Introduction
Metabolic syndrome is a global health problem of
increasing prevalence in Western, as well as Asian, countries
(1,2). In addition to its known association
with cardiovascular diseases (3),
recent evidence has suggested an association between the metabolic
syndrome and various types of cancer, including colorectal cancer
(CRC) (4,5).
Accumulating evidence suggested that visceral
obesity, insulin resistance and systemic inflammation may be
implicated in the pathophysiological link between metabolic
syndrome and CRC development (6).
Cytokines produced by adipose tissue may promote inflammation and
lead to subsequent adenomatous changes in the colonic epithelium
(7). Various studies demonstrated
an association between individual components of the metabolic
syndrome and colorectal adenoma, a pre-cancerous lesion of CRC
(8,9). In this study, we evaluated the
association between clinical profiles associated with the metabolic
syndrome and the occurrence of colorectal adenoma in Thai patients.
Furthermore, a metabolic risk scoring system was constructed, based
on clinical and laboratory items that exhibited a significant
association with this disease.
Patients and methods
Patient history
Patients aged >15 years who underwent a
colonoscopy at the NKC Institute of Gastroenterology and
Hepatology, Songklanagarind Hospital, between June, 2010 and
December, 2012, were enrolled in this study. Cases with known
colonic pathology, either colonic polyp or CRC, were excluded.
Medical history regarding a previous diagnosis of hypertension,
dyslipidemia, diabetes mellitus or cancer in a family member was
obtained through a structured interview. Lifestyle history included
tobacco smoking, alcohol consumption, vegetable consumption and
exercise. Anthropometric measurements were performed on the date of
the endoscopy. Blood pressure was measured twice with a 10-min
interval, using a manual sphygmomanometer.
Laboratory profiles
Laboratory profiles, including fasting blood sugar
(FBS), hemoglobin A1C (HbA1C), triglyceride, low- and high-density
lipoprotein, aspartate transaminase (SGOT), alanine transaminase
(SGPT) and uric acid levels were recorded on the morning of the
endoscopy. All colonoscopies were performed by or under the close
supervision of a colorectal surgeon. The endoscopist was blinded to
the metabolic history and laboratory results. Once a polyp was
detected, a biopsy sample was collected for histopathological
examination. A polyp was characterized as a cancer-associated polyp
(CAP) when it was found to be an adenomatous polyp including
elements of tubular, villous, tubulovillous or serrated adenoma.
Other types of polyp or carcinoma were excluded from the
association analysis.
Statistical analysis
Continuous data are presented as the means unless
stated otherwise. Non-continuous data are presented as numbers with
percentage values. The possible associations between demographic or
metabolic parameters and CAP were analyzed using the Chi-square
test and univariate logistic regression analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Demographic data
A total of 289 subjects underwent a colonoscopy at
our institute during the study period. Three cases of CRC were
excluded, leaving a total of 286 subjects (132 males and 154
females) for association analysis. The mean age of the patients was
52 years, with 45 cases (16%) aged >65 years. The mean body mass
index (BMI) of the patients was 23.4 kg/m2 (range,
13.3–50 kg/m2). The reasons for undergoing a colonoscopy
included hematochezia (101 cases, 35%), abdominal pain (50 cases,
18%), changes in bowel habits (45 cases, 16%), asymptomatic (49
cases, 17%), constipation (31 cases, 10%) and other (10 cases, 3%).
The overall polyp detection rate was 25% (72 out of the 286 cases).
CAP was detected in 56 cases (19.6%). The incidence of CAP was not
found to be associated with any symptoms that would lead the
physician to recommend a colonoscopy (Table I).
 | Table I.Association between metabolic
syndrome-related laboratory parameters and CAP. |
Table I.
