificed
mice treated with high dosage of matrine started ulcerating. The
results suggest that matrine may suppress the tumor growth of CRPC
at low dose but exhibit no antitumor effect at high dose due to
interruption of the homeostasis of mice.
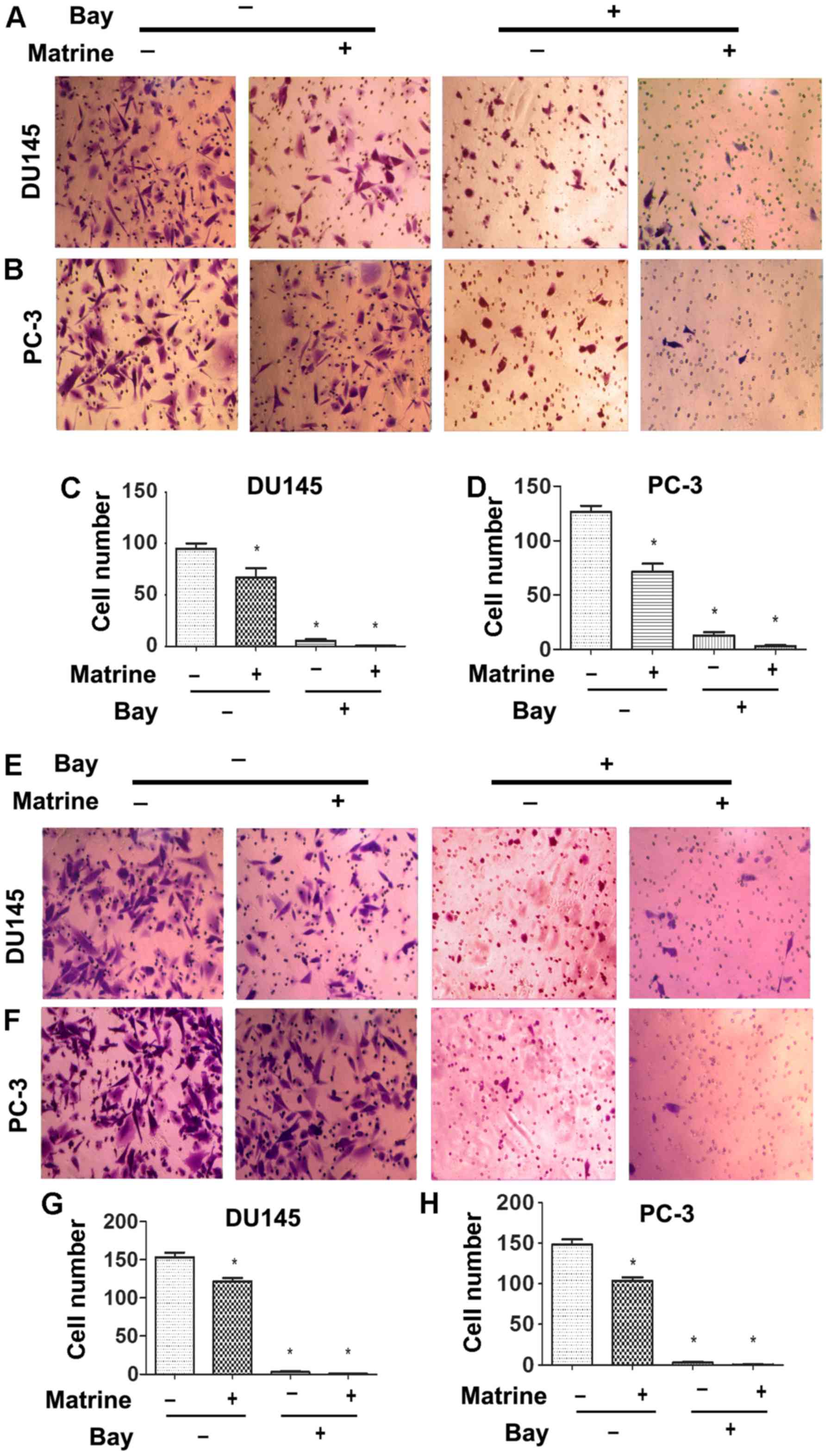
Matrine reduces the ability of migration
and invasion of CRPC cells
We further tested whether matrine has any effect on
the migration and invasion of CRPC cells. Cells were untreated or
treated with 0.5 g/l matrine to measure cell migration and
invasion. The migration rates of both DU145 and PC-3 cells were
significantly reduced when cells were treated with matrine for 48 h
(Fig. 3A–D). Invasion assay with
Transwell chambers coated with Matrigel indicated that the number
of either DU145 or PC-3 cells that invaded to the lower chamber
significantly decreased after cells were treated with matrine for
48 h (Fig. 3E–H). Therefore,
matrine effectively impairs the ability of migration and invasion
of CRPC cells.
