Introduction
Multiple myeloma (MM) is a plasma cell neoplasm,
characterized by an enlarged population of clonal B-cells in the
bone marrow (1), overproduction of
a monoclonal immunoglobulin (Ig) in the blood and/or urine, and
damage to end organs, including osteolytic bone lesions, renal
impairment, hypercalcemia and anemia (2,3).
In spite of advances in many therapeutic strategies,
MM remains an incurable hematologic neoplasm. Moreover, MM patients
are susceptible to relapse and drug resistance (2,4–6).
Therefore, a new treatment is needed for treating MM patients.
Arsenic trioxide (ATO; As2O3),
an FDA-approved drug for acute promyelocytic leukemia (APL), is a
well-known anticancer drug. It has been shown to be effective
against various solid cancer cell lines (7–9).
However, it has to be administered intravenously, and it is
questionable whether it can be the cornerstone of treatment for
solid tumors and other hematologic neoplasms (10).
KML001 (sodium metaarsenite; NaAsO2) is a
water-soluble, therefore, orally bioavailable, arsenical compound
(11–13). It has been suggested that one of
its mechanisms of action is to target the telomeres of malignant
cells (11).
In this study, using human MM cells and analyzing
the cell cycle, apoptosis, signaling pathways for cell growth,
telomere lengths, and DNA damage, we present the first evidence for
an antitumor action of MM.
Materials and methods
Cell lines and cell culture
The MM cell lines RPMI8226, U266, IM-9, MC/CAR,
KMS.11, and MM1.S were obtained from the ATCC (Rockville, MD, USA)
and maintained following a protocol provided by the ATCC. RPMI-1640
medium (Gibco-BRL, Gaithersburg, MD, USA) supplemented with 10%
(vol/vol) fetal bovine serum (FBS; Hyclone Labs, Inc., Logan, UT,
USA), 100 U/ml penicillin and 100 µg/ml streptomycin (Sigma
Chemical Co., St. Louis, MO, USA) was used to culture the cells in
tissue flasks. The cultures were maintained in a humidified
atmosphere with 5% CO2 at 37°C. The culture medium was
changed every 3–4 days.
Reagents
KML001 was acquired from Komipharm International
(Gyeonggi-Do, Korea). It was dissolved at 10−3 M in
distilled water and aliquots were stored at 4°C. The stock solution
was stable for more than a year, and was diluted with RPMI-1640
medium daily to obtain working concentrations.
As2O3 was purchased from Sigma-Aldrich Corp.
(St. Louis, MO, USA). A 5×10−2 M stock solution was
prepared in 5 M NaOH and aliquots were stored at 4°C.
In vitro cytotoxicity assays using
MTT
The in vitro cytotoxicity of KML001 for
myeloma cells was assessed with MTT
(3-[4,5-dimethylthiazol-2yl]-2,5-diphenyltetrazolium bromide) by
measuring dye absorption by living cells. In brief, cells
(1×104 cells/well) were seeded in 96-well plates and
incubated for 3 days at 37°C with 5% CO2. Then, 50
µl of MTT (Sigma) solution (2 mg/ml in PBS) was added to
each well and incubation continued for 4 h at 37°C. DMSO (200
µl/well) was added and the absorbance at 540 nm was recorded
using an iMark microplate absorbance reader (Bio-Rad, Hercules, CA,
USA).
In vitro apoptosis assay
Apoptosis was observed by staining cells with
Annexin V-FITC and PI labeling. Quantification of apoptosis of
cells was achieved by washing prepared cells twice with cold PBS
and re-suspending them in binding buffer (10 mM HEPES/NaOH, pH 7.4,
140 mM NaCl, 2.5 mM CaCl2) at 1×106 cells/ml.
One hundred microliters of the resulting suspension
(1×105 cells) was transferred to a 5-ml culture tube
containing 5 µl of Annexin V-FITC (Pharmingen, San Diego,
CA, USA) and 10 µl of 20 µg/ml PI (Sigma). The cells
were vortexed and allowed to incubate for 15 min at room
temperature in the dark. Following the addition of 400 µl of
binding buffer, the cells were analyzed by FACStar flow cytometry
(Becton-Dickinson, CA, USA).
