Introduction
Epithelial ovarian cancer is a fatal gynecological
malignancy, resulting in 295,414 new cases and 184,799 deaths
worldwide in 2018 (1), exhibiting
an upward trend (1,2). High-grade serous ovarian carcinoma
(HGSOC) is the most common subtype (70%) associated with a higher
degree of malignancy and a poorer prognosis (3). However, the molecular pathogenesis,
the mechanisms of molecular regulation and drug resistance
associated with HGSOC remain poorly characterized.
Protein regulator of cytokinesis-1 (PRC1), also
known as Ase1 (yeast)/MAP65 (plant), was first identified as a CDK
substrate in 1998 and was known as an essential microtubule
associated protein required for cytokinesis via the phosphorylation
of CDK1 (Cdc2/cyclin B) in early mitosis (4,5).
Previous studies have found that cells in which PRC1 is knocked
down can normally undergo interphase, prophase, prometaphase and
metaphase, and chromatin can be equally distributed in the anaphase
of mitosis; however, the structure of the central area of the
spindle appears to be abnormal at anaphase, leading to the aberrant
expression of cytokines and the formation of binuclear or
multinucleated cells (5,6). Therefore, an abnormal expression of
PRC1 can lead to aberrant cytokine expression, contributing to
tumorigenesis and tumor progression.
It has already been demonstrated that PRC1 is
upregulated in various types of tumor, such as hepatocellular
carcinoma (7), gastric carcinoma
(8) and pulmonary adenocarcinoma
(9,10). The overexpression of PRC1 has been
shown to significantly promoted the proliferation and metastasis of
hepatocellular carcinoma cells, and to be associated with early
recurrence and a poor patient outcome by regulating the oncogenic
effects of the Wnt signaling pathway (7). The knockdown of PRC1 has also been
shown to significantly suppress the proliferation, reduce monolayer
colony formation and to inhibit the invasive and migratory ability
of gastric carcinoma cells (8). To
date, however, at least to the best of our knowledge, the
expression, biological functions and prognosis significance of PRC1
in ovarian cancer have not yet been elucidated.
Materials and methods
Patients and tissue samples
All evaluated HGSOC and fallopian tube (FT) tissues
were collected from the Department of Obstetrics and Gynecology,
Qilu Hospital, Shandong University from 2006 to 2016. The 4
µm tissue microarrays (TMAs), including 210 cases of HGSOC
and 42 normal FT tissues, were designed in our laboratory. Fresh
tissues of 28 cases of HGSOC and 14 normal FT tissues were also
collected. Moreover, 18 specimens with chemosensitivity information
for second-line chemotherapy were obtained from the specimen
library. HGSOC tissues were obtained from patients who underwent
primary surgery and no neo-adjuvant chemotherapy was performed. The
normal FT tissues were obtained from patients who received
hysterectomy and salpingo-oophorectomies due to benign disease. The
clinicopathological characteristics of the patients are presented
in Table I. The accurate
assessment of the disease response was based on RECIST or GCIG
standards (11). Ethics approval
was obtained from the Ethics Committee of Shandong University, and
written informed consent was obtained from each patient.
 | Table IAssociation between PRC1 protein
expression and the clinicopathological characteristics of patients
with HGOSC. |
Table I
Association between PRC1 protein
expression and the clinicopathological characteristics of patients
with HGOSC.
| Characteristic | No. of
patients | PRC1 protein
expression (n)
|
|---|
| Low | High | P-value |
|---|
| Age (years) | | | | 0.333 |
| <55 | 97 | 42 | 55 | |
| ≥55 | 113 | 57 | 56 | |
| FIGO stage | | | | 0.282 |
| I-II | 38 | 21 | 17 | |
| III-IV | 172 | 77 | 95 | |
| CA-125 | | | | 0.870 |
| <500 U/ml | 54 | 25 | 29 | |
| ≥500 U/ml | 123 | 60 | 63 | |
| Ascites | | | | 0.893 |
| <500 ml | 72 | 33 | 39 | |
| ≥500 ml | 126 | 59 | 67 | |
| Residual
lesions | | | | 0.567 |
| <1 cm | 133 | 61 | 72 | |
| ≥1 cm | 77 | 32 | 45 | |
| Family history | | | | 0.891 |
| No | 161 | 74 | 87 | |
| Yes | 38 | 17 | 21 | |
| Platinum of first
line | | | | 0.019a |
| Sensitive | 87 | 40 | 47 | |
| Resistance | 7 | 0 | 7 | |
| Platinum of second
line | | | | 0.028a |
| Sensitive | 60 | 34 | 29 | |
| Resistance | 21 | 7 | 18 | |
| Primary
recurrence | | | | 0.127 |
| <6 months | 24 | 8 | 16 | |
| ≥6 months | 64 | 33 | 31 | |
| Secondary
recurrence | | | | 0.011a |
| <6 months | 16 | 5 | 11 | |
| ≥6 months | 15 | 12 | 3 | |
| Three-year
survival | | | | 0.006b |
| Alive | 89 | 55 | 34 | |
| Deceased | 54 | 20 | 34 | |
| Five-year
survival | | | | 0.003b |
| Alive | 72 | 47 | 25 | |
| Deceased | 71 | 28 | 43 | |
| BRCA mutation
status | | | | 0.019a |
| Pathogenic | 17 | 13 | 4 | |
|
Non-pathogenic | 35 | 14 | 21 | |
Cell lines and cell culture
The A2780 human ovarian cancer cell line was
originally established from the tumor tissue of an untreated
patient, and the cells grew as a monolayer and in suspension in
spinner cultures. The SKOV3 cell line was originally isolated from
the ascites of patients with ovarian tumors, and tumorigenesis in
nude mice resulted in a moderately differentiated adenocarcinoma
consistent with carcinoma in situ of the ovary. The
above-mentioned cells were cultured in RPMI-1640 medium and McCoy's
5A medium, respectively. 293T cells, which could continuously
express SV40 antigen, was used in the transfection experiments with
a high transfection efficiency, and was cultured in DMEM. The A2780
cell line was originally purchased from the European Collection of
Authenticated Cell Cultures (ECACC; Cat. no. 93112519). The SKOV3
and 293T cells were purchased from the American Type Culture
Collection (ATCC; Cat. nos. HTB-77 and CRL-11268, respectively).
All the culture media were supplemented with 10% fetal bovine serum
(FBS). All these cells were cultured in a humidified incubator
under standard culture conditions (37°C, 5% CO2).
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA from cells and tissues was isolated using
TRIZOL reagent (Invitrogen; Thermo Fisher Scientific) and cDNA was
synthesized using the PrimeScript RT reagent kit (Takara). qPCR was
performed using SYBR-Green qPCR master mix (Takara). The conditions
for PCR indluced 3 stages: Hold stage (95°C, 30 sec), PCR stage for
40 cycles (95°C, 5 sec and 60°C, 34 sec), melt curve stage (95°C,
15 sec; 60°C, 1 min and 95°C, 15 sec).GAPDH served as the
endogenous control. The primer sequences of PRC1 for RT-qPCR were
as follows: Forward primer, ACA CTC TGT GCA GCG AGT TAC; reverse
primer, TTC GCA TCA ATT CCA CTT GGG. The primer sequences of GAPDH
were as follows: Forward, ACA ACT TTG GTA TCG TGG AAG G and
reverse, GCC ATC ACG CCA CAG TTT C. The method of quantification
was relative quantification and ΔΔcq was calculated to analyze the
relative gene expression (12).
Plasmid construction and lentivirus
production
A lentivirus vector expressing shPRC1
(TRCN0000280715) was purchased from Sigma-Aldrich. siRNA was
synthesized by the GenePharma. The sequences were as follows: PRC1,
5′-CGC UGU UUA CUC AUA CAG U-3′; forkhead box protein M1 (FOXM1),
5′-GGA CCA CUU UCC CUA CUU UUU-3′ and negative control (NC), 5′-UUC
UCC GAA CGU GUC ACG UdT dT-3′.
Lentivirus was produced in 293T cells packaged by
psPAX2 and pMD2.G. The cells were infected with the 1 ml lentivirus
liquid for 24 h in the presence of polybrene (8 µg/ml), and
was selected with puromycin (2 µg/ml) for 1 week to acquire
stable expressing cell lines. siRNA manipulation was performed in
accordance with the instructions of the manufacturer of
Lipofectamine 2000 (Invitrogen™). The time duration between
transfection subsequent experimentation was approximately 10-14
h.
MTT assay
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT) assay was firstly used to measure the proliferative ability
of the A2780 cells subjected to PRC1 knockdown or overexpression
compared to the cells trans-fected with empty plasmid. The A2780
cells were incubated in RPMI-1640 medium supplemented with 10% FBS,
and were seeded in 96-well plates at concentrations of 1,000 cells
per well. Cell proliferation was monitored at different time points
(1-5 days). At fixed time points, 20 µl of MTT
(Sigma-Aldrich) solution were added to each well. Following
incubation for 4 h at 37°C, the supernatant was removed and 100
µl of DMSO (Sigma-Aldrich) were added to each well. The
absorbance at 490 nm was evaluated using a microplate reader
(Bio-Rad).
