Introduction
Lung cancer is one of the most common types of
cancer and the most common cause of cancer-related mortality
worldwide (1). The proportion of
small-cell lung cancer (SCLC) has decreased from 17.26% in 1986 to
12.95% in 2002 (2). In 2008,
~32,000 SCLC cases were diagnosed in the United States (3). SCLC is usually staged according to
the Veterans Administration Lung Study Group (VALSG) staging system
(4), according to which patients
are classified as having limited or extensive disease (LD and ED,
respectively). LD is defined as disease confined to one hemithorax,
in the absence of a malignant effusion, with disease that may be
encompassed in one radiation port. Disease that does not meet these
criteria is defined as ED. Despite a modest improvement in
survival, the outcome of SCLC remains poor. There are currently no
effective targeted agents that have been approved for the treatment
of SCLC (5) and chemotherapy is
the cornerstone of the treatment for SCLC. In ED SCLC, the most
commonly used initial combination chemotherapy regimens are
etoposide combined with cisplatin (EP), etoposide combined with
carboplatin (EC), irinotecan combined with cisplatin (IP) and
irinotecan combined with carboplatin (IC).
Approximately one-third of SCLC patients were
diagnosed with LD, which has a median survival time (MST) of 15–20
months (6). The standard clinical
practice is to combine chemotherapy and thoracic radiotherapy (TRT)
when treating patients with LD SCLC. A previous meta-analysis of 11
randomized trials including patients treated with chemotherapy and
TRT, demonstrated an improved 2-year survival of 5.4% and an
intrathoracic tumor control rate of 25.3% (7). EP plus accelerated hyperfractionated
thoracic radiotherapy (AHTRT) followed by 3 cycles of IP failed to
demonstrate a survival advantage over 4 cycles of EP plus AHTRT,
which remains the standard treatment for LD SCLC. An IP regimen
cannot be routinely recommended for LD SCLC (8). EP is superior to cyclophosphamide,
epirubicin and vincristine (CEV) in treating LD SCLC patients
(9). Previous studies on
cyclophosphamide-based therapy for LD SCLC failed to demonstrate
any survival benefit with the addition of TRT (10–11).
Carboplatin appears to be as effective as cisplatin and the EC
regimen was associated with significantly lower toxicity in
patients with SCLC (12).
Furthermore, a previous meta-analysis of individual patient data
reported no differences in efficacy between cisplatin and
carboplatin as first-line treatment for SCLC; however, the
incidence of hematological toxicity was higher with carboplatin and
that of non-hematological toxicity was higher with cisplatin
(13). In patients with LD SCLC,
the most commonly used initial combination chemotherapy regimens
are EP and EC. Concurrent TRT with chemotherapy has been considered
as the optimal treatment for LD SCLC (14–16).
However, the optimal initiation time for TRT has not been
definitively determined (16). The
limitations regarding early initiation of TRT are the potentially
enlarged radiation fields, due to initial planning for bulky
tumors, and toxicity. The aim of this meta-analysis was to
determine the optimal time for combining TRT with chemotherapy
(EP/EC) for the treatment of LD SCLC patients.
Patients and methods
Research objectives
The primary objective of this study was to compare
the effects of early and late concurrent TRT with EP/EC on overall
survival in LD SCLC. Furthermore, we aimed to evaluate early and
late TRT with chemotherapy regarding objective response and the
incidence of side effects.
Search strategy
The studies were selected from the following
databases: MEDLINE (1966 to present), the Cochrane Central Register
of Controlled Trials (CENTRAL, 2013, Issue 5), Embase (1974 to
present) and CINAHL (1982 to present). The Cochrane Lung Cancer
Groups Specialized Register was searched. The reference lists of
the identified studies were also searched for any additional
relevant studies. The electronic search for clinical trials was
complemented by a manual search of the following oncology journals:
Radiotherapy and Oncology (1985 to present); International Journal
of Radiation, Oncology, Biology and Physics (1985 to present);
Clinical Oncology (1999 to present); Lung Cancer (1985 to present);
Journal of Clinical Oncology (1985 to present); and Thorax (1985 to
present). The abstracts from the annual meetings of the American
Society of Clinical Oncology (ASCO) and the European Society for
Medical Oncology (ESMO) from 2000 onwards were hand-searched.
Colleagues, collaborators and other experts in the field were asked
to identify missing and unreported trials. The search was conducted
without language restrictions.
