Introduction
Breast cancer is one of the most frequently
diagnosed types of cancer and the second major cause of
cancer-related mortality among women worldwide (1,2). It
has been reported that 1 in 8 women may suffer from breast cancer
in her lifetime (3,4). Surgery, chemotherapy, radiotherapy,
endocrine therapy and targeted therapy present the main treatment
strategies for breast cancer. However, patients with breast cancer
always suffer from tumor recurrence and treatment resistance
(5,6). Although numerous studies have focused
on tumor suppressors and oncogenes for breast cancer and have
attempted to unveil the mechanisms responsible tumorigenesis,
development and metastasis, the accurate underlying molecular
mechanisms remain to be fully understood. Further studies of the
underlying molecular mechanisms of breast cancer are desirable, and
may aid in the development of novel clinical diagnostic and
therapeutic methods.
RNA binding motif protein 3 (RBM3), a member of the
highly conserved family of RNABPs, is a glycine rich protein and is
expressed in various human tissues (7-10).
RBM3 protein contains a RNA-recognition motif (RRM), through which
RBM3 protein can bind to both DNA and RNA (11,12).
RBM3 protein interacts with the untranslated regions (UTRs) of
mRNAs, resulting in either the stabilization or destabilization of
the mRNA (11,13). However, in fact, the exact genes
that can be regulated by RBM3 are limited. RBM3 contributes to the
response of cellular stressors, such as degenerative and hypoxic
conditions (7,14). Moreover, RBM3 has been documented
to promote cell proliferation and erythropoietic differentiation
(15,16). Recent studies have demonstrated
that RBM3 plays a promoting role in human colorectal cancer
(17,18) and prostate cancer (15). However, RBM3 has been identified to
be a tumor suppressor in human ovarian cancer (11), urothelial bladder cancer (19), malignant melanoma (8), and esophageal and gastric
adenocarcinoma (20). These data
suggest RBM3 plays differential roles in various human cancers and
exhibits tissue specificity. To the best of our knowledge, to date,
the role of RBM3 in human breast cancer remains unclear.
In the present study, we identified the
tumor-promoting role of RBM3 in human breast cancer. The
overexpression of RBM3 was observed in human breast cancer tissues
compared with adjacent non-tumor tissues. A high level of RBM3 was
found to be associated with both a low relapse-free survival (RFS)
and overall survival (OS) rates of breast cancer patients. In
patients with breast cancer, the expression of RBM3 was associated
with patient lymph node metastasis and a high tumor grade. RBM3 was
also found to promote the proliferation and metastasis of human
breast cancer cells. Moreover, RBM3 was determined to regulate the
expression of actin related protein 2/3 complex subunit 2
(ARPC2; a member of actin-related proteins that forms the
Arp2/3 complex). ARPC2 was also found to act as an oncogene
in breast cancer cells and to mediate the promoting role of RBM3 in
the proliferation and metastasis of breast cancer cells. Therefore,
RBM3 acts as an oncogene in human breast cancer cells and the
functional depletion of RBM3 may prove to be considered a potential
therapeutic strategy for breast cancer.
Materials and methods
Tissue samples and patients
A total of 103 human breast cancer tissues and 103
adjacent non-tumor tissues were collected from patients with breast
cancer who underwent surgery at Zhongda Hospital (Nanjing, China)
between 2010 and 2013. All these patients with breast cancer were
females. These tissues did not contain tissues from patients who
had received special therapies prior to surgery and patients with
other diseases. These patients with breast cancer were followed-up
for >60 months and their RFS and OS were documented. We also
collected the clinicopathological parameters of these patients with
breast cancer. Experiments related to the use of human tissues were
performed in accordance with The Code of Ethics of the World
Medical Association (Declaration of Helsinki) and local approval
(from the Institutional Review Boards of Southeast University) was
obtained prior to the commencement of this study. Informed consent
form was signed by each patient.
Immunohistochemistry
The protein levels of RBM3 and ARPC2 in the neutral
buffered formalin-fixed (room temperature for 24 h) and
paraffin-embedded tissue sections (4 µm) were examined by
immunohistochemistry using an Ultra Sensitive-SP kit (Maixin-Bio,
Fuzhou, China) as previously described (21,22).
RBM3 rabbit polyclonal antibody (1:100, 14363-1-AP; Proteintech
Group, Rosemont, IL, USA) and ARPC2 rabbit polyclonal antibody
(1:200, 15058-1-AP; Proteintech Group) were used. The sections
stained by immunohistochemistry were evaluated by two senior
pathologists using an Olympus microscope (Olympus, Tokyo, Japan)
independently. Positive signals of RBM3 or ARPC2 ≥20% were
designated as having a high expression of RBM3 or ARPC2 and
positive signals <20% were designated as having a low expression
of RBM3 or ARPC2, respectively.
Cell lines and cell culture
Human normal breast cells (MCF10A and HMEC) and
breast cancer cells (MCF7, T47D, MDA-MB-468, BT474, MDA-MB-231 and
BT549) were obtained from the American Type Culture Collection
(ATCC, Manassas, VA, USA). The MCF10A, MCF7, T47D, MDA-MB-231 and
BT549 cells were cultured using RPMI-1640 medium containing 10%
FBS. The HMEC cells were cultured using DMEM:F12 (1:1) medium
containing 10% FBS. The BT474 cells were cultured using DMEM medium
containing 10% FBS. The MDA-MB-468 cells were cultured using L-15
medium containing 10% FBS. All these cell lines were cultured using
T25 plates at 5% CO2 and 37°C in a humidified atmosphere
as recommended by ATCC.
Western blot analysis
Protein levels of RBM3 and ARPC2 in both fresh human
tissues and cell lines were detected by western blot analysis,
which was carried essentially as described in previous studies
(21,22). Briefly, 30 µg proteins were
resolved by 10% SDS-PAGE and then transferred onto nitrocellulose
(NC) membranes (Roche, USA). Membrane blocking was carried out at
room temperature for 1.5 h with 1% (w/v) BSA. The membranes were
then incubated with RBM3 rabbit polyclonal antibody (1:1,000,
14363-1-AP; Proteintech Group), ARPC2 rabbit polyclonal antibody
(1:1,000, 15058-1-AP; Proteintech Group) or β-actin mouse
monoclonal antibody (1:5,000, A1978, Sigma, St. Louis, MO, USA)
overnight at 4°C, respectively. The membranes were then incubated
with secondary antibody (1:50,000; Invitrogen/Thermo Fisher
Scientific, Waltham, MA, USA) at room temperature for 1.5 h. The
protein bands were examined by a chemiluminescence system (EMD
Millipore, Billerica, MA, USA). We used IPP6.0 software to analyze
the densi-tometry of blots according to standard methods. β-actin
was detected as a control.
Cell transfection
The shRNA plasmids, including si-RBM3#1, si-RBM3#2,
si-ARPC2#1, si-ARPC2#2 and si-NC were designed and synthesized by
GenePharma (Shanghai, China). The mammalian expression plasmid
pIRESneo3 (Invitrogen/Thermo Fisher Scientific) was used for the
construction of the ARPC2 overexpression plasmid, and the
Vec plasmid was used as a control. In this study, we used
Lipofectamine 2000 (Invitrogen/Thermo Fisher Scientific) for
transfection as previously described (21,22).
The sequences used in this study were as follows: si-RBM3#1,
5′-GGAGG GCUCAACUUUAACATT-3′; si-RBM3#2, 5′-GGACGUUC
CAGAGACUAUATT-3′; si-ARPC2#1, 5′-CATTGTGCATC AAGCTGGCTT-3′;
si-ARPC2#2, 5′-CACAGGTCCTCTTTA GCCATT-3′; siNC,
5′-UUCUCCGAACGUGUCACGUTT-3′. The subsequent experiments were
carried out 48 h later; the transfection efficiency was determined
by western blot analysis, as described above.
Cellular proliferation assays
The cellular proliferation assays performed in this
study included a cell counting assay, MTT assay and cell colony
formation assay, which were carried out as described in previous
studies (21,23).
For cell counting assay, the cells were plated into
6-well plates. The total cell number was counted every day for 5
days and cell growth curves were created for cell proliferation
analysis. For MTT assay, the cells were plated into 96-well plates
(2,000 cells per well). MTT evaluation was performed 96 h later.
