Introduction
Despite considerable progress in the management of
chemotherapy-induced nausea and vomiting (CINV), it remains one of
the most problematic adverse effects of chemotherapy among cancer
patients. Uncontrolled CINV can limit the dose intensity of
chemotherapy and severely compromise a patient's quality of life
(1). The occurrence of CINV depends
primarily on the dose and type of chemotherapeutic agent(s) used in
treatment strategies.
To the best of our knowledge, few previous studies
have addressed the efficacy of anti-emetic treatment in patients
receiving moderately emetogenic chemotherapy (MEC). It has been
demonstrated previously that a 5-hydroxytryptamine-3
(5-HT3) receptor antagonist (RA) plus a corticosteroid
have anti-emetic effects in patients receiving MEC (2–4). The
American Society of Clinical Oncology (ASCO) guidelines recommend a
three-drug combination of 5-HT3 RA, dexamethasone and
aprepitant (a neurokinin 1 RA) is administered prior to
highly-emetogenic chemotherapy, however, only a two-drug
combination of 5-HT3 RA with dexamethasone is
recommended for MEC. Aprepitant is only added to the anti-emesis
treatment for patients receiving anthracyclines and
cyclophosphamide (AC) (5). The
addition of aprepitant in patients receiving MEC with these agents
(AC-MEC) improves the prevention of CINV (6,7). According
to the National Comprehensive Cancer Network guidelines, aprepitant
is only recommended for patients receiving MEC regimens that
include agents such as carboplatin and irinotecan. However, the
characteristics of these patients are unclear, and there are no
randomized trials to support this strategy for non-AC MEC.
Furthermore, the Multinational Association of Supportive Care in
Cancer (MASCC) does not recommend the use of aprepitant in non-AC
MEC regimens (8). A phase III,
gender-stratified trial in 848 patients, demonstrated that
aprepitant significantly improves the primary endpoint of the study
(no vomiting) as well as the secondary endpoint, complete response
(CR), following MEC with AC or non-AC treatment regimens (7).
Colorectal cancer (CRC) is currently the third most
common cancer worldwide (9).
Approximately 20–25% of patients with the disease already have
metastases at the time of diagnosis, and 50–60% of the remaining
patients will go on to develop them (10,11). A
number of anti-cancer agents have demonstrated significant
antitumor activity in metastatic CRC (mCRC), including the systemic
drugs 5-fluorouracil (5-FU), irinotecan, oxaliplatin, and the oral
drug capecitabine. Different combinations of these drugs, such as
the FOLFOX [leucovorin (LV), 5-FU, and oxaliplatin], FOLFIRI (LV,
5-FU, and irinotecan) and XELOX regimens (oxaliplatin and
capecitabine), with or without a monoclonal antibody agent, are
known to improve outcomes in mCRC patients (12–15). In
terms of the adjuvant chemotherapy, oxaliplatin in combination with
FU, modulated by (LV) or capecitabine, is a standard therapy for
non-distant mCRC patients with positive (stage III) lymph nodes
(16–18). These three types of regimens are
classified as non-AC MEC for CRC. The current recommended therapy
for CRC patients receiving MEC is the combination of a
5-HT3 RA and dexamethasone (19–21).
In the present study, a multicenter, open-label,
randomized phase II study was conducted in order to evaluate the
efficacy of aprepitant in preventing CINV following oxaliplatin- or
irinotecan-based MEC (FOLFOX, XELOX or FOLFIRI) in CRC
patients.
Patients and methods
Study design and patients
The present multicenter, phase II, open-label,
randomized, parallel comparative study was conducted in a total of
18 institutions in Japan, as part of the Kagoshima Aprepitant Study
for Colon Cancer (KASCC). The trial was conducted between September
2011 and August 2013 following approval from each institution's
review board. Written, informed consent was obtained from all
patients, who were enrolled using an online registration system.
The patients with advanced or recurrent CRC were enrolled and
stratified according to their performance status (PS; 0 or 1–2),
institution, and chemotherapy regimen (FOLFOX, XELOX or FOLFIRI),
and then randomly assigned to the aprepitant (5-HT3 RA +
reduced-dose dexamethasone + aprepitant) or standard
(5-HT3+ dexamethasone) regimen group according to a
computer-generated, blinded allocation schedule. The study period
included the first course of chemotherapy for each patient.