In addition, the changes in the IC50
values of cisplatin, taxol and pegylated liposomal doxorubicin
(LPD) were evaluated in the A2780 cells subjected to PRC1 knockdown
or overexpression compared to the controls. The A2780 cells were
seeded in 96-well plates at concentrations of 4,000 cells per well
and were treated with gradually increasing concentrations of the
drugs (as shown in Fig. 5C-F).
Following incubation for 48 h at 37°C, 20 µl of MTT
(Sigma-Aldrich) solution were added to each well. The remaining
steps were the same as mentioned above.
Colony formation assay
Colony formation assay was used to measure the
proliferative ability of the A2780 cells subjected to PRC1
knockdown or overexpression compared to the controls. The A2780
cells were seeded in a 6-well plate with 1,000 cells per well, and
cultured at 37°C for 10-14 days. Colonies were fixed with methanol
for 15 min and stained with 0.5% crystal violet (Solarbio) for 20
min at room temperature. The number of colonies with >50 cells
was counted under a microscope (IX71, Olympus).
Cell cycle analysis
Cell cycle analysis was performed to verify whether
PRC1 regulates the cell cycle. The A2780 cells transfected with
PRC1 siRNA and the controls were harvested and fixed with 75%
ice-cold ethanol overnight at 4°C. Cell suspensions were stained
with 1 ml propidium iodide stock solution (MultiSciences Lianke
Biotech Co.) and analyzed using a FACScan flow cytometer (BD
Biosciences).
Transwell assays
Transwell assay was performed to confirm whether
RPC1 knockdown and overexpression affected the invasive and
migratory abilities of the A2780 and SKOV3 cells. In addition, it
was used to determine whether the silencing of PRC1 can reverse the
FOXM1-mediated enhancement of the metastatic abilities of the A2780
cells. The cells were re-suspended in serum-free medium, and 200
µl suspension containing 1-2×105 cells was seeded
into the upper chambers, and 700 µl culture medium
containing 20% fetal bovine serum was added to the lower
compartment. The filter membrane of invasion assays was coated with
diluted Matrigel (BD Biosciences). The cells that penetrated to the
bottom were fixed with methanol and stained with 0.5% crystal
violet (Solarbio) at room temperature for >30 min following
incubation under standard culture conditions (37°C, 5%
CO2). The incubation times for the migration of the
A2780 and SKOV3 cells were 24 and 10 h, and the times for invasion
was 36 and 18 h, respectively. A light microscope (IX71, Olympus)
was used to examine the cells and obtain images (×100
magnification).
Wound healing assay
To confirm whether PRC1 overexpression in A2780
cells facilitates the migratioryability compared with the control,
a wound healing assay was performed. The A2780 cells were seeded in
24-well plate at 3.0×105 per well. A 20 µl
pipette tip was used to scratch a line when the cells reached 100%
confluency as a monolayer. The cells were then cultured in
serum-free medium. The distance between the two edges was monitored
at 0 and 48 h following wound formation using a microscope (IX71,
Olympus).
Western blot analysis
The fresh tissues and cells were lysed with RIPA
buffer on ice. The BCA Protein Assay kit (Merck Millipore) was used
to detect the protein concentration of the samples. The mass of
protein loaded per lane was 50-90 µg. The sample proteins
were separated by SDS-PAGE (separating gel, 10%; stacking gel, 5%)
and electrotransferred onto a PVDF membrane (Merck Millipore).
After blocking with 5% non-fat milk, the membrane was incubated
overnight with primary antibodies at 4°C followed by appropriate
HRP-conjugated secondary antibodies for 2 h at room temperature the
following day. The ECL system (GE Healthcare) was used to detect
the protein bands, and β-actin was used as an endogenous control.
The primary antibodies in this study included: Anti-β-actin
(1:5,000, F3022, Sigma-Aldrich), anti-PRC1 (1:500, ab51248, Abcam),
anti-cyclin B1 (CCNB1; 1:1,000, ab32053, Abcam), anti-cyclin D1
(CCND1; 1:1,000, ab16663, Abcam), anti-Aurora B kinase (AURKB;
1:1,000, ab2254, Abcam), anti-p21 (1:1,000, ab109520, Abcam),
anti-E-Cadherin (1:1,000, #3195, Cell Signaling Technology),
anti-N-Cadherin (1:1,000, #13116, Cell Signaling Technology),
anti-matrix metalloproteinase (MMP)9 (1:1,000, ab38898, Abcam),
anti-Slug (1:1,000, ab27568, Abcam), anti-breast cancer 1 (BRCA1;
1:1,000, #9010, Cell Signaling Technology), anti-RAD51 (1:1,000,
ab113534, Abcam), anti-poly(ADP-Ribose) polymerase 1 (PARP1;
1:1,000, ab32138, Abcam), anti-c-Myc (1:1,000, ab32072, Abcam) and
anti-FOXM1 (1:1,000, ab207298, Abcam). The secondary antibodies
were purchased from KPL. Anti-rabbit IgG (5220-0336) was diluted at
1:4,000 and anti-mouse IgG (5220-0341) was diluted at 1:6,000.
ImageJ software (National Institutes of Health, 1.48v) was used to
calculate the relative density.
Immunohistochemical staining
Immunohistochemical staining was performed in 210
cases of HGSOC and 42 normal FT tissues to determine the expression
and clinical significance of PRC1 in HGSOC. In addition, the
immunohistochemical staining of PRC1 and FOXM1 in corresponding
HGSOC samples was analyzed to verify the association between PRC1
and FOXM1. The TMAs were incubated at 60°C for 1 h, and
subsequently deparaffinized in xylene and hydrated in a degraded
series of ethanol. The heat-mediated antigen retrieval in EDTA
buffer (pH 9.0), the inactivation of endogenous peroxidase activity
and the blocking of non-specific antigens were then performed
gradually. PRC1 antibody (1:100 dilution, HPA034521, Sigma-Aldrich)
was incubated with the slides at 4°C overnight followed by
secondary antibodies (SP-9000, ZSBG-BIO) incubation for 20 min at
room temperature. Then the sections were incubated with
streptavidin-peroxidase for 15 min at room temperature. The
staining was detected with the DAB detection system (Zhongshan
Biotechnology Co.). Two investigators blinded to the clinical data
evaluated the staining. Each sample had two duplicates and the
average scores were used as the final result.
Dual-luciferase reporter assay
To determine whether FOXM1 can regulate the
expression of PRC1 and identify the binding site, a dual-luciferase
reporter assay was performed. The 293T cells were plated in 96-well
plates and cultured for 24 h at 37°C, and transiently
co-transfected with PCMV-FOXM1C (RC202540, Origene), PGL4.26-PRC1
(E8441, Promega) and pRL-TK (E2241, Promega) using Lipofectamine
2000 (Invitrogen™). The luciferase activity was evaluated using the
Dual-Glo® Lu ciferase Assay System E2920 which was
supplied by Promega. After 24 h, 75 µl fresh medium were
added to the 96-well plates after the medium was removed. This was
followed by the addition of 75 µl lysis reagent and Firefly
luminescence was measured using a microplate reader (Bio-Rad) after
10 min. Subsequently, 75 µl Stop buffer were added to each
well and the Renilla luminescence was measured after 10 min.
The relative luciferase activity was determined by the ratio of
values between Firefly luminescence and Renilla
luminescence.
BRCA mutation detection
In order to verify whether PRC1 expression is
associated with germline BRCA mutation, germ-line BRCA genetic
testing was performed in 52 patients. Fresh blood of 6 ml was
extracted and sequenced using the NGS platform from Shanghai Topgen
Bio-pharm Co. Ltd. The BRCA1/2 panel (Morgen, China) was used which
covers the entire coding sequences of BRCA1 and BRCA2, including
10-50 bases of adjacent intronic sequence of each exon. The
variants were classified based on a highly accepted 5-class
classification (13).
Bioinformatics analyses
Oncomine (www.oncomine.org)
was used to visualize the differential expression of PRC1 in
ovarian cancer and control samples. TCGA RNA expression data of
ovarian serous cystadenocarcinoma were analyzed by the Cancer
Genomics Browser (https://genome-cancer.ucsc.edu). Kaplan Meier-plotter
(http://kmplot.com/analysis/) was used to
analyze overall survival and the progression-free survival of
patients as regards PRC1 expression in ovarian cancer. Gene
regulation website (www.gene-regulation.com) was used to analyze the
promoter of PRC1. Pearson's correlation analysis was used to
analyze the correlation of PRC1 and FOXM1 expression in TCGA
cohort.