Western blot analysis
Whole lysates (20 µg) were resolved on the
10% SDS-PAGE gels, and transferred to a nitrocellulose membranes
(Bio-Rad) by electro-blotting. Antibodies to CDK2, CDK4, CDK6,
cyclin A, cyclin B1, cyclin D1, cyclin E, p21, p27, Bcl-2, Bax,
caspase-3, -8 and -9, pChk1, pChk2, p65, p50 (Santa Cruz
Biotechnology, Santa Cruz, CA, USA), pSTAT1, STAT, pSTAT3, STAT3,
pSTAT5, pAKT, AKT, pmTOR, mTOR, pPTEN, PTEN, pP38, p38, pERK, ERK,
pJNK, JNK (Cell Signaling, MA, USA), and
p-γ-H2AX (Ser139) (Upstate Biotechnology, NY,
USA) were used for western blotting.
Immunoprecipitation (IP) and kinase
assays
Samples of 250 µg total protein were
incubated with anti-CDK2, anti-CDK4, and anti-CDK6 polyclonal
antibodies for 16 h at 4°C with continuous agitation. Protein
A/G-agarose (25 µl) was added to the mixtures and,
centrifuged at 1,200 rpm for 2 min, and the resulting pellets were
washed 3 times with extraction buffer to collect immune complexes.
SDS-polyacrylamide denaturing gels were used to resolve the immune
complexes; the resolved proteins, were then transferred to
nitrocellulose membranes, and probed with the anti-p27 antibody. An
ECL kit (Intron, Korea) was used to develop the blots.
For the kinase assay, immune complexes were washed
twice with kinase reaction buffer [50 mM Tris-Cl (pH 7.5), 10 mM
MgCl2 and 1 mM DTT]. CDK2, CDK4 and CDK6 kinase assays
with histone H1 as substrate were performed by mixing the
respective immune complexes with 5 µg of histone H1 and 1
µCi [γ-32P]ATP in 30 µl of kinase reaction
buffer. Kinase reactions were performed at 37°C for 30 min and
terminated with 2X SDS-PAGE loading buffer. After boiling for 5
min, the reaction products were separated electrophoretically on
12% SDS-PAGE gels, and analyzed by autoradiography to detect
phosphorylated proteins.
Quantitative hTERT real-time PCR
Total RNA was isolated using TRI reagent (Molecular
Research Center, Inc., Cincinnati, OH, USA), and cDNAs were
synthesized from 1 µg of total RNA using SuperScript-II
Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) with random
hexamers. Quantitative PCR was performed with a Lightcycler 480 II
(Roche Diagnostics Corp.) using SYBR Green I as a double-strand
DNA-specific binding dye. Quantitative hTERT real-time PCR was
performed as described previously (14).
TRF length analysis
A TeloTAGG telomere restriction fragment (TRF)
length kit was used, according to the instructions provided by the
manufacturer (Roche Diagnostics GmbH, Mannhein, Germany). In brief,
10 µg of genomic DNA was digested with a 6-fold excess of
restriction enzymes HinfI and RsaI at 37°C for 2 h.
The resulting DNA digest was fractionated by electrophoresis on a
0.8% agarose gel and transferred to a nylon membrane by southern
blotting. The DNA was fixed to the membrane by UV-crosslinking (120
mJ), and the immobilized DNA fragments were hybridized with
digoxigenin-labelled telomere-specific probes (3′-TTAGGG-5′). The
resulting hybrids were detected with anti-digoxigenin coupled to
alkaline phosphatase, which metabolizes the chemiluminescent
substrate, CDP-Star. The membrane was then exposed to X-ray
film.
Statistical analysis
Data are presented as means ± SDs, and were analyzed
with Student's t-test. Probability values of <0.05 were
considered to be statistically significant.