Chemotherapy regimen
The following chemotherapy agents were administered
intravenously (i.v.) or orally (per os; p.o.): mFOLFOX6 (LV
200 mg/m2 i.v. over 2 h, prior to 5-FU day 1, 5-FU 400
mg/m2 i.v. bolus day 1, followed by 2,400
mg/m2 i.v. over 46 h, and oxaliplatin 85
mg/m2 i.v. day 1 in a 2-week cycle); XELOX (oxaliplatin
130 mg/m2 on day 1, followed by oral capecitabine 1,000
mg/m2 twice daily on days 1–14, in a 3-week cycle);
FOLFIRI (LV 400 mg/m2 i.v. over 2 h, prior to 5-FU day 1
and 5-FU 400 mg/m2 i.v. bolus day 1, and then 2,400
mg/m2 i.v. over 46 h and irinotecan 180 mg/m2
i.v. over 90 min day 1 in a 2-week cycle).
Treatment administration
Patients in the standard-regimen group received
5-HT3 RA and dexamethasone 9.9 mg by i.v. on day 1,
followed by oral dexamethasone 4 mg twice daily on days 2 and 3.
Patients in the aprepitant-regimen group received oral aprepitant
125 mg plus i.v. 5-HT3 RA and dexamethasone 6.6 mg on
day 1, and oral aprepitant 80 mg plus oral dexamethasone 2 mg twice
daily on days 2 and 3. 5-HT3 RAs were administered by
i.v. over 30-min prior to chemotherapy. Aprepitant was administered
orally at 125 mg on day 1 prior to chemotherapy, and 80 mg each on
days 2 and 3. Dexamethasone was administered by i.v. over 30-min in
combination with the 5-HT3 RA, prior to chemotherapy
(Table I).
 | Table I.Outline of the standard and aprepitant
treatment regimens. |
Table I.
Outline of the standard and aprepitant
treatment regimens.
| Regimen group | Day 1 | Day 2 (p.o.) | Day 3 (p.o.) |
|---|
| Standard |
|
|
|
|
5-HT3
RAsa | Administered |
|
|
|
Dexamethasone | 9.9 mg i.v. | 8 mg | 8 mg |
| Aprepitant |
|
|
|
|
5-HT3
RAsa | Administered |
|
|
|
Dexamethasone | 6.6 mg i.v. | 4 mg | 4 mg |
|
Aprepitant | 125 mg p.o. | 80 mg | 80 mg |
Endpoints and investigation
methods
The total study period was from the initiation of
chemotherapy until day 5. The primary endpoints of the study were
the proportions of patients who achieved CR (defined as no emetic
episodes and no use of rescue therapy) during the overall phase
(0–120 h post-chemotherapy), the acute phase (0–24 h
post-chemotherapy), and the delayed phase (24–120 h
post-chemotherapy) of the first planned chemotherapy cycle.
Secondary endpoints were: i) Complete protection (CP, defined as no
emesis, no rescue therapy, and no more than moderate nausea), and
ii) the proportion of patients without emetic episodes or nausea,
and with no more than moderate nausea during the overall, acute and
delayed phases, and time to treatment failure (i.e., time to first
emetic episode or time to administration of rescue therapy,
whichever occurred first).
Patient diaries were used to record any emetic
episodes, nausea, or rescue anti-emetics in daily (24 h) intervals.
The presence or absence of CINV was recorded and graded according
to the common terminology criteria for adverse events (CTCAE) from
the National Cancer Institute, version 4.0 (available at:
http://ctep.cancer.gov/protocolDevelopment/). Grade 1
or higher was considered as positive for CINV. Patients recorded
the most severe nausea intensity during the previous 24 h period,
based on a 4-point scale (0, none; 1, mild; 2, moderate; 3,
severe).