Statistical analysis
Statistical analysis was carried out using SPSS 23
software. The differences between continuous data were analyzed
using a Student's t-test, and the comparisons between multiple
groups were performed by one-way ANOVA, and Fishers' Least
Significant Difference (LSD) was used as a post hoc test. The
association between PRC1 expression and the clinical
characteristics of the patients were analyzed using the Chi-square
test. Multivariate cox regression analysis was used to analyze the
association between clinical prognostic markers and overall
survival. Overall survival analysis was performed by Kaplan-Meier
and the log-rank test. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
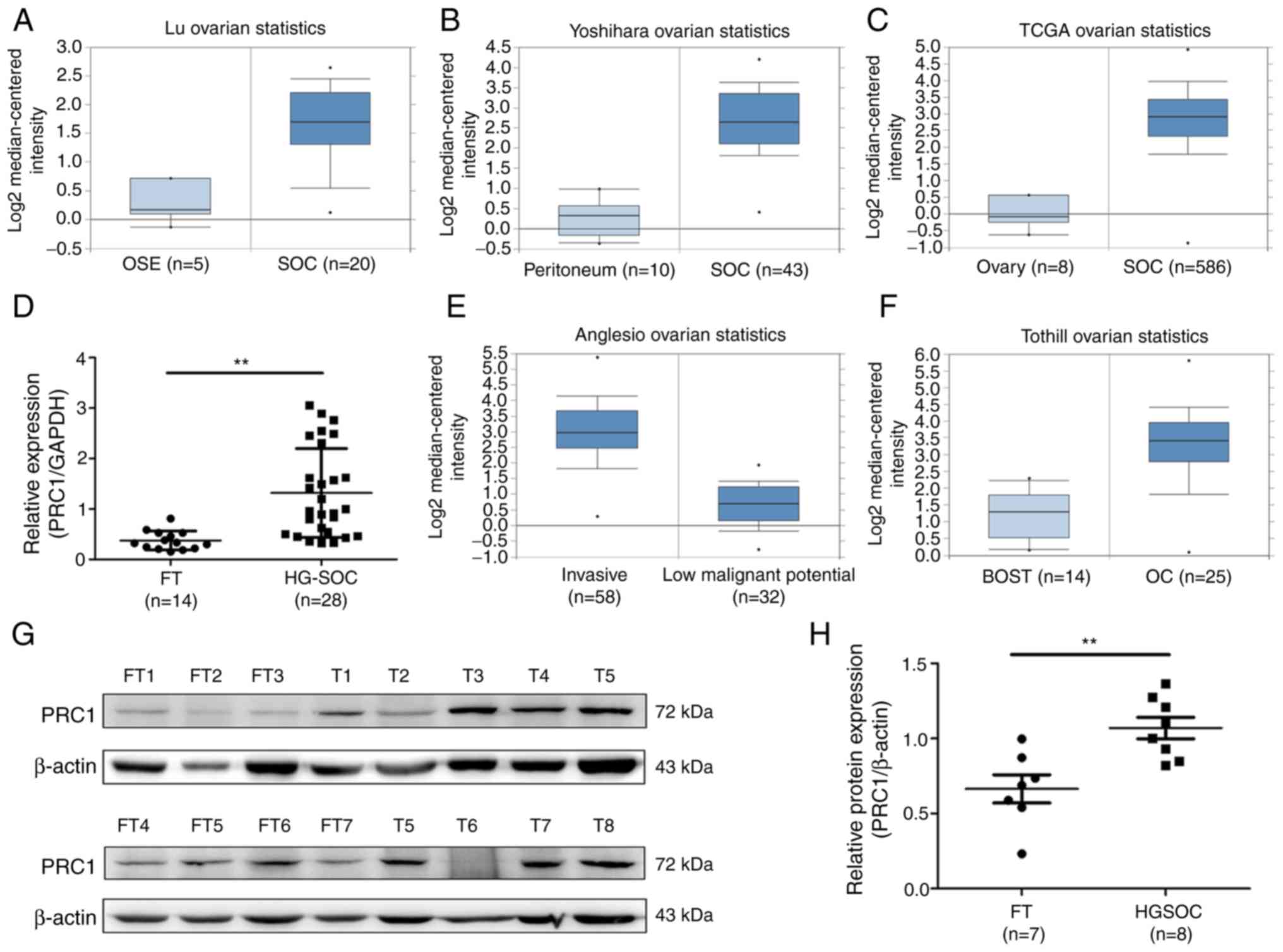
PRC1 is overexpressed in HGSOC
To determine the expression of PRC1 in HGSOC, the
publicly accessible database Oncomine and TCGA cohort were employed
to analyze PRC1 mRNA expression, and it was observed that PRC1 mRNA
expression in serous ovarian carcinoma (SOC) was significantly
higher compared with that in normal ovary tissues or normal
peritoneum tissues (Fig. 1A-C). It
was also found that the expression of PRC1 was positively
associated with the malignancy of ovarian tumors (Fig. 1E and F). To confirm PRC1
overexpression in HGSOC, RT-qPCR was performed to measure PRC1
expression in normal FT (n=14) and HGSOC (n=28) tissues, and it was
observed that the PRC1 mRNA level was significantly higher in HGSOC
(Fig. 1D). Subsequently, western
blot analysis was performed to determine the protein expression
levels and the relative protein expression of PRC1 was calculated
in normal FT (n=7) and HGSOC (n=8) tissues. The results obtained
were similar to those for mRNA expression (Fig. 1G and H). All these results verified
that PRC1 was markedly overexpressed in HGSOC tissues at both the
mRNA and protein level, and suggested that PRC1 plays an important
role in ovarian cancer development and biological
characteristics.
Association between PRC1 expression and
clinicopathological parameters
To further determine the expression and clinical
significance of PRC1 in HGSOC, an immunohistochemistry assay was
conducted to examine PRC1 protein expression in 210 HGSOC tissues
and 42 normal FT tissues. As shown in Fig. 2E, PRC1 staining was mainly
distributed in the cytoplasm, and the expression of the PRC1 was
significantly upregulated in HGSOC tissues. To better understand
the significance of PRC1 in HGSOC, the association between the
expression of PRC1 and the clinicopathological parameters of 210
patients was analyzed. The results revealed that the overexpression
of PRC1 was significantly associated with platinum-based
chemotherapy sensitivity and the secondary recurrence intervals
(P<0.05). Among the 95 patients with FIGO stage III-IV disease
who received satisfactory cytoreductive surgery, 7 patients were
resistant to platinum-based chemotherapy, and the staining of these
7 patients, surprisingly, revealed a high expression of PRC1. For
second-line chemotherapy, 5 patients had a direct progression of
the disease following partial remission among the 18 drug-resistant
cases with PRC1 high expression. The primary and secondary
recurrence interval and the mean recurrence interval were 12.1
vs. 10.2 months (P>0.05) and 7.3 vs. 4.9 months
(P<0.05), respectively. In total, 52 cases of germline BRCA
mutations were summarized; 17 cases were BRCA mutation carriers,
and 13 of these 17 patients had a low expression of PRC1, with a
P-value of 0.019 (Table I).
However, no significant association was observed between the
expression of PRC1 and the age of the patients, FIGO stage, the
CA-125 level, ascites volume, residual lesions, family history and
the primary recurrence interval (P>0.05). All the
above-mentioned data confirmed that PRC1 played a major role in
drug resistance and recurrence of HGSOC.