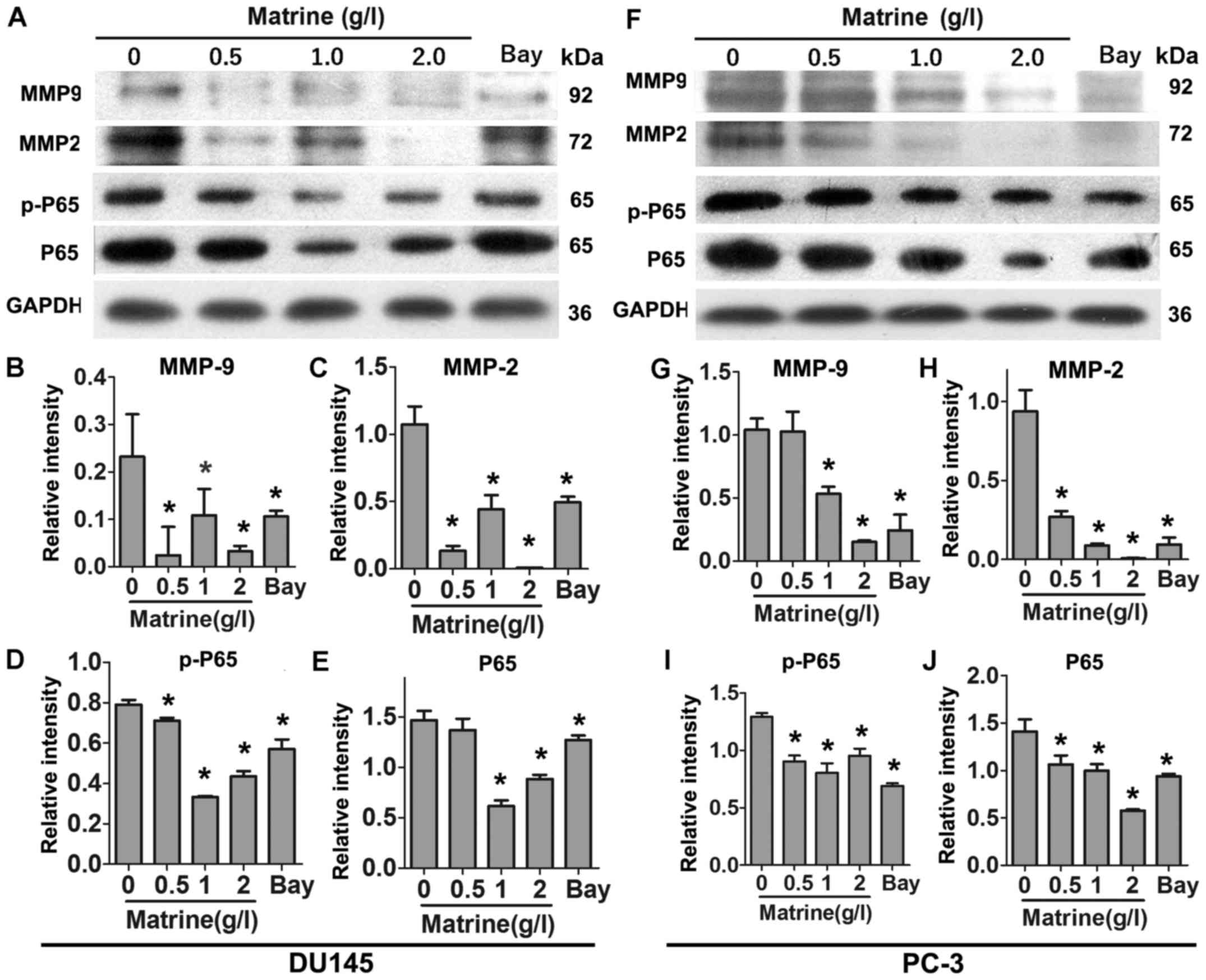
Matrine reduces the expression levels of
MMP-9 and MMP-2 and impairs NF-κB pathway in CRPC cells
Since MMPs play important roles in the invasion of
cancer cells. We examined the expression levels of MMP-9 and MMP-2
in CRPC cells exposed to different concentrations of matrine by
immunoblotting. Similar to NF-κB inhibitor Bay, matrine reduced the
expression levels of MMP-9 and MMP-2 in DU145 cells in a
concentration-dependent manner (Fig.