Briefly, 100 µl 5 mg/ml MTT (A100793, Sangon Biotech,
Shanghai, China) was added into 1 ml cell culture medium; the cell
culture medium in 96-well plates was then changed into the upper
medium (normal medium containing MTT) and the cells were incubated
in an incubator with 5% CO2 at 37°C for 1.5 h,
discarding the MTT medium and adding 200 µl DMSO per well
into the 96-well plates. This was followed by mixing for uniformity
and detection of the absorbance at OD570. The absorbance at OD570
was measured using a Multiskan FC spectrometer (Thermo Fisher
Scientific). For cell colony formation assay, cells were plated
into 6-well plates (1,000 per well). Cell colony formation was
calculated 10-15 days later. Briefly, this was carried out by the
addition of 200 µl 4% formaldehyde per well into the 6-well
plates and incubation for 20 min. The cells were then washed with
PBS and 100 µl 0.1% crystal violet (A100528, Sangon Biotech)
were added per well into the 6-well plates followed by incubation
for 20 min at room temperature and washing with water; images were
captured using an Canon camera 1500D (Canon, Tokyo, Japan)
(magnification, ×200) and the cell colony numbers were counted (by
the researcher according to the images).
Cellular metastasis assays
Cellular metastasis assays used in this study
included cell migration assay and cell invasion assay which were
carried out in 24-well 8-µm pore Transwell chambers (Corning
Inc., Corning, NY, USA). For cell invasion assay, the upper
chambers were coated with Matrigel (BD Biosciences, San Jose, CA,
USA). The upper chambers uncoated for cell migration assay. The
upper chambers were seeded with 2×104 cells in medium
containing 0.1% FBS. The lower chambers were added with medium with
5% FBS. For the cell migration assay, MCF7 cells were examined
after 24 h, and MDA-MB-231 cells were examined after 12 h. For the
cell invasion assay, MCF7 cells were examined after 48 h, and
MDA-MB-231 cells were examined after 24 h. The lower chambers (for
both cell migration assay and cell invasion assaay) were stained
with crystal violet (A100528, Sangon Biotech) for 20 min. Images
were captured using an Olympus microscope (20X objective; Olympus)
(magnification, ×200) and the cell numbers were counted (by the
researcher according to the images).
Reverse transcription-quantitative PCR
(RT-qPCR)
The mRNA levels of human epidermal growth factor
receptor 2 (HER2), MYC, ARPC2, signal transducer and
activator of transcription 3 (STAT3), phosphatase and tensin
homolog (PTEN), FOS, cyclin D1 (CCND1), epidermal
growth factor receptor (EGFR), progesterone receptor
(PGR) and trefoil factor 1 (TFF1) were examined by
RT-qPCR, which was performed as previously described (21,23).
GAPDH was used as a control. Briefly, an RT kit
(PrimeScript™ RT reagent kit, DRR037A; Takara, Dalian, China) was
used for the RT process, and the conditions were 37°C for 15 min
and 85°C for 5 sec. A qPCR kit [SYBR Premix Ex Taq™ II (Perfect
Real Time), DRR081A, Takara] was used for the qPCR process; the
conditions were as follows: 95°C for 10 min, 40 cycles of 95°C for
5 sec, 60°C for 30 sec, dissolution curve analysis at 95°C for 15
sec, 60°C for 30 sec and 95°C for 15 sec. The primers used in this
study were as follows: HER2 forward, 5′-GTCTGGAC
GTGCCAGTGTG-3′ and reverse, 5′-CATCTGGGAACTCAA GCAGG-3′; MYC
forward, 5′-CTGGTGCTCCATGAGGA GAC-3′ and reverse,
5′-GCACCTCTTGAGGACCAGTG-3′; ARPC2 forward,
5′-GACGACGATGTGGTCATTGG-3′ and reverse, 5′-CAATGTTGTCACCCACAGCG-3′;
STAT3 forward, 5′-CAGTTCTCCTCCACCACCAAG-3′ and reverse,
5′-GGTCAATGATATTGTCCAGCCAG-3′; PTEN forward,
5′-ATCAAGAGGGATAAAACACCATG-3′ and reverse,
5′-ATCTGACACAATGTCCTATTGCC-3′; FOS forward,
5′-GCAGACCGAGATTGCCAAC-3′ and reverse, 5′-GATCAAGGGAAGCCACAGAC-3′;
CCND1 forward, 5′-CGTGGCCTCTAAGATGAAGG-3′ and reverse,
CTGGCATTTTGGAGAGGAAG; EGFR forward,
5′-CAGCTTCTTGCAGCGATACAG-3′ and reverse,
5′-CTGGTAGTGTGGGTCTCTGCTG-3′; PGR forward,
5′-CTGACACCTCCAGTTCTTTGC-3′ and reverse,
5′-TCTCCATCCTAGACCAAACACC-3′; TFF1 forward,
5′-AGCAGAGAGGAGGCAATGG-3′ and reverse, 5′-GGATAGAAGCACCAGGGGAC-3′;
and GAPDH forward, 5′-TGCACCACCAACTGCTTAGC-3′ and reverse,
GGCATGGACTGTGGTCATGAG-3′. The RT-qPCR results were quantified using
2−∆∆Cq method (24).
Luciferase reporter assay
Luciferase reporter assay was performed in this
study using a Dual Luciferase Reporter Assay System (Promega Corp.,
Madison, WI, USA) as previously described (21,23).
Luciferase reporter plasmids (Promega Corp.) containing the
ARPC2 wild-type 3′untranslated region (UTR) was constructed.
ARPC2-3′UTR: CTTGGGAATAA GAGGAGGAAGCGGCTGGCAACTGAAGGCTGGAACA
CTTGCTACTGGATAATCGTAGCTTTTAATGTTGCGCC
TCTTCAGGTTCTTAAGGGATTCTCCGTTTTGGTTCCA
TTTTGTACACGTTTGGAAAATAATCTGCAGAAACGA
GCTGTGCTTGCAAAGACTTCATAGTTCCCAAGAATT
AAAAAAAAAAAAAAAAGAATTCCACTTGATCAACT
TAATTCCTTTTCTTTATCTTCCCTCCCTCACTTCCCTT
TTCTCCCACCCTCTTTTCCAAGCTGTTTCGCTTTGCA
ATATATTACTGGTAATGAGTTGCAGGATAATGCAGTC
ATAACTTGTTTTCTCCTAAGTATTTGAGTTCAAAACT
CCTGTATCTAAAGAAATACGGTTGGGGTCATTAATA
AAGAAAATCTTTCTATCTTACATGAGAA.
Co-transfection with siRNAs and luciferase reporter
plasmid (ARPC2-3′UTR) was carried out in 1×106
cells at 6-well plates using Lipofectamine 2000 (Invitrogen/Thermo
Fisher Scientific), according to the manufacturer’s instructions.
After 48 h cells were washed with PBS for twice and lysed using
lysis buffer (Promega Corp.). A total of 20 µl cell extract
and 100 µl luciferase assay reagent were mixed together at
room temperature. The Firefly luciferase activity of this mixture
was then quantified using a Dual Luciferase Reporter Assay System
(Promega Corp.).
mRNA decay assay
mRNA decay assay was carried out in this study to
analyze the interaction between RBM3 protein and ARPC2 mRNA. The
cells were treated with actinomycin D (10 µg/ml) for 0, 2,
4, 6 and 8 h, and the mRNA levels of ARPC2 were examined by
RT-qPCR. GAPDH was detected as a control.
Ribonucleoprotein (RNP)
immunoprecipitation (IP) RT-PCR (RNP-IP RT-PCR)
In this study, the binding between RBM3 protein and
ARPC2 mRNA were analyzed using RNP-IP RT-PCR, which was
carried out essentially as previously described (25). Fifty million cells were collected
per sample, and lysates were used for IP for 12 h at 4°C in the
presence of excess (50 µg) IP antibody (IgG, anti-RBM3). RNA
in IP materials was used in RT followed by PCR and qPCR analysis to
detect the presence of ARPC2 and GAPDH mRNAs.