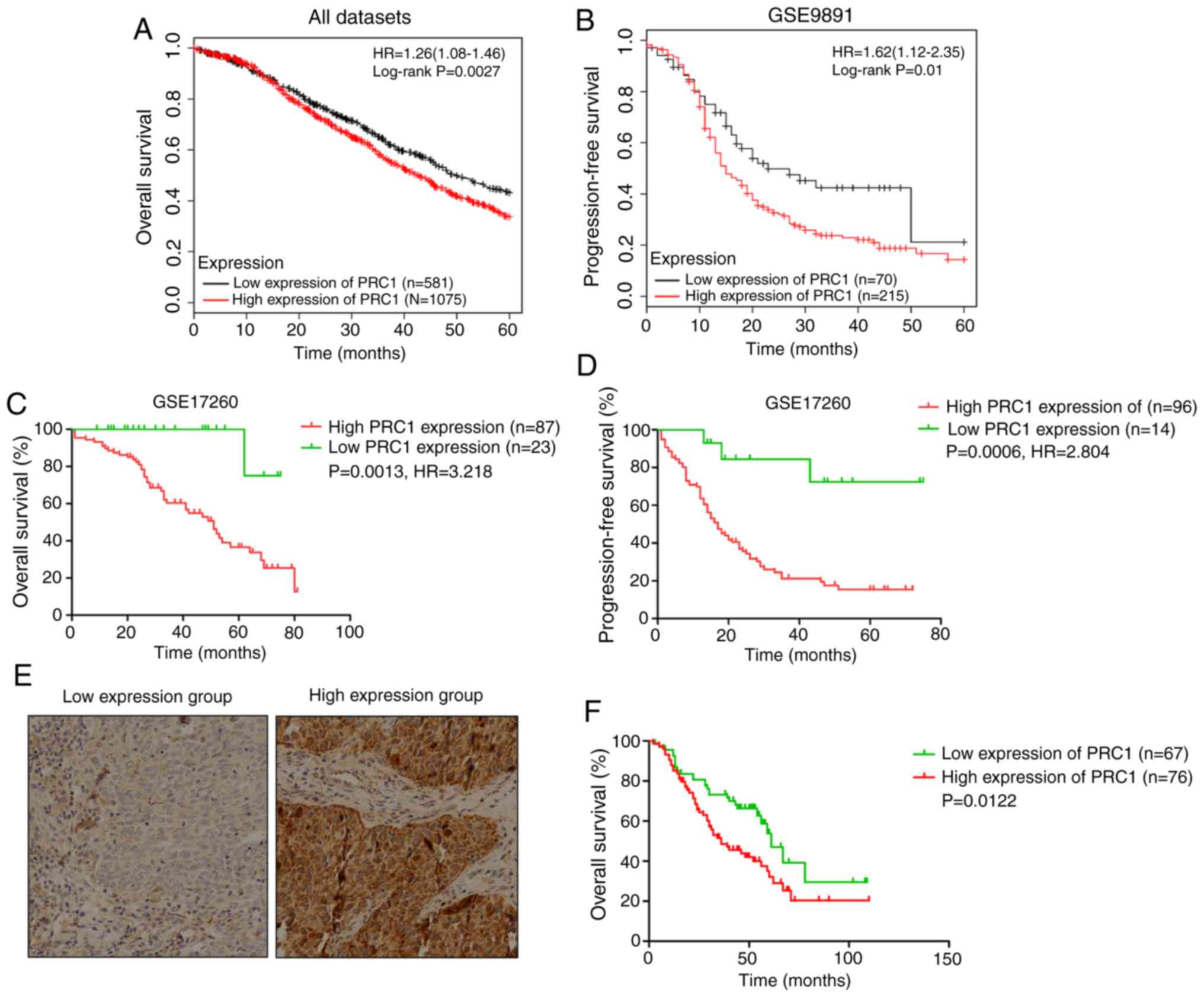
PRC1 contributes to a poor prognosis of
patients with HGSOC
Kaplan Meier-plotter was used to investigate the
effects of PRC1 expression on clinical prognosis, and the results
revealed that the expression of PRC1 was negatively associated with
the survival time (Fig. 2A-D). In
total, 143 patients with HGSOC with complete survival information
were analyzed to evaluate the importance of PRC1 overexpression in
predicting HGSOC clinical outcomes according to immunohistochemical
staining. It is found that patients with an elevated PRC1
expression had extremely significant poor outcomes in the 3-year
survival rate, 5-year survival rate (Table I) and overall survival (Fig. 2F). Notably, multivariate Cox
regression analysis revealed that a high expression of PRC1 protein
was a unique independent prognostic factor for patients with HGSOC
(Table II). These results provide
evidence that a high expression of PRC1 indicates a worse prognosis
of patients with HGSOC.
 | Table IIMultivariate analysis of PRC1 protein
levels and other clinical prognostic markers related to OS in
HGSOC. |
Table II
Multivariate analysis of PRC1 protein
levels and other clinical prognostic markers related to OS in
HGSOC.
| Item | OS
|
|---|
| HR (95% Cl) | P-value |
|---|
| Age (≥55/<55
years) | 0.735
(0.412-1.312) | 0.298 |
| FIGO stage
(III-IV/I-II) | 0.818
(0.360-1.868) | 0.631 |
| CA-125
(≥500/<500 U/ml) | 1.040
(0.592-1.827) | 0.890 |
| Ascites
(≥500/<500 ml) | 0.652
(0.337-1.263) | 0.205 |
| Residual lesions
(≥1/<1 cm) | 0.657
(0.381-1.134) | 0.131 |
| PRC1 level
(high/low) | 1.970
(1.147-3.384) | 0.014a |
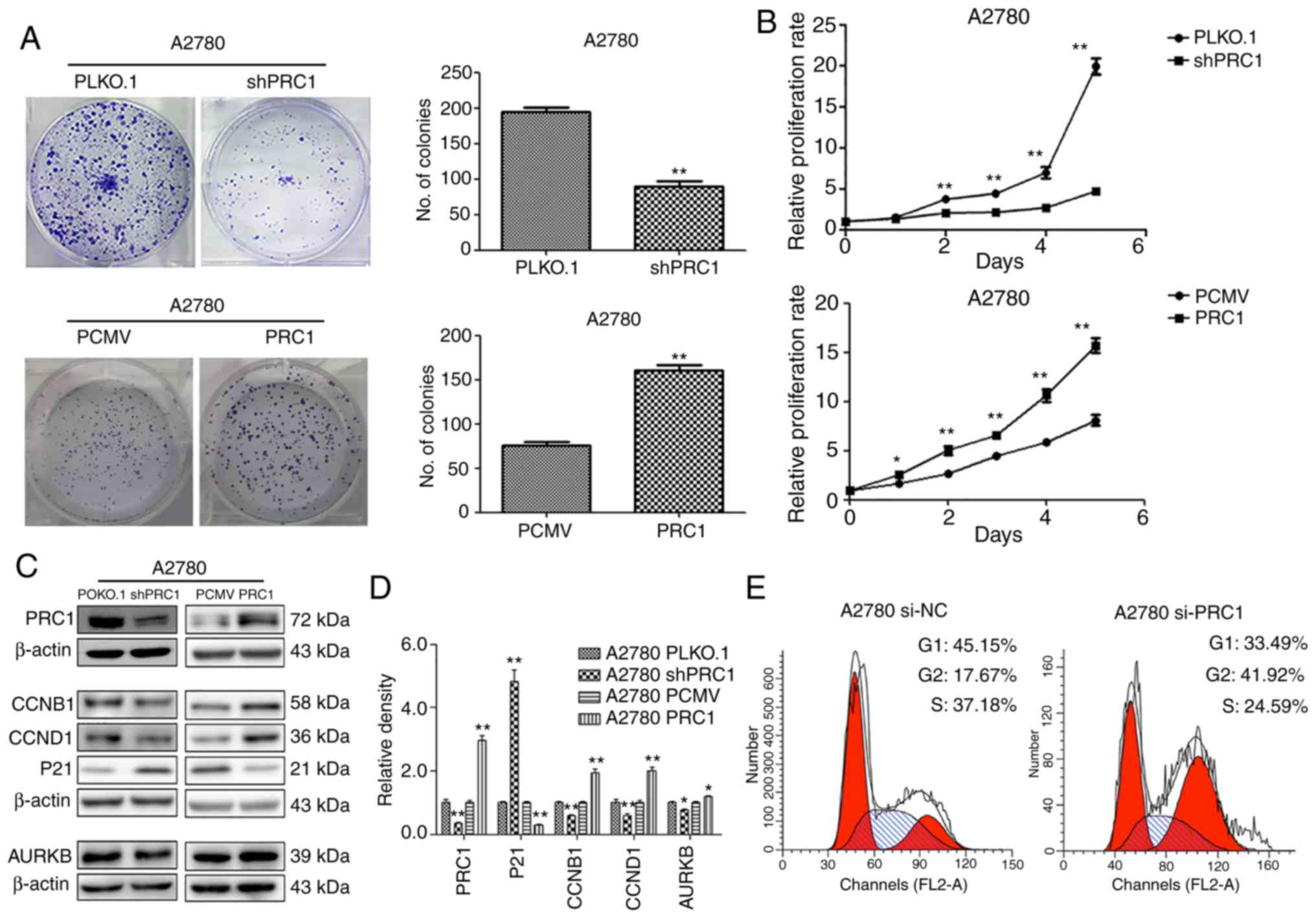
PRC1 promotes ovarian cancer cell
proliferation in vitro
To evaluate the biological function of PRC1 in
ovarian cancer, stable ovarian cancer cell lines with PRC1
overexpression and knockdown were established. The effect of PRC1
on the proliferative ability of the A2780 cells was then examined.
Both growth curve analysis and colony formation assay demonstrated
that PRC1 knockdown markedly inhibited cell growth and that PRC1
overexpression significantly enhanced cell proliferation (Fig. 3A and B). To further verify whether
PRC1 regulates the cell cycle, PRC1 was knocked down in A2780 cells
using siRNA and the changes in the cell cycle were then examined
using fluorescence-activated cell sorting (FACS) analysis. As shown
in Fig. 3E, PRC1 depletion
significantly increased the percentage of cells in the G2 phase and
decreased the percentage of cells in the G1 phase. In addition, the
results of western blot analysis revealed that the CCNB1, CCND1 and
AURKB expression levels were decreased and p21 expression was
increased following PRC1 knockdown, while the corresponding
expression levels were reversed with PRC1 overexpression in A2780
cells (Fig. 3C and D). All these
results suggested that PRC1 promoted ovarian cancer proliferation
in vitro.