4A–C). NF-κB is reported as a key signaling molecule that
mediates the expression of MMPs (11). We hypothesized that NF-κB signaling
pathway might also be affected by matrine. As shown in Fig. 4A, D and E, the expression levels of
p-P65 and P65 in DU145 cells were markedly downregulated upon
treatment with matrine for 48 h. The same trends were observed in
PC-3 cells (Fig. 4F–J).
Collectively, these results demonstrate that matrine suppresses the
expression of MMP-9 and MMP-2 and inactivates NF-κB signaling
pathway in CRPC cells.
Matrine inhibits migration and invasion
of CRPC cells by suppressing MMP-9 and MMP-2 through NF-κB
pathway
In addition, NF-κB is involved in the control of
migration and invasion of tumor cells. We hypothesized that the
suppression of migration and invasion of CRPC cells may be caused
by downregulation of NF-κB. Combined treatment of NF-κB inhibitor
Bay and matrine caused a synergistic reduction in migration and
invasion of both in DU145 and PC-3 cells (Fig. 3). We further investigated whether
the inhibitory effects of matrine on cell invasion and expression
of MMP-9 and MMP-2 were correlated with the inhibition of NF-κB
pathway. Either DU145 or PC-3 cells were cultured in the absence or
presence of 0.5 g/l matrine, 10 µM Bay and/or 10 ng/ml TNF-α
for 48 h. Consistently, the levels of p-P65 and P65 proteins were
significantly reduced when DU145 cells were treated with matrine
and/or Bay (Fig. 5A–C).
Consequently, the expression levels of MMP-9 and MMP-2 in DU145
cells were significantly reduced upon exposure to matrine and/or
Bay (Fig. 5A, D and E). When NF-κB
pathway was activated by TNF-α, matrine significantly reduced
expression levels of p-P65 and MMP-9 in DU145 cells (Fig. 5F–J). However, expression levels of
MMP-2 were not significantly changed when DU145 cells were cultured
in the presence of both matrine and TNF-α, suggesting different
regulatory mechanisms for MMP-9 and MMP-2. Similar results were
obtained when the tested cell line was changed to PC-3 (Fig. 5K–T). Therefore, inhibitory effects
of matrine on migration and invasion of CRPC cells act by reducing
levels of MMP-9 and MMP-2 through the NF-κB pathway.
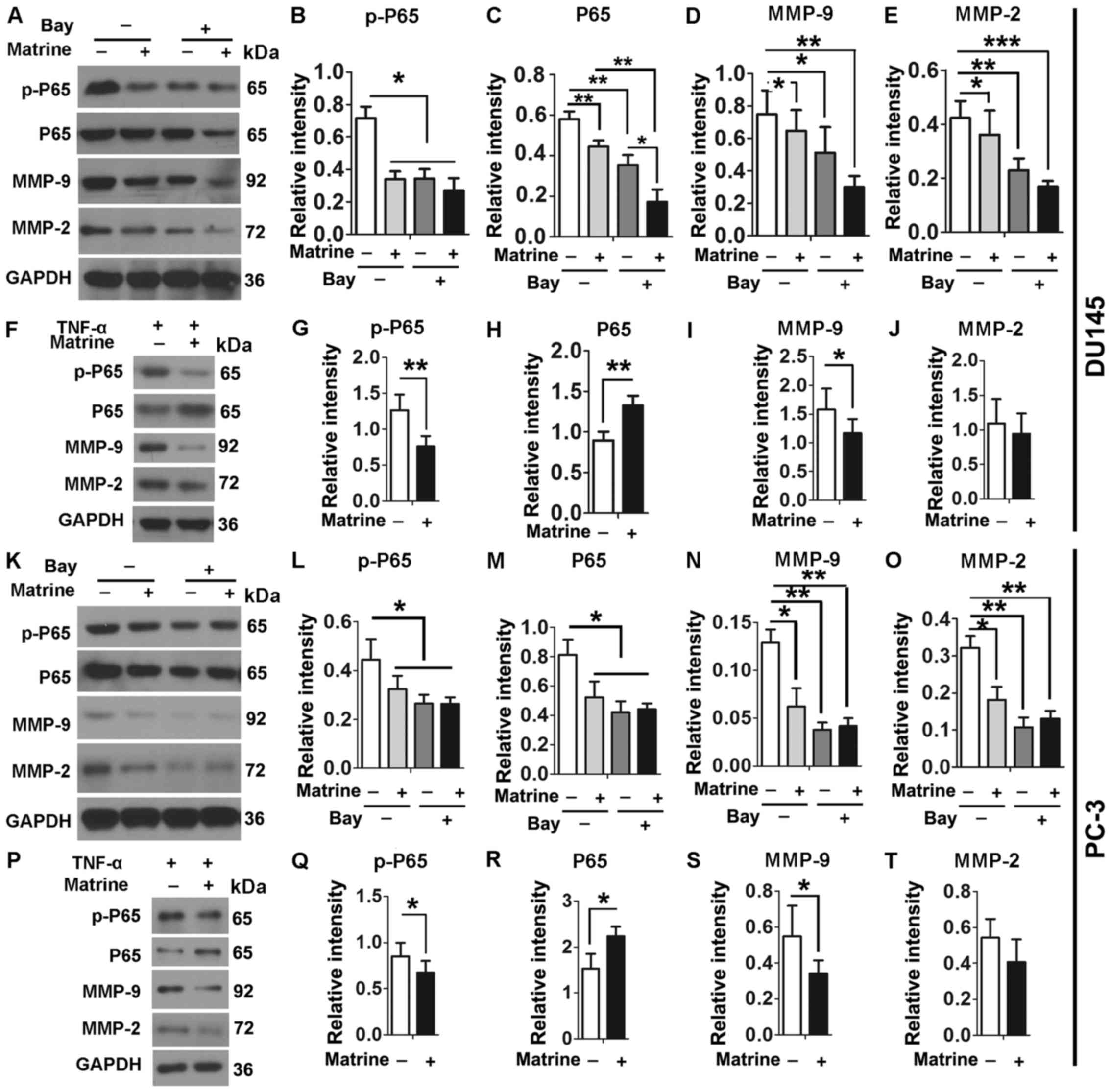 | Figure 5Matrine reduces the levels of MMP-2
