Introduction
Two paramount topics are central to the ongoing
cancer research, namely the initiation and metastasis of cancer.
The mechanism underlying cancer development remains debated upon
and several mechanisms have been suggested, including gene mutation
(1), disorganization of tissue
structure (2,3), or genome and chromosome scrambling
(4). As regards metastasis, there is
also significant debate regarding whether metastasis is an
inherited or acquired trait (5) and
whether epithelial-to-mesenchymal transition is a prerequisite, an
important first step for both invasion and metastasis (6), or merely an artificial phenomenon, i.e.,
a mirage (7). At the era of
reductionism, scientists investigating cancer are focusing their
efforts on elucidating the molecular mechanisms underlying the
migration of cancer cells, revealing a complex process involving at
least hundreds, if not thousands of molecules. The aim of this
study was to address the issue of metastasis from a level beyond
individual molecules, in order to determine the reason behind
cancer cells moving out of their original location. We hypothesize
that, by elucidating the molecular mechanisms cancer uses to
metastasize, we may be able to design strategies to inhibit the
process of metastasis. Alternatively, by understanding why cancer
metastasizes, we may be able to alleviate the factors that induce
migration of cancer cells.
Metastasis - predetermined or acquired
genetic trait
The question as to why cancer metastasizes may
appear simplistic for the average scientist, who may categorize
this trait as a basal, innate instinct caused by genetic mutation.
The prevailing theory of cancer is that it all begins with a single
or even a series of genetic mutations. If metastasis is a trait of
cancer, then it must also share the same origin. However, when
considering cellular growth and development in the embryonic stage
of any organism, a number of embryonic cells, such as primordial
germ cells and neurocrest cells, naturally migrate over long
distances to their final location, without any genetic mutations.
Furthermore, evidence-based medicine requires evidence to support
all theories. However, no single gene has yet been identified as
responsible for metastasis, even after numerous genome-wide
sequencing analyses.
Suzuki and Tarin (8)
suggested that metastasis is an acquired trait, based on the
significant differences in the gene expression profile between
primary and lymph node metastatic breast cancer observed through
microarrays. However, other researchers may rebut by pointing out
that the metastasis originated in the primary site and not in the
lymph node per se (9).
Necrosis and apoptosis are associated with
metastasis
When investigating the reasons for cancer
metastasis, we must begin with known facts and premises. Cancer
cells are living, reproducing cells that are capable of movement if
not bound or restricted by other cells or structures. Cancer cells
possess a certain amount of autonomy after leaving their site of
origin within the epithelium. However, although cancer cells are
capable of movement, this ability is put to use when these cells
require sustenance or when they must avoid danger.
Necrosis is crucial for the diagnosis
of malignancy
In diagnostic pathology, the presence and extent of
necrosis are important references for the diagnosis of malignancy.
Although there is no proven explanation for the cause of necrosis,
the most plausible explanation is that the tumor overgrows the
ability of the circulatory system to supply sufficient nutrients.
In fact, extensive necrosis is a common indicator of metastasis.