Anti-RBM3 antibody was used to capture the RBM3 protein-ARPC2 mRNA
complex, and the ARPC2 mRNA levels were detected by RT-qPCR. IgG
was used as a control. The mRNA levels of the negative control
genes, HER2 and MYC, were also detected. The RBM3 rabbit polyclonal
antibody (1:100, 14363-1-AP, Proteintech Group), HER2 rabbit
polyclonal antibody (1:100, 18299-1-AP, Proteintech Group) and IgG
antibody (1:100, 16402-1-AP, Proteintech Group) were used in this
experiment.
Biotin pulldown assay
In this study, biotin pulldown assay was performed
to examine the binding region of RBM3 protein to ARPC2 mRNA.
Different biotinylated transcript regions were synthesized using
the MAXIScript T7 kit (Invitrogen/Thermo Fisher Scientific). The
protein-mRNA binding complexes were isolated using
Streptavidin-coupled Dynabeads (Invitrogen/Thermo Fisher
Scientific). Western blot analysis was performed to detect the
proteins in the protein-mRNA binding complexes.
Statistical analyses
All the data shown in the figures represent the
average of 3 independently repeated experiments. For cellular
proliferation and metastasis assays, RT-qPCR and luciferase
reporter assay, one-way analysis of variance followed by the
Bonferroni or Tamhane post hoc tests were used. Kaplan-Meier curves
were created for the analyses of patient RFS and OS, and the
log-rank test was also used. We have re-valuated the correlation
between RBM3 and ARPC2 using GraphPad Prism 7.03 software. The
association between the patient clinicopathological parameters and
RBM3 expression was analyzed using Pearson’s Chi-square
(χ2) test. The correlation between RBM3 and ARPC2
expression was determined using the Spearman’s rank correlation
test. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
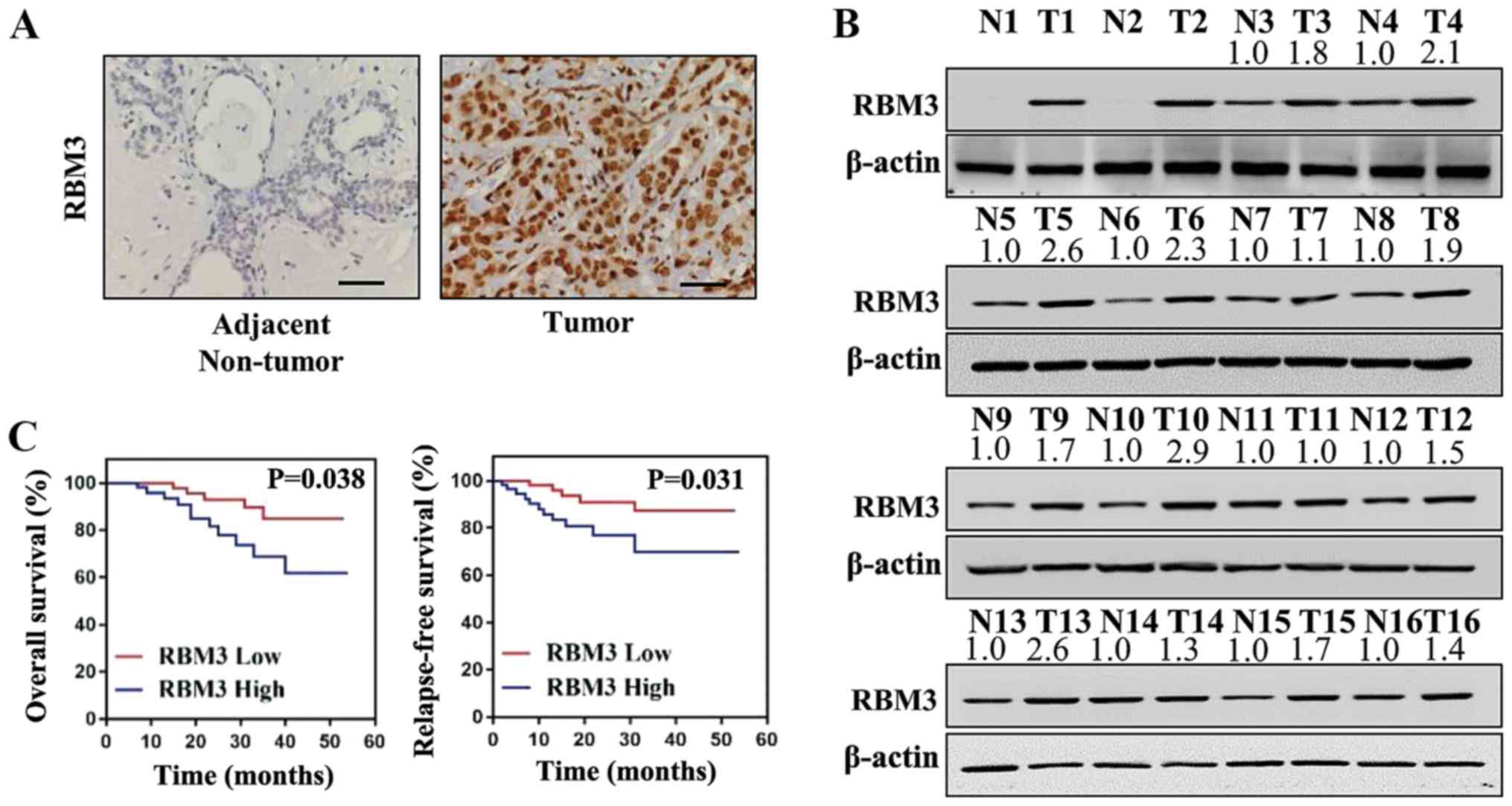
Expression of RBM3 in human tissues from
patients with breast cancer
Breast cancer tissues and adjacent non-tumor tissues
were collected from patients and the protein levels of RBM3 were
examined by immunohistochemistry. As shown in Fig. 1A, the protein levels of RBM3 were
markedly higher in the human breast cancer tissues compared with
the adjacent non-tumor tissues. For further analysis, the
expression levels of RBM3 in 16 pairs of tumor tissues and adjacent
non-tumor tissues were detected by western blot analysis.
Concordantly, the protein levels of RBM3 were markedly higher in
the breast cancer tissues compared with the adjacent non-tumor
tissues (Fig. 1B). Thus, RBM3 was
found to be overexpressed in the breast cancer tissues compared
with the normal adjacent non-tumor tissues.
Association of RBM3 expression with
survival rates and clinicopathological parameters of patients with
breast cancer
The patients with breast cancer were followed-up for
>60 months and the association of RBM3 expression with the
patient survival rates was analyzed. As shown by the Kaplan-Meier
curves, both the OS rate (P=0.038) and RFS rate (P=0.031) were
markedly lower in the patients with breast cancer with a high level
of RBM3 expression compared with the patients with a low level of
RBM3 (Fig. 1C). For further
analysis, the association of RBM3 expression with the
clinicopathological parameters of these breast cancer patients was
examined. As shown in Table I, the
expression of RMB3 in these breast cancer tissues was positively
associated with lymph node metastasis (P=0.015) and tumor grade
(P=0.005). However, no significant association was observed between
RMB3 expression and the age, tumor size or tumor stage of these
patients with breast cancer (P>0.05). Therefore, a high
expression level of RBM3 was associated with poor a prognosis,
lymph node metastasis and a high tumor grade in the patients with
breast cancer.
 | Table IAssociation of RBM3 expression with
the clinicopathological parameters of patients with breast
cancer. |
Table I
Association of RBM3 expression with
the clinicopathological parameters of patients with breast
cancer.
