Introduction
Lymphomas are the third most common type of cancer
identified during childhood and adolescence, with 60% of those
being mature B-cell non-Hodgkin’s lymphoma (B-NHL). Primary central
nervous system malignant lymphomas (PCNSLs) comprise <2% of
malignant lymphomas. B-NHL is usually of B-cell origin (1–4).
First-line chemotherapy is found to be effective in the majority of
children diagnosed with B-NHL. Although long-term cure rates are
75% for high-risk disease (5),
relapses occur in ∼20% of the patients, almost always within a year
from diagnosis (6). Relapsed or
refractory B-NHL has a poor prognosis. CD20 is expressed in >98%
of childhood B-NHL and increasingly a chimeric anti-CD20 monoclonal
antibody, rituximab, is being used at relapse (7,8).
Although rituximab is commonly used as a first-line therapy in
adults, the effect of rituximab in children with B-NHL has yet to
be adequately investigated (9–12).
Three B-NHL cases were investigated in this case study to determine
the efficacy of rituximab-containing regimens on relapsed
B-NHL.
Case reports
Patient 1
A 16-year-old male was admitted to the Department of
Pediatric Oncology complaining of recurrent abdominal pain and
distention for the previous 2 months. Physical examination revealed
that the abdomen was distended but non-tender, with an immobile,
painless and hard mass, 5×8 cm in diameter, located on the right
side of the abdomen. Results of bone marrow (BM) examination,
hematologic and basic metabolic analysis were normal. Abdominal
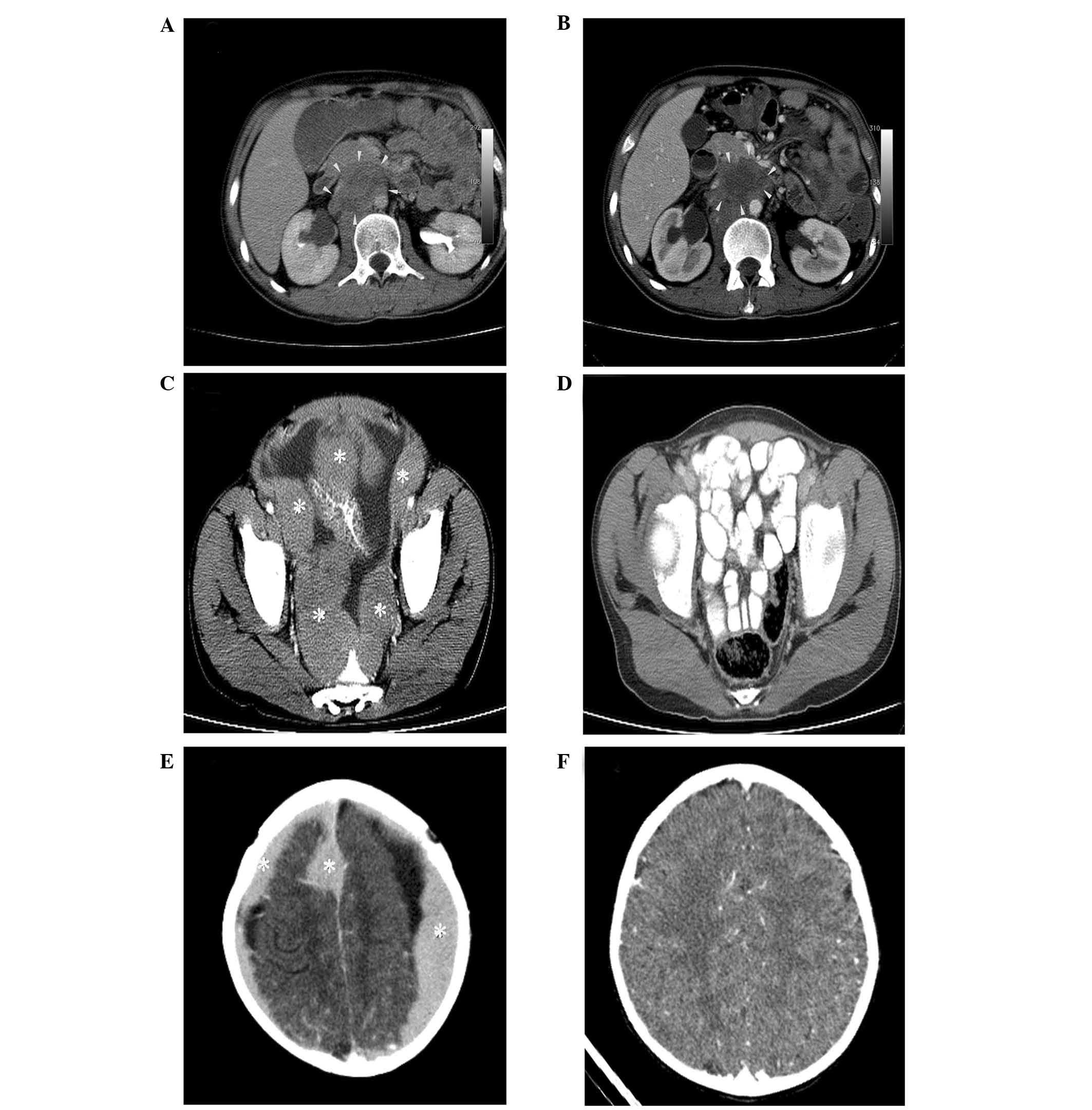
computed tomography (CT) scan confirmed multiple retroperitoneal
lymph nodes and a mass, 6×10×11 cm in a diameter, surrounding the
superior mesenteric artery (Fig.
1A). Burkitt lymphoma was diagnosed. Positive CD20, CD10, bcl6
and negative CD30, CD3, CD5 and TdT were reported by
immunohistochemical examination. After 4 months of
