Introduction
Breast cancer (BC) heterogeneity is associated with
diverse causal factors, such as heredity, environmental exposure,
hormonal impregnation and infectious agents. Among these factors,
viruses are regularly implicated in the pathogenesis of cancer,
although the results of different studies have been inconsistent
(1). In particular, the Epstein
Barr virus (EBV) is clearly associated with nasopharyngeal cancer,
but a causal relationship with BC was also demonstrated in the
mid-1990s (2). The reported
incidence of EBV-positive BC varies according to the technique used
and the targeted viral genomic regions (1). We previously demonstrated the
presence of EBV in ≤ 30% of BC specimens (3, 4). Of
note, EBV-related BC were found to be associated with more
aggressive patterns (4).
A previous study published by Perou et al
using genomic profiling has made a major contribution to the
understanding of BC heterogeneity (5). A new classification is currently
available, based on 5 expression signatures revealing distinct
patterns. The BC subtypes clearly reflect groups with different
outcomes and response to chemotherapy (6). Using clinicopathological factors,
such as hormone receptors (HRs), human epidermal growth receptor 2
(HER2) and grade or Ki67, this intrinsic BC classification may be
determined in routine practice (7).
Several questions regarding the significance of EBV
in BC remain challenging and the association with oncogenesis
remains obscure. An adverse outcome for EBV-positive BC was
previously suggested (8, 9). The purpose of our study was to
evaluate the prognosis of EBV-positive BC. In addition, we
incorporated the BC subtype classification in order to refine the
evaluation of the prognosis.
Patients and methods
Patients and methods
All the BCs investigated were identified through a
prospective institutional database search. All the patients with
primary BC had undergone surgery at the Department of Obstetrics
and Gynaecology, Conception Hospital, Marseille, France. BC tissue
samples were prospectively collected between 1981 and 1998. The
indications according to the department protocols for adjuvant
treatment were based on the tumour size, patient age, grading, HR
status and nodal status. Patients who had undergone conservative
treatment had also received breast radiotherapy. Radiotherapy had
also been delivered to the internal mammary lymph nodes in cases
with centrally or internally located tumours and to the
supraclavicular and internal mammary lymph nodes in cases with
positive axillary lymph nodes. Hormone therapy had been
administered for 5 years to all HR-positive tumours. The steroid HR
(oestrogen and progesterone receptor) status had initially been
determined biochemically in cytosol fractions and then expressed
quantitatively as fmol/mg protein (Abbott Laboratories, Diagnostic
Division, Chicago, IL, USA). The EBV status had been determined
using quantitative polymerase chain reaction (qPCR). The protocol
was previously described (3,
4) and approved by Johi and
Buehring (1), who consider our
analytical chain as one of 4/30 EBV-positive studies that
convincingly demonstrated the presence of EBV in BC.
Statistical analysis
The differences in baseline characteristics between
the EBV-negative and EBV-positive subgroups were summarized and
compared using the Chi-square test (categorical variables) or the
Student's t-test (continuous variables). Survival rates [overall
survival (OS) and disease-free survival (DFS)] were measured from
the date of surgery to the time of disease-related death or to the
first clinical or radiographic evidence of recurrent disease. We
plotted Kaplan-Meier curves for DFS and used the log-rank test to
determine the univariate significance of the variables. All the
analyses were performed using the SPSS software package version 21
(SPSS, Inc., Chicago, IL, USA). P-values < 0.05 were considered
to indicate statistically significant differences. All the reported
P-values are two-sided.
