Introduction
The brain is the most vulnerable organ to ischemic
infringement. Cerebral ischemia reperfusion injury is a recognized
complication of restoring blood flow in the ischemic brain tissue
(1). During brain ischemia and
reperfusion injury neurons undergo apoptosis (2,3).
Various mechanisms have been proposed for the pathophysiology of
cerebral ischemia-reperfusion injury, including glutamate release,
ATP depletion, anoxic depolarization, the generation of reactive
oxygen and nitrogen species, inflammatory cytokines, and matrix
metalloproteinases (MMPs) (4–7).
Matrix metalloproteinase (MMP)-3 is involved in
neuroinflammation (8,9), cell apoptosis (10), extracellular matrix (ECM)
degradation (11), and the
cleavage and activation of other MMPs, as well as shedding of death
receptors (12,13). It is rapidly upregulated upon
cerebral ischemia in rats, mice, and primates including humans
(14). There is evidence that
MMP-3 is involved in blood brain barrier (BBB) breakdown and
neuronal death (9,15), while it was also implicated in
microglial activation and inflammation in a Parkinson’s disease
model (16). Since MMP-3
participates in apoptotic signaling, the latter may be reversed by
pharmacological inhibition, gene knockdown and gene knockout of
MMP-3 in vitro (17,18).
It was reported that MMP-3 is regulated by the nuclear factor
(NF)-κB (19).
Resveratrol (Res) or
trans-3,4′,5-trihydroxystilbene, is a natural phytoalexin
found in plants that has neuroprotective, anticancer and
anti-inflammatory effects (20–22).
Res was also reported to have anti-oxidant properties, for example
the ability to modulate nitric oxide (NO) metabolism (23), chemopreventive activity (24), and the ability to regulate the
expression of MMP-3 (19). Res
also exerted protective effects against brain injury induced by
ischemia-reperfusion in gerbils (25). The beneficial neuroprotective
effects of Res may be due to its antiplatelet aggregation and
vasodilating effect, its antioxidant activity or the combination of
the above. Our previous study demonstrated that cerebral
ischemia-reperfusion injury induces the expression of MMP-9 in mice
(26). However, whether Res can
act as a MMP-3 inhibitor in cerebral ischemia remains unknown.
In this context, the present study investigated the
effects of Res on injury induced by oxygen-glucose deprivation
(OGD), as well as the underlying mechanism, in primary cortical
neuron cultures.
Materials and methods
Preparation of mouse cortical
cultures
All procedures used in this study complied with the
Guide for the Care and Use of Laboratory Animals of the Xijing
Hospital. Cultures of mouse cortical neurons were prepared using
methods similar to those previously reported (27). Timed-pregnant (13–15 days) Balb/C
mice were anesthetized with halothane and sacrificed by cervical
dislocation. After dissection, cortical neurons were dispersed by
trituration and digestion in 0.25% trypsin (Sigma-Aldrich, St.
Louis, MO, USA) for 30 min at 37°C. Then, the cell suspension was
centrifuged at 4°C for 5 min at 250 g, and resuspended in
dissociating medium [Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 10% fetal bovine serum (Gibco-BRL, Carlsbad, CA,
USA), 2 mM L-glutamine, 10 mM HEPES and 44 mM glucose (all from
Sigma-Aldrich)]. Cells were plated on poly-L-lysine-coated culture
plates at a density of 1×106 cells/ml. Twenty-four hours
later, the medium was replaced by Neurobasal medium consisting of
2% B27® Supplement, 0.5 mM L-glutamine, and 25 μM
glutamate (all from Sigma-Aldrich) to minimize glial growth. At 7
days of growth, one-half of the medium was replaced with new
Neurobasal medium. Experiments were performed on cultures following
14–16 days of incubation.
Simulation of ischemia and reperfusion
injury in vitro
OGD was used as an in vitro model of
ischemia. For OGD, the medium was removed and stored separately.
Cultures were rinsed three times with phosphate-buffered saline
(PBS), and low-glucose DMEM with 2% B27 ® Supplement was
added. Cultures were then transferred to a humidified chamber kept
in a 37°C incubator and subjected to an anaerobic environment of
95% N2-5% CO2 for 3 h. Oxygen concentration
was maintained at 0.5–1.0%, which was monitored by an oxygen
analyzer (MSA, Pittsburgh, PA, USA), throughout the experiment. OGD
was terminated by the replacement of stored medium and by returning
the cultures to a standard incubator maintained at 37°C in a 5%
CO2 atmosphere for 21 h of reoxygenation. Control cells
were not subjected to OGD and were grown at 37°C in an atmosphere
containing 5% CO2.
Treatment with Res
A fresh solution of Res was prepared from a stock of
100 mM Res in 50% dimethylsulfoxide (DMSO) (both from
Sigma-Aldrich), and was diluted in PBS to reach the desired final
concentration (10, 25, 50 and 100 μM) for treatment. Res treatment
controls (vehicle group) received the same amount of DMSO without
Res. The duration of treatment was from OGD until the end of the
experiment.