Results
Cytotoxic effect of KML001 on multiple
myeloma cell lines
Significant dose-dependent inhibition of cell growth
was observed in all of seven cell lines after treatment of KML001
for 72 h (Fig. 1A). KML001 at
5×10−8 M exhibited >50% inhibition of growth in all
cell lines. As2O3 was less effective than
KML001 in four MM cell lines: RPMI-1640, U266, IM-9 and MC/CAR
(Fig. 1B). KML001 inhibited the
proliferation of U266 cells in a time-dependent manner, while
As2O3 did not (Fig. 1C and D).
Levels of cell cycle-regulatory molecules
in KML001-treated U266 cells
Levels of CDK2, CDK4, CDK6, cyclin A, cyclin B1,
cyclin D1 and cyclin E were reduced in KML001-treated U266 cells,
but unaltered in As2O3-treated cells (data
not shown). Levels of p21 and p27, which negatively regulate
various cyclin/CDK complexes, increased in a time-dependent manner
(Fig. 2), suggesting that KML001
inhibited cell cycle arrest in U266 cells.
Association of p21 and p27 with CDK2,
CDK4, and CDK6 in KML001-treated U266 cells
Since western blot analysis showed that KML001
induced marked accumulation of CDK inhibitors (p21 and p27), we
determined whether the p21 and p27 proteins induced by KML001
(5×10−8 M for 72 h) could be detected as complexes with
CDKs. As shown in Fig. 3A, CDK
complexes immunoprecipitated from KML001-treated U266 cells with
anti-CDK2, -CDK4 and -CDK6 antibodies contained larger amounts of
p21 and p27 proteins than those immunoprecipitated from untreated
cells. This suggest that the p21 and p27 proteins inhibit CDK2,
CDK4 and CDK6 activities by direct binding to these CDKs.
CDK-associated kinase activity in
KML001-treated U266 cells
In order to determine whether binding of the CDK
inhibitors (p21 and p27) resulted in the inhibition of CDK
activity, in vitro CDK activity assays were performed. A
dramatic decrease in CDK2-, CDK4- and CDK6-associated kinase
activities was observed in KML001-treated U266 cells (Fig. 3B). These results suggest that
KML001 inhibits the G1 phase cell cycle arrest via reducing CDK2-,
CDK4-, and CDK6 kinase activities through the binding of p21 and
p27 proteins.
Induction of apoptosis by KML001 in U266
cells
In order to determine whether KML001 treatment
induced apoptosis in U266 cells, apoptosis was detected by FACS
analysis. As shown in Fig. 4A,
treatment of U266 cells with KML001 led to an increase in the
proportion of Annexin V-FITC-stained cells. With 10−7 M
KML001, 59.7% of the cells were either apoptotic or dead.
With regard to changes in Bcl-2 family members, Bax
was increased by treatment with KML001, and Bcl-2 decreased
(Fig. 4B). These observations
suggested that apoptosis might be mediated by activation of the
pro-apoptotic protein Bax and downregulation of the anti-apoptotic
protein Bcl-2. We therefore measured the activation of caspases by
KML001. As shown in Fig. 4B, the
cleaved forms of initiator caspase-3 and -8 increased while
procaspase-9 declined. Thus KML001-induced apoptosis was
accompanied by a reduction of Bcl-2 level and increased caspase
activities.
Effect of KML001 on cell signaling
One of the most important oncogenic signaling
pathways involves STAT proteins. To investigate the role of STAT
molecules in KML001-treated U266 cells, STAT proteins were
determined by immunoblotting. STAT proteins levels were not changed
by KML001 treatment, but the phosphorylated forms of STAT1 and 3
declined in a dose-dependent manner. Regarding the Akt signaling
pathway, KML001 decreased p-Akt and p-mTOR levels. In addition,
p-PTEN increased in a dose-dependent manner, and pERK (p44/42)
(Fig. 5A) and the p65 and p50
subunits of NF-κB (Fig. 5B)
decreased. These results suggested that the cytotoxic effect of
KML001 is mediated through a number of oncogenic signaling
pathways.
Effect of KML001 on telomere-related DNA
damage signals
Uncapped telomeres are recognized as DNA damage.