| Parameter | No. | RBM3 expression [n
(%)]
| P-value | χ2 |
|---|
| Low | High |
|---|
| Age (years) | | | | | |
| ≤50 | 53 | 25 (47.2) | 28 (52.8) | 0.851 | 0.363 |
| >50 | 50 | 23 (46.0) | 27 (50.0) | | |
| Tumor size
(cm) | | | | | |
| ≤2 | 30 | 19 (63.3) | 11 (36.6) | 0.396 | 0.508 |
| >2 | 73 | 45 (61.6) | 28 (38.4) | | |
| Lymph node
metastasis | | | | | |
| No | 46 | 30 (65.2) | 16 (34.8) | 0.031 | 4.035 |
| Yes | 57 | 28 (49.1) | 29 (50.9) | | |
| Tumor grade | | | | | |
| I-II | 64 | 44 (68.8) | 20 (31.2) | 0.054 | 6.116 |
| III | 39 | 11 (28.2) | 28 (71.8) | | |
| Tumor stage | | | | | |
| I-II | 75 | 51 (68.0) | 24(32.0) | 0.034 | 0.011 |
| III-IV | 28 | 19 (67.9) | 9 (32.1) | | |
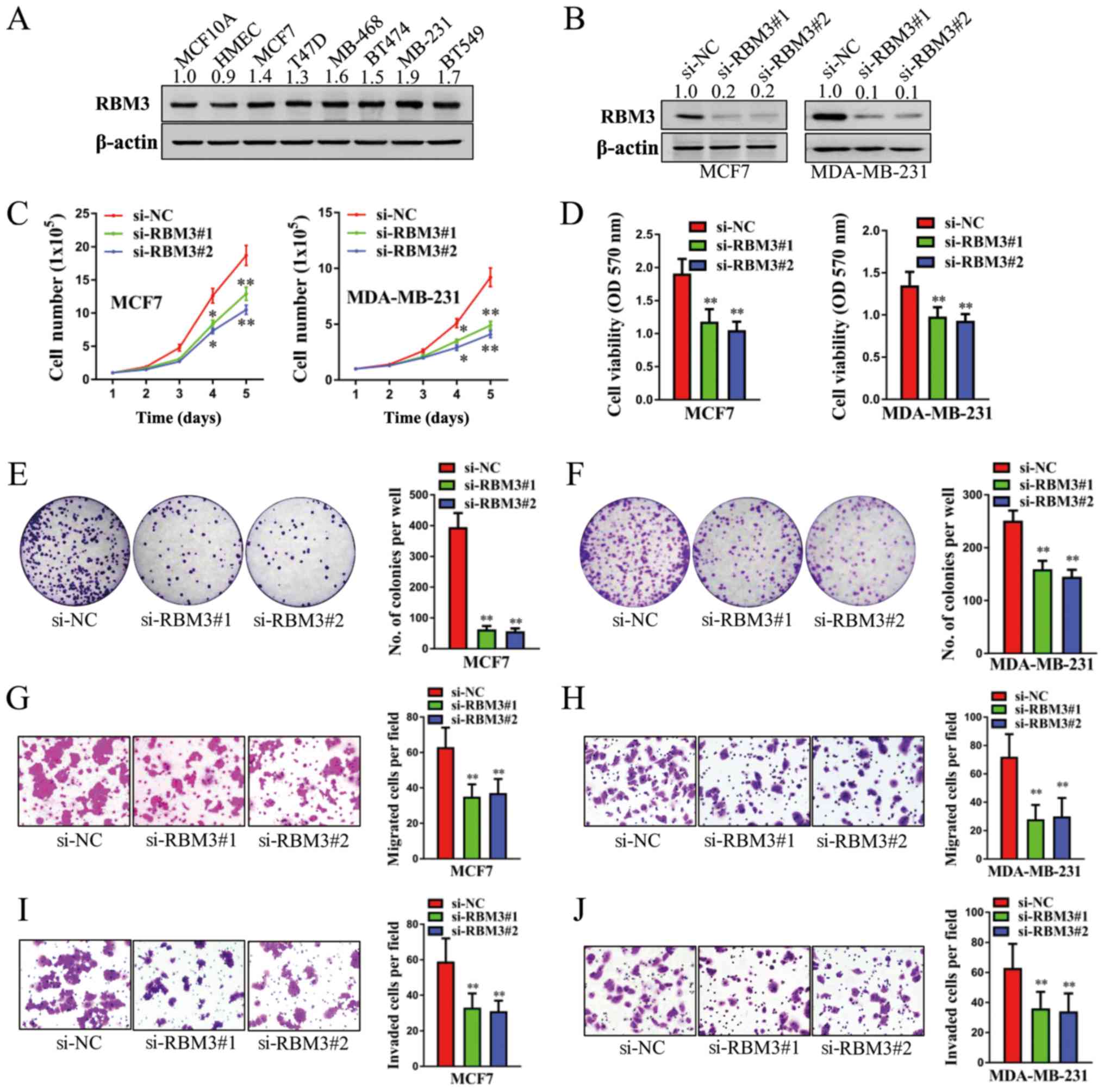
RBM3 promotes the proliferation and
metastasis of human breast cancer cells
We then examined the expression levels of RBM3 in
human normal breast cells (MCF10A and HMEC) and breast cancer cells
(MCF7, T47D, MDA-MB-468, BT474, MDA-MB-231 and BT549) by western
blot analysis. As shown in Fig.
2A, the protein levels of RBM3 were markedly higher in the 6
breast cancer cells than in the 2 normal breast cells. The MCF7 and
MDA-MB-231 were selected for further functional analyses. As shown
in Fig. 2B, the stable knockdown
of RBM3 led to a marked decrease in the protein levels of RBM3. As
determined by cell counting assay, transfection with both si-RBM3#1
and si-RBM3#2 significantly decreased the cell total number over a
period of 5 days in both the MCF7 and MDA-MB-231 cells (Fig. 2C). Concordantly, the viability of
both the MCF7 and MDA-MB-231 cells in which RBM3 was silenced
decreased significantly, as determined by MTT assay (Fig. 2D). Moreover, the knockdown of RBM3
markedly decreased cell colony formation in both the MCF7 and
MDA-MB-231 cells (all P<0.01; Fig.
2E and F). To investigate the role of RBM3 in the metastasis of
human breast cancer cells, cell migration assay and invasion assay
were carried out. As shown in Fig.
2G-J, both migration and invasion decreased significantly in
both the MCF7 and MDA-MB-231 cells in which RBM3 was silenced (all
P<0.01). Thus, these findings indicated that the depletion of
RBM3 suppressed both the proliferation and metastasis of human
breast cancer cells. Therefore, RBM3 plays a promoting role,
contributing to the proliferation and metastasis of human breast
cancer cells.
ARPC2 is regulated by RBM3 in human
breast cancer cells
To identify the downstream mechanisms of action of
RBM3 in human breast cancer cells, we selected several candidate
genes (including HER2, MYC, ARPC2, STAT3, PTEN, FOS, CCND1,
EGFR, PGR and TFF1) and performed RT-qPCR assay in the
MCF7 cells. Among these genes, the mRNA levels of ARPC2
decreased markedly in the MCF7 si-RBM3#1 and MCF7 si-RBM3#2 cells
compared with the MCF7 si-NC cells. However, the mRNA levels of
other candidate genes demonstrated no significant changes (Fig. 3A). To confirm the results of
RT-qPCR, the protein levels of RBM3 and ARPC2 were examined in the
MCF7 and MDA-MB-231 cells. Concordantly, the protein levels of RBM3
decreased markedly following transfection with si-RBM3, and the
protein levels of ARPC2 also decreased markedly in both the
RBM3-silenced MCF7 and MDA-MB-231 cells (Fig. 3B). Therefore, ARPC2 was
proven to be regulated by RBM3 in human breast cancer cells.