Criteria for study selection
Studies eligible for inclusion in this meta-analysis
were randomized controlled clinical trials that compared early to
late concurrent TRT, fully published in journals and relevant
scientific meetings, for which full details were available. The
patients were required to have histologically and cytologically
confirmed LD SCLC. Radiotherapy was administered concurrently with
chemotherapy and the chemotherapeutic regimen was EP/EC.
Early thoracic radiotherapy (ERT) was defined as
initiating irradiation within 30 days after the initiation of
chemotherapy (17). Late thoracic
radiotherapy (LRT) was defined as initiating irradiation after 30
days following the initiation of chemotherapy.
Data extraction
The identified randomized clinical trials were
assessed to determine whether they met the inclusion criteria by
three independent reviewers (Lu HY, Fang L and Wang XJ). The
quality of the methods and the key outcomes were evaluated against
predetermined criteria. Two reviewers (Lu HY and Fang L)
independently extracted data to ensure validity. Discrepancies were
resolved by consulting a third reviewer (Cai JF). The following
data were collected from the manuscript:patient gender, age,
performance status at the time of randomization, chemotherapy
regimen, induction treatment that resulted in a complete response,
date of radiotherapy initiation, presence of brain or other
metastases and locoregional recurrence.
Statistical analysis
Data analysis was performed with Rev Man 5.2
software, provided by The Cochrane Collaboration. Weighted risk
ratios (RRs) and their 95% confidence intervals (CIs) were
calculated according to the Mantel-Haenszel method (18). The results were assessed for
heterogeneity at a significance level of P<0.05, according to
the methods of DerSimonian and Laird (19). We performed a sensitivity analysis
to detect potential heterogeneity; if there was no evidence of
heterogeneity, a fixed-effects model was used, whereas if
heterogeneity existed, a random-effects model was used.
Results
Study selection
The characteristics of the included randomized
controlled trials are summarized in Table I. The study of Park et al
(16) was a phase III trial of
concurrent TRT with either the first or the third cycle of EP
chemotherapy in order to determine the optimal timing of TRT for LD
SCLC. The study of Skarlos et al (15) was a phase II randomized comparison
of early vs. late hyperfractionated TRT concurrently with EC
chemotherapy in LD SCLC. The study of Jeremic et al
(20) was a randomized comparison
of initial vs. delayed AHTRT concurrently with EP chemotherapy for
LD SCLC. Other studies were not eligible for inclusion in this
meta-analysis, as the chemotherapeutic regimen was not EP/EC
(21–24) and study 9104 was not considered
eligible due to administration of sequential TRT in only half of
the patients (14).
 | Table ICharacteristics of the included
studies. |
Table I
Characteristics of the included
studies.
| Trials (refs.) | Patient no.
(excluded) | Median age at E/L
(years) | Chemotherapy | TRT schedule | PCI E/L |
|---|
| Skarlos et al
(15) | 86 (5) | 61/60 | EC | 45 Gy (1.5 × 2 daily
× 15) | Yes, only if CR
41.0/57.0% |
| Park et al
(16) | 222 (3) | 60/61 | EP | 52.5 Gy/25 fx (2.1,
once daily) | Yes, only if CR or PR
49.5/55.6% |
| Jeremic et al
(20) | 107 (4) | 59/59 | EP | 54 Gy (1.5 × 2 daily
× 18) | Yes, only if CR or PR
98.0/84.0% |
Comparison of early and late concurrent
TRT
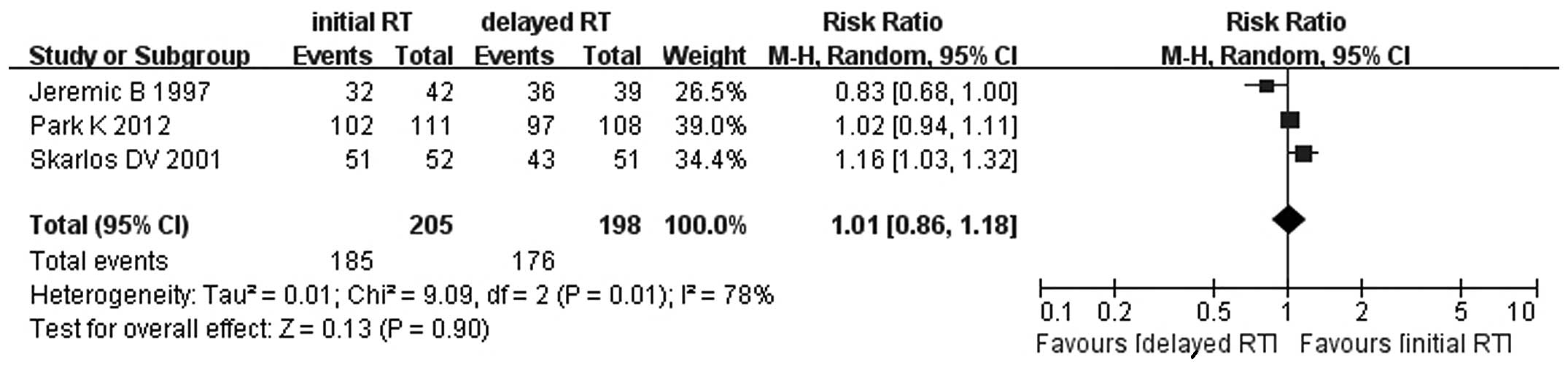
There were no significant differences in the
objective response between early and late concurrent TRT (RR=1.01,
95% CI: 0.86–1.18, P=0.90) (Fig.