Results
Tumour characteristics
Our data demonstrated that the tumours in 38 of the
117 patients (32.5%) exhibited positivity for EBV. The
characteristics of the study population are summarized in Table I. The majority of the tumours were
T1 (58.1%), node-negative (56.4%), grade I-II and HR+. The
clinicopathological characteristics according to the EBV status are
presented in Table II. A
significant correlation was observed between viral positivity and
grading in the univariate analysis. Thus, the frequency of grade
III tumours was higher among EBV-positive BC cases. The oestrogen
receptor status was of borderline significance. When BC was
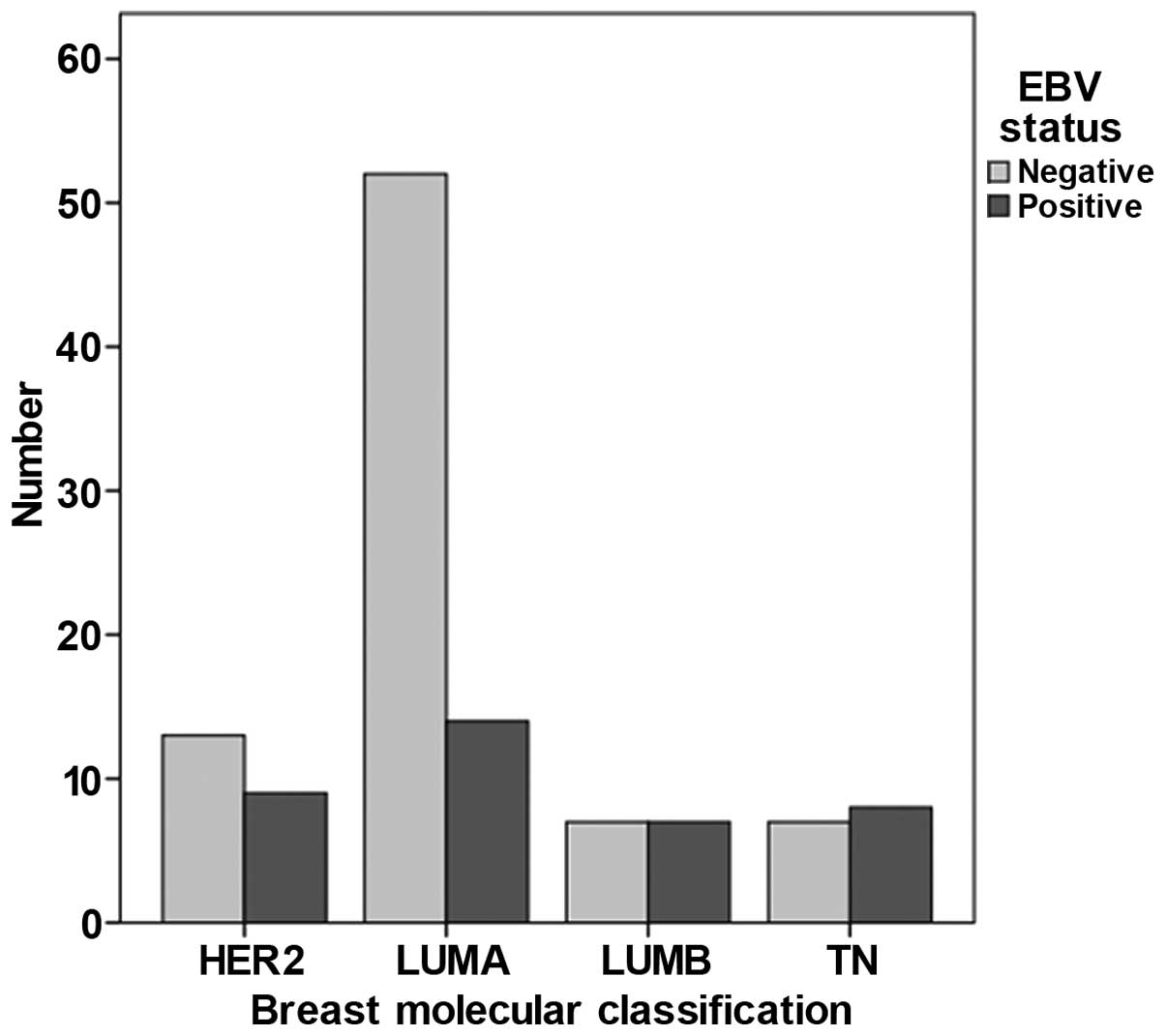
classified according to subtypes (Fig.
1), triple-negative (TN), HER2 and luminal B (lumB) tumours
were more frequent among EBV-positive BC cases (P=0.02).
 | Table I.Patient and tumour
characteristics. |
Table I.
Patient and tumour
characteristics.
| Characteristics | Patient no. (%) (n
=117) |
|---|
| Age at diagnosis,
years [Median (range)] | 57 (36–79) |
|
<50 | 37 (31.6) |
| ≥50 | 80 (68.4) |
| p Tumour size |
|
| T1 | 68 (58.1) |
| T2 | 37 (31.6) |
| T3 | 12 (10.3) |
| Lymph node
status |
|
|
Negative | 66 (56.4) |
|
Positive | 51 (43.6) |
| SBR grade |
|
| I-II | 79 (67.5) |
| III | 38 (32.5) |
| Oestrogen receptor
status |
|
|
Positive | 89 (76.1) |
|
Negative | 28 (23.9) |
| Progesterone receptor
status |
|
|
Positive | 77 (65.8) |
|
Negative | 40 (34.2) |
| HER2
overexpression | 22 (18.8) |
| EBV positivity | 38 (32.5) |
 | Table II.Patient characteristics according to
the EBV status of the tumours. |
Table II.