Statistical analysis
The outcomes in both groups were analyzed using
χ2 tests for primary endpoints, secondary endpoints and
patients' characteristics by treatment regimen group. Two-sided
two-sample t-tests were used where appropriate. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patients
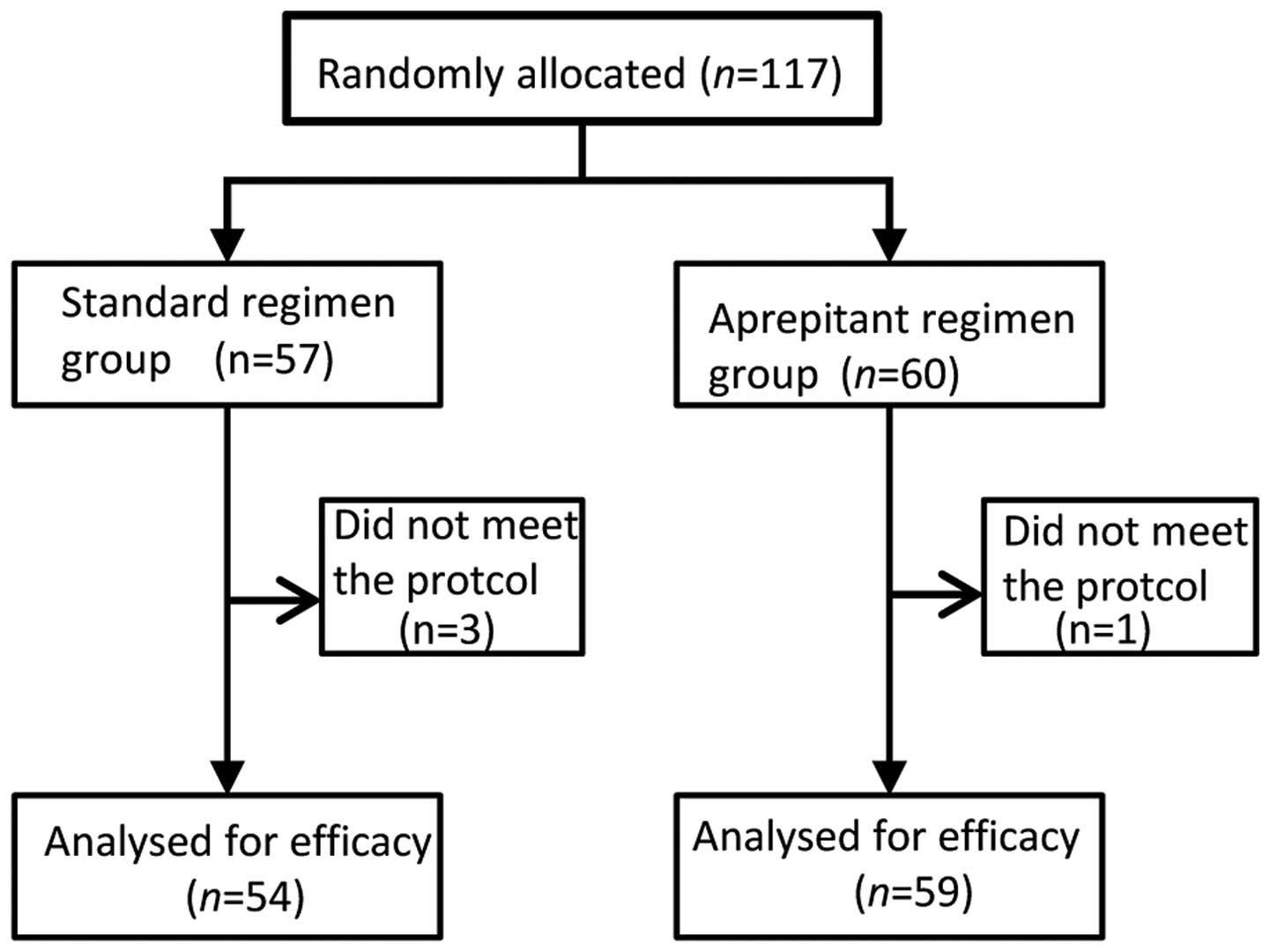
A total of 117 patients were randomly assigned to
one of the two treatment arms (Fig.