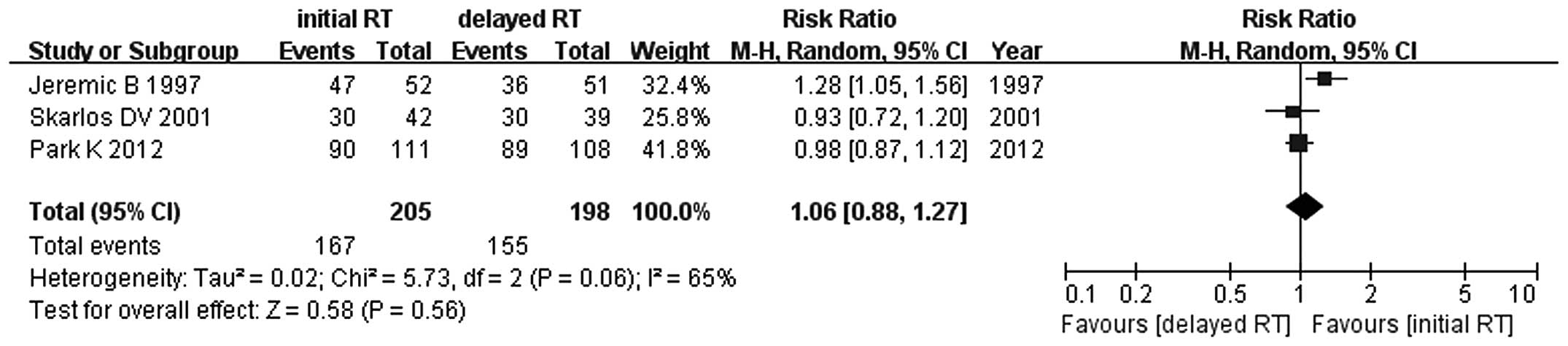
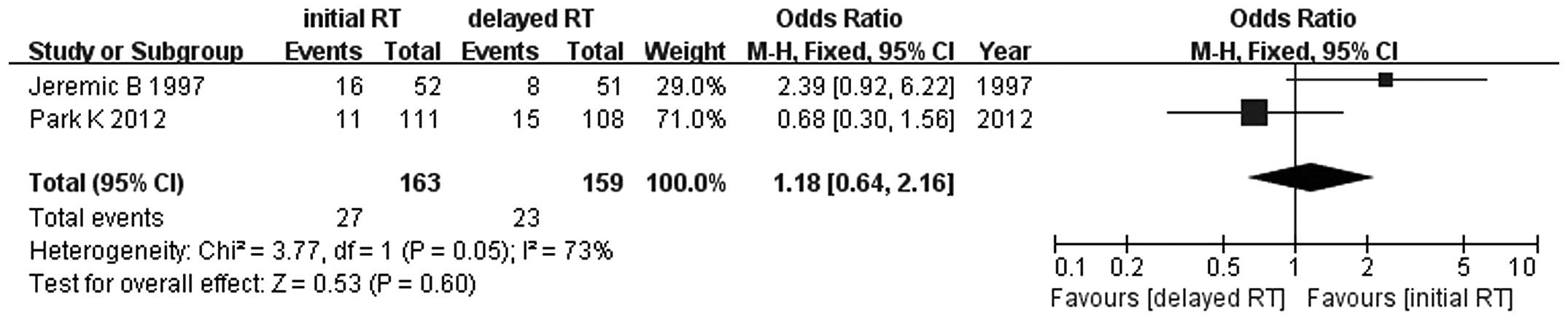
1). Similar results were observed for 1-, 2-, 3- and 5-year
survival rates between early and late concurrent TRT (RR=1.06, 95%
CI: 0.88–1.27, P=0.56; RR=1.15, 95% CI: 0.77–1.71, P=0.49; RR=0.90,
95% CI: 0.66–1.22, P=0.49; and RR=1.18, 95% CI: 0.64–2.16, P=0.60,
respectively) (Figs. 2–5). Since the study of Skarlos et
al (15) did not provide the
5-year survival rate, only the data from Park et al
(16) and Jeremic et al
(20) were analyzed.