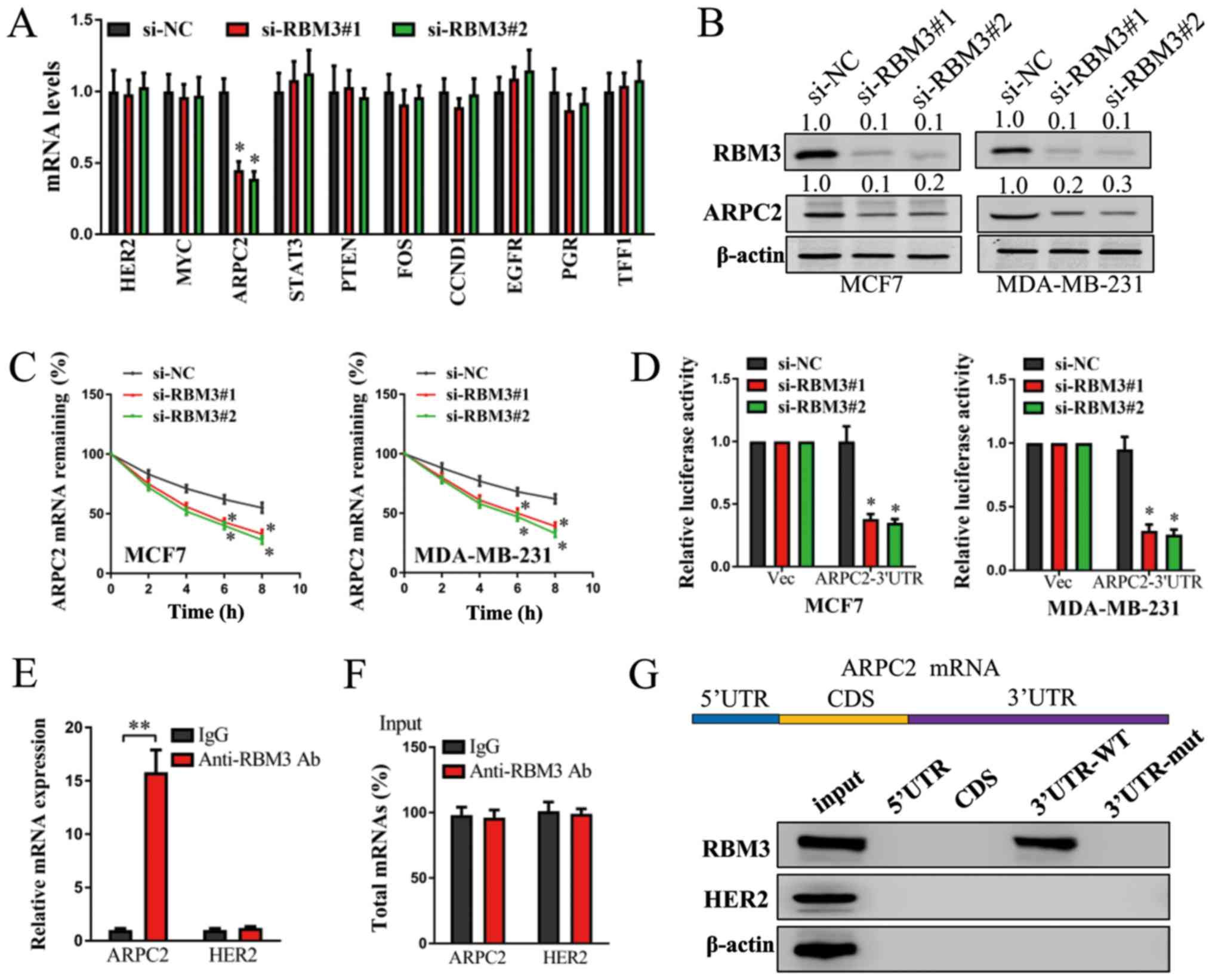 | Figure 3ARPC2 is regulated by RBM3 in human
breast cancer cells. (A) mRNA levels of HER2, MYC, ARPC2, STAT3,
PTEN, FOS, CCND1, EGFR, PGR and TFF1 were detected in
MCF7 cells following the stable knockdown of RBM3 by RT-qPCR.
GAPDH expression was detected as a control. (B) Protein
levels of RBM3 and ARPC2 in MCF7 and MDA-MB-231 cells following the
stable knockdown of RBM3 were detected by western blot analysis.
β-actin was detected as control. (C) MCF7 and MDA-MB-231 cells
following the stable knockdown of RBM3 were treated with
actinomycin D (10 µg/ml) for 0, 2, 4, 6 and 8 h, and the
mRNA levels of ARPC2 were examined by RT-qPCR. GAPDH
expression was detected as a control. (D) Luciferase reporter
activities were examined in MCF7 and MDA-MB-231 cells following the
stable knockdown of RBM3. (E) Ribonucleoprotein (RNP)
immunoprecipitation (IP) assay was carried out in MCF7 cells. mRNA
levels of ARPC2 and negative control genes HER2 were
captured using anti-RBM3 antibody or control IgG and were examined
by RT-qPCR. (F) Total input mRNAs of ARPC2 and negative
control genes HER2 were also examined. (G) Biotinylated RNA
pulldown assay was performed in MCF7 cells. Cell lysates were
incubated with biotinylated different regions of ARPC2 mRNA.
The interactions between different mRNA regions of ARPC2 and
RBM3 protein were examined by western blot analysis. Protein levels
of RBM3, HER2 and β-actin in input cell lysates were examined as
controls. *P<0.05 and **P<0.01 compared
to the negative control (si-NC) group. RBM3, RNA binding motif
protein 3; ARPC2, actin related protein 2/3 complex subunit 2. |
For further analysis regarding the mechanisms of the
regulation of ARPC2 by RBM3, mRNA decay assay, luciferase
reporter assay, RNP IP assay and biotin pulldown assay were
performed. As shown in Fig. 3C,
the mRNA decay rate of ARPC2 increased markedly in both the
RBM3-silenced MCF7 and MDA-MB-231 cells. Moreover, the silencing of
RBM3 significantly decreased the level of ARPC2 3′UTR
luciferase reporter activity in both the MCF7 and MDA-MB-231 cells
(Fig. 3D). As shown by RNP IP
assay, anti-RBM3 antibody significantly enriched the ARPC2
mRNA compared with the control IgG group (Fig. 3E). No significant change was
observed in the negative control genes HER2. Moreover, no
significant differences were observed in the total level of
ARPC2 and HER2 mRNA between the anti-RBM3 group and
IgG group (Fig. 3F). In addition,
biotin pulldown assay revealed that RBM3 directly bound to the
3′UTR region of ARPC2, whereas it did not bind to other regions of
ARPC2 mRNA necessarily (Fig.
3G). Thus, ARPC2 is regulated by RBM2 in a
post-transcriptional 3′UTR-binding manner.
ARPC2 promotes the proliferation and
metastasis of human breast cancer cells
Following the procedures described above, we
continued to examine the protein levels of ARPC2 in human normal
breast cells (MCF10A and HMEC) and breast cancer cells (MCF7, T47D,
MDA-MB-468, BT474, MDA-MB-231 and BT549). Similar to RBM3, the
protein levels of ARPC2 were markedly higher in the 6 breast cancer
cells compared with the 2 normal breast cells (Fig. 4A). Transfection with
si-ARPC2#1 or si-ARPC2#2 markedly decreased the protein
levels of ARPC2 in both the MCF7 and MDA-MB-231 cells (Fig. 4B). The silencing of ARPC2
significantly decreased the total cell number over a period 5 days
(Fig. 4C), cell viability (as
examined by MTT assay) (Fig. 4D),
cell colony formation (Fig. 4E and
F), cell migration (Fig. 4G and
H) and cell invasion (Fig. 4I and
J) in both the MCF7 and MDA-MB-231 cells. Therefore,
ARPC2 also promotes the proliferation and metastasis of
human breast cancer cells.