and MMP-9 via NF-κB signaling pathway in CRPC cells. (A–T)
Representative immunoblot results (A, F, K and P) and plots of
relative intensity of p-P65 (B, G, L and Q), P65 (C, H, M and R),
MMP-9 (D, I, N and S) and MMP-2 (E, J, O and T) in DU145 (A–J) or
PC-3 cells (K–T) in the absence or presence of 0.5 g/l matrine in
the absence or presence of 10 µM Bay (A–E and K–O), or in
the absence or presence of 0.5 g/l matrine in the presence of 10
ng/ml TNF-α for 48 h (F–J and P–T). Relative intensity of proteins
are their ratios to levels of GAPDH. *P≤0.05;
**P≤0.01; and ***P≤0.001. |
Discussion
Naturally-occurring phytochemicals play important
roles in the prevention and treatment of cancers (19,20).
As a naturally-occurring phytochemical, matrine has been reported
to exhibit anticancer effects on different malignancies such as
lung cancer, breast cancer, and castration-sensitive prostate
cancer (15–17). In our previous study, we identified
the inhibitory ability of matrine in CRPC cells in vitro.
This study investigated the mechanisms of anti-metastatic effects
of matrine against CRPC cells. Our data showed that matrine
inhibited the viability of CRPC cells time- and dose-dependently.
In vivo study further demonstrated that matrine inhibited
the growth of CRPC tumors in nude mice. Transwell analysis showed
that matrine also inhibited the migration and invasion of CRPC
cells dose-dependently through NF-κB pathway. The underlying
mechanism is potentially that suppressing NF-κB activities causes
reduction in levels of MMP-9 and MMP-2. These data imply that
matrine is a potent candidate for CRPC treatment.
PCa mainly depends on the presence of androgens for
growth and survival, and blocking androgen secretion or activity
represents the most effective initial treatment (2). However, after the initial surgical or
medical ablation, most PCa will progress to CRPC stage with
increased malignancy in 2–3 years and need for second-line therapy
(3,21). Although cytotoxic drugs and
recently approved drugs for more efficient blockade of androgen
signaling are available, there is still a need for new and more
efficient treatment strategies in CRPC (22). Matrine has been reported to inhibit
the proliferation and invasion of different malignancies (23–25).