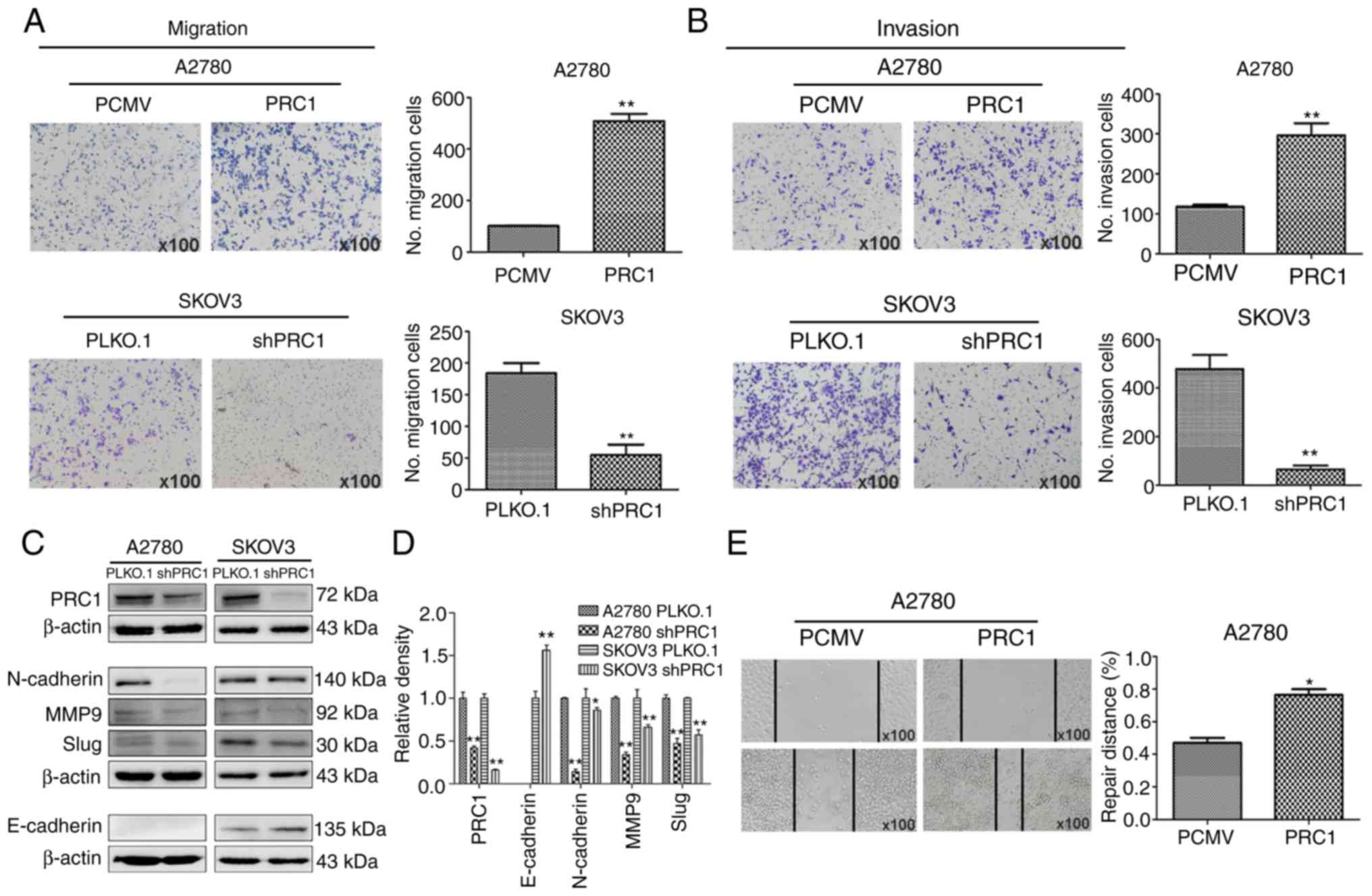
PRC1 promotes the migration and invasion
of ovarian cancer cells in vitro
A wound healing assay was first performed to
determine the effects of PRC1 expression on the metastasis of
ovarian cancer cells, and the results reveled that PRC1
overexpression in A2780 cells significantly facilitated the
migratory ability of the cells compared with the control (Fig. 4E). Transwell assay also revealed
that the migratory and invasive ability of the cells was
significantly inhibited in the PRC1-depleted cells; on the contrary
however, the metastatic ability was enhanced in the
PRC1-overexpressing cells (Fig. 4A and
B). Finally, the levels of epithelial-mesenchymal transition
(EMT) markers were measured by western blot analysis, as shown in
Fig. 4C and D. The expression of
E-Cadherin, N-Cadherin, MMP9 and Slug was decreased following PRC1
knockdown. Taken together, all these data suggest an important role
of PRC1 in the migratory and invasive ability of ovarian cancer
cells via EMT.
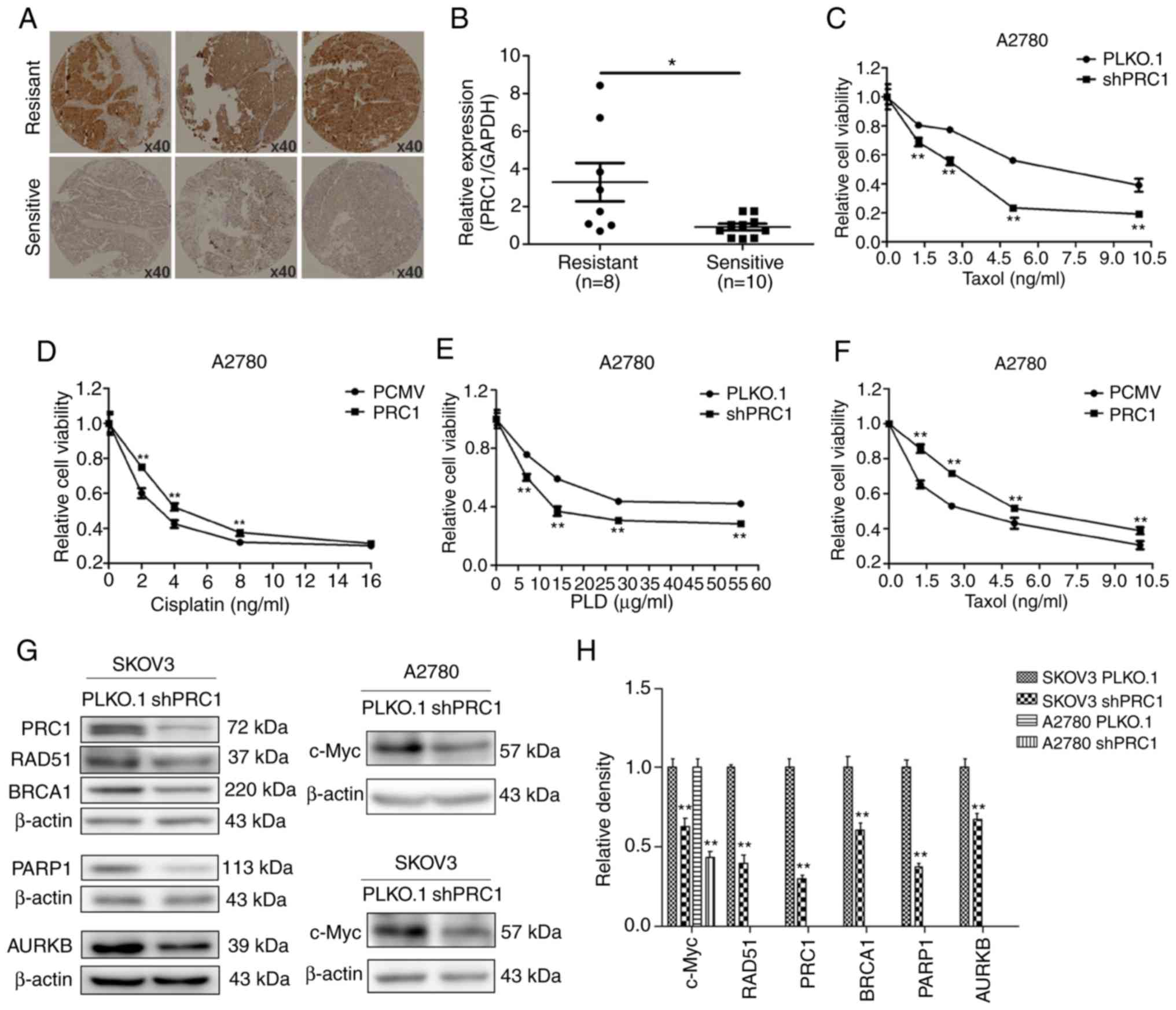
PRC1 promotes the multi-drug resistance
of ovarian cancer cells in vitro
Immunohistochemical staining was first applied to
examine the expression of PRC1 in HGSOC samples with second-line
treatment. As shown in Fig. 5A,
the expression of PRC1 in the resistant samples was higher than in
the sensitive ones. In total, 38.3% (18/47) patients with a high
expression of PRC1 were resistant to platinum-based chemotherapy,
while only 17.1% (7/41) of patients with a low expression of PRC1
were resistant (Table I). The mRNA
expression level of PRC1 in the partial samples with second-line
treatment described above was further investigated; it was found
that 10 patients were sensitive to platinum, 8 were
platinum-resistant, and the expression of PRC1 was evidently higher
in the samples of resistant patients (Fig. 5B). Finally, MTT assay was performed
to evaluate the changes in the IC50 values of cisplatin,
taxol and LPD, and the results revealed that PRC1 knockdown
significantly enhanced the sensitivity of the chemotherapeutic
drugs (Fig. 5C-F). In addition, it
was also found that PRC1 knockdown downregulated BRCA1, RAD51,
PARP1 and c-Myc expression in SKOV3 cells and the expression of
c-Myc was also downregulated in the A2780 cells (Fig. 5G and H). These results confirmed
that PRC1 played an important role in the drug resistance of
ovarian cancer.