For example, axillary lymph node metastasis was detected in a case
of intracapsular carcinoma of the mammary gland. This type of
lesion is usually considered as in situ carcinoma, which is
rarely associated with metastasis. In fact, lymph node metastasis
is not rare in the comedo type of ductal carcinoma in situ
of the breast, which is characterized by central necrosis. The
strong association of tumor necrosis with metastasis indicates that
cell death per se or a factor closely asociated with cell
death, such as lack of blood supply, is a strong stimulator of
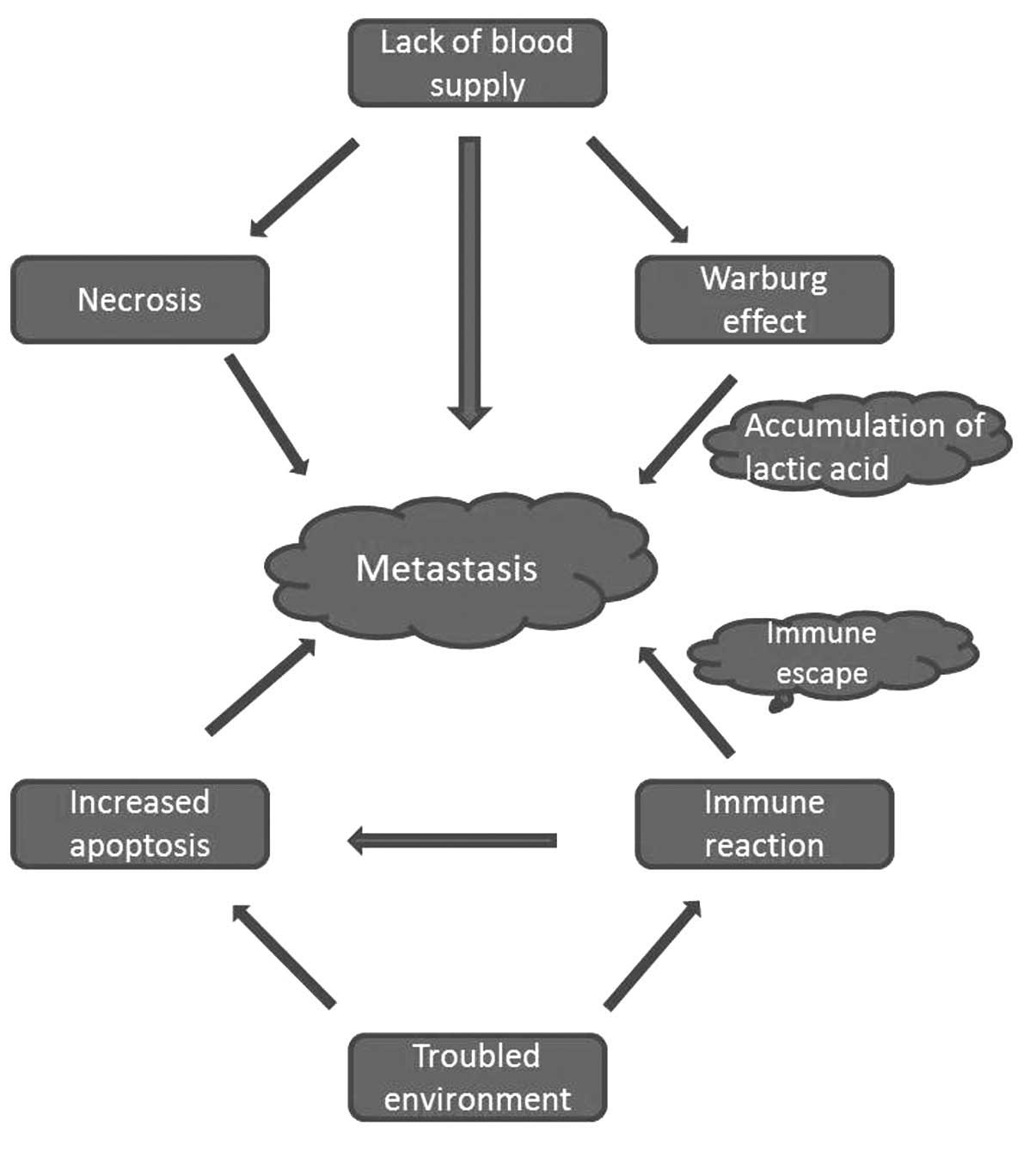
cancer metastasis (Fig. 1).
Increased apoptosis is associated with
a higher grade of malignancy and poorer clinical outcome
Despite the widely accepted hypothesis that cancer
cells are characterized by ‘resistance to apoptosis’ (10), malignant tumors display in fact an
even higher occurrence of apoptosis compared with corresponding
benign tumors or normal tissues. Pathological studies have
repeatedly demonstrated that increased apoptosis is associated with
a higher grade of malignancy and poor clinical outcome (11–15).