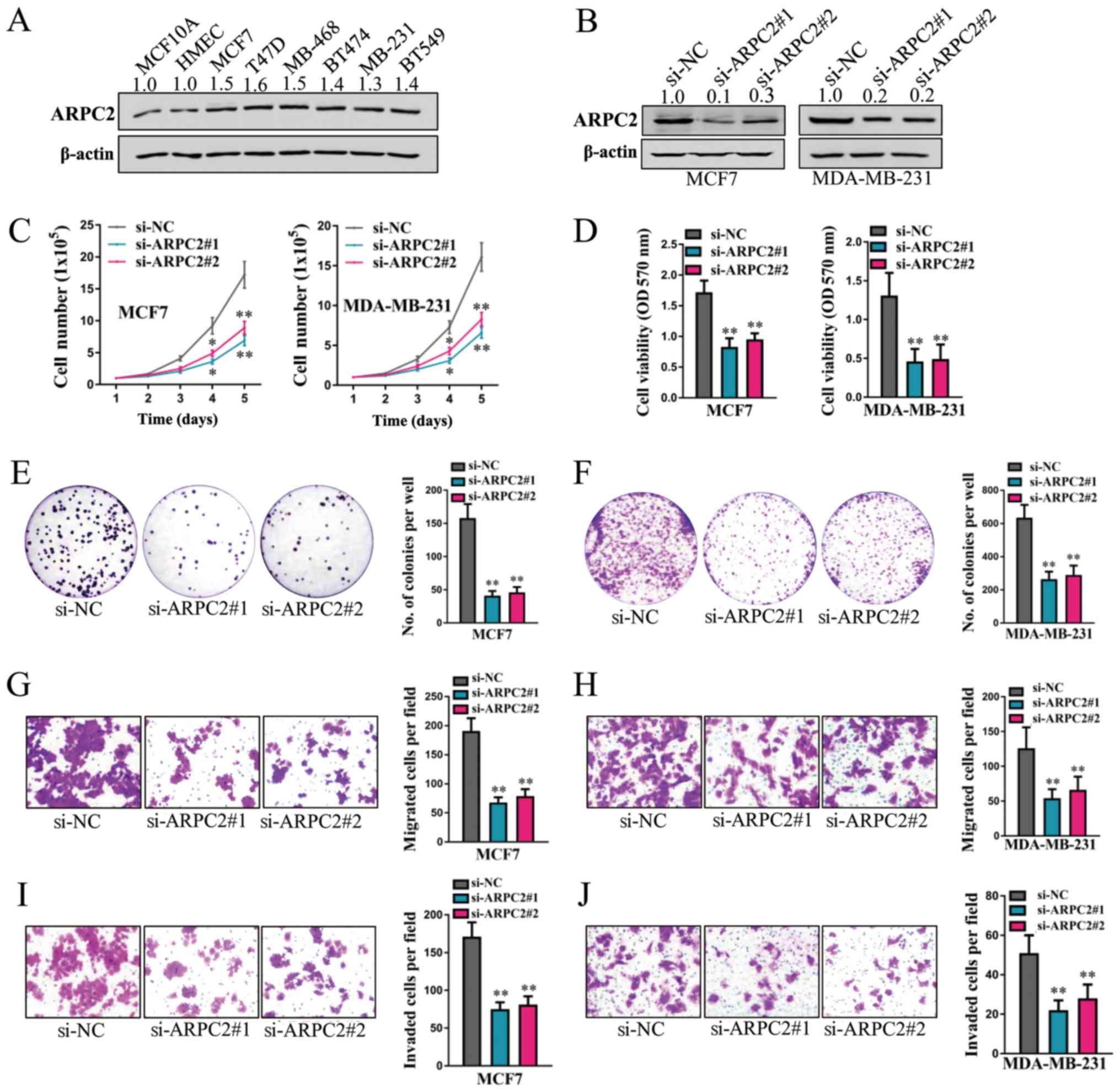 | Figure 4ARPC2 promotes the proliferation and
metastasis of human breast cancer cells. (A) Protein levels of
ARPC2 in parental MCF10A, HMEC, MCF7, T47D, MDA-MB-468, BT474,
MDA-MB-231 and BT549 cells were examined by western blot analysis.
(B) Protein levels of ARPC2 in MCF7 and MDA-MB-231 cells following
the stable knockdown of ARPC2 were detected by western blot
analysis. β-actin expression was detected as a control. (C) Cell
counting assay (1.0×105 cells per well were plated into
6-well plates and cell total number were counted every day for 5
days). (D) MTT assay (2,000 cells per well were plated into 96-well
plates and MTT evaluation was performed 96 h later); (E and F) cell
colony formation assay (1,000 cells per well were plated into
6-well plates and cell colony formation was calculated 10-15 days
later); (G and H) cell migration assay (1×105 cells per
well were plated and cell migration was examined 24 h later for
MCF7 cells and 12 h later for MDA-MB-231 cells); and (I and J) cell
invasion assay (1×105 cells per well were plated and
cell invasion was examined 48 h later for MCF7 cells and 24 h later
for MDA-MB-231 cells) were performed in MCF7 and MDA-MB-231 cells
following the stable knockdown of ARPC2. *P<0.05 and
**P<0.01 compared to the negative control (si-NC)
group. RBM3, RNA binding motif protein 3; ARPC2, actin related
protein 2/3 complex subunit 2. |
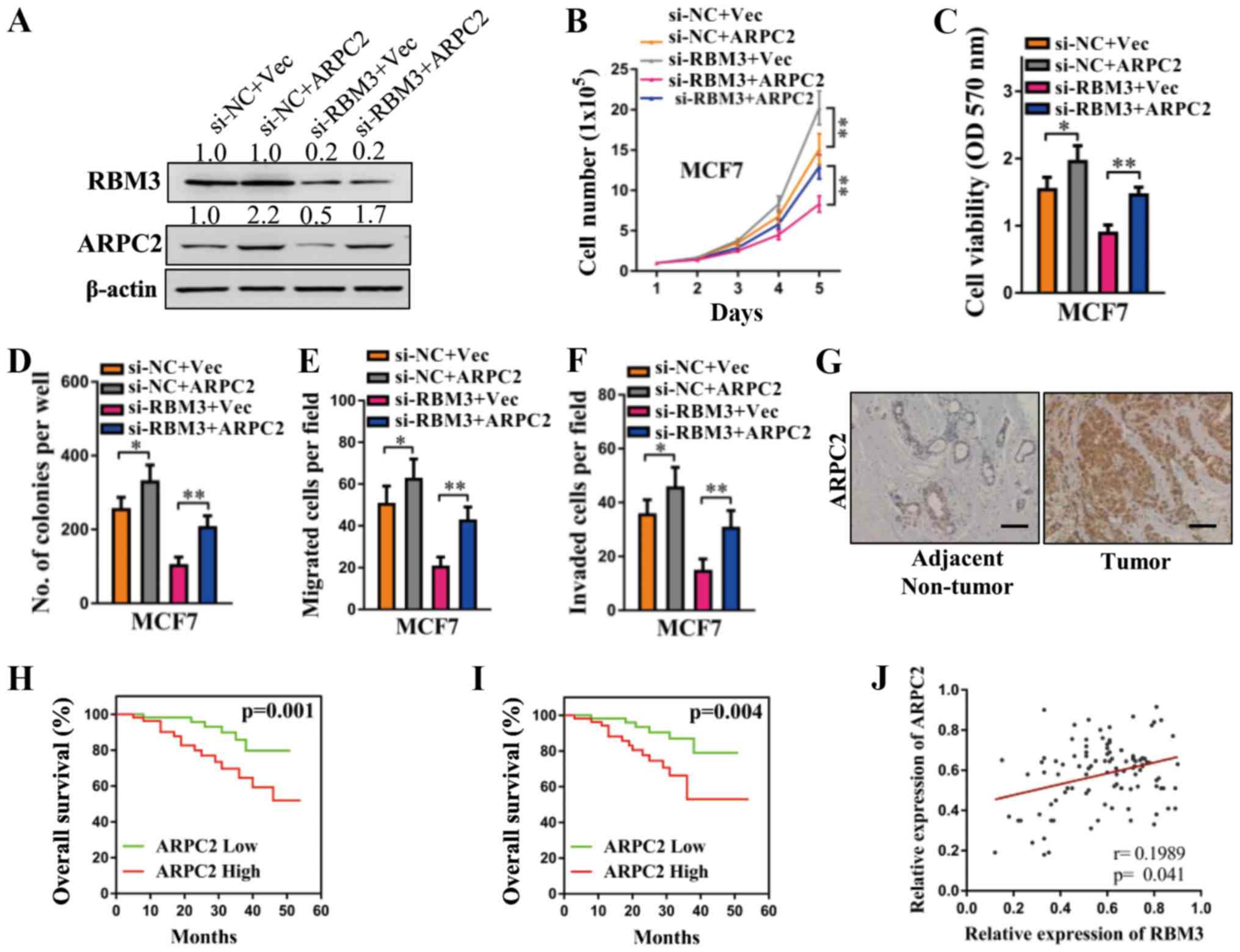
RBM3 promotes the proliferation and
metastasis of human breast cancer cells through ARPC2
To determine whether ARPC2 mediates the
promoting effects of RBM3 on the proliferation and metastasis of
human breast cancer cells, we re-introduced ARPC2 without
its 3′UTR. The restoration of ARPC2 in the MCF7-si-NC and
MCF7-siRBM3 cells was confirmed by western blot analysis (Fig. 5A). Concordant with the results
obtained in our above-mentioned analyses, the enforced expression
of ARPC2 significantly increased the total cell number, cell
viability (as examined by MTT assay), cell colony formation, cell
migration and cell migration in the MCF7 cells (Fig. 5B-F). The silencing of RBM3 markedly
decreased the total cell number, cell viability, cell colony
formation, cell migration and cell migration in MCF7 cells.