Patient characteristics according to
the EBV status of the tumours.
| Characteristics | EBV-positive
(n=38) | EBV-negative
(n=79) | P-value |
|---|
| Age, years, no.
(%) |
|
| 0.20 |
|
<50 | 9 (23.7) | 28 (35.4) |
|
| ≥50 | 29 (76.3) | 51 (64.6) |
|
| p Tumour size
(%) |
|
| 0.65 |
| T1 | 21 (55.3) | 47 (59.5) |
|
| T2 | 14 (36.8) | 23 (29.1) |
|
| T3 | 3 (7.9) | 9 (11.4) |
|
| Lymph node status,
no. (%) |
|
| 0.33 |
|
Negative | 19 (50 .0) | 47 (59.5) |
|
|
Positive | 19 (50 .0) | 32 (40.5) |
|
| SBR grade, no.
(%) |
|
| 0.02 |
| I-II | 20 (52.6) | 59 (74.7) |
|
| III | 18 (47.4) | 20 (25.3) |
|
| Oestrogen receptor
status, no. (%) |
|
| 0.07 |
|
Positive | 25 (65.8) | 64 (81 .0) |
|
|
Negative | 13 (34.2) | 15 (19.0) |
|
| Progesterone receptor
status, no. (%) |
|
| 0.21 |
|
Positive | 22 (57.9) | 55 (69.6) |
|
|
Negative | 16 (42.1) | 24 (30.4) |
|
| HER2 overexpression,
no. (%) | 9 (23.7) | 13 (16. 4) | 0.35 |
| TK, no. (%) |
|
| 0.03 |
| Low | 25 (65.8) | 66 (83.5) |
|
| High | 13 (34.2) | 13 (16.5) |
|
| BC subtypes, no.
(%) |
|
|
|
| Luminal
A | 14 (36.8) | 52 (65.8) |
|
| Luminal
B | 7 (18.4) | 7 (8.9) |
|
|
Triple-negative | 8 (21.1) | 7 (8.9) |
|
| HER2 | 9 (23.7) | 13 (16.4) |
|
Survival
The results of the Kaplan-Meier estimation of OS and
DFS revealed no difference in OS between EBV-positive and
EBV-negative tumours (P=0.49). The probability of 5-year OS in
patients with EBV-negative BC was 91.7% [95% confidence interval
(CI): 82.4-96.2%], whereas it was 87.3% (95% CI : 69.6-95.1%) in
patients with EBV-positive BC. Similarly, no difference was
observed in DFS (P=0.19). The probability of 5-year DFS in patients
with EBV-negative BC was 78.3% (95% CI : 66.5-86.4%) vs. 64.5% (95%
CI : 44.7-78.7%) in patients with EBV-positive BC (data not
shown).
When these survival rates were stratified according
to the molecular phenotype, the DFS rates differed among BC
subtypes (P=0.002), but the difference did not reach statistical
significance when they were stratified according to the EBV status
(P=0.08 for EBV-negative and 0.06 for EBV-positive BC). The OS
rates were similar among BC subtypes (P= 0.50) and when they were
stratified according to the EBV status (P=0.16 and P=0.67 for
EBV-positive and -negative cases, respectively) (data not
shown).
The 5-year DFS in patients with EBV-positive BC was
67.5% (95% CI: 45.4-100%) for lumA, 41.7% (95% CI: 14.7-100%) for
lumB, 46.9% (95% CI: 21.5-100%) for HER2 and 100% for TN BC. The
5-year DFS in patients with EBV-negative BC was 84.2% (5% CI:
74.1-95.8%) for lumA, 71.4% (5% CI: 44.7-100%) for lumB, 55.6% (5%
CI: 32.5-95%) for HER2 and 85.7% (5% CI: 63.3-100%) for TN BC (data
not shown).
According to the results of the Cox univariate
analysis, EBV was not associated with OS [hazard ratio (HR)= 1.48,
P=0.49], in contrast to tumour size (HR = 9.77, P=0.001) or nodal
status (HR = 3.98, P=0.02). In this univariate analysis, EBV was
not significantly correlated with DFS (HR = 1.33, P=0.61), but was
significantly associated with tumour size (HR = 1.46, P<0.001)
and nodal positivity (HR = 3.5, P=0.03) (data not shown).
Discussion
Several converging studies have demonstrated the
presence of EBV in BC. However, despite evidence on the presence of
EBV in BC specimens, an oncogenic role for EBV has yet to be
established and the significance of EBV-positive BC has not been
fully elucidated.
Virus-related BC has a poor prognosis, particularly
when multiple viruses are detected in breast specimens (8). We previously demonstrated that
EBV-positive BC exhibits more aggressive characteristics (3, 4).
