Introduction
Osteosarcoma is the most frequent primary osteogenic
tumor with a prevalence of 1–3 cases per million people (1). Resection of tumor (with negative
surgical margin) and systemic administration of chemotherapy agents
have significantly improved the long-term prognosis in osteosarcoma
patients, with 5-year survival rates being 60–70% (2). Osteosarcoma patients are able to receive
limb-salvage surgeries with improved postoperative function.
However, complications, including infection, local
recurrence, wound dehiscence, pathological fracture and prosthetic
loosening, were frequently reported in osteosarcoma patients
undergoing limb-salvage surgeries (3,4). Of note,
infection is deemed to be one of the most significant complications
with an incidence of 5.3–13% (2,3,5,6). Despite
this, severities of postoperative infections vary from mild to
severe extents, and deep infection was an important reason for
readmission, revision surgery and even amputation (7,8). Notably,
the association of postoperative infection and improved survival in
osteosarcoma patients has been observed in several preceding
studies, reporting that infection could be associated with
prolonged survival in canines and humans with osteosarcoma,
however, the outcomes were inconsistent (2,9,10). Jeys et al (9) revealed that the 10-year survival for
infected osteosarcoma patients was 84.5% compared to 62.3% in
non-infected patients, and no difference was detected between the
two groups in terms of histological responses to chemotherapy.
However, Lee et al (2) noted
no survival difference between the infected patients and
non-infected group following matching for prognostic factors, which
suggested that the reported positive effect on survival rate could
be due to other clinical characteristics of infected patients.
However, no studies have carried out further clinical observations
nor laboratory experiments relating to the infection-survival
association in osteosarcoma, although this association has been
reported in a wide variety of cancer, showing that certain types of
malignancies are possibly sensitive to immune effects associating
with infection, while others are not (11–13).
Sensitivity of osteosarcoma to infection could be associated with
the potential efficacy of immunotherapy as a treatment for this
disease, since infection involves a cascade of cellular events and
inflammatory transducers, the elucidation of the association of the
infection and survival in osteosarcoma patients will possibly bring
reconsideration and increasing attention to immunotherapy for
osteosarcoma. The aim of the present study was to determine whether
inflammation has positive or negative impacts on survival in
osteosarcoma patients and explore what could be obtained from this
association.
Patients and methods
Inclusion and exclusion criteria
Diagnosis of Enneking IIB osteosarcoma according to
the Enneking staging system (14) is
determined in every patient in the present cohort between 1991 and
2012 in the Department of Orthopaedic Surgery (General Hospital of
Jinan Military Region, Jinan, China). All the patients underwent
limb-salvage surgeries along with neo-adjuvant chemotherapy. The
mean follow-up period was 5.1 years (range 0.5–19.8 years), the
minimum follow-up period was 5 years unless the patient succumbed
or was lost to the cohort. Patients with the following
characteristics were excluded: Infection that developed >1 year
postoperatively, recurrence/metastasis and mortality that developed
before infection or within 1 year postoperatively, previous surgery
on the tumor location, patients who did not receive chemotherapy
treatment and patients lost to follow-up. Finally, 125 patients
were enrolled in the cohort (Table
I).
 | Table I.Comparison of the clinical patient
data with and without infection. |
Table I.
Comparison of the clinical patient
data with and without infection.