This study initially investigated whether matrine had similar
effects on CRPC. Results proved that matrine did reduce the
viability of DU145 and PC-3 cells time- and dose-dependently.
Moreover, matrine inhibited migration and invasion of the CRPC
cells. These results may be translated into the development of
novel treatment approach with matrine. To further confirm the
hypothesis of such a novel treatment modality, we conducted in
vivo analyses to demonstrate that matrine decreased the growth
of tumor xenografts in nude mice inoculated with CRPC cells by
inhibiting cell proliferation.
Tumor metastasis involves a complex process and
various cellular physiological changes. Cancer cell invasion is the
first step for metastasis, which is characterized by increasing
cell motility caused by alterations in cell-cell and cell-ECM
interactions (26). Apoptotic
defects may cause cells to be resistant to cell death induced by
such alterations, which may promote cancer cell invasion (27). Apoptosis is a type of programmed
cell death that is characterized by cell membrane blebbing, cell
shrinkage, nuclear fragmentation, chromatin condensation, and
chromosomal DNA fragmentation (28). After initiating invasion, cancer
cells undergo various stresses that may trigger apoptosis by the
extrinsic and the intrinsic pathways (29). However, even with the successful
micro-metastases, there is only ~0.01% of tumor cells that
ultimately develop into macro-metastases in their destined organs.
Such inefficiency may be closely related to the stress-induced
apoptosis because of changed cell environment (30). Many small molecules targeting
apoptotic pathways, such as ABT-263, and GX15-070, have been
developed for cancer therapy (31). Other studies indicated that matrine
exerts anticancer effects by inducing apoptosis in different
malignancies such as cholangiocarcinoma, medulloblastoma and lung
cancer (23–25). Studies also showed that matrine
exhibits anti-metastatic effects by inducing apoptosis in lung
cancer and breast cancer (16,32).
Matrine induces apoptosis by decreasing the expression of Bcl-2 and
increasing expression of Bax. Apoptosis may block metastatic
dissemination by killing misplaced cells. Apoptotic resistance
induced by the loss of cell-cell and cell-ECM contacts may promote
metastatic progression (27).
The ECM is composed of heterogeneous macromolecules
and provides structural support to cells. It stores rich
angiogenesis promoters and inhibitors (6). The degradation or breakdown of the
ECM is a critical step in tumor invasion, leading to the separation
of the intercellular matrix to promote metastasis. MMPs, which are
a family of structurally related zinc-dependent endo-peptidases,
are collectively capable of degrading essentially all components of
ECM including collagens, gelatin and proteoglycan (8,33).
Among MMPs, MMP-2 and MMP-9 were reported to play the most
important roles for cancer invasion and metastasis (34,35).
Previous studies have reported that matrine can reduce the
expression of MMP-2 and MMP-9 in tumor cells (32,36,37).
NF-κB, promoting expression of MMP-2 and MMP-9, has previously been
reported as a downstream target of matrine in cancer cells
(32,36,37).
Our study indicated that matrine significantly reduces the
expression levels of MMP-2 and MMP-9, implying that matrine may
inhibit the invasion of DU145 and PC-3 cells by downregulating the
expression and activity of MMP-2 and MMP-9. Further analysis on the
signaling pathway regulating MMP-2 and MMP-9 suggested that matrine
significantly decreased the expression levels of NF-κB subunit p65
and p-p65 that are located within the nuclei of DU145 and PC-3
cells. All results indicated that NF-κB-MMP-2/9 is a key signaling
pathway by which matrine regulates the invasive and metastatic
abilities of CRPC cells.
In conclusion, this study provides evidence that
matrine is capable of inhibiting the proliferation, migration and
invasion of CRPC cells. The invasion is most likely inhibited by
reducing expression levels of MMP-2 and MMP-9 through inactivation
of NF-κB pathway. A novel potential therapeutic application of
matrine for anti-metastatic therapy of CRPC is revealed.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (nos. 81472382 and 81672550), the
National Natural Science Foundation of China for Young Scientists
Grant (no. 81101947), the Guangdong Province Natural Science
Foundation (no. 2014A030313079), the Fundamental Research Funds for
the Central Universities (no. 14ykpy19), the Guangdong Province
Science and Technology for Social Development Project (no.