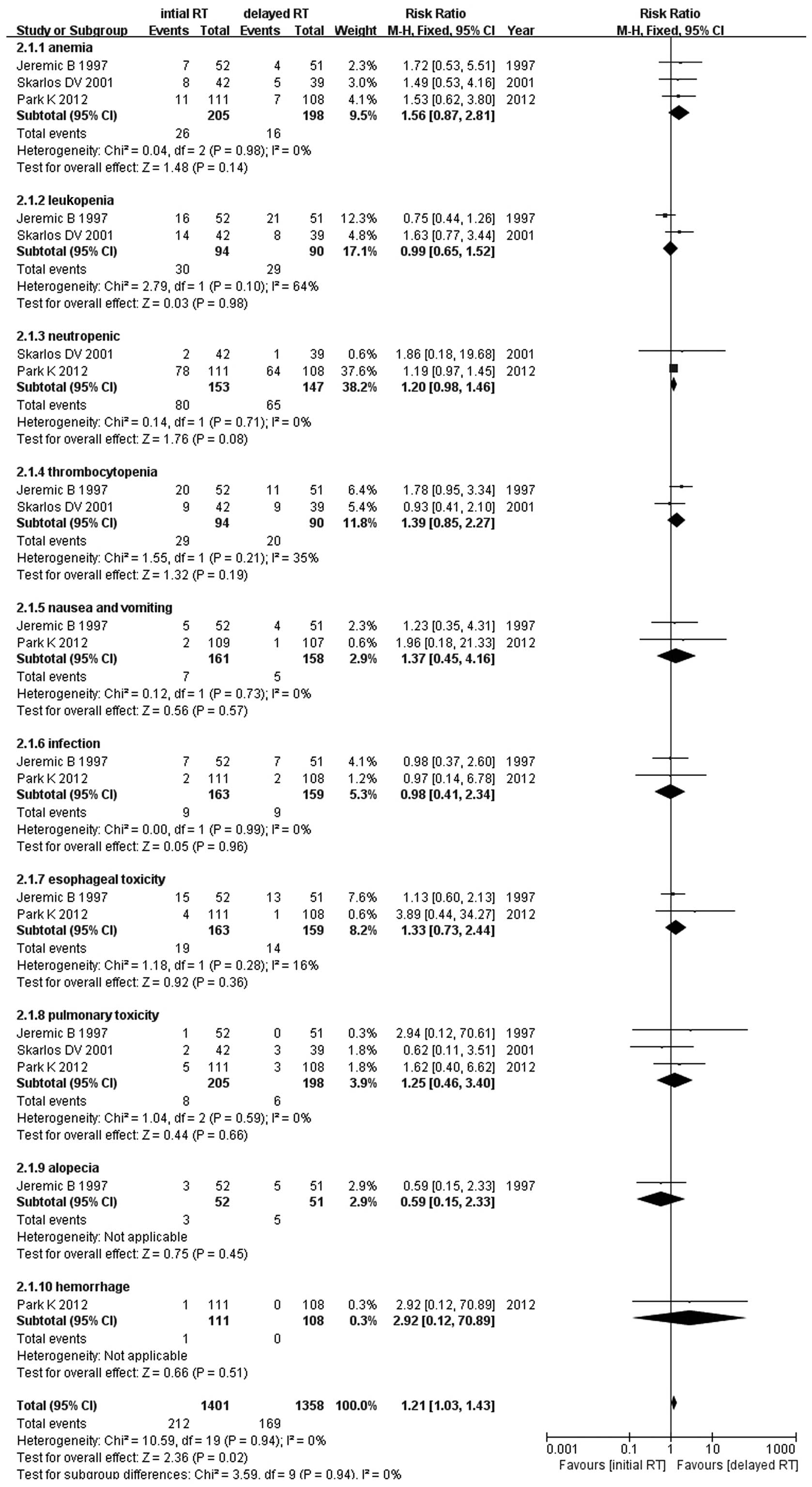
Adverse events
The incidence of grade 3–4 adverse events, such as
anemia, leukopenia, neutropenia, thrombocytopenia, nausea and
vomiting, infection, esophageal toxicity, pulmonary toxicity,
alopecia and hemorrhage, was higher with early compared to that
with late concurrent TRT (RR=1.21, 95% CI: 1.03–1.43, P=0.02)
(Fig. 6). There were no
significant differences for each grade 3–4 adverse event, such as
anemia, leukopenia and neutropenia (Fig. 6).