| Characteristics | Infected | Non-infected | P-value |
|---|
| Age, years | 21±11 | 19±8 | 0.52 |
| Gender, n (%) |
| Male | 3 (50.0) | 77 (64.7) | 0.75 |
|
Female | 3 (50.0) | 42 (35.3) | |
| Tumor site, n
(%) |
|
Femur | 1 (16.7) | 62 (52.6) | 0.04 |
|
Tibia | 4 (66.7) | 37 (31.0) | |
|
Fibula | 1 (16.6) | 11 (8.6) | |
|
Other | – | 10 (7.8) | |
| Chemotherapy regimen,
n (%) |
| DIA | 5 (83.3) | 83 (70.7) | 1.00 |
| MMIA | 1 (16.7) | 27 (22.4) | |
|
Other | – | 9 (6.9) | |
| Types of surgery, n
(%) |
|
Prosthesis | 3 (50.0) | 46 (38.3) | 0.51 |
|
Biological reconstruction | 3 (50.0) | 73 (61.7) |
| Response to
chemotherapy, n (%) |
| Good | 4 (66.7) | 70 (58.6) | 0.65 |
| Poor | 2 (33.3) | 49 (41.4) | |
|
Prosthetic
loosening/fracture | 1 (16.7) | 8 (6.7) | 1.00 |
|
Amputation | 3 (50.0) | 2 (1.7) | 0.00 |
|
Recurrence | 1 (16.7) | 28 (23.5) | 1.00 |
|
Metastasis | – | 59 (49.6) | 0.03 |
|
Mortality | – | 65 (54.6) | 0.00 |
|
Total | 6 (4.8) | 119 (95.2) | |
Method
Deep infection was confirmed if the patients had
clinical evidence of infection (including fever, pain, abscess,
elevated white blood cell counts and elevated C-creative protein)
with microbial culture within the wound location or histology
compatible with infection at surgery. According to this standard,
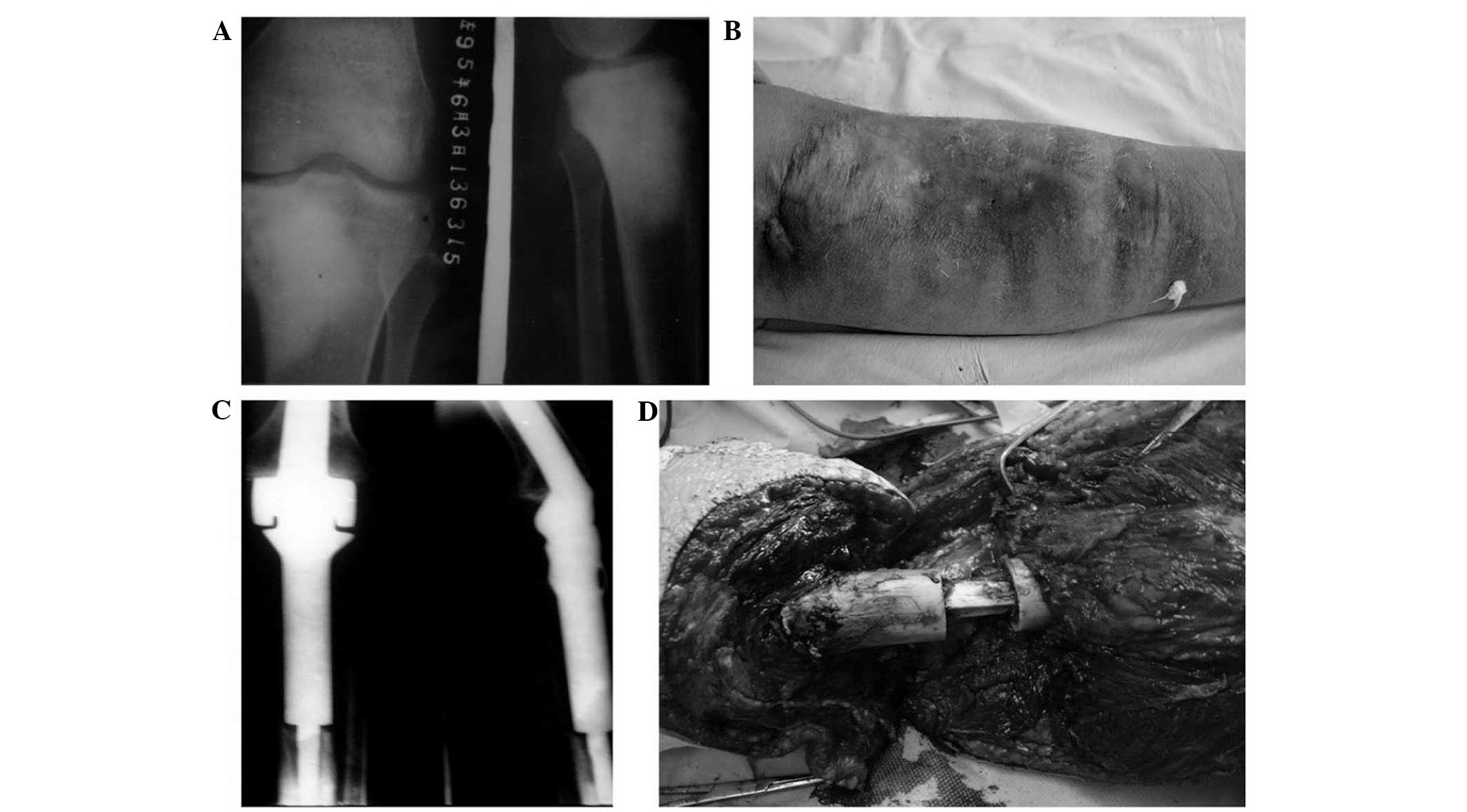
postoperative deep infection was determined in 6 patients (Fig. 1), treatment of infection included
debridement and drainage (6 patients, 100%), revision surgery (1
patient, 16.7%) and amputation (4 patients, 66.7%). There was no
evidence of systemic infection, and all the infections were
localized. Perioperative infection (1 patient, 16.7%) was
determined if it occurred within 2 months postoperatively,
infections occurring >2 months postoperatively were late
infections (5 patients, 83.3%). Infections of >3 months were
chronic infections (6 patients, 100%).
A total of 97 patients (77.6%) underwent
cisplatin-doxorubicin-isofamide therapy, the remaining patients
received high-dose methotrexate-doxorubicin-isofamide chemotherapy