NHL-Berlin-Frankfurt-Muenster (BFM) 95 chemotherapy protocol, the
patient noted backache. BM examination revealed diffuse L3 type
lymphoblasts. Additionally, the CT scan showed that the size of the
intra-abdominal mass did not decrease. The patient received
intravenous rituximab (375 mg/m2/dose/once every 3
weeks) for six doses and Ifosfamide, Carboplatin and Etoposide
(ICE). The control CT scan revealed that the size of the mass
decreased and central necrosis was evident (Fig. 1B). Although in complete remission
at least 12 months following chemotherapy, the patient succumbed
due to systemic progression of severe sepsis.
Patient 2
A 14-year-old male presented with a 1-month history
of abdominal pain and distension. He noted a 7-pound weight loss
during the previous two weeks. On physical examination, the abdomen
was distended, although soft and non-tender, with hypoactive bowel
sounds and shifting dullness. No organomegaly was noted. Results of
BM examination, as well as hematologic and metabolic tests were
normal. Abdominal CT scan revealed enlarged multiple
lymphadenopathies, of which the largest was 6 cm in diameter, in
the mesenteric region and peritoneal surfaces with ascites
(Fig. 1C). Flow cytometry showed
that the ascites fluid was 90% positive for CD45 and 80% for CD20.
Burkitt lymphoma was diagnosed and NHL-BFM 95 chemotherapy protocol
was administered. After 6 months of chemotherapy, the abdominal and
the maxillary mass gradually decreased. At the end of the
chemotherapy, complete remission was achieved (Fig. 1D). One month later, the patient
complained of chest pain and a 4×3 cm solid mass on the right 4th
rib was detected in the thorax CT scan. Fine-needle aspiration
confirmed B-cell lymphoma. ICE and intravenous rituximab (375
mg/m2/dose/once every 3 weeks) for 6 weeks were given
and remission was achieved again. After 2 years of follow-up, the
patient is still in complete remission.