Association between metabolic
syndrome-related laboratory parameters and CAP.
| Parameters | Cases (n=286) | CAP
| P-value |
|---|
| Absent (%) | Present (%) |
|---|
| Total | 286 | 230 (80.4) | 56 (19.6) | |
| Age (years) | | | | <0.01 |
| <65 | 241 | 203 (84.2) | 38 (15.8) | |
| >65 | 45 | 27 (60.0) | 18 (40.0) | |
| Gender | | | | 0.21 |
| Male | 132 | 102 (77.3) | 30 (22.7) | |
| Female | 154 | 128 (83.1) | 26 (16.9) | |
| History of
hypertension | | | | <0.01 |
| No | 226 | 194 (85.8) | 32 (14.2) | |
| Yes | 60 | 36 (60.0) | 24 (40.0) | |
| History of
dyslipidemia | | | | <0.01 |
| No | 255 | 213 (83.5) | 42 (16.5) | |
| Yes | 31 | 17 (54.8) | 14 (45.1) | |
| History of DM | | | | <0.01 |
| No | 265 | 218 (82.3) | 47 (17.7) | |
| Yes | 21 | 12 (57.1) | 9 (42.9) | |
| Hypertension | | | | 0.02 |
| No | 231 | 192 (83.1) | 39 (16.9) | |
| Yes | 55 | 38 (69.1) | 17 (30.9) | |
| BMI
(kg/m2) | | | | 0.02 |
| ≤23.4 | 148 | 127 (85.8) | 21 (14.2) | |
| >23.4 | 138 | 103 (74.6) | 35 (25.4) | |
| Waist circumference
(in.) | | | | |
| Male | | | | 0.82 |
| ≤35 | 116 | 90 (77.6) | 26 (22.4) | |
| >35 | 16 | 12 (75.0) | 4 (25.0) | |
| Female | | | | 0.02 |
| ≤32 | 122 | 106 (86.9) | 16 (13.1) | |
| >32 | 32 | 22 (68.6) | 10 (31.3) | |
| Hip circumference
(in.) | | | | |
| Male | | | | 0.56 |
| ≤37 | 102 | 80 (78.4) | 22 (21.6) | |
| >37 | 30 | 22 (73.3) | 8 (26.7) | |
| Female | | | | 0.07 |
| ≤37 | 111 | 96 (86.5) | 15 (13.5) | |
| >37 | 43 | 32 (74.4) | 11 (25.6) | |
Univariate and multivariate analysis of
the association between metabolic profiles and CAP
On univariate analysis, a clinical history of
chronic disease, including hypertension, diabetes mellitus and
dyslipidemia was significantly associated with the occurrence of
CAP (Table I). The anthropometric
parameters that exhibited an association with CAP were high blood
pressure and BMI >23.4 kg/m2. In females, a high
waist and hip circumference (>32 and >37 inches,
respectively) were also significantly associated with CAP. None of
the lifestyle history items were found to be associated with
CAP.
The laboratory profiles that were associated with
CAP included FBS, HbA1C, hepatic transaminases and uric acid levels
(Table II). Notably, lipid
profiles did not exhibit a significant correlation with the
occurrence of polyps. The parameters that were found to be
association with CAP are summarized along with their odds ratios
(ORs) in Table III.
 | Table II.Association between metabolic
syndrome-related laboratory parameters and CAP. |
Table II.
Association between metabolic
syndrome-related laboratory parameters and CAP.
| Parameter | Cases (n=286) | CAP
| P-value |
|---|
| Absent (%) | Present (%) |
|---|
| FBS (mg%) | | | | <0.01 |
| ≤110 | 263 | 218 (82.9) | 45 (17.1) | |
| >110 | 23 | 12 (52.2) | 11 (47.8) | |
| HbA1C (%) | | | | <0.01 |
| ≤7.0 | 274 | 224 (81.8) | 50 (18.3) | |
| >7.0 | 12 | 6 (50.0) | 6 (50.0) | |
| HDL (mg%) | | | | 0.44 |
| ≤34.9 | 19 | 14 (73.7) | 5 (26.3) | |
| >34.9 | 267 | 216 (80.9) | 51 (19.1) | |
| LDL (mg%) | | | | 0.241 |
| ≤160 | 164 | 128 (78.0) | 36 (22.0) | |
| >160 | 122 | 102 (83.6) | 20 (16.4) | |
| TG (mg%) | | | | 0.973 |
| ≤200 | 255 | 205 (80.4) | 50 (19.6) | |
| >200 | 31 | 25 (80.7) | 6 (19.3) | |
| SGOT (IU/l) | | | | <0.01 |
| ≤40 | 262 | 216 (82.4) | 46 (17.6) | |
| >40 | 24 | 14 (58.3) | 10 (41.7) | |
| SGPT (IU/l) | | | | <0.01 |
| ≤50 | 268 | 200 (82.6) | 68 (17.9) | |
| >50 | 18 | 10 (55.6) | 8 (44.4) | |
| Uric acid
(mg%) | | | | <0.01 |
| ≤7 | 213 | 180 (84.5) | 33 (15.5) | |
| >7 | 73 | 50 (68.5) | 23 (31.5) | |
 | Table III.Parameters significantly associated
with CAP. |
Table III.