regimen (Table II). Histological
responses were graded according to percentage of tumor necrosis,
where good response consists of grade III and IV (necrosis of ≥90%)
and grades I and II (necrosis of <90%) indicated poor responses
(15). Resections of tumors were
performed according to established principles of surgical margins
for osteosarcoma, reconstructive methods included tumor
endoprostheses, allograft-prosthetic composites and biological
reconstruction. A negative tumor margin was also determined by
histological findings (16).
Prophylactic antibiotics (penicillin or cephalosporin) were
administered within 30 min before skin incision and were
discontinued within 24 h of the end of surgery recommendations by
the American Academy of Othopaedic Surgeons (17). Therapeutic regimens for postoperative
infections covered a variety of intravenous antibiotics, including
penicillin, cephalosporin, quinolones, aminoglycosides and
clindamycin. Two-agent antibiotic regimens were administered in 5
patients (83.3%), while the remaining patients received
single-agent antibiotic therapy (16.7%).
 | Table II.Chemotherapeutic regimen for
osteosarcoma patients. |
Table II.
Chemotherapeutic regimen for
osteosarcoma patients.
| Type of
chemotherapya | Order of agents in
one episode of chemotherapy | Dose and duration of
agents | Total number of
episodes |
|---|
| DDP-ADM-IFO | DDP was administered
firstly, after an interval of 1 week, ADM + IFO were used | | 2 preoperative + 6
postoperative |
| DDP | | 120 mg/m2,
1/day × 1, 4–6 h/time | |
|
ADM | | 30
mg/m2, 1/day × 3 | |
|
IFO | | 2.0
g/m2, 1/day × 5 | |
| MTX-ADM-IFO | MTX was
administered weekly for the first 2 weeks, after an interval of 1
week, ADM + IFO were used | | 2 preoperative + 6
postoperative |
|
MTX | | 8–12
g/m2 × 12, 4–6 h/time, at interval of 6 h | |
|
ADM | | 30
mg/m2, 1/day × 3 | |
|
IFO | | 2.0
g/m2, 1/day × 5 | |
Statistical analysis was performed using SPSS 13.0
(SPSS Inc., Chicago, IL, USA). χ2 test, Fisher's test
and student's t-test were carried out in univariate analysis.