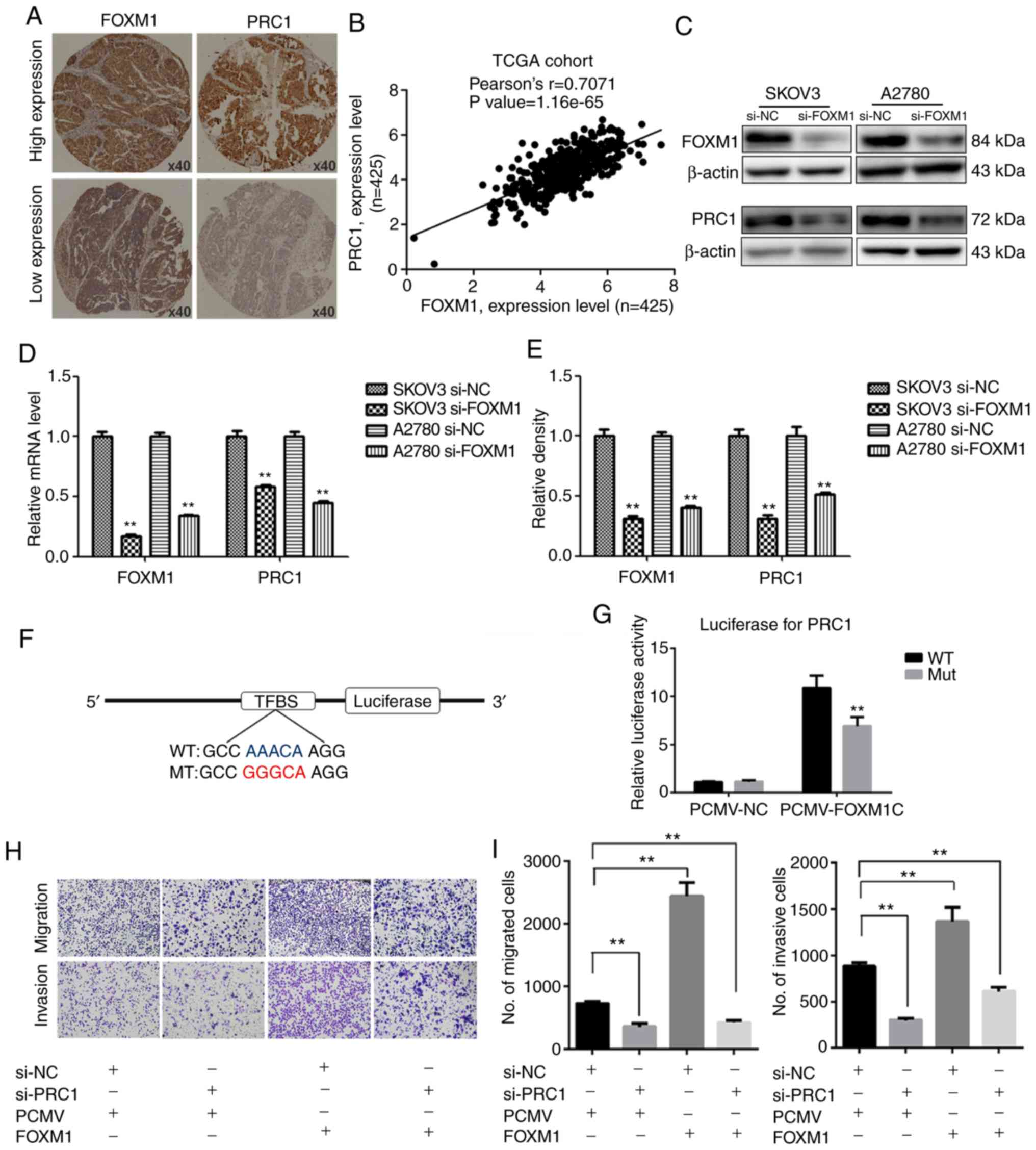
FOXM1 activates the expression of PRC1
through binding to its promoter directly
The promoter of PRC1 was analyzed using a gene
regulation website (www.gene-regulation.com), and it was found that a
number of transcription factors could bind to its promoter,
including FOXM1. The expression of PRC1 was found to positively
correlate with the expression of FOXM1 in the TCGA cohort analysis
(Fig. 6B). It was found that the
expression of PRC1 was markedly decreased at both the mRNA and
protein level following FOXM1 knockdown (Fig. 6C-E). The results of
immunohistochemical staining of corresponding HGSOC samples
revealed that the expression of PRC1 was consistent with the
expression of FOXM1 (Fig. 6A). The
results of luciferase report assay revealed that FOXM1 activated
the expression of PRC1 by directly binding to the promoter of PRC1
and PRC1 luciferase activity was decreased when the binding site
was mutated (Fig. 6F and G). To
determine whether PRC1 serves as a downstream target of FOXM1, PRC1
was knocked down in FOXM1-overexpressing cells. Transwell assay was
then used to determine whether the silencing of PRC1 could reverse
the FOXM1-mediated increase in the metastatic ability of A2780
cells. As shown in Fig. 6H and I,
the silencing of PRC1 inhibited the FOXM1-mediated promotion of the
migratory and invasive ability of the ovarian cancer cells. These
results thus suggested that the overexpression of PRC1 was ascribed
from the regulation of FOXM1, and it may serve as an important
executor.
Discussion
Although ovarian cancer is not the most common
malignant tumor of gynecological cancers, it is the most lethal,
and despite advances being made in surgical and chemotherapy
management, ovarian cancer mortality has remained virtually
unaffected (14,15). PARP inhibitors targeting BRCA
pathogenic mutations are the only major breakthrough made in the
treatment of ovarian cancer in recent decades; however, only
approximately 20% of patients can benefit from these (16). At present, there is still no
systematic mechanism for the pathogenesis, metastasis,
drug-resistance mechanisms of ovarian cancer. In this study, it was
verified that PRC1 was significantly overexpressed in HGSOC,
particularly in patients without BRCA pathogenic mutations, and
that its overexpression was strongly associated with the
sensitivity of platinum-based chemotherapy and poor prognosis of
patients. In addition, we further demonstrated that PRC1 was
essential for the function of ovarian cancer cells. As a key target
gene of FOXM1, PRC1 is expected to be a novel therapeutic target of
ovarian cancers, particularly for patients without BRCA pathogenic
mutations.
Proliferation is an important characteristic of life
activity, and the manifestation of cellular level is cell division.
The accurate entry of cells into the growth and division cycle is a
prerequisite for maintaining normal cell proliferation and genomic
stability. Cytokinesis is a physical separation of two daughter
cells during cell division and is the final stage of the cell
cycle. However, the tetraploid and chromosomes instability caused
by failure of accurate cell division can promote tumorigenesis and
development (17,18). Thus far, the key role of PRC1 has
been demonstrated in a variety of malignancies associated with the
p53 and Wnt signaling pathways (7-10).
However, there is no relevant study available to date on PRC1 in
ovarian cancer; to the best of our knowledge, the current study is
the first study to describe the key role of PRC1 in ovarian cancer
both as regards the clinicopathological features and the mechanisms
involved.