Formation of the phosphorylated form of
γ-H2AX represents a very early event in DNA
damage signaling of double-strand breaks (15,16).
Therefore, p-γ-H2AX is a hallmark of the
cellular response to DNA double strand breaks (DSB). We found that
p-γ-H2AX increased significantly at 24 h
KML001 treatment (Fig. 6). DNA
damaging agents cause single strand DNA (ssDNA) and double-strand
break (DSB) lesions, which initiate activation of ATR-Chk1 and
ATM-Chk2, respectively (17,18).
Phosphorylated Chk1 and Chk2 increased significantly in 24
h-treated U266 cells (Fig. 6).
Effect of KML001 on hTERT expression and
telomere length
Telomerase activity has been found in >85% of
human tumors. We asked whether KML001 altered the level of the mRNA
or the catalytic subunit of telomerase, human telomere reverse
transcriptase (hTERT). Real-time PCR analysis, showed that hTERT
mRNA expression decreased in 12 h-treated U266 cells (Fig. 7).
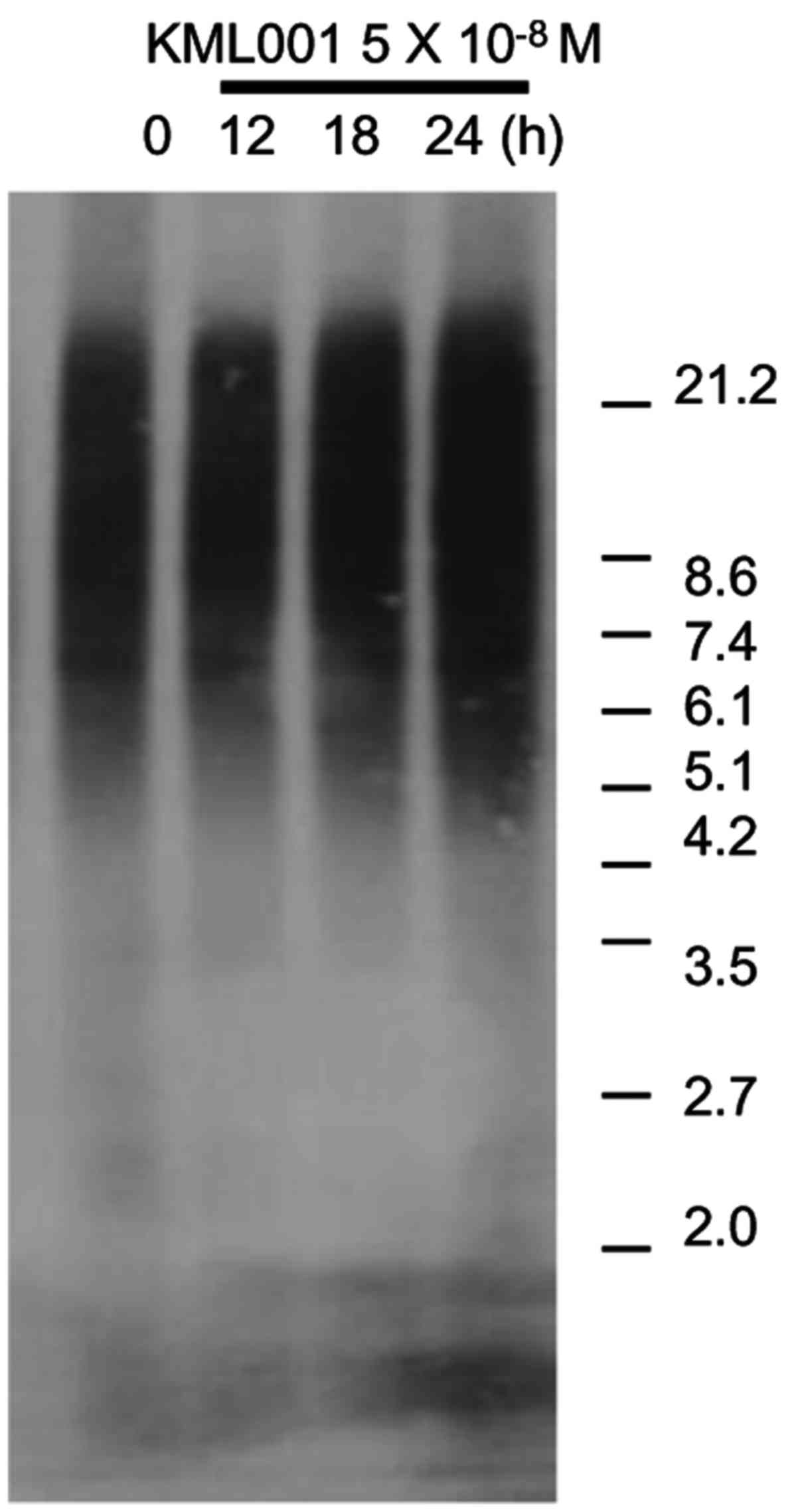
It has been shown that KML001 binds to human
telomeric sequences and causes rapid erosion of the telomeres of
cancer cells (19). Using southern
blots of terminal restriction fragements (TRFs) we investigated the
effect of KML001 on U266 telomeres. We observed no reduction of TRF
length by, KML001 treatment for up to 24 h (Fig. 8).
Discussion
We showed above that KML001 inhibited the growth all
7 MM cell lines in a dose-dependent manner and
As2O3 was less effective. We also observed
that KML001 inhibited the growth of U266 cells by inducing G1
arrest and apoptosis, and reduced levels of cyclin D1, cyclin E,
CDK2, CDK4, and CDK6.
KML001 treatment increased levels of the CDK
inhibitors p21 and p27, which bound to CDK2, CDK4, and CDK6
proteins and lowered CDK2, CDK4, and CDK6 kinase activities.
Taken together, our results suggest that KML001
causes G1 cell cycle arrest via inhibition of CDK2, CDK4, and CDK6
kinase activities by p21 and p27.
We observed apoptosis using Annexin V/PI double