P<0.05 was considered to indicate a statistically significant
difference. End points in Kaplan-Meier analysis were
recurrence/metastasis and mortality. Cox regression model was
applied for multivariate analysis.
Results
A total of 6 patients had postoperative deep
localized infections (4.8%, Table
III). Mean postoperative time of infection was 6.8±4.0 months
(1–12 months). One patient (16.7%) had perioperative infection and
5 patients (83.3%) had late infections. Bacterial culture indicated
Staphylococcus aureus (4 patients, 66.7%) and
Staphylococcus epidermidis (2 patients, 33.3%). Clinical
characteristics of infected and non-infected patients are compared
in Table I. The patients had no
statistical significance in chemotherapy regimens (P=0.01) and
histological response (P=0.65), infected patients were exposed to
lower risks for metastasis (P=0.03) and mortality (P<0.001).
There was no association of amputation and tumor recurrence
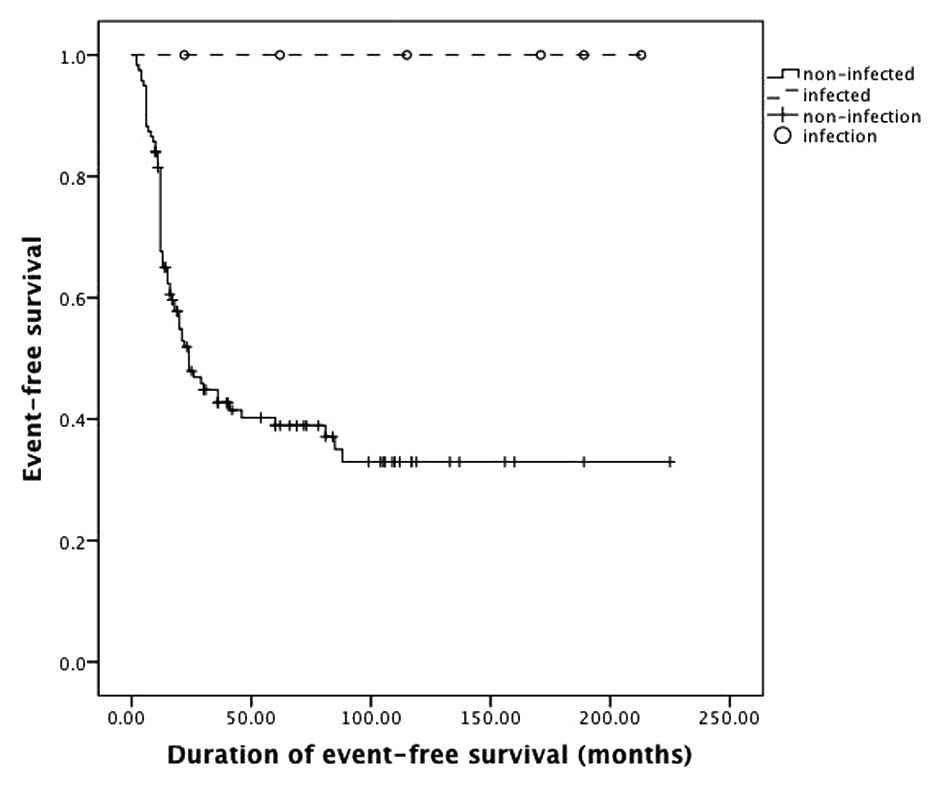
(P=0.33), metastasis (P=0.06) and mortality (P=0.67). The 5-year
survival rate and event-free survival rate of infected patients
were 100%; 5-year survival rate of non-infected patients was 54%
and event-free survival rate was 43% (Figs 2 and 3).