1). Of these patients, one in the aprepitant regimen group and
three in the standard regimen group were excluded from the efficacy
analyses because the anti-emetic regimen was deemed to have been
changed, and did not meet the inclusion protocol for the present
study. Thus, in total, 113 patients were included in the full
analysis set. Both treatment groups had similar baseline
demographics.
The majority of patients (94.7%) received
oxaliplatin-based chemotherapy. Patient baseline characteristics,
including known risk factors for CINV (female, history of alcohol
use, morning sickness, motion sickness, or prior CINV), were
similar between the two treatment groups (Table II).
 | Table II.Patient characteristics. |
Table II.
Patient characteristics.
|
Characteristics | Standard regimen
group (n=54) | Aprepitant regimen
group (n=59) | Comparison
test |
|---|
| Age, mean years ±
SD | 63.48±10.23 | 66.46±9.81 | n.s.a |
| Gender
(male/female) | 30/24 | 34/25 | n.s. |
| Smoking
(no/yes) | 41/12 | 44/15 | n.s. |
| Alcoholic
drinks/week |
|
|
|
|
0/1/2–3/>4 | 34/3/3/14 | 34/6/10/8 | n.s. |
| History of motion
sickness |
|
|
|
|
(no/yes) | 47/7 | 49/10 | n.s. |
| Chemotherapy
regimen |
|
|
|
|
(FOLFOX/FOLFIRI/XEROX) | 25/3/26 | 19/3/37 | n.s. |
| 5-HT3
RAs |
|
|
|
|
Granisetron/ondansetron | 17/4/8/25 | 13/2/7/37 | n.s. |
|
Azasetron/palonosetron |
|
|
|
Efficacy
The percentages of patients with CR in the overall,
acute, and delayed phases for each treatment are shown in Fig. 2. The CR rates in the overall, and
delayed phases were similar in the standard and aprepitant regimen
groups (overall phase: 79.6% (43/54) and 79.7% (47/59); acute
phase: 94.4% (51/54) and 94.9% (56/59); delayed phase: 79.6%