staining in KML001-treated U266 cells. Apoptotic cells increased in
a time-dependent manner and KML001 induced downregulation of
anti-apoptotic Bcl-2 and upregulation of pro-apoptotic Bax in a
time- and dose-dependent manner.
Apoptosis is initiated by caspases, a large family
of cysteine protease enzymes (20). It requires the activation of
initiator caspases (caspase-8 and -9) and executioner caspases
(caspases-3 and -7). Cleaved-caspase-8, -9 and -3 were detected in
KML001-treated U266 cells. Taken together, these observations
provide strong KML001 induces apoptosis of U266 cells.
Key signaling pathways in cancer include the STAT,
the MAPK and PI3K/Akt pathway. In this study, the phosphorylated
forms of STAT1 and 3 were decreased in 2 h KML001-treated cells,
the phosphorylated forms of ERK decreased, phosphorylated PTEN
increased and phosphorylated Akt/mTOR decreased.
NF-κB/Rel proteins are dimers composed of p65 and
p50 subunits involved in various cell signaling pathways including
survival, proliferation and inflammation. We showed previously that
the expression of p65 and p50 was reduced in hematologic cancer
cells after exposure to KML001 (14,21).
In this study, we demonstrated that p65 and p50 levels were reduced
by exposure to KML001.
Telomeres, the nucleoprotein caps, are composed of
the telomeric repeat sequences (TTAGGG)n at the ends of
eukaryotic chromosomes that protect chromosomes from degradation,
end-to-end fusion, and recombination (22,23).
As a result, telomere uncapping can cause a cellular response to
DNA double strand breaks (DSB). Phosphorylation of
γ-H2AX at Ser139 is an early event in DSB-DDR
(DNA damage response) (15,16).
In the present study, phosphorylated γ-H2AX
increased significantly in U266 cells following KML001
treatment.
DNA damaging agents generate SSB and/or DSB lesions.
Typically, ssDNA initiates ATR-Chk1 activation, and DSB lesion are
initiated by ATM-Chk2 activation (17,18).