2013B021800107), 2015 Guangzhou City Scientific Research Projects
(201510010298), the International Science and Technology
Cooperation Project of Guangdong Province Science and Technology
Plan (no. 2016A050502020) and the Chinese National Scholarship to
Hai Huang; the Natural Science Foundation of Guangdong Province
(nos. 2015A030310091 and 2016A030313185), and the Medical
Scientific Research Foundation of Guangdong Province (no. A2015027)
to Kaiwen Li; and NCI R01CA142862 to Leyuan Liu. This study was
also supported by Grant [2013]163 from Key Laboratory of Malignant
Tumor Molecular Mechanism and Translational Medicine of Guangzhou
Bureau of Science and Information Technology.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Limonta P and Manea M:
Gonadotropin-releasing hormone receptors as molecular therapeutic
targets in prostate cancer: Current options and emerging
strategies. Cancer Treat Rev. 39:647–663. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lassi K and Dawson NA: Update on
castrate-resistant prostate cancer: 2010. Curr Opin Oncol.
22:263–267. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Acar O, Esen T and Lack NA: New
therapeutics to treat castrate-resistant prostate cancer. Sci World
J. 2013:3796412013. View Article : Google Scholar
|
|
5
|
Su Z, Yang Z, Xu Y, Chen Y and Yu Q:
Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol
Cancer. 14:482015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jain RK: Molecular regulation of vessel
maturation. Nat Med. 9:685–693. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen K, Zhang S, Ji Y, Li J, An P, Ren H,
Liang R, Yang J and Li Z: Baicalein inhibits the invasion and
metastatic capabilities of hepatocellular carcinoma cells via
down-regulation of the ERK pathway. PLoS One. 8:e729272013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Page-McCaw A, Ewald AJ and Werb Z: Matrix
metalloproteinases and the regulation of tissue remodelling. Nat
Rev Mol Cell Biol. 8:221–233. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Björklund M and Koivunen E:
Gelatinase-mediated migration and invasion of cancer cells. Biochim
Biophys Acta. 1755:37–69. 2005.PubMed/NCBI
|
|
10
|
Yang SF, Hsieh YS, Lin CL, Hsu NY, Chiou
HL, Chou FP and Chu SC: Increased plasma levels of urokinase
plasminogen activator and matrix metalloproteinase-9 in nonsmall
cell lung cancer patients. Clin Chim Acta. 354:91–99. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bilandzic M, Wang Y, Ahmed N, Luwor RB,
Zhu HJ, Findlay JK and Stenvers KL: Betaglycan blocks metastatic
behaviors in human granulosa cell tumors by suppressing
NFκB-mediated induction of MMP2. Cancer Lett. 354:107–114. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yong J, Wu X and Lu C: Anticancer advances
of matrine and its derivatives. Curr Pharm Des. 21:3673–3680. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang W, You RL, Qin WJ, Hai LN, Fang MJ,
Huang GH, Kang RX, Li MH, Qiao YF, Li JW, et al: Anti-tumor
activities of active ingredients in compound Kushen injection. Acta
Pharmacol Sin. 36:676–679. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun N, Wang ZW, Wu CH, Li E, He JP, Wang
SY, Hu YL, Lei HM and Li HQ: Antiviral activity and underlying
molecular mechanisms of Matrine against porcine reproductive and
respiratory syndrome virus in vitro. Res Vet Sci. 96:323–327. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shao H, Yang B, Hu R and Wang Y: Matrine
effectively inhibits the proliferation of breast cancer cells
through a mechanism related to the NF-κB signaling pathway. Oncol
Lett. 6:517–520. 2013.PubMed/NCBI
|
|
16
|
Zhang Y, Zhang H, Yu P, Liu Q, Liu K, Duan
H, Luan G, Yagasaki K and Zhang G: Effects of matrine against the
growth of human lung cancer and hepatoma cells as well as lung
cancer cell migration. Cytotechnology. 59:191–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen K, Hu Z, Wang T, Guo H and Ye Z:
Inhibitory effect of matrine on the expression of PSA and AR in
prostate cancer cell line LNCaP. J Huazhong Univ Sci Technolog Med
Sci. 28:697–699. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Q, Lai Y, Wang C, Xu G, He Z, Shang X,
Sun Y, Zhang F, Liu L and Huang H: Matrine inhibits the
proliferation, invasion and migration of castration-resistant
prostate cancer cells through regulation of the NF-κB signaling
pathway. Oncol Rep. 35:375–381. 2016.