Parameters significantly associated
with CAP.
| Parameters | OR | 95% CI |
|---|
| Age >65
years | 3.30 | 1.67–6.50 |
| History of
hypertension | 4.04 | 2.14–7.65 |
| History of DM | 3.48 | 1.39–8.73 |
| History of
dyslipidemia | 4.18 | 1.91–9.12 |
| BMI >23.4
kg/m2 | 2.06 | 1.13–3.75 |
|
Hypertensiona | 2.20 | 1.13–4.29 |
| FBS >110
mg% | 4.44 | 1.84–10.69 |
| HbA1C >7% | 4.48 | 1.39–14.47 |
| SGOT >40
IU/l | 3.35 | 1.40–8.02 |
| SGPT >50
IU/l | 3.67 | 1.38–9.78 |
| Uric acid >7
mg% | 2.51 | 1.35–4.65 |
On multivariate analysis, three factors were found
to be independently associated with CAP: age >60 years (OR=3.9,
95% CI: 2.0–7.4), FBS >110 mg% (OR=2.9; 95% CI: 1.1–7.4) and
uric acid >7 mg% (OR=2.0; 95% CI: 1.0–3.9).
Construction and validation of metabolic
scoring system
Six metabolic items were selected to construct a
metabolic scoring system that may accurately predict the occurrence
of CAP in patients scheduled for a colonoscopy, irrespective of
their age and presenting symptoms. This metabolic risk scoring
system is presented in Table IV.
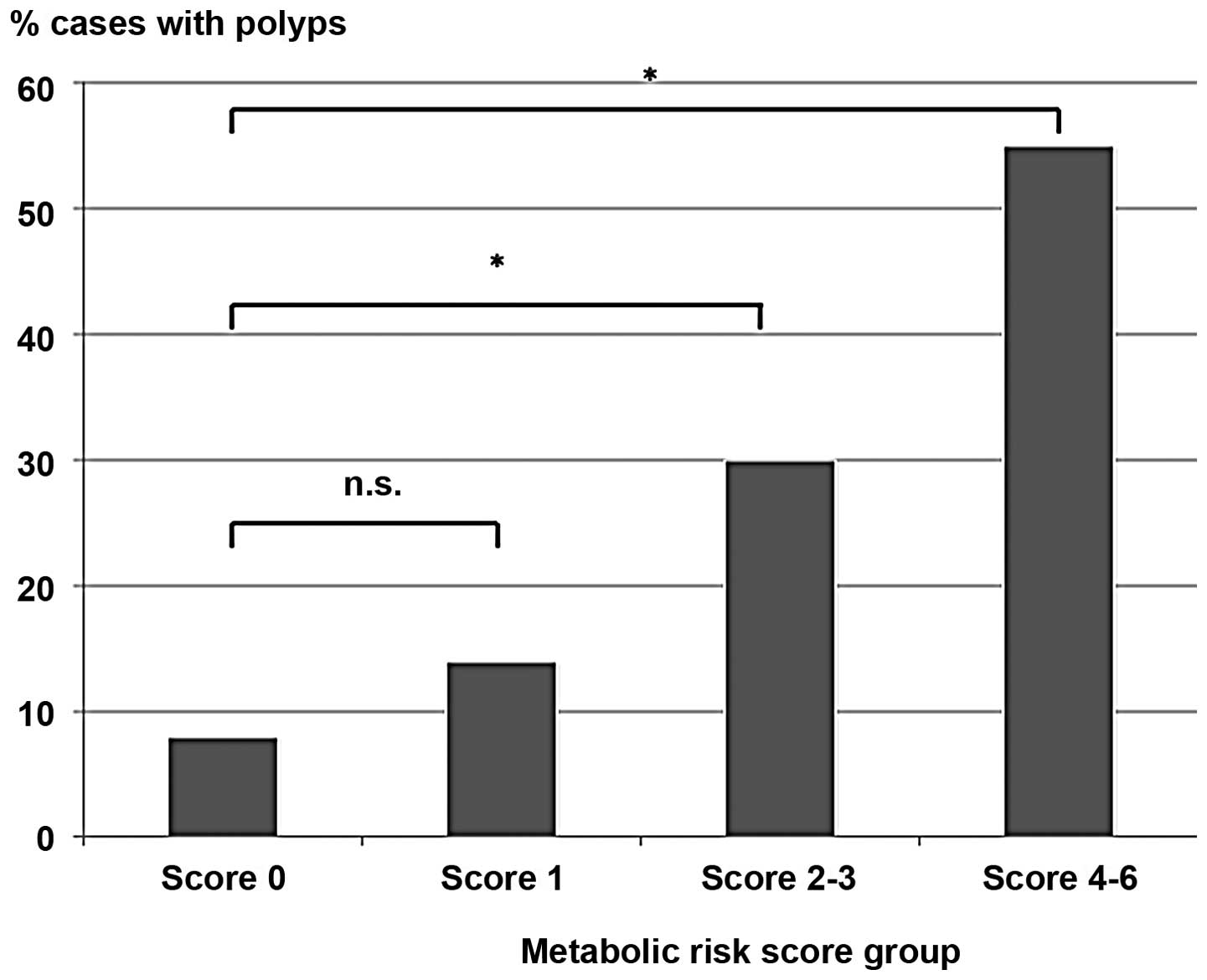
When the score was validated with the cases, it was observed that
moderate (2–3) and high scores (4–6) were
significantly associated with CAP at ORs of 4.9 (95% CI: 2.0–12.0)
and 13.7 (95% CI: 4.4–42.9), respectively (Table V, Fig.
1). Taking into consideration the age of the patients as an
interacting factor, the subgroup analysis demonstrated that the
metabolic score was associated with CAP only in the subgroup aged
<65 years, but not in that aged ≥65 years. In the <65-year
subgroup, the OR for moderate and high metabolic scores was
increased to 5.6 (95% CI: 1.8–17.9) and 39.0 (95% CI: 8.2–186.6),
respectively (Table V).
 | Table IV.Metabolic risk score constructed by
clinical history, anthropometric measurements and laboratory
profiles. |
Table IV.
Metabolic risk score constructed by
clinical history, anthropometric measurements and laboratory
profiles.
| Clinical
history | Anthropometric and
laboratory parameters | Points |
|---|