In this study, KML001 significantly increased the levels of
phosphorylated Chk1 and Chk2 in U266 cells, suggesting that it
might impair the telomeres.
Telomerase activity has been found in 85–90% of
human tumors. However, it is not found in most normal human somatic
cells, which lack hTERT (24,25).
Previous studies showed that arsenic inhibits the transcription of
hTERT (26). We also confirmed
that the mRNA level of hTERT decreased as early as 12 h after
treatment of KML001 in U266 cells, and we showed previously that
KML001 induced telomere restriction fragment (TRF) length
shortening in non-Hodgkin's lymphoma cells (14). However, we detected no restriction
fragment (TRF) length shortening in U266 cells after exposure to
KML001 for 72 h. Wang et al reported that an oligonucleotide
telomerase template antagonist induced telomeric shortening
following 14-day treatment of multiple myeloma (27). Therefore, the absence of telomeric
shortening in our results might be due to the shorter treatment (24
h) with KML001.
We showed previously that KML001 had no severe side
effects in nude mice. In addition, non-Hodgkin's lymphoma patients
treated with KML001 showed no severe side effects except for grade
I/II anorexia and nausea (14). In
unpublished clinical study we treated 3 MM patients who had
relapsed and were refractory to conventional and high-dose
chemotherapies with KML001, and obtained partial responses (data
not shown).
In conclusion, we have demonstrated that KML001
inhibits the proliferation of human multiple myeloma cell lines and
that it acts by inducing cell cycle arrest, triggering apoptosis
and inhibiting various cell signaling pathways. Consequently,
KML001 could be an effective agent for treating MM patients.
Acknowledgments
This study was conducted in the Hanyang University
Hospital Clinical Laboratory, and this study was supported by
Komipharm International Co., Ltd., Gyeonggi-do, Korea.
References
|
1
|
Hallek M, Bergsagel PL and Anderson KC:
Multiple myeloma: Increasing evidence for a multistep
transformation process. Blood. 91:3–21. 1998.PubMed/NCBI
|
|
2
|
Palumbo A and Anderson K: Multiple
myeloma. N Engl J Med. 364:1046–1060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raab MS, Podar K, Breitkreutz I,
Richardson PG and Anderson KC: Multiple myeloma. Lancet.
374:324–339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy
MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust
JA, et al: Improved survival in multiple myeloma and the impact of
novel therapies. Blood. 111:2516–2520. 2008. View Article : Google Scholar
|
|
5
|
Laubach J, Richardson P and Anderson K:
Multiple myeloma. Annu Rev Med. 62:249–264. 2011. View Article : Google Scholar
|
|
6
|
Mahindra A, Laubach J, Raje N, Munshi N,
Richardson PG and Anderson K: Latest advances and current
challenges in the treatment of multiple myeloma. Nat Rev Clin
Oncol. 9:135–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ling YH, Jiang JD, Holland JF and
Perez-Soler R: Arsenic trioxide produces polymerization of
microtubules and mitotic arrest before apoptosis in human tumor
cell lines. Mol Pharmacol. 62:529–538. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Uslu R, Sanli UA, Sezgin C, Karabulut B,
Terzioglu E, Omay SB and Goker E: Arsenic trioxide-mediated
cytotoxicity and apoptosis in prostate and ovarian carcinoma cell
lines. Clin Cancer Res. 6:4957–4964. 2000.
|
|
9
|
Zhang TC, Cao EH, Li JF, Ma W and Qin JF:
Induction of apoptosis and inhibition of human gastric cancer
MGC-803 cell growth by arsenic trioxide. Eur J Cancer.