Furthermore, overexpression of the anti-apoptotic protein Bcl-2 is
an indicator of a favorable prognosis in breast cancer (16–20),
colorectal cancer (21,22) and non-small-cell lung cancer (NSCLC)
(23). Conversely, overexpression of
the cell death receptor CD95 is associated with poor clinical
outcome in solid tumors, such as renal cell carcinoma and melanoma
(24,25).
The results described in the abovementioned studies
appear to be contradictory. However, this may not be the case. In
the biosphere, long lifespan and high fecundity are two mutually
non-cooperative genetic traits. Organisms with short lifespans must
correspondingly exhibit high fecundity (26). Otherwise, they would be considered
unfit by Darwinian standards and find it difficult to propagate.
Conversely, organisms with long lifespans must exhibit lower
fecundity, otherwise they would dominate the biosphere and disrupt
the balance of species. Cancer cells are autonomous cells that have
control over their own lives. Therefore, increased cell death,
either through necrosis or apoptosis, would stimulate the
proliferation of surviving cells. Conversely, extending the
lifespan of cancer cells would inhibit cell growth, as demonstrated
by the overexpression of Bcl-2 (27–29).
Similarly, knockdown of apoptosis-promoting proteins also resulted
in the inhibition of tumor growth, as in the case of CD95 (30), caspase-3 (31) and c-Jun N-terminal kinases (32).
The increased apoptosis associated with poor
clinical outcome is not merely attributed to the fast growth of
cancer. In fact, it appears reasonable to hypothesize that, under
conditions of increased cell death, surviving cells are likely to
move away (Fig. 1).
A recently published study reported solid evidence
supporting this hypothesis. An inhibitor of the inhibitor of
apoptosis protein, which was designed for the treatment of cancer
through inducing apoptosis, was found to facilitate the metastasis
of breast cancer cells to bone tissues (33). Should this hypothesis prove to be
correct, a number of studies are expected to be published reporting
similar findings, particularly since several drugs that induce
apoptosis are currently in the stage of clinical trial.
Immune reaction/inflammation stimulates
metastasis
The association between immune reaction and cancer
is an interesting paradox. For several years, immunosurveillance
has been considered an important barrier for carcinogenesis and a
number of studies and clinical cares aim to prevent and treat
cancer by enhancing the immune system. However, inflammation, which
is an immune reaction, is widely accepted as a facilitator of
carcinogenesis and cancer metastasis.
Although several studies have demonstrated that
cytokines, such as interleukins and other cytokines released by
immunocytes, are able to promote cancer cell proliferation
(34), we hypothesized that the
immune reaction against cancer cells per se is aimed at
destroying these cells. However, the dead cells resulting from
immune reactions may further stimulate cell proliferation and
cancer metastasis (35). When under
attack, the natural response of any organism is to defend itself or
escape; cancer cells tend to escape (Fig.
1).
‘Immune escape’ has been described as a hallmark of
cancer by Hanahan and Weinberg (10).
In fact, escape from attack is a natural response in the biosphere
rather than the patent of cancer cells. The immune escape
techniques used by cancer cells include downregulation of the
expression of major histocompatibility complex molecules, by which
cancer cells try to become invisible to immune cells. The other
important strategy is escape. It has been demonstrated that
macrophages and other immunocytes promote cancer cell metastasis
(36). Therefore, we agree with Prehn
and Prehn (37) that
immunosuppression may be a better approach to treating cancer
compared with immunostimulation.
Warburg effect and metastasis
The predilection of cancer cells to engage in a high
rate of glycolysis, even under conditions of adequate oxygen
supply, is referred to as the Warburg effect and was first
described by the famous German biochemist Otto Warburg in 1924
(38). Approximately 90 years after
its discovery, the Warburg effect has again attracted significant
attention in the field of cancer research (38). A number of researchers suggest that
glycolysis renders cancer cells superior to their normal peers
regarding proliferation (39).