The log-rank test indicated that the total survival rate (P=0.01)
and event-free survival (P=0.01) rate of infected patients were
significantly higher than those without infection.
 | Table III.Clinical characteristics and
prognosis of infected patients. |
Table III.
Clinical characteristics and
prognosis of infected patients.
| No. | Gender | Age at surgery,
years | Tumor site | Types of
surgery | Management of
infection | Prognosis | Time of
infection | Event-free
survival, months | Survival,
months |
|---|
| 1 | Male | 33 | Distal femur | Prosthesis | Debridement | Survival | Late | 115 | 115 |
| 2 | Female | 18 | Proximal tibia | Inactivation and
re-implantation | Amputation | Survival | Late | 171 | 171 |
| 3 | Female | 17 | Proximal tibia | Inactivation and
re-implantation | Debridement | Survival | Perioperative | 62 | 62 |
| 4 | Female | 10 | Proximal tibia | Inactivation and
re-implantation | Amputation | Survival | Late | 22 | 22 |
| 5 | Male | 16 | Proximal tibia | Inactivation and
re-implantation | Amputation | Survival | Late | 189 | 189 |
| 6 | Male | 22 | Proximal tibia | Resection of
tumor | Amputation | Survival | Late | 24 | 237 |
A variety of factors indicated by single-variate
analysis and clinical observation were included in COX regression
analysis. These factors comprised amputation, postoperative
infection, chemotherapeutic regimen, response to chemotherapy,
tumor recurrence and metastasis. Results showed that tumor
metastasis was an independent risk factor for survival (P<0.001,
95 % confidence interval, 1.59–3.98). Patients with evidence of
metastasis were exposed to a risk of mortality that was 15.6 times
as high as those without metastasis.
Discussion
In 1891, William B. Coley injected streptococcal
organisms into a patient with unresectable sarcoma and sequential
infection in this patient resulted in shrinking of the tumor. Since
then, Coley used a series of heat-inactivated streptococci and
Bacillus prodigiosus (known as Coley's toxins) in the
treatment for cancer (18). However,
Coley's toxins did not significantly improve long-term survival in
patients with cancer, and development of chemotherapy and radiation
therapy caused Coley's toxins to gradually disappear from use
(19). In recent decades,
immunotherapy has drawn increasing attention as an adjuvant therapy
for human malignancies, including osteosarcoma, showing that
Coley's principles of treatment for cancer is correct and that
certain malignant tumors are sensitive to an enhanced immune system
while others are not. However, it remains unclear whether infection
is associated with improved survival in osteosarcoma patients and
what role the immune system could play in this process. These
results have shown that patients with postoperative deep infections
had improved survival compared to the uninfected patients, despite
that the majority of the postoperative infections resulted in
amputation and/or postponed chemotherapy.
The importance of the present clinical observation
consists in that osteosarcoma is possibly sensitive to an enhanced
immune system associated with infection, indicating that
immunotherapy could be a valuable therapeutic route for
osteosarcoma. However, the underlying mechanisms remain unclear.
Preceding studies showed that infection possibly plays antitumor
roles by enhancing cellular immune system, as elevated levels of
tumor necrosis factor-α concomitant with infection stimulated the
innate immune system in patients, resulting in enhanced antitumor
effects (18). Buddingh et al
(20) reported that chemoresistant
osteosarcoma cells are susceptible to lysis of
interleukin-15-induced natural killing (NK) cells, and the results
indicated the potential antitumor effects of NK cells or NK
cell-activating agents in high-grade osteosarcoma patients. In
addition to these findings, infection may play antitumor roles
through other routes; in vivo studies have shown that
infection is associated with angio-suppressive effects in tumors,
yet further explorations remain to be carried out to elucidate this
connection (21).