In this study, it was confirmed that PRC1 promotes
the proliferation, invasion, migration and multi-drug resistance of
ovarian cancer cells. The data indicated that PRC1 knockdown was
significantly related to mitotic-related genesm including cyclin
B1, cyclin D1, p21 and AURKB. Therefore, it was concluded that PRC1
promotes the proliferation of cancer cells through the
cytokinesis-related function. Furthermore, AURKB, one of the few
effective targets for the treatment of ovarian cancer, was closely
related to PRC1 expression. The overexpression or amplification of
AURKB is generally detected in a number of of human cancers, such
as breast cancer (19), ovarian
cancer (20-23), gastrointestinal cancer (24) and other tumors (25-29)
and is associated with drug resistance and a poor prognosis. c-Myc
has been reported in most types of human malignancies (30,31),
and integrated genome analysis of ovarian carcinoma using the TCGA
project have revealed that c-Myc is one of the 8 common genes that
are amplified in 30-60% of human ovarian carcinomas at the somatic
level (32,33). Targeting c-Myc in
platinum-resistant ovarian cancer has been confirmed as a potential
therapeutic method (34). In
summary, PRC1 knockdown can enhance the sensitivity of
chemotherapeutic drugs by downregulating the expression of AURKB
and c-Myc.
EMT is a process through which epithelial cells lose
cell polarity and homogenous adhesion, and gain migratory and
invasive properties (35). In
recent studies, EMT has been confirmed to be associated with drug
resistance in hepatic carcinoma (36-38),
colorectal cancer (39-41), gastric cancer (42), breast cancer (43,44)
and non-small cell lung cancer (45). This study confirmed that PRC1
expression was closely related to E-cadherin, N-cadherin, MMP9 and
Slug, which participated in the important process of EMT, and
mediated the invasion, migration and drug resistance of ovarian
cancer cells.
More importantly, this study obtained results from
the analysis of clinical data which are worthy of mention. It is
well known that chemo-resistance causes disease relapse and
metastasis, remaining the main obstacle to cancer therapy. Previous
studies have indicated that the aberrant expression of PRC1 may
point to biochemical recurrence and a poor prognosis in lung
squamous cell carcinoma (9) and
hepatic carcinoma (7). In this
study, it was confirmed that PRC1 over-expression led to
platinum-based chemo-resistance both in the first line and second
line. PRC1 overexpression shortened the recurrence interval, and
furthermore, PRC1 was an independent risk factor of overall
survival. Although the experiments in this study confirmed that
PRC1 was associated with metastasis in vitro, there was no
significant difference found in the FIGO stage, and this may be
related to the failure of the early diagnosis of ovarian cancer.
All these results suggest that PRC1 may prove to be a therapeutic
target with great potential.
FOXM1 is a tumorigenic transcription factor of the
forkhead family, and has been confirmed to play a crucial role in
the proliferation and progression of multiple tumor cells. FOXM1 is
overexpressed in >20 human tumors and promotes tumor cell
proliferation, invasion, metastasis and the chemo-resistant
processes of ovarian cancers (46-50).
In addition, the overexpression of FOXM1 has been shown to
significantly reduce the survival of ovarian cancer patients
(51). In this study, it was
confirmed that PRC1 was a direct downstream gene of FOXM1 via mRNA
and protein expression verification and luciferase assay.
Furthermore, this study demonstrated that the silencing PRC1
inhibited the invasive and migratory ability of
FOXM1-overexpressing ovarian cancer cells.
In conclusion, this study verified the expression
pattern, molecular mechanisms and clinical information of PRC1 in
HGSOC, and confirmed that the upregulated expression of PRC1
enhanced the proliferation, invasion, migration and multi-drug
resistance of ovarian cancer cells in vitro. Clinical data
analysis also confirmed the key role of PRC1 in tumor resistance,
recurrence and a poor prognosis. The study thus indicated that PRC1
may prove to be a promising molecular target for ovarian cancer,
and small molecule inhibitors targeting PRC1 may have desirable
anticancer effects.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81874107, 81572554 and
81572559), and the Program for Interdisciplinary Basic Research of
Shandong University (grant no. 2018JC014).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HB and YL contributed to the experimental work,
figures and drafting of the manuscript. CJ made substantial
contributions to the design of the study and data analysis. HY, XW
assisted with the experiments and data analysis. JC, YW and YM
assisted with acquisition and analysis of clinical information. YZ
and BK designed and supervised all the experiments. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Ethics approval was obtained from the Ethics
Committee of Shandong University, and written informed consent was
obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nik NN, Vang R, Shih Ie M and Kurman RJ:
Origin and pathogenesis of pelvic (ovarian, tubal, and primary
peritoneal) serous carcinoma. Annu Rev Pathol. 9:27–45. 2014.
View Article : Google Scholar
|
|
4
|
Jiang W, Jimenez G, Wells NJ, Hope TJ,
Wahl GM, Hunter T and Fukunaga R: PRC1: A human mitotic
spindle-associated CDK substrate protein required for cytokinesis.
Mol Cell. 2:877–885. 1998. View Article : Google Scholar
|
|
5
|
Mollinari C, Kleman JP, Jiang W, Schoehn
G, Hunter T and Margolis RL: PRC1 is a microtubule binding and
bundling protein essential to maintain the mitotic spindle midzone.
J Cell Biol. 157:1175–1186. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kurasawa Y, Earnshaw WC, Mochizuki Y,
Dohmae N and Todokoro K: Essential roles of KIF4 and its binding
partner PRC1 in organized central spindle midzone formation. EMBO
J. 23:3237–3248. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen J, Rajasekaran M, Xia H, Zhang X,
Kong SN, Sekar K, Seshachalam VP, Deivasigamani A, Goh BK, Ooi LL,
et al: The microtubule-associated protein PRC1 promotes early
recurrence of hepatocellular carcinoma in association with the
Wnt/β-catenin signalling pathway. Gut. 65:1522–1534. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang B, Shi X, Xu G, Kang W, Zhang W,
Zhang S, Cao Y, Qian L, Zhan P, Yan H, et al: Elevated PRC1 in
gastric carcinoma exerts oncogenic function and is targeted by
piperlongumine in a p53-dependent manner. J Cell Mol Med.
21:1329–1341. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhan P, Xi GM, Liu HB, Liu YF, Xu WJ, Zhu
Q, Zhou ZJ, Miao YY, Wang XX, Jin JJ, et al: Protein regulator of
cytokinesis-1 expression: Prognostic value in lung squamous cell
carcinoma patients. J Thorac Dis. 9:2054–2060. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhan P, Zhang B, Xi G, Wu Y, Liu HB, Liu
YF, Xu WJ, Zhu QQ, Cai F, Zhou ZJ, et al: PRC1 contributes to
tumorigenesis of lung adenocarcinoma in association with the
Wnt/β-catenin signaling pathway. Mol Cancer. 16:1082017. View Article : Google Scholar
|
|
11
|
Rustin GJ, Vergote I, Eisenhauer E,
Pujade-Lauraine E, Quinn M, Thigpen T, du Bois A, Kristensen G,
Jakobsen A, Sagae S, et al: Definitions for response and
progression in ovarian cancer clinical trials incorporating RECIST
1.1 and CA 125 agreed by the Gynecological Cancer Intergroup
(GCIG). Int J Gynecol Cancer. 21:419–423. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
13
|
Plon SE, Eccles DM, Easton D, Foulkes WD,
Genuardi M, Greenblatt MS, Hogervorst FB, Hoogerbrugge N, Spurdle
AB and Tavtigian SV: Sequence variant classification and reporting:
Recommendations for improving the interpretation of cancer
susceptibility genetic test results. Hum Mutat. 29:1282–1291. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vaughan S, Coward JI, Bast RC Jr, Berchuck
A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R,
Etemadmoghadam D, et al: Rethinking ovarian cancer: Recommendations
for improving outcomes. Nat Rev Cancer. 11:719–725. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi T, Wang P, Xie C, Yin S, Shi D, Wei C,
Tang W, Jiang R, Cheng X, Wei Q, et al: BRCA1 and BRCA2 mutations
in ovarian cancer patients from China: Ethnic-related mutations in
BRCA1 associated with an increased risk of ovarian cancer. Int J
Cancer. 140:2051–2059. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujiwara T, Bandi M, Nitta M, Ivanova EV,
Bronson RT and Pellman D: Cytokinesis failure generating
tetraploids promotes tumorigenesis in p53-null cells. Nature.
437:1043–1047. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Steigemann P, Wurzenberger C, Schmitz MH,
Held M, Guizetti J, Maar S and Gerlich DW: Aurora B-mediated
abscission checkpoint protects against tetraploidization. Cell.
136:473–484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Jiang C, Li H, Lv F, Li X, Qian
X, Fu L, Xu B and Guo X: Elevated Aurora B expression contributes
to chemo-resistance and poor prognosis in breast cancer. Int J Clin
Exp Pathol. 8:751–757. 2015.
|
|
20
|
Hetland TE, Nymoen DA, Holth A, Brusegard
K, Flørenes VA, Kærn J, Tropé CG and Davidson B: Aurora B
expression in metastatic effusions from advanced-stage ovarian
serous carcinoma is predictive of intrinsic chemotherapy
resistance. Hum Pathol. 44:777–785. 2013. View Article : Google Scholar
|
|
21
|
Beussel S, Hasenburg A, Bogatyreva L,
Hauschke D, Werner M and Lassmann S: Aurora-B protein expression is
linked to initial response to taxane-based first-line chemotherapy
in stage III ovarian carcinoma. J Clin Pathol. 65:29–35. 2012.