However, these decreases were abated by the enforced expression of
ARPC2 (Fig. 5B-F). Therefore,
ARPC2 mediates the promoting effects of RBM3 on the proliferation
and metastasis of human breast cancer cells.
Expression of ARPC2 in tissues from
patients with breast cancer and the association of ARPC2 with
patient survival rates
For further analysis, the protein levels of ARPC2 in
tissues form patients with breast cancer were examined. As shown in
Fig. 5G, the breast cancer tissues
exhibited higher levels of ARPC2 compared with the adjacent
non-tumor tissues, as determined by immunohistochemistry. Moreover,
the association between ARPC2 expression and the patient survival
rates in the breast cancer patients was also analyzed by the
Kaplan-Meier method. The patients with breast cancer with a high
level of ARPC2 exhibited both a lower OS rate (P=0.001) and RFS
rate (P=0.004) compared with the patients with a low level of ARPC2
(Fig. 5H and I). Hence, the breast
cancer tissues overexpressed ARPC2 compared with normal breast
tissues, and a high level of ARPC2 was associated with poor
prognosis in patients with breast cancer. Moreover, the correlation
between RBM3 and ARPC2 expression was analyzed. As shown in
Fig. 5J, a positive correlation
was observed between the expression levels of RBM3 and ARPC2 in the
breast cancer tissues.
Discussion
In this study, we systematically examined the
promoting role of RBM3 in human breast cancer cells and tissues. A
total of 60 breast cancer tissues and adjacent non-tumor tissues
were collected, and RBM3 was found to be overexpressed in the tumor
tissues compared with the normal tissues. Patients with a high
level of RBM3 had markedly poorer OS (P=0.038) and RFS (P=0.031)
rates compared with the patients with a low level of RBM3. The
expression level of RBM3 was positively associated with patient
lymph node metastasis and tumor grade, but exhibited no significant
association with patient age, tumor size or tumor stage. Compared
with normal breast cells, RBM3 exhibited a higher expression level
in breast cancer cells. The silencing of RBM3 with siRNAs markedly
decreased cell proliferation (as detected by cell counting assay,
MTT assay and cell colony formation assay) and cell metastasis (as
detected by cell migration assay and invasion assay) in the MCF7
and MDA-MB-231 cells. Concordantly, it has been documented that
RBM3 plays a tumor-promoting role through the prevention of mitotic
catastrophe and increasing stem cell behaviors via the regulation
of β-catenin in human colorectal cancer cells (17,18).
Sakurai et al reported that RBM3 promoted the development of
colitis-associated cancer (26).
Zeng et al reported that RBM3 interfering with CD44 variant
splicing enhanced the stem-like properties of human prostate cancer
cells and acted as a tumor promoter (15). In the study by Karnevi et
al, RBM3 was found to promote periampullary adenocarcinoma,
including pancreatic cancer and to be associated with a poor
prognosis of patients (27).
However, a high level of RBM3 has also been reported to be
associated with an improved survival and the decreased expression
of RBM3 has been shown to be associated with tumor progression and
a poor prognosis of patients with intestinal-type gastric cancer
(28), testicular non-seminomatous
germ cell cancer (29), esophageal
and gastric adenocarcinoma (20),
epithelial ovarian cancer (11),
urothelial bladder cancer (14)
and malignant melanoma (8). These
studies demonstrate the tissue specificity of RBM3 in different
types of human cancer.
In this study, for the downstream pathway analysis,
ARPC2 was determined to be positively regulated by RBM3. ARPC2 is a
member of actin-related proteins forming the ARP2/3 complex, which
contributes to the generation of the branched actin filament
network responsible for pushing forward the leading edge of motile
eukaryotic cells (30,31). The ARPC2 complex contributes to
cell growth, actin nucleation and endocytosis (32). In the present study, we determined
that RBM3 positively regulated the expression of ARPC2 by RT-qPCR
and western blot analysis. Furthermore, RBM3 was identified to
interact with the mRNA of ARPC2 and to regulate the
expression of ARPC2 through a post-transcriptional pathway by using
mRNA decay assay and luciferase reporter assay. Moreover, we
identified the interacted region between RBM3 protein and
ARPC2 mRNA is the 3′UTR region of ARPC2 by using
RNP-IP RT-PCR assay and biotin pulldown assay. In breast cancer
cells, ARPC2 was found to promote both cell proliferation and
metastasis. Combinatorial cell functional experiments revealed that
ARPC2 mediated the promoting role of RBM3 in human breast cancer
cells. As reported previously, the silencing of the ARP2/3 complex
decreased the migration of pancreatic cancer cells (33). Zhang et al reported that
ARPC2 promoted the proliferation and invasion of the human gastric
cancer cell line, MKN-28; ARPC2 exhibited a higher expression in
gastric cancer tissues than in normal gastric tissues; ARPC2 was
significantly associated with a large tumor size, lymph node
invasion, and a high tumor stage of gastric cancer patients;
ARPC2-positive patients exhibited lower RFS and OS rates compared
with ARPC2-negative patients (34). These data all support our present
results. In addition, we determined that the protein levels of
ARPC2 were markedly higher in breast cancer tissues compared with
adjacent non-tumor tissues. Patients with a high level of ARPC2
exhibited markedly poorer OS (P=0.001) and RFS (P=0.004) rates
compared with patients with a low level of ARPC2. Therefore, ARPC2
is also an important oncogene in human breast cancer cells. The
RBM3-ARPC2 pathway plays an important role in human breast
cancer.
In conclusion, this study systematically examined
the oncogenic role of RBM3 in human breast cancer cells. A high
expression level of RBM3 was found to be associated with worse
outcomes of breast cancer patients. In the downstream pathway
analysis, RBM3 was found to regulate ARPC2 in a positive manner,
which also played an oncogenic role in human breast cancer cells.
Furthermore, the regulatory effects were mediated by
post-transcriptional 3′UTR binding. In addition, ARPC2 mediated the
promoting role of RBM3 in the proliferation and metastasis of
breast cancer cells. A high expression level of ARPC2 was also
associated with worse survival rates of breast cancer patients.
Thus, in this study, it was identified that RBM3 was associated
with ARPC2 and were both tumor promoters, and thus may be used as
biomarkers for breast cancer therapy. It was also identified that
RBM3 correlates with ARPC2 in breast cancer and both promote the
tumor progression. The findings of this study may aid in the
identification and use of novel biomarkers for breast cancer
therapy in the future.
Funding
This study was supported by the Special Project of
Clinical Medicine Science and Technology of Jiangsu Science and
Technology Agency (grant no. BL2012064).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors’ contributions
PC, XY and HX maintained all of the cell cultures
and ran qRT-PCR and western blots. PC designed and performed shRNA
experiment. XY and XL helped with Clinical tissue samples
collection. PC and ZJ conceived the study and drafted statistical
methods. PC and HX wrote the manuscript. ZJ provided funding for
the experiments performed in the manuscript. All authors have read
and approved the manuscript for publication.
Ethics approval and consent to
participate
Experiments related to the use of human tissues were
performed in accordance with The Code of Ethics of the World
Medical Association (Declaration of Helsinki) and local approval
(from the Institutional Review Boards of Southeast University) was
obtained prior to the commencement of this study. Informed consent
form was signed by each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Chen D, Si W, Shen J, Du C, Lou W, Bao C,
Zheng H, Pan J, Zhong G, Xu L, et al: miR-27b-3p inhibits
proliferation and potentially reverses multi-chemoresistance by
targeting CBLB/GRB2 in breast cancer cells. Cell Death Dis.