(43/54) and 79.7% (47/59), respectively.
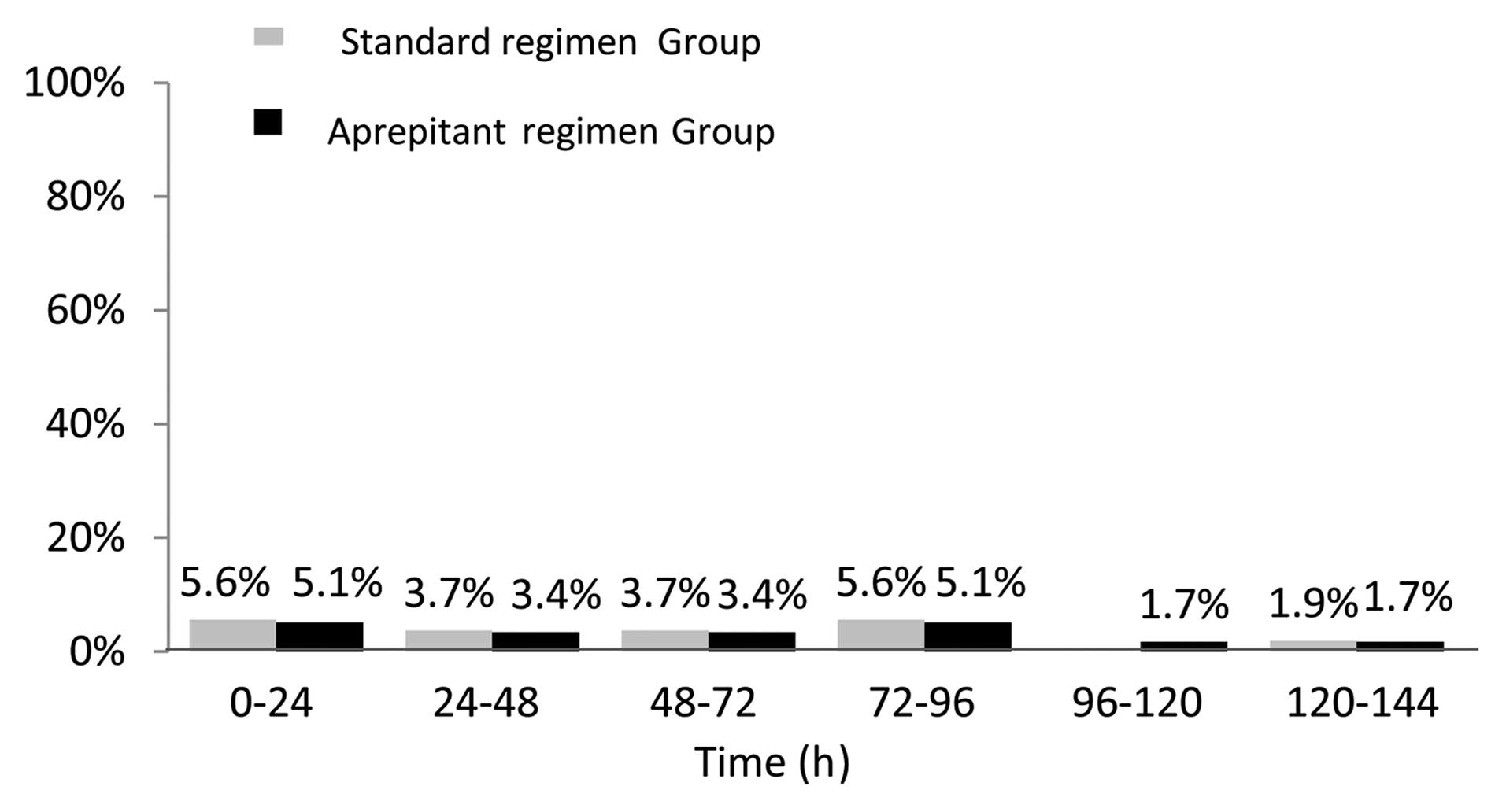
There were no significant differences between the
aprepitant- and standard-regimen groups in terms of the following
predefined secondary endpoints: The proportion of patients without
emetic episodes, with no nausea, with no more than moderate nausea
during the overall, acute and delayed phases, and the time to
treatment failure (Table III and
Fig. 3).
 | Table III.Percentage of patients reaching
efficacy endpoints by study phase and treatment group. |
Table III.
Percentage of patients reaching
efficacy endpoints by study phase and treatment group.
|
| Acute phase | Delayed phase |
|
|
|---|
|
|
|
|
|---|
| Endpoint | Standard regimen
group, % (n=54) | Aprepitant regimen
group, % (n=59) | Standard regimen
group,% (n=54) | Aprepitant regimen
group,% (n=59) |
|---|
| Complete
response | 94.4 | 94.9 | 79.6 | 79.7 |
| Complete
protection | 94.4 | 93.2 | 79.6 | 78.0 |
| No vomiting | 94.4 | 98.3 | 81.5 | 86.4 |
| No nausea | 96.3 | 89.8 | 68.5 | 64.4 |
| No significant
nauseaa | 100.0 | 98.3 | 88.9 | 91.3 |
Tolerability
The adverse events reported following treatment are
summarized in Table IV. The overall
incidences of adverse events were similar in both groups. The
incidences of leucopenia and neutropenia were similar in both
treatment groups. Grade 3–4 neutropenia, defined by the National
Cancer Institute toxicity criteria, occurred in 11 patients (20.7%)
in the aprepitant group and 15 patients (25.4%) in the standard
group. The neutrophil counts were similar in the two treatment
groups (Table IV).
 | Table IV.Patients with specific clinical
adverse events of incidence over 5%, in at least one treatment
group. |
Table IV.