When the advances in immunotherapy of osteosarcoma
are reviewed, interferon (IFN)-α has obtained considerable
attention as an adjuvant treatment of osteosarcoma. Evidence from
fundamental research has indicated that IFN played direct antitumor
and/or indirect immune roles, particularly in osteosarcoma. IFN
signaling was found to be intact in the periphery blood of
osteosarcoma patients, while in other malignancies (such as
melanoma), the signaling is impaired suggesting that osteosarcoma
patients could be sensitive to treatment of IFN (22). A cooperative clinical trial being
conducted by European and American Osteosarcoma Study Group-1 is
currently the largest prospective study associated with
immunotherapy of osteosarcoma patients. Patients with good
responses to chemotherapy (tumor necrosis rate ≥90% by histological
findings at surgery) underwent maintenance treatment of IFN-α as an
adjuvant treatment to chemotherapy (methotrexate, cisplatin and
doxorubicin). However, no evidence has shown that IFN-α had
significant roles in improving the survival rate of osteosarcoma
patients (22,23). The discrepancy between clinical trials
and laboratory findings may be associated with various confounding
factors. Additionally, an enhanced immune system is associated with
a cascade of immune cells and cytokines, which could be more
complicated than the effects induced by one type of inflammatory
cytokine. Therefore, further research with a wider spectrum of
immune cells and cytokines is required.
In recent years, antitumor effects relating to tumor
associated macrophages (TAM) and neutrophils (TAN) are gaining
increasing attention. Preceding studies suggested that TAM and TAN
are integrated in the regulation of innate and adaptive immune
responses. Strong evidence showed protumoral macrophages could be
stimulated by IFN and become antitumoral cells attracting T helper
1 lymphocytes to the microenvironment of cancer (24,25). Of
note, neutrophils have long been considered to be terminal effector
cells playing a major role in inflammation and resistance against
microbes. Various studies have shown that neutrophils could play an
important role in tumor growth and progression. However, in the
analogy with macrophages, neutrophils have double-edged roles in
tumor progression. Neutrophils could be driven by transforming
growth factor-β to acquire a protumoral phenotype, and by contrast,
it could play antitumoral roles through cytotoxic and
anti-angiogenic effects (26,27). Based on the outcomes of the present
study, osteosarcoma could be sensitive to an immune system enhanced
by infection. In addition, macrophages and neutrophils are
important effector cells in infection, and studies in this area
could offer valuable information in the treatment of osteosarcoma.
However, current studies associated with TAM and TAN in
osteosarcoma are limited.
Another important aspect associated with
postoperative infection is local high temperature within the wound.
Over the past decades, therapy applying thermal effects has been
widely utilized as an adjuvant treatment route of malignant tumor,
and the thermoablative technique is a typical example in this
field. In clinical practice, thermal ablation comprises of
radiofrequency ablation, microwave ablation, high-intensity focused
ultrasound and laser-induced thermotherapy (28). The strengths of thermal ablation are
not only its advantages as a surgical technique, but also the
immunomodulation by thermotherapy in cancers. Fundamental studies
and clinical observations have shown that thermal ablation plays
important roles in modulating immune system in cancer patients. In
addition, different thermoablative techniques have shown
immunostimulating effects with similar immune cells and transducers
profiles, however, studies associated with the immune effects
aroused by thermal therapy for osteosarcoma patients are extremely
scarce (28). In clinical practice,
microwave ablation is currently used in the treatment of
osteosarcoma, and in certain cases, inactivated bone tumor remained
in situ instead of being entirely resected. As a result,
postoperative exudation, redness and swelling of incisions were
frequently observed. Microbiological culture excluded localized
infection in all the patients, and the prognosis of these patients
is favorable thus far. We believe the patients who underwent
thermotherapy in the Department of Orthopaedic Surgery experienced
a situation imitating postoperative wound infection (with similar
clinical presentations). Thermoablative techniques and inactivated
tumor tissues in vivo could possibly cause a wide variety of
immune response, and this will have impacts on the long-term
survival of osteosarcoma patients, however, further studies are
required to explore the immune effects by thermotherapy in
osteosarcoma and to determine its efficacy in the treatment of
osteosarcoma patients. Currently the related studies are
scarce.