Discussion
EP and EC are the standard first-line
chemotherapeutic regimens for SCLC. IP or IC may also be used as
first-line chemotherapy for ED SCLC, but not for LD SCLC (8,25).
The addition of TRT has improved the survival of LD SCLC patients.
It was previously demonstrated that TRT combined with EP is more
effective for LD SCLC compared to radiotherapy and the
hematological toxicity was more severe in the concurrent arm
(14). Concurrent
chemoradiotherapy is preferable to sequential therapy in patients
with SCLC with a good performance status. The preliminary results
indicated that irradiated postchemotherapy tumor extent and omitted
elective nodal irradiation did not decrease locoregional control in
the study arm compared to the control arm with prechemotherapy
tumor extent and the overall survival difference was not
statistically significant between the two arms (26). Over two-thirds of patients who
succumbed to lung cancer in the United States are aged >65 years
(27). Elderly patients tolerate
concurrent TRT poorly and toxicity must be considered for
concurrent TRT in LD SCLC. It is important for LD SCLC patients to
decrease the toxicity of concurrent TRT in order to complete the
treatment schedule. Previous meta-analyses suggested that patients
with LD SCLC may benefit from early TRT, with a significant
difference if the overall treatment time of TRT is <30 days
(17,28–30);
however, the chemotherapy regimens in some clinical trials in those
articles were not EP/EC. One trial demonstrated that TRT (52.5 Gy,
once daily) initiated with the third cycle of chemotherapy resulted
in survival outcomes and complete response rates comparable to
those of TRT initiated with the first cycle of chemotherapy, with a
lower frequency of febrile neutropenia (16).
In this meta-analysis, we demonstrated that there
were no significant differences between early and late concurrent
TRT regarding the 1-, 2-, 3- and 5-year survival rates and
objective response rate, whereas the overall incidence of grade 3–4
adverse events was lower with late concurrent TRT. Elderly patients
or patients with co-existing diseases should be treated with extra
caution. Early concurrent TRT may result in enlarged irradiation
fields, due to initial planning for bulky tumors. The balance
between therapeutic effects and treatment-related toxicities should
also be considered. Decreasing toxicity lowers the overall
treatment cost and saves on medical resources. In order to deliver
adequate radiation doses to the tumor, while respecting normal
tissue dose constraints, the use of advanced technologies,
including image-guided radiation therapy (IGRT),
intensity-modulated radiation therapy (IMRT)/volumetric-modulated
arc therapy (VMAT), four-dimensional (4-D) CT and/or PET-CT, is
considered appropriate. Modern radiotherapy, including accurate
target definition and conformal radiotherapy planning, may help
maximize tumor control and minimize treatment-related toxicity.
In conclusion, we demonstrated that late concurrent
TRT with EP/EC chemotherapy is suitable for LD SCLC patients,
particularly elderly patients or those with bulky tumors. However,
further studies on concurrent TRT with EP/EC chemotherapy for LD
SCLC are required in order to increase overall survival rates and
decrease treatment-related toxicity.
Acknowledgements
This study was funded by Project 81202806 supported
by the National Natural Science Foundation of China and the
Zhejiang Province Medical Science Fund Project of China (grant nos.
2012KYB034 and 2012RCB004).