Other authors corroborated our findings (2, 8);
however, the effect on survival has not been extensively
investigated. The subgroup analyses as a function of the BC
phenotype confirmed that the EBV status exerted no effect on
survival outcome (DFS or OS). Of note, in EBV-positive tumours, DFS
was of borderline significance, with an adverse prognostic outcome
for lumB and HER2 tumours. LumB and HER2 tumours were previously
demonstrated to carry a poor prognosis (6).
There were certain limitations to our study. Our
series was small, information was lacking on the adjuvant therapies
administered and the treatments received were heterogeneous. In
particular, the unavailability of trastuzumab therapy when the
patients were treated may have significantly affected the prognosis
of HER2 tumours.
In response to published comments by Khan G et
al (10) on our previous study
(4) and in order to understand the
discrepancies regarding the detection of viral genomic DNA in BC,
it is important to take into consideration the viral genomic load
and the amount of DNA in the samples tested. Similarly, in tests
for gene mutations in somatic samples, we cannot consider the
limits of the detection method without taking into account the
total amount of DNA tested. In this study, we demonstrated that the
presence of EBV did not exert an adverse effect on the prognosis,
or on OS and DFS. This finding is quite surprising, given the
greater representation of high-grade and HER2 tumours, but
significance was not demonstrated when survival estimates were
stratified according to the EBV status (P=0.08 for EBV-negative and
0.06 for EBV-positive tumours), although the values were of
borderline significance. It is noteworthy that TN, HER2 and lumB
tumours were more frequent among EBV-positive BC cases (P=0.02).
The implication of EBV in BC requires further investigation, which
may be facilitated by the improvement of droplet digital PCR. The
sensitivity of this technology is well suited to address these
questions and, above all, the presence or absence of viral genome
in tumours of epithelial origin.
The presence of EBV in BC is frequently reported and
has been associated with more aggressive forms. However, the
prognostic significance of EBV presence remains unclear. Future
studies are required to identify the association of EBV presence
and activity at the DNA level, as it has not been clearly
determined whether EBV acts as an oncogene in BC.
Acknowledgements
The authors would like to thank Lorna Saint Ange for
editing the manuscript.
References
|
1
|
Joshi D and Buehring GC: Are viruses
associated with human breast cancer? Scrutinizing the molecular
evidence. Breast Cancer Res Treat. 135:1–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Labrecque LG, Barnes DM, Fentiman IS and
Griffin BE: Epstein-Barr virus in epithelial cell tumors: a breast
cancer study. Cancer Res. 55:39–45. 1995.PubMed/NCBI
|
|
3
|
Fina F, Romain S, Ouafik L, Palmari J, Ben
Ayed F, Benharkat S, Bonnier P, Spyratos F, Foekens JA, Rose C,
Buisson M, Gérard H, Reymond MO, Seigneurin JM and Martin PM:
Frequency and genome load of Epstein-Barr virus in 509 breast
cancers from different geographical areas. Br J Cancer. 84:783–790.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mazouni C, Fina F, Romain S, Ouafik L,
Bonnier P, Brandone JM and Martin PM: Epstein-Barr virus as a
marker of biological aggressiveness in breast cancer. Br J Cancer.
104:332–337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE,
Børresen-Dale AL, Brown PO and Botstein D: Molecular portraits of
human breast tumours. Nature. 406:747–752. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rouzier R, Perou CM, Symmans WF, Ibrahim
N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P,
Morandi P, Fan C, Rabiul I, Ross JS, Hortobagyi GN and Pusztai L:
Breast cancer molecular subtypes respond d ifferently to
preoperative chemotherapy. Clin Cancer Res. 11:5678–5685. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ: Role: Panel membersS tr ategies for
subtypes - dealing with the diversity of breast cancer: highlights
of the St. Gallen International Expert Consensus on the Primary
Therapy of Early Breast Cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsai JH, Hsu CS, Tsai CH, Su JM, Liu YT,
Cheng MH, Wei JC, Chen FL and Yang CC: Relationship between viral
factors, axillary lymph node status and survival in breast cancer.
J Cancer Res Clin Oncol. 133:13–21. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aguayo F, Khan N, Koriyama C, González C,
Ampuero S, Padilla O, Solís L, Eizuru Y, Corvalán A and Akiba S:
Human papillomavirus and Epstein-Barr virus infections in breast
cancer from chile. Infect Agent Cancer. 6:72011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khan G, Philip PS and Al Ashari M: Is
Epstein-Barr virus associated with aggressive forms of breast
cancer? Br J Cancer. 104:1362–1363. 2011. View Article : Google Scholar : PubMed/NCBI
|