Although the present study is inherent to several
limitations of observational research, including that this is a
single-institute retrospective study with a relatively small sample
size (particularly the infected patients), the outcome has shown
that postoperative infection was likely to improve the survival
rate of osteosarcoma patients. However, the association between
infection and survival rate of osteosarcoma patients remains to be
elucidated. A wide spectrum of immune cells and transducers has
shown potential in the treatment of osteosarcoma. Immune effects
induced by thermal therapy also showed a possibility of exploring
new treatment modalities. However, studies associated with these
fields are limited and therefore, further studies are required to
elucidate the association of the immune system and survival rate in
osteosarcoma patients to improve the prognosis of these
patients.
Glossary
Abbreviations
Abbreviations:
|
DIA
|
cisplatin-doxorubicin-ifosfamide
|
|
MMIA
|
methotrexate-doxorubicin-ifosfamide
|
|
IFN
|
interferon
|
|
IL
|
interleukin
|
|
TNF
|
tumour necrosis factor
|
|
TGF
|
transforming growth factor
|
|
NK
|
natural killing
|
References
|
1
|
Agarwal M, Anchan C, Shah M, Puri A and
Pai S: Limb salvage surgery for osteosarcoma: Effective low-cost
treatment. Clin Orthop Relat Res. 459:82–91. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee JA, Kim MS, Kim DH, Lim JS, Park KD,
Cho WH, Song WS, Lee SY and Jeon DG: Postoperative infection and
survival in osteosarcoma patients. Ann Surg Oncol. 16:147–151.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li X, Moretti VM, Ashana AO and Lackman
RD: Perioperative infection rate in patients with osteosarcomas
treated with resection and prosthetic reconstruction. Clin Orthop
Relat Res. 469:2889–2894. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shin DS, Weber KL, Chao EY, An KN and Sim
FH: Reoperation for failed prosthetic replacement used for limb
salvage. Clin Orthop Relat Res. 358:53–63. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gorelick MH, Owen WC, Seibel NL and Reaman
GH: Lack of association between neutropenia and the incidence of
bacteremia associated with indwelling central venous catheters in
febrile pediatric cancer patients. Pediatr Infect Dis J.
10:506–510. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hingorani P, Seidel K, Krailo M,
Mascarenhas L, Meyers P, Marina N, Conrad EU and Hawkins DS: Body
mass index (BMI) at diagnosis is associated with surgical wound
complications in patients with localized osteosarcoma: A report
from the Children's Oncology Group. Pediatr Blood Cancer.
57:939–942. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wirganowicz PZ, Eckardt JJ, Dorey FJ,
Eilber FR and Kabo JM: Etiology and results of tumor endoprosthesis
revision surgery in 64 patients. Clin Orthop Relat Res. 358:64–74.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pan KL, Chan WH, Ong GB, Premsenthil S,
Zulkarnaen M, Norlida D and Abidin Z: Limb salvage in osteosarcoma
using autoclaved tumor-bearing bone. World J Surg Oncol.
10:1052012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeys LM, Grimer RJ, Carter SR, Tillman RM
and Abudu A: Post operative infection and increased survival in
osteosarcoma patients: Are they associated? Ann Surg Oncol.
14:2887–2895. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lascelles BD, Dernell WS, Correa MT,
Lafferty M, Devitt CM, Kuntz CA, Straw RC and Withrow SJ: Improved
survival associated with postoperative wound infection in dogs
treated with limb-salvage surgery for osteosarcoma. Ann Surg Oncol.