Patients with specific clinical
adverse events of incidence over 5%, in at least one treatment
group.
| Adverse event | Standard regimen
group, n (%) | Aprepitant regimen
group, n (%) |
|---|
| Anorexia | 26 (48.1) | 26 (44.1) |
| Fatigue | 8
(14.8) | 6
(10.2) |
| Diarrhea | 3 (5.6) | 2 (3.4) |
| Constipation | 4 (7.4) | 1 (1.7) |
| Oral mucositis | 3 (5.6) | 1 (1.7) |
| Leukopenia (Grade
3–4) | 11 (20.4) | 12 (20.3) |
| Neutropenia (Grade
3–4) | 11 (20.4) | 15 (25.4) |
|
Thrombocytopenia | 7
(13.0) | 4 (6.8) |
| Total | 54 (100) | 59 (100) |
Discussion
CINV is an unpleasant adverse effect of MEC in
patients with CRC, and may limit the efficacy of the treatment for
this disease. The prevention and treatment of CINV are therefore
important considerations for CRC patients, as well as those with
other cancers. To the best of our knowledge, the present study
provides the first report of a randomized trial to evaluate the
efficacy of triple therapy that incudes aprepitant (with
dexamethasone and a 5-HT3 RA), for the prevention of
CINV in CRC patients receiving oxaliplatin or irrinotecan-based
MEC.
MEC-induced vomiting in the acute phase of treatment
is known to be well-controlled by 5-HT3 RA (22,23).
However, delayed vomiting and nausea are still poorly controlled
during MEC, resulting in negative patient attitudes towards
treatment and hindering the continuation of MEC. The present study
investigated the addition of aprepitant to dexamethasone in the
delayed phase, to determine if it could improve outcomes in CRC
patients receiving MEC. However, our results revealed there were no
significant differences between the standard and aprepitant regimen
in terms of complete suppression of vomiting, CR and CP rates,
incidences of no vomiting and no nausea, no significant nausea, and
time to treatment failure either overall, or in the acute or
delayed phase. Similarly, there were no notable differences in
adverse events between the standard and aprepitant regimens.
The MASCC (24) and
ASCO guidelines (25) recommend
palonosetron as the preferred 5-HT3 RA for non-AC MEC
regimens, and thus, the use of palonosetron instead of granisetron
may improve delayed CINV in this setting. Moreover, a recent study
from the Rochester Cancer Center demonstrated that delayed nausea
was significantly improved by the administration of additional
dexamethasone on days 2 and 3; however, there was no difference
between palonosetron and granisetron during highly-emetogenic
chemotherapy or MEC (26). The
difference between palonosetron and granisetron would be expected
to be small. As noted previously, the suggested optimum dose of
dexamethasone for standard prophylaxis is 20 mg in combination with
a 5-HT3 RA (27).
The addition of dexamethasone to 5-HT3 RA
has been reported to improve total control rates by 9.8–13.4% at 24
h, and by 4.7–8.7% at 48 h (22,28).
However, while corticosteroids (dexamethasone) are recommended for
treating delayed nausea and vomiting, their side effects remain a
concern for many clinical oncologists (29). In the present study, the dexamethasone
dose was 9.9 mg on day l, and 8 mg p.o. on days 2 and 3. Aprepitant
is a substrate and inhibitor of CYP3A4, known to increase plasma
dexamethasone concentrations (30).
Therefore, to achieve comparable plasma levels of dexamethasone in
the presence of aprepitant, the dose of dexamethasone was 6.6 mg
i.v. on day l and 4 mg p.o. on days 2 and 3 in the aprepitant
regimen. Both the i.v. and p.o. doses of dexamethasone could
therefore be reduced when combined with aprepitant, in comparison
to the standard regimen for MEC. The lower dose of dexamethasone in
the aprepitant regimen may therefore help to reduce the side
effects associated with long-term corticosteroids administration
during MEC in patients with CRC, and may therefore also help to
maintain the quality of life in these patients.
In conclusion, the present study demonstrates that
aprepitant in combination with a 5-HT3 RA and reduced
dose of corticosteroid was well tolerated and effective for
preventing CINV associated with MEC in Japanese patients with
CRC.
References
|
1
|
Oo TH and Hesketh PJ: Drug insight: New
anti-emetics in the management of chemotherapy-induced nausea and
vomiting. Nat Clin Pract Oncol. 2:196–201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soukop M, McQuade B, Hunter E, Stewart A,
Kaye S, Cassidy J, Kerr D, Khanna S, Smyth J and Coleman R:
Ondansetron compared with metoclopramide in the control of emesis
and quality of life during repeated chemotherapy for breast cancer.