Abbreviations:
|
TRT
|
thoracic radiotherapy
|
|
EP
|
etoposide and cisplatin
|
|
EC
|
etoposide and carboplatin
|
|
LD
|
limited-disease
|
|
SCLC
|
small-cell lung cancer
|
|
ERT
|
early thoracic radiotherapy
|
|
VALSG
|
Veterans Administration Lung Study
Group
|
|
ED
|
extensive-disease
|
|
IP
|
irinotecan and cisplatin
|
|
IC
|
irinotecan and carboplatin
|
|
MST
|
median survival time
|
|
AHTRT
|
accelerated hyperfractionated thoracic
radiotherapy
|
|
CEV
|
cyclophosphamide, epirubicin and
vincristine
|
|
ASCO
|
American Society of Clinical
Oncology
|
|
ESMO
|
European Society for Medical
Oncology
|
|
LRT
|
late thoracic radiotherapy
|
|
RR
|
risk ratio
|
|
CI
|
confidence interval
|
|
IGRT
|
image-guided radiation therapy
|
|
IMRT
|
intensity-modulated radiation
therapy
|
|
VMAT
|
volumetric-modulated arc therapy
|
|
4-D
|
four-dimensional
|
References
|
1
|
Ferlay J, Shin HR, Bray F, et al:
Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int
J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Govindan R, Page N, Morgensztern D, et al:
Changing epidemiology of small-cell lung cancer in the United
States over the last 30 years: analysis of the surveillance,
epidemiologic, and end results database. J Clin Oncol.
24:4539–4544. 2006.PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar
|
|
4
|
Patel AM, Dunn WF and Trastek VF: Staging
systems of lung cancer. Mayo Clin Proc. 68:475–482. 1993.
View Article : Google Scholar
|
|
5
|
Lu HY, Wang XJ and Mao WM: Targeted
therapies in small cell lung cancer (Review). Oncol Lett. 5:3–11.
2013.
|
|
6
|
Hanna NH and Einhorn LH: Small-cell lung
cancer: state of the art. Clin Lung Cancer. 4:87–94. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Warde P and Payne D: Does thoracic
irradiation improve survival and local control in limited-stage
small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol.
10:890–895. 1992.PubMed/NCBI
|
|
8
|
Kubota K, Hida T, Ishikura S, et al:
Etoposide and cisplatin versus irinotecan and cisplatin in patients
with limited-stage small-cell lung cancer treated with etoposide
and cisplatin plus concurrent accelerated hyperfractionated
thoracic radiotherapy (JCOG0202): a randomised phase 3 study.
Lancet Oncol. 15:106–113. 2014. View Article : Google Scholar
|
|
9
|
Sundstrom S, Bremnes RM, Kaasa S, et al:
Cisplatin and etoposide regimen is superior to cyclophosphamide,
epirubicin, and vincristine regimen in small-cell lung cancer:
results from a randomized phase III trial with 5 years’ follow-up.
J Clin Oncol. 20:4665–4672. 2002.PubMed/NCBI
|
|
10
|
Johnson DH, Bass D, Einhorn LH, et al:
Combination chemotherapy with or without thoracic radiotherapy in
limited-stage small-cell lung cancer: a randomized trial of the
Southeastern Cancer Study Group. J Clin Oncol. 11:1223–1229.
1993.
|
|
11
|
Osterlind K, Hansen HH, Hansen HS, et al:
Chemotherapy versus chemotherapy plus irradiation in limited small
cell lung cancer. Results of a controlled trial with 5 years
follow-up. Br J Cancer. 54:7–17. 1986.PubMed/NCBI
|
|
12
|
Skarlos DV, Samantas E, Kosmidis P, et al:
Randomized comparison of etoposide-cisplatin vs.
etoposide-carboplatin and irradiation in small-cell lung cancer. A
Hellenic Co-operative Oncology Group study. Ann Oncol. 5:601–607.
1994.PubMed/NCBI
|
|
13
|
Rossi A, Di Maio M, Chiodini P, et al:
Carboplatin- or cisplatin-based chemotherapy in first-line
treatment of small-cell lung cancer: the COCIS meta-analysis of
individual patient data. J Clin Oncol. 30:1692–1698. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takada M, Fukuoka M, Kawahara M, et al:
Phase III study of concurrent versus sequential thoracic
radiotherapy in combination with cisplatin and etoposide for
limited-stage small-cell lung cancer: results of the Japan Clinical
Oncology Group Study 9104. J Clin Oncol. 20:3054–3060. 2002.