12:1073–1083. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sottnik JL, U'Ren LW, Thamm DH, Withrow SJ
and Dow SW: Chronic bacterial osteomyelitis suppression of tumor
growth requires innate immune responses. Cancer Immunol Immunother.
59:367–378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Noor S, Thornormoethsson HS, Zervas CT, et
al: Limb versus life-the outcomes of osteosarcoma in Cambodia. Int
Orthop. 38:579–585. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ando K, Heymann MF, Stresing V, Mori K,
Rédini F and Heymann D: Current therapeutic strategies and novel
approaches in osteosarcoma. Cancers (Basel). 5:591–616. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jeon DG, Song WS, Kong CB, Cho WH, Cho SH,
Lee JD and Lee SY: Role of surgical margin on local recurrence in
high risk extremity osteosarcoma: A case-controlled study. Clin
Orthop Surg. 5:216–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prokuski L: Prophylactic antibiotics in
orthopaedic surgery. J Am Acad Orthop Surg. 16:283–293.
2008.PubMed/NCBI
|
|
18
|
Wiemann B and Starnes CO: Coley's toxins,
tumor necrosis factor and cancer research: A historical
perspective. Pharmacol Ther. 64:529–564. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Werneke U: A guide to using complementary
alternative medicines in cancer. Nurs Times. 101:32–35.
2005.PubMed/NCBI
|
|
20
|
Buddingh EP, Schilham MW, Ruslan SE,
Berghuis D, Szuhai K, Suurmond J, Taminiau AH, Gelderblom H, Egeler
RM, Serra M, et al: Chemotherapy-resistant osteosarcoma is highly
susceptible to IL-15-activated allogeneic and autologous NK cells.
Cancer Immunol Immunother. 60:575–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thomas-Tikhonenko A and Hunter CA:
Infection and cancer: The common vein. Cytokine Growth Factor Rev.
14:67–77. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Buddingh EP, Ruslan SE, Berghuis D,
Gelderblom H, Anninga JK, Hogendoorn PC, Egeler RM, Schilham MW and
Lankester AC: Intact interferon signaling in peripheral blood
leukocytes of high-grade osteosarcoma patients. Cancer Immunol
Immunother. 61:941–947. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Whelan JS, Bielack SS, Marina N, et al:
EURAMOS-1, an international randomised study for osteosarcoma:
Results from pre-randomisation treatment. Ann Oncol. 26:407–414.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
De Palma M, Mazzieri R, Politi LS, Pucci
F, Zonari E, Sitia G, Mazzoleni S, Moi D, Venneri MA, Indraccolo S,
et al: Tumor-targeted interferon-alpha delivery by Tie2-expressing
monocytes inhibits tumor growth and metastasis. Cancer Cell.
14:299–311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Galdiero MR, Bonavita E, Barajon I,
Garlanda C, Mantovani A and Jaillon S: Tumor associated macrophages
and neutrophils in cancer. Immunobiology. 218:1402–1410. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matsushima H, Geng S, Lu R, Okamoto T, Yao
Y, Mayuzumi N, Kotol PF, Chojnacki BJ, Miyazaki T, Gallo RL, et al:
Neutrophil differentiation into a unique hybrid population
exhibiting dual phenotype and functionality of neutrophils and
dendritic cells. Blood. 121:1677–1689. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Geng S, Matsushima H, Okamoto T, Yao Y, Lu
R, Page K, Blumenthal RM, Ward NL, Miyazaki T and Takashima A:
Emergence, origin, and function of neutrophil-dendritic cell
hybrids in experimentally induced inflammatory lesions in mice.
Blood. 121:1690–1700. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Haen SP, Pereira PL, Salih HR, Rammensee
HG and Gouttefangeas C: More than just tumor destruction:
Immunomodulation by thermal ablation of cancer. Clin Dev Immunol.
2011:1602502011. View Article : Google Scholar : PubMed/NCBI
|