9:1882018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo J, Gong G and Zhang B: miR-539 acts as
a tumor suppressor by targeting epidermal growth factor receptor in
breast cancer. Sci Rep. 8:20732018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chu C, Liu X, Bai X, Zhao T, Wang M, Xu R,
Li M, Hu Y, Li W, Yang L, et al: MiR-519d suppresses breast cancer
tumorigenesis and metastasis via targeting MMP3. Int J Biol Sci.
14:228–236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tartari F, Santoni M, Pistelli M and
Berardi R: Healthcare cost of HER2-positive and negative breast
tumors in the United States (2012-2035). Cancer Treat Rev.
60:12–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gottesman MM and Ling V: The molecular
basis of multidrug resistance in cancer: The early years of
P-glycoprotein research. FEBS Lett. 580:998–1009. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pilotte J, Dupont-Versteegden EE and
Vanderklish PW: Widespread regulation of miRNA biogenesis at the
Dicer step by the cold-inducible RNA-binding protein, RBM3. PLoS
One. 6:e284462011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jonsson L, Bergman J, Nodin B, Manjer J,
Pontén F, Uhlén M and Jirström K: Low RBM3 protein expression
correlates with tumour progression and poor prognosis in malignant
melanoma: An analysis of 215 cases from the Malmö Diet and Cancer
Study. J Transl Med. 9:1142011. View Article : Google Scholar
|
|
9
|
Derry JM, Kerns JA and Francke U: RBM3, a
novel human gene in Xp11.23 with a putative RNA-binding domain. Hum
Mol Genet. 4:2307–2311. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jögi A, Brennan DJ, Rydén L, Magnusson K,
Fernö M, Stål O, Borgquist S, Uhlen M, Landberg G, Påhlman S, et
al: Nuclear expression of the RNA-binding protein RBM3 is
associated with an improved clinical outcome in breast cancer. Mod
Pathol. 22:1564–1574. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ehlén A, Brennan DJ, Nodin B, O’Connor DP,
Eberhard J, Alvarado-Kristensson M, Jeffrey IB, Manjer J,
Brändstedt J, Uhlén M, et al: Expression of the RNA-binding protein
RBM3 is associated with a favourable prognosis and cisplatin
sensitivity in epithelial ovarian cancer. J Transl Med. 8:782010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wright CF, Oswald BW and Dellis S:
Vaccinia virus late transcription is activated in vitro by cellular
heterogeneous nuclear ribonucleoproteins. J Biol Chem.
276:40680–40686. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burd CG and Dreyfuss G: Conserved
structures and diversity of functions of RNA-binding proteins.
Science. 265:615–621. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kita H, Carmichael J, Swartz J, Muro S,
Wyttenbach A, Matsubara K, Rubinsztein DC and Kato K: Modulation of
poly-glutamine-induced cell death by genes identified by expression
profiling. Hum Mol Genet. 11:2279–2287. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeng Y, Wodzenski D, Gao D, Shiraishi T,
Terada N, Li Y, Vander Griend DJ, Luo J, Kong C, Getzenberg RH, et
al: Stress-response protein RBM3 attenuates the stem-like
properties of prostate cancer cells by interfering with CD44
variant splicing. Cancer Res. 73:4123–4133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baghdoyan S, Dubreuil P, Eberlé F and
Gomez S: Capture of cytokine-responsive genes (NACA and RBM3) using
a gene trap approach. Blood. 95:3750–3757. 2000.PubMed/NCBI
|
|
17
|
Venugopal A, Subramaniam D, Balmaceda J,
Roy B, Dixon DA, Umar S, Weir SJ and Anant S: RNA binding protein
RBM3 increases β-catenin signaling to increase stem cell
characteristics in colorectal cancer cells. Mol Carcinog.
55:1503–1516. 2016. View Article : Google Scholar
|
|
18
|
Sureban SM, Ramalingam S, Natarajan G, May
R, Subramaniam D, Bishnupuri KS, Morrison AR, Dieckgraefe BK,
Brackett DJ, Postier RG, et al: Translation regulatory factor RBM3
is a proto-oncogene that prevents mitotic catastrophe. Oncogene.
27:4544–4556. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boman K, Segersten U, Ahlgren G, Eberhard
J, Uhlén M, Jirström K and Malmström PU: Decreased expression of
RNA-binding motif protein 3 correlates with tumour progression and
poor prognosis in urothelial bladder cancer. BMC Urol. 13:172013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jonsson L, Hedner C, Gaber A, Korkocic D,
Nodin B, Uhlén M, Eberhard J and Jirström K: High expression of
RNA-binding motif protein 3 in esophageal and gastric
adenocarcinoma correlates with intestinal metaplasia-associated
tumours and independently predicts a reduced risk of recurrence and
death. Biomark Res. 2:112014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding K, Wu Z, Wang N, Wang X, Wang Y, Qian
P, Meng G and Tan S: MiR-26a performs converse roles in
proliferation and metastasis of different gastric cancer cells via
regulating of PTEN expression. Pathol Res Pract. 213:467–475. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Q, Yan H, Tao SQ, Wang XN, Mou L, Chen
P, Cheng XW, Wu WY and Wu ZS: XIAP 3′-untranslated region as a
ceRNA promotes FSCN1 function in inducing the progression of breast
cancer by binding endogenous miR-29a-5p. Oncotarget. 8:16784–16800.
2017.PubMed/NCBI
|
|
23
|
Ding K, Tan S, Huang X, Wang X, Li X, Fan
R, Zhu Y, Lobie PE, Wang W and Wu Z: GSE1 predicts poor survival
outcome in gastric cancer patients by SLC7A5 enhancement of tumor
growth and metastasis. J Biol Chem. 293:3949–3964. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Zhuang R, Rao JN, Zou T, Liu L, Xiao L,
Cao S, Hansraj NZ, Gorospe M and Wang JY: miR-195 competes with HuR
to modulate stim1 mRNA stability and regulate cell migration.
Nucleic Acids Res. 41:7905–7919. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sakurai T, Kashida H, Komeda Y, Nagai T,
Hagiwara S, Watanabe T, Kitano M, Nishida N, Fujita J and Kudo M:
Stress response protein RBM3 promotes the development of
colitis-associated cancer. Inflamm Bowel Dis. 23:57–65. 2017.
View Article : Google Scholar
|
|
27
|
Karnevi E, Dror LB, Mardinoglu A, Elebro
J, Heby M, Olofsson SE, Nodin B, Eberhard J, Gallagher W, Uhlén M,
et al: Translational study reveals a two-faced role of RBM3 in
pancreatic cancer and suggests its potential value as a biomarker
for improved patient stratification. Oncotarget. 9:6188–6200.
2017.
|
|
28
|
Ye F, Jin P, Cai X, Cai P and Cai H: High
RNA-binding motif protein 3 (RBM3) expression is independently
associated with prolonged overall survival in intestinal-type
gastric cancer. Med Sci Monit. 23:6033–6041. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Olofsson SE, Nodin B, Gaber A, Eberhard J,
Uhlén M, Jirström K and Jerkeman M: Low RBM3 protein expression
correlates with clinical stage, prognostic classification and
increased risk of treatment failure in testicular non-seminomatous
germ cell cancer. PLoS One. 10:e01213002015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Robinson RC, Turbedsky K, Kaiser DA,
Marchand JB, Higgs HN, Choe S and Pollard TD: Crystal structure of
Arp2/3 complex. Science. 294:1679–1684. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Svitkina TM and Borisy GG: Arp2/3 complex
and actin depolymerizing factor/cofilin in dendritic organization
and treadmilling of actin filament array in lamellipodia. J Cell
Biol. 145:1009–1026. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Daugherty KM and Goode BL: Functional
surfaces on the p35/ARPC2 subunit of Arp2/3 complex required for
cell growth, actin nucleation, and endocytosis. J Biol Chem.
283:16950–16959. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rauhala HE, Teppo S, Niemelä S and
Kallioniemi A: Silencing of the ARP2/3 complex disturbs pancreatic
cancer cell migration. Anticancer Res. 33:45–52. 2013.
|
|
34
|
Zhang J, Liu Y, Yu CJ, Dai F, Xiong J, Li
HJ, Wu ZS, Ding R and Wang H: Role of ARPC2 in human gastric
cancer. Mediat Inflamm. 2017:54328182017. View Article : Google Scholar
|