However, glycolysis produces large amounts of lactic acid, thereby
significantly increasing the acidity of the surrounding
environment. Therefore, cancer cells tend to move away from this
hostile environment (Fig. 1). It was
previously demonstrated that low local pH stimulated cancer
invasion and metastasis (40); by
neutralizing the acidic pH, the occurrence of invasion and
metastasis was reduced (41).
Promotion of blood circulation vs.
metastasis
In traditional Chinese medicine, the cause of cancer
was considered to be ‘blood stasis’. Consequently, the guiding
principle of cancer treatment in traditional medicine is to promote
blood circulation. Over several decades, promoting blood
circulation with Chinese medicine combined with radiotherapy has
been used to enhance the efficacy of radiotherapy, based on the
hypothesis that increased blood flow provides more oxygen to the
tissues, which is critical for radiotherapy. Indeed, the efficacy
of radiotherapy was significantly increased. However, the
metastasis rate was also increased. Thus, clinical oncologists in
China are quite resistant to the use of treatments aimed at
promoting blood circulation, which is consistent with the concept
that blood vessels provide pathways for cancer to metastasize.
It would appear that anti-angiogenesis may be used
to starve cancer cells, as well as to inhibit their metastasis.
Unexpectedly, however, anti-angiogenesis has also been found to
stimulate cancer metastasis (42–44). It
may appear puzzling that cancer cell metastasis may be stimulated
by promoting blood circulation as well as by inhibiting
angiogenesis. The explanation for this phenomenon lies with the
fact that the promotion of blood circulation was not applied alone,
but rather was used together with radiotherapy, which kills or
injures cancer cells and forces them to metastasize. Promoting
blood circulation provides the cells with the means to metastasize
at the right time. If promotion of blood circulation was used
alone, it would not have triggered cancer cell metastasis. Indeed,
it was demonstrated that improving blood circulation reduced cancer
metastasis (45). However, the
problem with using Chinese medicine to promote blood circulation
alone, is that it would be difficult to detect a tumor size
reduction or disappearance; this may be difficult for physicians
and patients to accept.
Regarding anti-angiogenesis, as mentioned above,
blocking blood circulation would deprive cancer cells of their
means for survival, which would naturally invoke a metastasis
response. Furthermore, blocking angiogenesis would result in
further apoptosis, invoking a higher degree of glycolysis and
accumulation of lactic acid. All these phenomena, in turn, would
stimulate cancer cell migration. Despite the lower availability of
blood vessels, the cancer cells may invade further and use the
lymphatics and blood vessels in the stroma to evade.
Clinical implications
Metastasis accounts for >90% of cancer-related
mortality. Therefore, reducing metastasis is the key to curtailing
the rate of death from cancer. The quandary is that there is
currently no method effective in blocking or impeding metastasis.
By contrast, almost all available treatment approaches, including
surgical resection, have the potential of stimulating the
metastatic growth of cancer. Therefore, rather than investigating
methods of eliminating cancer cells, we should be looking into
methods for inhibiting cancer growth and metastasis. Instead of
starving cancer cells by inhibiting angiogenesis, it may be
preferable to ‘feed’ cancer cells by promoting blood circulation;
and instead of inducing apoptosis of cancer cells by targeting the
anti-apoptotic proteins, it may be preferable to prolong the
lifespan of cancer cells through overexpression of these proteins,
as living with cancer may be preferable to dying from cancer.
Acknowledgements
The authors would like to thank Jiasen Wang, Rice
University, USA, for helping with the language improvement and
William CS Cho, Queen Elizabeth Hospital, Hong Kong, for
constructive comments and suggestions.
References
|
1
|
Vaux DL: In defense of the somatic
mutation theory of cancer. Bioessays. 33:341–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sonnenschein C and Soto AM: Somatic
mutation theory of carcinogenesis: Why it should be dropped and
replaced. Mol Carcinog. 29:205–211. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Soto AM and Sonnenschein C: The tissue
organization field theory of cancer: a testable replacement for the
somatic mutation theory. Bioessays. 33:332–340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duesberg P and Rasnick D: Aneuploidy, the
somatic mutation that makes cancer a species of its own. Cell Motil
Cytoskeleton. 47:81–107. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang LJ, Li H, Zhang LY, et al:
Metastasis: inherent vs acquired phenotype. Med Hypotheses.