Oncology. 49:295–304. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sigsgaard T, Herrstedt J, Andersen LJ,
Havsteen H, Langer SW, Kjaerbøl AG, Lund H, Kjaer M and
Dombernowsky P: Granisetron compared with predonisolone plus
metopimazine as anti-emetic prophylaxis during multiple cycles of
moderately emetogenic chemotherapy. Br J Cancer. 80:412–418. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sigsgaard T, Herrstedt J, Handberg J,
Kjaer M and Dombernowsky P: Ondansetron plus metopimazine compared
with ondansetron plus metopimazine plus prednisolone as anti-emetic
prophylaxis in patients receiving multiple cycles of moderately
emetogenic chemotherapy. J Clin Oncol. 19:2091–2097.
2001.PubMed/NCBI
|
|
5
|
American Society of Clinical Oncology.
Kris MG, Hesketh PJ, Somerfield MR, Feyer P, Clark-Snow R, Koeller
JM, Morrow GR, Chinnery LW, Chesney MJ, Gralla RJ and Grunberg SM:
American society of clinical oncology guideline for anti-emetics in
oncology: Update 2006. J Clin Oncol. 24:2932–2947. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Warr DG, Hesketh PJ, Gralla RJ, Muss HB,
Herrstedt J, Eisenberg PD, Raftopoulos H, Grunberg SM, Gabriel M,
Rodgers A, et al: Efficacy and tolerability of aprepitant for the
prevention of chemotherapy-induced nausea and vomiting in patients
with breast cancer after moderately emetogenic chemotherapy. J Clin
Oncol. 23:2822–2830. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rapoport BL, Jordan K, Boice JA, Taylor A,
Brown C, Hardwick JS, Carides A, Webb T and Schmoll HJ: Aprepitant
for the prevention of chemotherapy-induced nausea and vomiting
associated with a broad range of moderately emetogenic
chemotherapies and tumor types: A randomized, double-blind study.
Support Care Cancer. 18:423–431. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roila F, Warr D, Aapro M, Clark-Snow RA,
Einhorn L, Gralla RJ, Herrstedt J, Saito M and Tonato M: Delayed
emesis: Moderately emetogenic chemotherapy (single-day chemotherapy
regimens only). Support Care Cancer. 19(Suppl 1): S57–S62. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer
in. 2008 GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Van Cutsem E and Oliveira J: ESMO
Guidelines Working Group: Advanced colorectal cancer: ESMO clinical
recommendations for diagnosis, treatment and follow-up. Ann Oncol.
20(Suppl 4): S61–S63. 2009.
|
|
11
|
Yoo PS, Lopez-Soler RI, Longo WE and Cha
CH: Liver resection for metastatic colorectal cancer in the age of
neoadjuvant chemotherapy and bevacizumab. Clin Colorectal Cancer.
6:202–207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Gramont A, Figer A, Seymour M, Homerin
M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer
G, et al: Leucovorin and fluorouracil with or without oxaliplatin
as first-line treatment in advanced colorectal cancer. J Clin
Oncol. 18:2938–2947. 2000.PubMed/NCBI
|
|
13
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cassidy J, Tabernero J, Twelves C, Brunet
R, Butts C, Conroy T, Debraud F, Figer A, Grossmann J, Sawada N, et
al: XELOX (capecitabine plus oxaliplatin): Active first-line
therapy for patients with metastatic colorectal cancer. J Clin
Oncol. 22:2084–2091. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
André T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil, and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
André T, Boni C, Navarro M, Tabernero J,
Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F
and de Gramont A: Improved overall survival with oxaliplatin,
fluorouracil, and leucovorin as adjuvant treatment in stage II or
III colon cancer in the MOSAIC trial. J Clin Oncol. 27:3109–3116.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haller DG, Tabernero J, Maroun J, de Braud
F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K and
Schmoll HJ: Capecitabine plus oxaliplatin compared with
fluorouracil and folinic acid as adjuvant therapy for stage III
colon cancer. J Clin Oncol. 29:1465–1471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prevention of chemotherapy- and
radiotherapy-induced emesis: Results of the perugia consensus
conference. Antiemetic subcommittee of the multinational
association of supportive care in cancer (MASCC). Ann Oncol.