|
|
19
|
Madka V and Rao CV: Anti-inflammatory
phytochemicals for chemoprevention of colon cancer. Curr Cancer
Drug Targets. 13:542–557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Parikh NR, Mandal A, Bhatia D, Siveen KS,
Sethi G and Bishayee A: Oleanane triterpenoids in the prevention
and therapy of breast cancer: Current evidence and future
perspectives. Phytochem Rev. 13:793–810. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mohler JL, Armstrong AJ, Bahnson RR,
Boston B, Busby JE, D'Amico AV, Eastham JA, Enke CA, Farrington T,
Higano CS, et al: Prostate cancer, Version 3.2012: Featured updates
to the NCCN guidelines. J Natl Compr Canc Netw. 10:1081–1087.
2012.PubMed/NCBI
|
|
22
|
Sridhar SS, Freedland SJ, Gleave ME,
Higano C, Mulders P, Parker C, Sartor O and Saad F:
Castration-resistant prostate cancer: From new pathophysiology to
new treatment. Eur Urol. 65:289–299. 2014. View Article : Google Scholar
|
|
23
|
Yang N, Han F, Cui H, Huang J, Wang T,
Zhou Y and Zhou J: Matrine suppresses proliferation and induces
apoptosis in human cholangiocarcinoma cells through suppression of
JAK2/STAT3 signaling. Pharmacol Rep. 67:388–393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Niu H, Zhang Y, Wu B, Zhang Y, Jiang H and
He P: Matrine induces the apoptosis of lung cancer cells through
downregulation of inhibitor of apoptosis proteins and the Akt
signaling pathway. Oncol Rep. 32:1087–1093. 2014.PubMed/NCBI
|
|
25
|
Zhou K, Ji H, Mao T and Bai Z: Effects of
matrine on the proliferation and apoptosis of human medulloblastoma
cell line D341. Int J Clin Exp Med. 7:911–918. 2014.PubMed/NCBI
|
|
26
|
Horak CEBJ, Bouadis A and Steeg PS:
Metastasis-the evasion of apoptosis. Apoptosis, cell signaling, and
human diseases. Mol Mech. 1:63–96. 2007.
|
|
27
|
Zörnig M, Hueber A, Baum W and Evan G:
Apoptosis regulators and their role in tumorigenesis. Biochim
Biophys Acta. 1551:F1–F37. 2001.PubMed/NCBI
|
|
28
|
Burgess DJ: Apoptosis: Refined and lethal.
Nat Rev Cancer. 13:792013. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Verbrugge I, Johnstone RW and Smyth MJ:
SnapShot: Extrinsic apoptosis pathways. Cell. 143:11921192
e1191–1192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luzzi KJ, MacDonald IC, Schmidt EE,
Kerkvliet N, Morris VL, Chambers AF and Groom AC: Multistep nature
of metastatic inefficiency: Dormancy of solitary cells after
successful extravasation and limited survival of early
micrometastases. Am J Pathol. 153:865–873. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bai L and Wang S: Targeting apoptosis
pathways for new cancer therapeutics. Annu Rev Med. 65:139–155.
2014. View Article : Google Scholar
|
|
32
|
Yu P, Liu Q, Liu K, Yagasaki K, Wu E and
Zhang G: Matrine suppresses breast cancer cell proliferation and
invasion via VEGF-Akt-NF-kappaB signaling. Cytotechnology.
59:219–229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Parks WC, Wilson CL and López-Boado YS:
Matrix metalloproteinases as modulators of inflammation and innate
immunity. Nat Rev Immunol. 4:617–629. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar
|
|
35
|
Yeh CB, Hsieh MJ, Hsieh YH, Chien MH,
Chiou HL and Yang SF: Antimetastatic effects of norcantharidin on
hepatocellular carcinoma by transcriptional inhibition of MMP-9
through modulation of NF-κB activity. PLoS One. 7:e310552012.
View Article : Google Scholar
|
|
36
|
Li Y, Zhang ZN, Zhao HM, Tong ZC, Yang J,
Wang H and Liang XJ: Matrine inhibits the invasive properties of
human osteosarcoma cells by downregulating the ERK-NF-κB pathway.
Anticancer Drugs. 25:1035–1043. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun B and Xu M: Matrine inhibits the
migratory and invasive properties of nasopharyngeal carcinoma
cells. Mol Med Rep. 11:4158–4164. 2015.PubMed/NCBI
|