| Obesity | BMI >23.4
kg/m2 | 1 |
| Hypertension | History of
hypertension, or SBP >140 mmHg, or DBP >90 mmHg | 1 |
| DM | History of DM, or
FBS >110 mg%, or HbA1C >7% | 1 |
| Dyslipidemia | History of
dyslipidemia | 1 |
| Transaminitis | SGOT >40 IU/l;
SGPT >50 IU/l | 1 |
| Hyperuricemia | Serum uric acid
>7 mg% | 1 |
| Total | - | 6 |
 | Table V.Odds ratios of metabolic risk score,
compared between all patients and the subgroup of patients aged
<65 years. |
Table V.
Odds ratios of metabolic risk score,
compared between all patients and the subgroup of patients aged
<65 years.
| Patient
subgroup | Metabolic risk
score
|
|---|
| 0 | 1 | 2–3 | 4–6 |
|---|
| All patients | Reference | 1.8 (0.7–4.9) | 4.9 (2.0–12.0) | 13.7
(4.4–43.0) |
| Subgroup <65
years | Reference | 2.3 (0.7–8.1) | 5.6 (1.8–17.9) | 39.0
(8.2–186.6) |
Discussion
The detection and removal of an adenomatous polyps
has been proven to be an effective screening tool that reduces
CRC-related mortality (10,11).
However, the standard protocol recommends that screening is
initiated after the age of 50 years. Previous studies have
suggested that there is a certain degree of correlation between
metabolic syndrome and colorectal adenoma, a precancerous lesion of
CRC (12–14), our study aimed to develop a risk
determinant for younger patients who may benefit from screening on
the basis of their metabolic profiles and associated clinical
history.
The general adenoma detection rate of 20% for both
sexes, 23% in males and 17% in females, is in line with standard
quality indicators in colonoscopic practice (15). Our data confirmed a certain degree
of correlation between individual clinical parameters associated
with metabolic syndrome and colorectal adenoma and demonstrated
that the association was stronger in the subgroup of patients aged
<65 years. This finding may be explained by the fact that age
exerts a significant effect on the incidence of CRC. When the
factor of age was subtracted, the association between other
parameters and the disease became more apparent.