Patient 3
A previously healthy 4.5-year-old girl was admitted
with a 1-month history of headache and seizures. Her physical
examination was normal. BM examination, routine blood chemistry and
hematologic parameters had no abnormality. The CT revealed multiple
homogeneously enhancing dural mass lesions (Fig. 1E). There was no evidence of
lymphoma in any other anatomic location. After partial excision,
immunohistochemistry revealed highly expressed HLA-DR, CD19, CD20,
CD38 and CD79a. B-NHL was diagnosed. The patient was administered
intensive chemotherapy with NHL-BFM 95 chemotherapy protocol.
However, at the fourth month of treatment, right maxillary swelling
was identified. The mass, 4×3×5 cm, was evident on maxillofacial
MRI scan. Diffuse L3 type lymphoblasts were observed with fine
needle aspiration. Two doses of rituximab (375
mg/m2/dose/once every 3 weeks) were given. The control
CT scan revealed that the cranial tumor completely regressed
(Fig. 1F). However, the patient’s
status deteriorated gradually due to sepsis. Consequently acute
respiratory distress syndrome and multi-organ failure developed.
The patient ultimately succumbed one year after the initiation of
chemotherapy of causes unrelated to central nervous system lymphoma
(CNSL).
Discussion
Although an improvement in the treatment outcome for
children with high-grade B-cell lymphomas has been noted, the
prognosis of relapsed or refractory B-cell lymphomas is poor
(10). Rituximab has shown good
clinical activity in the treatment of CD20-positive B-cell
lymphomas. It has also been reported that rituximab provides the
opportunity for combining to chemotherapy combinations and
increasing the overall and disease-free survival (11–15).
Our first case received rituximab in addition to ICE after relapsed
B-NHL was diagnosed. One month after completion of the
chemotherapy, the tumor size was found to have markedly decreased
and there were no blasts in BM. This case supports a potential role
of rituximab in children with established relapsed or refractory
B-NHL.
The Children’s Oncology Group reported that the
toxicity of combination with ICE and rituximab was acceptable in
children with recurrent or refractory B-NHL (10). Rituximab in combination with ICE
was well tolerated and proved to be effective, with no major side
effects. The abdominal mass completely recovered with NHL-BFM 95
chemotherapy protocol in the second case. Following rapid detection
of a new mass on the patient’s right 4th rib, rituximab in
combination with ICE was administered and complete remission was
achieved. The patient remains in complete remission after 2 years
of completing combined therapy. Taken together, for patients who do
not achieve complete remission or relapse following reinduction
chemotherapy, rituximab combined with intensive chemotherapy could
provide a beneficial role in the treatment of B-NHL. Although the
number of patients in this study was limited, the follow-up of a
larger group of patients could aid in determining the benefits of
rituximab in children with B-NHL.
Large-group studies have reported that <1% of
PCNSLs, either B- or T-cell, occur in patients under 18 years of
age (4,11,16,17).
Due to the insufficient number of prospective studies, it is not
easy to determine the best therapeutic option for this rare tumor
(18). Rituximab was found to be
effective in adults with secondary CNSLs (19). When no remission was achieved, the
cranial tumor almost completely recovered with rituximab in our
case. Although we obtained a remission in only one patient, better
understanding of rituximab in pediatric PCNSLs should be emphasized
with large-scale studies. PCNSLs tended to occur more frequently in
immunodeficient children (17,18).
Our case was not immunosuppressive. Therefore, the proper time to
apply rituximab is while being unresponsive to current pediatric
protocols in relapsed/refractory B-NHL.
This case series has demonstrated that early
treatment with rituximab alone or combined with intensive
chemotherapy could play a significant role in improving the outcome
of relapsed B-cell lymphomas in the pediatric age group.
Nevertheless, the identification of clinical efficacy and safety of
rituximab as a monotheraphy or combination chemotherapy should be
investigated in a large pediatric series.
References
|
1.
|
Bilić E, Femenić R, Konja J, et al: CD20
positive childhood B-non Hodgkin lymphoma (B-NHL): morphology,
immunophenotype and a novel treatment approach: a single center
experience. Coll Antropol. 34:171–175. 2010.PubMed/NCBI
|
|
2.
|
Molyneux EM, Rochford R, Griffin B, et al:
Burkitt’s lymphoma. Lancet. 379:1234–1244. 2012.
|
|
3.
|
Akbayram S, Doğan M, Akgün C, et al: Use
of rituximab in three children with relapsed/refractory Burkitt
lymphoma. Target Oncol. 5:291–294. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Alasaad T and Barr R: Successful treatment
of multiply relapsed lymphoma with rituximab as a single agent.