35:1258–1263. 1999. View Article : Google Scholar
|
|
10
|
Litzow MR: Arsenic trioxide. Expert Opin
Pharmacother. 9:1773–1785. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Phatak P, Dai F, Butler M, Nandakumar MP,
Gutierrez PL, Edelman MJ, Hendriks H and Burger AM: KML001
cytotoxic activity is associated with its binding to telomeric
sequences and telomere erosion in prostate cancer cells. Clin
Cancer Res. 14:4593–4602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Woo SR, Ham Y, Kang W, Yang H, Kim S, Jin
J, Joo KM and Nam DH: KML001, a telomere-targeting drug, sensitizes
glioblastoma cells to temozolomide chemotherapy and radiotherapy
through DNA damage and apoptosis. BioMed Res Int. 2014:7474152014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang MH, Kim HT, Lee KT, Yang S, Lee JK,
Lee KH and Rhee JC: KML001 inhibits cell proliferation and invasion
in pancreatic cancer cells through suppression of NF-κB and VEGF-C.
Anticancer Res. 34:3469–3474. 2014.PubMed/NCBI
|
|
14
|
Yoon JS, Hwang DW, Kim ES, Kim JS, Kim S,
Chung HJ, Lee SK, Yi JH, Uhm J, Won YW, et al: Anti-tumoral effect
of arsenic compound, sodium metaarsenite (KML001), in non-Hodgkin's
lymphoma: An in vitro and in vivo study. Invest New Drugs. 34:1–14.
2016. View Article : Google Scholar
|
|
15
|
d'Adda di Fagagna F, Reaper PM,
Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G,
Carter NP and Jackson SP: A DNA damage checkpoint response in
telomere-initiated senescence. Nature. 426:194–198. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rogakou EP, Pilch DR, Orr AH, Ivanova VS
and Bonner WM: DNA double-stranded breaks induce histone H2AX
phosphorylation on serine 139. J Biol Chem. 273:5858–5868. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bartek J and Lukas J: DNA damage
checkpoints: From initiation to recovery or adaptation. Curr Opin
Cell Biol. 19:238–245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jazayeri A, Falck J, Lukas C, Bartek J,
Smith GC, Lukas J and Jackson SP: ATM- and cell cycle-dependent
regulation of ATR in response to DNA double-strand breaks. Nat Cell
Biol. 8:37–45. 2006. View
Article : Google Scholar
|
|
19
|
Zhang B, Suer S, Livak F, Adediran S,
Vemula A, Khan MA, Ning Y and Hussain A: Telomere and microtubule
targeting in treatment-sensitive and treatment-resistant human
prostate cancer cells. Mol Pharmacol. 82:310–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thornberry NA and Lazebnik Y: Caspases:
Enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoon JS, Kim ES, Park BB, Choi JH, Won YW,
Kim S and Lee YY: Anti-leukemic effect of sodium metaarsenite
(KML001) in acute myeloid leukemia with breaking-down the
resistance of cytosine arabinoside. Int J Oncol. 46:1953–1962.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blackburn EH: Telomeres and telomerase:
Their mechanisms of action and the effects of altering their
functions. FEBS Lett. 579:859–862. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moyzis RK, Buckingham JM, Cram LS, Dani M,
Deaven LL, Jones MD, Meyne J, Ratliff RL and Wu JR: A highly
conserved repetitive DNA sequence, (TTAGGG)n, present at the
telomeres of human chromosomes. Proc Natl Acad Sci USA.
85:6622–6626. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Masutomi K, Yu EY, Khurts S, Ben-Porath I,
Currier JL, Metz GB, Brooks MW, Kaneko S, Murakami S, DeCaprio JA,
et al: Telomerase maintains telomere structure in normal human
cells. Cell. 114:241–253. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shay JW and Gazdar AF: Telomerase in the
early detection of cancer. J Clin Pathol. 50:106–109. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chou WC, Hawkins AL, Barrett JF, Griffin
CA and Dang CV: Arsenic inhibition of telomerase transcription
leads to genetic instability. J Clin Invest. 108:1541–1547. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang ES, Wu K, Chin AC, Chen-Kiang S,
Pongracz K, Gryaznov S and Moore MA: Telomerase inhibition with an
oligonucleotide telomerase template antagonist: In vitro and in
vivo studies in multiple myeloma and lymphoma. Blood. 103:258–266.
2004. View Article : Google Scholar
|