We investigated fundamental clinical parameters,
such as history of chronic diseases, with the hypothesis that,
under certain circumstances, these parameters may be more revealing
compared to laboratory tests. One example from our study that
confirmed this hypothesis was the case of dyslipidemia, for which
the medical history, but not the lipid profiles, indicated an
association with CAP in our patients. The metabolic risk score was
then constructed to cover all the aspects of the metabolic
syndrome, by using less objective data, such as medical history,
and more objective items, including anthropometric measurements and
laboratory profiles. Our positive findings indicated that further
validation of our approach in another set of subjects is
required.
Unlike other cohorts (14,15),
we did not identify a significant association between serum lipid
profiles and CAP detection. This may be attributed to the
relatively smaller size of our study compared to earlier studies.
Furthermore, a number of our patients were receiving medication for
dyslipidemia. Under such circumstances, it may be more useful to
investigate other markers of visceral obesity that are not
interfered with by treatment, such as serum adipokines.
In conclusion, our study confirmed a correlation
between individual parameters in the metabolic syndrome and the
occurrence of colorectal adenoma in Thai subjects. Furthermore,
this study constructed a metabolic risk scoring system that may
help identify patients at risk of having CAP.
Acknowledgements
The authors would like to thank the
staff at the NKC Institute of Gastroenterology and Hepatology for
their contribution in conducting this study.
References
|
1.
|
Zimmet P, Magliano D, Matsuzawa Y, Alberti
G and Shaw J: The metabolic syndrome: a global public health
problem and a new definition. J Atheroscler Thromb. 12:295–300.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Chen M, He M, Min X, et al: Different
physical activity subtypes and risk of metabolic syndrome in
middle-aged and older Chinese people. PLoS One. 8:e532582013.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Eckel RH, Grundy SM and Zimmet PZ: The
metabolic syndrome. Lancet. 365:1415–1428. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Esposito K, Chiodini P, Colao A, Lenzi A
and Giugliano D: Metabolic syndrome and risk of cancer: a
systematic review and meta-analysis. Diabetes Care. 35:2402–2411.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Aleksandrova K, Nimptsch K and Pischon T:
Influence of obesity and related metabolic alterations on
colorectal cancer risk. Curr Nutr Rep. 2:1–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Pais R, Silaghi H, Silaghi AC, Rusu ML and
Dumitrascu DL: Metabolic syndrome and risk of subsequent colorectal
cancer. World J Gastroenterol. 15:5141–5148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Guffey CR, Fan D, Singh UP and Murphy EA:
Linking obesity to colorectal cancer: recent insights into
plausible biological mechanisms. Curr Opin Clin Nutr Metab Care.
16:595–600. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Wang YY, Lin SY, Lai WA, Liu PH and Sheu
WH: Association between adenomas of rectosigmoid colon and
metabolic syndrome features in a Chinese population. J
Gastroenterol Hepatol. 20:1410–1415. 2005. View Article : Google Scholar
|
|
9.
|
Liao KF, Lai HC, Lai SW, Cheng KC and Lin
CH: Association between rectosigmoid adenomas and cardiovascular
risk factors: a hospital-based, cross-sectional study. Ann Acad Med
Singapore. 38:630–636. 2009.PubMed/NCBI
|
|
10.
|
Winawer SJ, Zauber AG, Ho MN, et al:
Prevention of colorectal cancer by colonoscopic polypectomy. The
National Polyp Study Workgroup N Engl J Med. 329:1977–1981. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Walsh JM and Terdiman JP: Colorectal
cancer screening: scientific review. JAMA. 289:1288–1296. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Yang MH, Rampal S, Sung J, et al: The
association of serum lipids with colorectal adenomas. Am J
Gastroenterol. 108:833–841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ulaganathan V, Kandiah M, Zalilah MS, et
al: Colorectal cancer and its association with the metabolic
syndrome: a Malaysian multi-centric case-control study. Asian Pac J
Cancer Prev. 13:3873–3877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kim BC, Shin A, Hong CW, et al:
Association of colorectal adenoma with components of metabolic
syndrome. Cancer Causes Control. 23:727–735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Rex DK, Petrini JL, Baron TH, et al:
Quality indicators for colonoscopy. Am J Gastroenterol.
101:873–885. 2006.PubMed/NCBI
|