Pediatr Blood Cancer. 55:356–358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Attarbaschi A, Dworzak M, Steiner M, et
al: Outcome of children with primary resistant or relapsed
non-Hodgkin lymphoma and mature B-cell leukemia after intensive
first-line treatment: a population-based analysis of the Austrian
Cooperative Study Group. Pediatr Blood Cancer. 44:70–76. 2005.
View Article : Google Scholar
|
|
6.
|
Bay A, Dogan M, Acikgoz M and Oner AF:
Rituximab in a child with relapsed Burkitt lymphoma. Pediatr Blood
Cancer. 49:2182007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Culić S, Culić V, Armanda V, et al:
Anti-CD20 monoclonal antibody (Rituximab) for therapy of
mediastinal CD20-positive large B-cell non-Hodgkin lymphoma with a
local tumor extension into the lung of a 10-year-old girl. Pediatr
Hematol Oncol. 20:339–344. 2003.PubMed/NCBI
|
|
8.
|
Meinhardt A, Burkhardt B, Zimmermann M, et
al: Phase II window study on rituximab in newly diagnosed pediatric
mature B-cell non-Hodgkin’s lymphoma and Burkitt leukemia. J Clin
Oncol. 28:3115–3121. 2010.PubMed/NCBI
|
|
9.
|
Corbacioglu S, Eber S, Gungor T, et al:
Induction of long-term remission of a relapsed childhood B-acute
lymphoblastic leukemia with rituximab chimeric anti-CD20 monoclonal
antibody and autologous stem cell transplantation. J Pediatr
Hematol Oncol. 25:327–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Griffin TC, Weitzman S, Weinstein H, et
al: A study of rituximab and ifosfamide, carboplatin, and etoposide
chemotherapy in children with recurrent/refractory B-cell (CD20+)
non-Hodgkin lymphoma and mature B-cell acute lymphoblastic
leukemia: a report from the Children’s Oncology Group. Pediatr
Blood Cancer. 52:177–181. 2009.PubMed/NCBI
|
|
11.
|
Boyle EM and Morschhauser F: Ongoing
development of monoclonal antibodies and antibody drug-conjugates
in lymphoma. Curr Oncol Rep. 13:386–397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Leget GA and Czuczman MS: Use of
rituximab, the new FDA approved antibody. Curr Opin Oncol.
10:548–551. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Song KW, Mollee P, Patterson B, et al:
Pure red cell aplasia due to parvovirus following treatment with
CHOP and rituximab for B-cell lymphoma. Br J Haematol. 119:125–127.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Quartier P, Brethon B, Philippet P, et al:
Treatment of childhood autoimmune haemolytic anaemia with
rituximab. Lancet. 358:1511–1513. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zecca M, Nobili B, Ramenghi U, et al:
Rituximab for the treatment of refractory autoimmune hemolytic
anemia in children. Blood. 101:3857–3861. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Campo E, Swerdlow SH, Harris NL, et al:
The 2008 WHO classification of lymphoid neoplasms and beyond:
evolving concepts and practical applications. Blood. 117:5019–5032.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Bhagavathi S and Wilson JD: Primary
central nervous system lymphoma. Arch Pathol Lab Med.
132:1830–1834. 2008.PubMed/NCBI
|
|
18.
|
Abla O and Weitzman S: Primary central
nervous system lymphoma in children. Neurosurg Focus. 21:E82006.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kawano N, Ochiai H, Yoshida S, et al:
Clinical features and treatment outcomes of isolated secondary
central nervous system lymphomas in Miyazaki Prefecture. Int J Clin
Oncol. 17:336–340. 2012. View Article : Google Scholar : PubMed/NCBI
|