9:811–819. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gralla RJ, Osoba D, Kris MG, Kirkbride P,
Hesketh PJ, Chinnery LW, Clark-Snow R, Gill DP, Groshen S, Grunberg
S, et al: Recommendations for the use of anti-emetics:
Evidence-based, clinical practice guidelines. American society of
clinical oncology. J Clin Oncol. 17:2971–2994. 1999.PubMed/NCBI
|
|
21
|
Herrstedt J, Aapro MS, Roila F and Kataja
VV: ESMO, Guidelines Task Force: ESMO minimum clinical
recommendations for prophylaxis of chemotherapy-induced nausea and
vomiting (NV). Ann Oncol. 16(Suppl 1): i77–i79. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perez EA, Hesketh P, Sandbach J, Reeves J,
Chawla S, Markman M, Hainsworth J, Bushnell W and Friedman C:
Comparison of single-dose oral granisetron versus intravenous
ondansetron in the prevention of nausea and vomiting induced by
moderately emetogenic chemotherapy: A multicenter, double-blind,
randomized parallel study. J Clin Oncol. 16:754–760.
1998.PubMed/NCBI
|
|
23
|
Jordan K, Hinke A, Grothey A, Voigt W,
Arnold D, Wolf HH and Schmoll HJ: A meta-analysis comparing the
efficacy of four 5-HT3-receptor antagonists for acute
chemotherapy-induced emesis. Support Care Cancer. 15:1023–1033.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roila F, Herrstedt J, Aapro M, Gralla RJ,
Einhorn LH, Ballatori E, Bria E, Clark-Snow RA, Espersen BT, Feyer
P, et al: Guideline update for MASCC and ESMO in the prevention of
chemotherapy- and radiotherapy-induced nausea and vomiting: Results
of the Perugia consensus conference. Ann Oncol. 21(Suppl 5):
v232–v243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Basch E, Prestrud AA, Hesketh PJ, Kris MG,
Feyer PC, Somerfield MR, Chesney M, et al: Antiemetics: American
society of clinical oncology clinical practice guideline update. J
Clin Oncol. 29:4189–4198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roscoe JA, Heckler CE, Morrow GR, Mohile
SG, Dakhil SR, Wade JL and Kuebler JP: Prevention of delayed
nausea: A University of Rochester cancer center community clinical
oncology program study of patients receiving chemotherapy. J Clin
Oncol. 30:3389–3395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Double-blind, dose-finding study of four
intravenous doses of dexamethasone in the prevention of
cisplatin-induced acute emesis. Italian group for anti-emetic
research. J Clin Oncol. 16:2937–2942. 1998.PubMed/NCBI
|
|
28
|
Gralla RJ, Navari RM, Hesketh PJ, Popovic
W, Strupp J, Noy J, Einhorn L, Ettinger D, Bushnell W and Friedman
C: Single-dose oral granisetron has equivalent anti-emetic efficacy
to intravenous ondansetron for highly emetogenic cisplatin-based
chemotherapy. J Clin Oncol. 16:1568–1573. 1998.PubMed/NCBI
|
|
29
|
Vardy J, Chiew KS, Galica J, Pond GR and
Tannock IF: Side effects associated with the use of dexamethasone
for prophylaxis of delayed emesis after moderately emetogenic
chemotherapy. Br J Cancer. 94:1011–1015. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hesketh PJ, Grunberg SM, Gralla RJ, Warr
DG, Roila F, de Wit R, Chawla SP, Carides AD, Ianus J, Elmer ME, et
al: The oral neurokinin-l antagonist aprepitant for the prevention
of chemotherapy-induced nausea and vomiting: A multinational,
randomized, double-blind, placebo-controlled trial in patients
receiving high-dose cisplatin-the aprepitant protocol 052 study
group. J Clin Oncol. 21:4112–4119. 2003. View Article : Google Scholar : PubMed/NCBI
|