74:874–876. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weinberg RA: Mechanisms of malignant
progression. Carcinogenesis. 29:1092–1095. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tarin D, Thompson EW and Newgreen DF: The
fallacy of epithelial mesenchymal transition in neoplasia. Cancer
Res. 65:59962005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suzuki M and Tarin D: Gene expression
profiling of human lymph node metastases and matched primary breast
carcinomas: clinical implications. Mol Oncol. 1:172–180. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weinberg RA: Is metastasis predetermined?
Mol Oncol. 1:263–264, 265–266. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lipponen P, Aaltomaa S, Kosma VM and
Syrjanen K: Apoptosis in breast cancer as related to
histopathological characteristics and prognosis. Eur J Cancer 30A.
2068–2073. 1994. View Article : Google Scholar
|
|
12
|
Lipponen PK and Aaltomaa S: Apoptosis in
bladder cancer as related to standard prognostic factors and
prognosis. J Pathol. 173:333–339. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lipponen P: Apoptosis in breast cancer:
relationship with other pathological parameters. Endocr Relat
Cancer. 6:13–16. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishimura R, Nagao K, Miyayama H, et al:
Apoptosis in breast cancer and its relationship to
clinicopathological characteristics and prognosis. J Surg Oncol.
71:226–234. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sinicrope FA, Hart J, Hsu HA, Lemoine M,
Michelassi F and Stephens LC: Apoptotic and mitotic indices predict
survival rates in lymph node-negative colon carcinomas. Clin Cancer
Res. 5:1793–1804. 1999.PubMed/NCBI
|
|
16
|
Lee KH, Im SA, Oh DY, et al: Prognostic
significance of bcl-2 expression in stage III breast cancer
patients who had received doxorubicin and cyclophosphamide followed
by paclitaxel as adjuvant chemotherapy. BMC Cancer. 7:632007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ali HR, Dawson SJ, Blows FM, et al: A
Ki67/BCL2 index based on immunohistochemistry is highly prognostic
in ER-positive breast cancer. J Pathol. 226:97–107. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Callagy GM, Pharoah PD, Pinder SE, et al:
Bcl-2 is a prognostic marker in breast cancer independently of the
Nottingham prognostic index. Clin Cancer Res. 12:2468–2475. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Callagy GM, Webber MJ, Pharoah PD and
Caldas C: Meta-analysis confirms BCL2 is an independent prognostic
marker in breast cancer. Bmc Cancer. 8:1532008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rolland P, Spendlove I, Madjd Z, et al:
The p53 positive Bcl-2 negative phenotype is an independent marker
of prognosis in breast cancer. Int J Cancer. 120:1311–1317. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Poincloux L, Durando X, Seitz JF, et al:
Loss of Bcl-2 expression in colon cancer: a prognostic factor for
recurrence in stage II colon cancer. Surg Oncol. 18:357–365. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sinicrope FA, Hart J, Michelassi F and Lee
JJ: Prognostic value of bcl-2 oncoprotein expression in stage II
colon carcinoma. Clin Cancer Res. 1:1103–1110. 1995.PubMed/NCBI
|
|
23
|
Tomita M, Matsuzaki Y, Edagawa M, Shimizu
T, Hara M and Onitsuka T: Prognostic significance of bcl-2
expression in resected pN2 non-small cell lung cancer. Eur J Surg
Oncol. 29:654–657. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
MacherGoeppinger S, Bermejo JL, Wagener N,
et al: Expression and prognostic relevance of the death receptor
CD95 (Fas/APO1) in renal cell carcinomas. Cancer Lett. 301:203–211.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ugurel S, Rappl G, Tilgen W and Reinhold
U: Increased soluble CD95 (sFas/CD95) serum level correlates with
poor prognosis in melanoma patients. Clin Cancer Res. 7:1282–1286.