View Article : Google Scholar
|
|
15
|
Skarlos DV, Samantas E, Briassoulis E, et
al: Randomized comparison of early versus late hyperfractionated
thoracic irradiation concurrently with chemotherapy in limited
disease small-cell lung cancer: a randomized phase II study of the
Hellenic Cooperative Oncology Group (HeCOG). Ann Oncol.
12:1231–1238. 2001. View Article : Google Scholar
|
|
16
|
Park K, Sun JM, Kim SW, et al: Phase III
trial of concurrent thoracic radiotherapy (TRT) with either the
first cycle or the third cycle of cisplatin and etoposide
chemotherapy to determine the optimal timing of TRT for
limited-disease small cell lung cancer. J Clin Oncol. 30(Suppl):
abstract 7004. 2012.
|
|
17
|
De Ruysscher D, Pijls-Johannesma M,
Vansteenkiste J, et al: Systematic review and meta-analysis of
randomised, controlled trials of the timing of chest radiotherapy
in patients with limited-stage, small-cell lung cancer. Ann Oncol.
17:543–552. 2006.
|
|
18
|
Higgins JPT and Green S: Cochrane Handbook
for Systematic Reviews of Interventions Version 5.1.0 [updated
March 2011]. The Cochrane Collaboration; 2011
|
|
19
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jeremic B, Shibamoto Y, Acimovic L, et al:
Initial versus delayed accelerated hyperfractionated radiation
therapy and concurrent chemotherapy in limited small-cell lung
cancer: a randomized study. J Clin Oncol. 15:893–900. 1997.
|
|
21
|
Work E, Nielsen OS, Bentzen SM, et al:
Randomized study of initial versus late chest irradiation combined
with chemotherapy in limited-stage small-cell lung cancer. Aarhus
Lung Cancer Group. J Clin Oncol. 15:3030–3037. 1997.PubMed/NCBI
|
|
22
|
Spiro SG, James LE, Rudd RM, et al; London
Lung Cancer Group. Early compared with late radiotherapy in
combined modality treatment for limited disease small-cell lung
cancer: a London Lung Cancer Group multicenter randomized clinical
trial and meta-analysis. J Clin Oncol. 24:3823–3830. 2006.
View Article : Google Scholar
|
|
23
|
Murray N, Coy P, Pater JL, et al:
Importance of timing for thoracic irradiation in the combined
modality treatment of limited-stage small-cell lung cancer. The
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 11:336–344. 1993.PubMed/NCBI
|
|
24
|
Perry MC, Herndon JE III, Eaton WL and
Green MR: Thoracic radiation therapy added to chemotherapy for
small-cell lung cancer: an update of Cancer and Leukemia Group B
Study 8083. J Clin Oncol. 16:2466–2467. 1998.PubMed/NCBI
|
|
25
|
Jiang J, Liang X, Zhou X, et al: A
meta-analysis of randomized controlled trials comparing
irinotecan/platinum with etoposide/platinum in patients with
previously untreated extensive-stage small cell lung cancer. J
Thorac Oncol. 5:867–873. 2010. View Article : Google Scholar
|
|
26
|
Hu X, Bao Y, Zhang L, et al: Omitting
elective nodal irradiation and irradiating postinduction versus
preinduction chemotherapy tumor extent for limited-stage small cell
lung cancer: interim analysis of a prospective randomized
noninferiority trial. Cancer. 118:278–287. 2012. View Article : Google Scholar
|
|
27
|
Gridelli C, Perrone F and Monfardini S:
Lung cancer in the elderly. Eur J Cancer. 33:2313–2314. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
De Ruysscher D, Pijls-Johannesma M,
Bentzen SM, et al: Time between the first day of chemotherapy and
the last day of chest radiation is the most important predictor of
survival in limited-disease small-cell lung cancer. J Clin Oncol.
24:1057–1063. 2006.PubMed/NCBI
|
|
29
|
Pijls-Johannesma M, De Ruysscher D,
Vansteenkiste J, et al: Timing of chest radiotherapy in patients
with limited stage small cell lung cancer: a systematic review and
meta-analysis of randomised controlled trials. Cancer Treat Rev.
33:461–473. 2007. View Article : Google Scholar
|
|
30
|
Huncharek M and McGarry R: A meta-analysis
of the timing of chest irradiation in the combined modality
treatment of limited-stage small cell lung cancer. Oncologist.
9:665–672. 2004. View Article : Google Scholar : PubMed/NCBI
|