View Article : Google Scholar
|
|
22
|
Davidson B, Nymoen DA, Elgaaen BV, Staff
AC, Tropé CG, Kærn J, Reich R and Falkenthal TE: BUB1 mRNA is
significantly co-expressed with AURKA and AURKB mRNA in
advanced-stage ovarian serous carcinoma. Virchows Arch.
464:701–707. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen YJ, Chen CM, Twu NF, Yen MS, Lai CR,
Wu HH, Wang PH and Yuan CC: Overexpression of Aurora B is
associated with poor prognosis in epithelial ovarian cancer
patients. Virchows Arch. 455:431–440. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Honma K, Nakanishi R, Nakanoko T, Ando K,
Saeki H, Oki E, Iimori M, Kitao H, Kakeji Y and Maehara Y:
Contribution of Aurora-A and -B expression to DNA aneuploidy in
gastric cancers. Surg Today. 44:454–461. 2014. View Article : Google Scholar
|
|
25
|
Tuncel H, Shimamoto F, Kaneko Guangying,
Qi H, Aoki E, Jikihara H, Nakai S, Takata T and Tatsuka M: Nuclear
Aurora B and cytoplasmic Survivin expression is involved in lymph
node metastasis of colorectal cancer. Oncol Lett. 3:1109–1114.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takeshita M, Koga T, Takayama K, Ijichi K,
Yano T, Maehara Y, Nakanishi Y and Sueishi K: Aurora-B
overexpression is correlated with aneuploidy and poor prognosis in
non-small cell lung cancer. Lung Cancer. 80:85–90. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fadri-Moskwik M, Weiderhold KN, Deeraksa
A, Chuang C, Pan J, Lin SH and Yu-Lee LY: Aurora B is regulated by
acetylation/deacetylation during mitosis in prostate cancer cells.
FASEB J. 26:4057–4067. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Premkumar DR, Jane EP and Pollack IF:
Cucurbitacin-I inhibits Aurora kinase A, Aurora kinase B and
survivin, induces defects in cell cycle progression and promotes
ABT-737-induced cell death in a caspase-independent manner in
malignant human glioma cells. Cancer Biol Ther. 16:233–243. 2015.
View Article : Google Scholar :
|
|
29
|
Diaz RJ, Golbourn B, Shekarforoush M,
Smith CA and Rutka JT: Aurora kinase B/C inhibition impairs
malignant glioma growth in vivo. J Neurooncol. 108:349–360. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vita M and Henriksson M: The Myc
oncoprotein as a therapeutic target for human cancer. Semin Cancer
Biol. 16:318–330. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baker VV, Borst MP, Dixon D, Hatch KD,
Shingleton HM and Miller D: c-myc amplification in ovarian cancer.
Gynecol Oncol. 38:340–342. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cancer Genome Atlas Research Network:
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Prathapam T, Aleshin A, Guan Y, Gray JW
and Martin GS: p27Kip1 mediates addiction of ovarian cancer cells
to MYCC (c-MYC) and their dependence on MYC paralogs. J Biol Chem.
285:32529–32538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Reyes-Gonzalez JM, Armaiz-Peña GN, Mangala
LS, Valiyeva F, Ivan C, Pradeep S, Echevarría-Vargas IM,
Rivera-Reyes A, Sood AK and Vivas-Mejía PE: Targeting c-MYC in
platinum-resistant ovarian cancer. Mol Cancer Ther. 14:2260–2269.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesen-chymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Y, Zeng S, Ma J, Deng G, Qu Y, Guo C
and Shen H: Nestin overexpression in hepatocellular carcinoma
associates with epithelial-mesenchymal transition and
chemoresistance. J Exp Clin Cancer Res. 35:1112016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu Q, Wang R, Yang Q, Hou X, Chen S, Hou
Y, Chen C, Yang Y, Miele L, Sarkar FH, et al: Chemoresistance to
gemcitabine in hepatoma cells induces epithelial-mesenchymal
transition and involves activation of PDGF-D pathway. Oncotarget.
4:1999–2009. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ju BL, Chen YB, Zhang WY, Yu CH, Zhu DQ
and Jin J: miR-145 regulates chemoresistance in hepatocellular
carcinoma via epithelial mesenchymal transition. Cell Mol Biol
(Noisy-le-Grand). 61:12–16. 2015.
|
|
39
|
Lee TY, Liu CL, Chang YC, Nieh S, Lin YS,
Jao SW, Chen SF and Liu TY: Increased chemoresistance via Snail-Raf
kinase inhibitor protein signaling in colorectal cancer in response
to a nicotine derivative. Oncotarget. 7:23512–23520.
2016.PubMed/NCBI
|
|
40
|
Li J, Liu H, Yu J and Yu H:
Chemoresistance to doxorubicin induces epithelial-mesenchymal
transition via upregulation of transforming growth factor beta
signaling in HCT116 colon cancer cells. Mol Med Rep. 12:192–198.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li Y, Huang S, Li Y, Zhang W, He K, Zhao
M, Lin H, Li D, Zhang H, Zheng Z and Huang C: Decreased expression
of LncRNA SLC25A25-AS1 promotes proliferation, chemore-sistance,
and EMT in colorectal cancer cells. Tumour Biol. 37:14205–14215.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Feng S, Zheng Z, Feng L, Yang L, Chen Z,
Lin Y, Gao Y and Chen Y: Proton pump inhibitor pantoprazole
inhibits the proliferation, selfrenewal and chemoresistance of
gastric cancer stem cells via the EMT/β-catenin pathways. Oncol
Rep. 36:3207–3214. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jiang L, He D, Yang D, Chen Z, Pan Q, Mao
A, Cai Y, Li X, Xing H, Shi M, et al: MiR-489 regulates
chemoresistance in breast cancer via epithelial mesenchymal
transition pathway. FEBS Lett. 588:2009–2015. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hu SH, Wang CH, Huang ZJ, Liu F, Xu CW, Li
XL and Chen GQ: miR-760 mediates chemoresistance through inhibition
of epithelial mesenchymal transition in breast cancer cells. Eur
Rev Med Pharmacol Sci. 20:5002–5008. 2016.PubMed/NCBI
|
|
45
|
Jin Z, Guan L, Song Y, Xiang GM, Chen SX
and Gao B: MicroRNA-138 regulates chemoresistance in human
non-small cell lung cancer via epithelial mesenchymal transition.
Eur Rev Med Pharmacol Sci. 20:1080–1086. 2016.PubMed/NCBI
|
|
46
|
Westhoff GL, Chen Y and Teng NNH:
Targeting Foxm1 improves cytotoxicity of paclitaxel and cisplatinum
in platinum-resistant ovarian cancer. Int J Gynecol Cancer.
27:887–894. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tassi RA, Todeschini P, Siegel ER, Calza
S, Cappella P, Ardighieri L, Cadei M, Bugatti M, Romani C, Bandiera
E, et al: FOXM1 expression is significantly associated with
chemotherapy resistance and adverse prognosis in non-serous
epithelial ovarian cancer patients. J Exp Clin Cancer Res.
36:632017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wen N, Wang Y, Wen L, Zhao SH, Ai ZH, Wang
Y, Wu B, Lu HX, Yang H, Liu WC and Li Y: Overexpression of FOXM1
predicts poor prognosis and promotes cancer cell proliferation,
migration and invasion in epithelial ovarian cancer. J Transl Med.
12:1342014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhao F, Siu MK, Jiang L, Tam KF, Ngan HY,
Le XF, Wong OG, Wong ES, Gomes AR, Bella L, et al: Overexpression
of forkhead box protein M1 (FOXM1) in ovarian cancer correlates
with poor patient survival and contributes to paclitaxel
resistance. PLoS One. 9:e1134782014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chiu WT, Huang YF, Tsai HY, Chen CC, Chang
CH, Huang SC, Hsu KF and Chou CY: FOXM1 confers to
epithelial-mesenchymal transition, stemness and chemoresistance in
epithelial ovarian carcinoma cells. Oncotarget. 6:2349–2365. 2015.
View Article : Google Scholar :
|
|
51
|
Jin C, Liu Z, Li Y, Bu H, Wang Y, Xu Y,
Qiu C, Yan S, Yuan C, Li R, et al: PCNA-associated factor
P15PAF, targeted by FOXM1, predicts poor prognosis in
high-grade serous ovarian cancer patients. Int J Cancer.
143:2973–2984. 2018. View Article : Google Scholar : PubMed/NCBI
|