2001.PubMed/NCBI
|
|
26
|
Partridge L, Gems D and Withers DJ: Sex
and death: what is the connection? Cell. 120:461–472. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Knowlton K, Mancini M, Creason S, Morales
C, Hockenbery D and Anderson BO: Bcl-2 slows in vitro breast cancer
growth despite its antiapoptotic effect. J Surg Res. 76:22–26.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pietenpol JA, Papadopoulos N, Markowitz S,
Willson JK, Kinzler KW and Vogelstein B: Paradoxical inhibition of
solid tumor cell growth by bcl2. Cancer Res. 54:3714–3717.
1994.PubMed/NCBI
|
|
29
|
O'Reilly LA, Huang DC and Strasser A: The
cell death inhibitor Bcl-2 and its homologues influence control of
cell cycle entry. Embo J. 15:6979–6990. 1996.PubMed/NCBI
|
|
30
|
Chen L, Park SM, Tumanov AV, et al: CD95
promotes tumour growth. Nature. 465:492–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang Q, Li F, Liu X, et al: Caspase
3-mediated stimulation of tumor cell repopulation during cancer
radiotherapy. Nat Med. 17:860–866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hui L, Zatloukal K, Scheuch H, Stepniak E
and Wagner EF: Proliferation of human HCC cells and chemically
induced mouse liver cancers requires JNK1-dependent p21
downregulation. J Clin Invest. 118:3943–3953. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang C, Davis JL, Zeng R, et al:
Antagonism of inhibitor of apoptosis proteins increases bone
metastasis via unexpected osteoclast activation. Cancer Discov.
3:212–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lippitz BE: Cytokine patterns in patients
with cancer: a systematic review. Lancet Oncol. 14:e218–e228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang RA, Li QL, Li ZS, et al: Apoptosis
drives cancer cells proliferate and metastasize. J Cell Mol Med.
17:205–211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fidler IJ: Macrophages and metastasis - a
biological approach to cancer therapy. Cancer Res. 45:4714–4726.
1985.PubMed/NCBI
|
|
37
|
Prehn RT and Prehn LM: Cancer
immunotherapy by immunosuppression. Theor Biol Med Model. 7:452010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Thompson CB: Rethinking the regulation of
cellular metabolism. Cold Spring Harb Symp Quant Biol. 76:23–29.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bonuccelli G, Tsirigos A, WhitakerMenezes
D, et al: Ketones and lactate ‘fuel’ tumor growth and metastasis:
Evidence that epithelial cancer cells use oxidative mitochondrial
metabolism. Cell Cycle. 9:3506–3514. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Estrella V, Chen T, Lloyd M, et al:
Acidity generated by the tumor microenvironment drives local
invasion. Cancer Res. 73:1524–1535. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ibrahim HA, Cornnell HH, Coelho RML, et
al: Reduction of metastasis using a non-volatile buffer. Clin Exp
Metastasis. 28:841–849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ebos JM, Lee CR, CruzMunoz W, Bjarnason
GA, Christensen JG and Kerbel RS: Accelerated metastasis after
short-term treatment with a potent inhibitor of tumor angiogenesis.
Cancer Cell. 15:232–239. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
PaezRibes M, Allen E, Hudock J, et al:
Antiangiogenic therapy elicits malignant progression of tumors to
increased local invasion and distant metastasis. Cancer Cell.
15:220–231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu XD, Sun HC, Xu HX, et al:
Antiangiogenic therapy promoted metastasis of hepatocellular
carcinoma by suppressing host-derived interleukin-12b in mouse
models. Angiogenesis. 16:809–820. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang WQ, Liu L, Sun HC, et al: Tanshinone
IIA inhibits metastasis after palliative resection of
hepatocellular carcinoma and prolongs survival in part via vascular
normalization. J Hematol Oncol. 5:692012. View Article : Google Scholar : PubMed/NCBI
|