Treatment with inhibitors
Pyrrolidine dithiocarbamate (PDTC) is a specific
NF-κB inhibitor. PDTC (10 μM; Sigma-Aldrich) was added 15 min prior
to Res treatment. Next, Res was added to cultures at a final
concentration of 50 μM. The vehicle group cultures received the
same amount of the carrier solvent (0.1% DMSO) without Res. The
duration of the treatment was from OGD until the end of the
experiment.
Treatment with NO donor
NOC-18 (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) is a diazeniumdiolate (NONOate) NO donor designed
to release NO at a slower rate. NOC-18 (500 μM) was added during
Res treatment.
Cell viability assay
The effects of Res on OGD-induced cytotoxicity were
examined by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
uptake assay, with a commercial kit purchased from Sigma-Aldrich.
Neuronal primary cultures were grown on 96-well plates at a density
of 2×105 cells/cm2. At 14 days of growth,
cells were subjected to OGD and reoxygenation. Different
concentrations of Res were added to the medium. After 21 h of
reoxygenation, MTT was added to the cells at a final concentration
of 0.5 mg/ml, and the plates were incubated for 4 h at 37°C. The
insoluble formazan product was then precipitated by centrifugation,
the supernatant removed, and the crystals were dissolved in 100 μl
DMSO. Absorbance at 570 nm was measured using a microplate reader
(Bio-Rad, Hercules, CA, USA). The ratio of absorbance of treated
cells to that of the control cells was calculated, and was used to
represent the percentage of growth inhibition.
Apoptosis assay
Cell apoptosis was assayed by flow cytometry with
the Annexin V-FITC Apoptosis Detection kit (Sigma-Aldrich). Cells
were subjected to OGD and reoxygenation, and different
concentrations of Res were added to the medium. Specifically,
1×106 single cells per sample were collected following a
3-h OGD, were reoxygenated for 21 h and were washed twice with PBS
buffer; Annexin V/FITC was then added. After incubation for 10 min
at room temperature in the dark, the cells were washed and
resuspended; propidium iodide was then added to a final
concentration of 1 mg/l. Stained cells were analyzed using a
FACSCalibur instrument (Becton Dickinson, Mountain View, CA,
USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
The level of MMP-3 mRNA was semi-quantified
using RT-PCR. Total RNA was extracted using the TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA), and dissolved in
nuclease-free water, according to the manufacturer’s instructions.
Reverse transcription was performed with oligo(dT) primers and the
First-Strand cDNA Synthesis kit (Invitrogen Life Technologies). The
synthesized first-strand cDNA (1 μl) was next subjected to PCR
amplification using the following program: 94°C for 30 sec; 56°C
for 1 min; 72°C for 1 min. A total of 35 cycles were performed for
the amplification of MMP-3 and 30 cycles for the
housekeeping gene β-actin, which served as the control. The
last cycle was followed by 10 min of elongation at 72°C. Primer
pairs for the amplification of the mouse MMP-3 and
β-actin genes (231 and 242 bp, respectively) were the
following: MMP-3 forward, 5′-GTACCAACCTATTCCTGGTTGC-3′, and
reverse, 5′-CCAGAGAGTTAGATTTGGTGGG-3′; β-actin forward,
5′-AACCCTAAGGCCAACCGTGAAAAG-3′, and reverse,
5′-TCATGAGGTAGTCTGTCAGGT-3′. PCR products were visualized on 1.5%
agarose gels stained with ethidium bromide ausing a UV
Transilluminator 2000 (#170-7942; Bio-Rad). Semi-quantitative
analysis was conducted using a computerized densitometric imager
(Gel Doc™ XR+, Bio-Rad).
Western blot analysis
Protein concentrations were determined using the
Bradford assay (Bio-Rad). The same amount of total proteins (30
μg/lane) was loaded into each lane, electrophoresed and transferred
onto nitrocellulose membranes at 80 V for 1 h. After blocking for 4
h in 5% skim milk, the membrane was incubated overnight at 4°C with
primary antibodies (dilution, 1:1,000) targeting MMP-3, inducible
NO synthase (iNOS), NF-κB, β-actin, B-cell lymphoma 2 (Bcl-2),
Bcl-2-associated X protein (Bax) (all from Sigma-Aldrich), and
caspase-3 (monoclonal antibody; Cell Signaling Technology, Inc.,
Danvers, MA, USA). Next, the membrane was incubated with the
corresponding secondary antibody (goat anti-rat IgG; diluted with
PBS at 1:1,000; Sigma-Aldrich) at room temperature for 1 h.
Finally, the proteins were detected using the standard enhanced
chemiluminescence ECL method using a kit purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
Intracellular NO measurement
NO levels were estimated with the Griess assay. We
used the Griess Reagent system (Promega Corp., Madison, WI, USA)
and measured the total nitrate and nitrite concentrations at 550 nm
in a SmartSpec Plus Spectrophotometer (#170-2525; Bio-Rad).
Data analysis
Unless otherwise stated, all experiments were
performed with triplicate samples and repeated at least three
times. Results were presented as the means ± SD. Statistical
comparisons between groups were performed using one-way ANOVA
followed by Student’s t-tests. P<0.05 or <0.01 was considered
to indicate a statistically significant difference.
Results
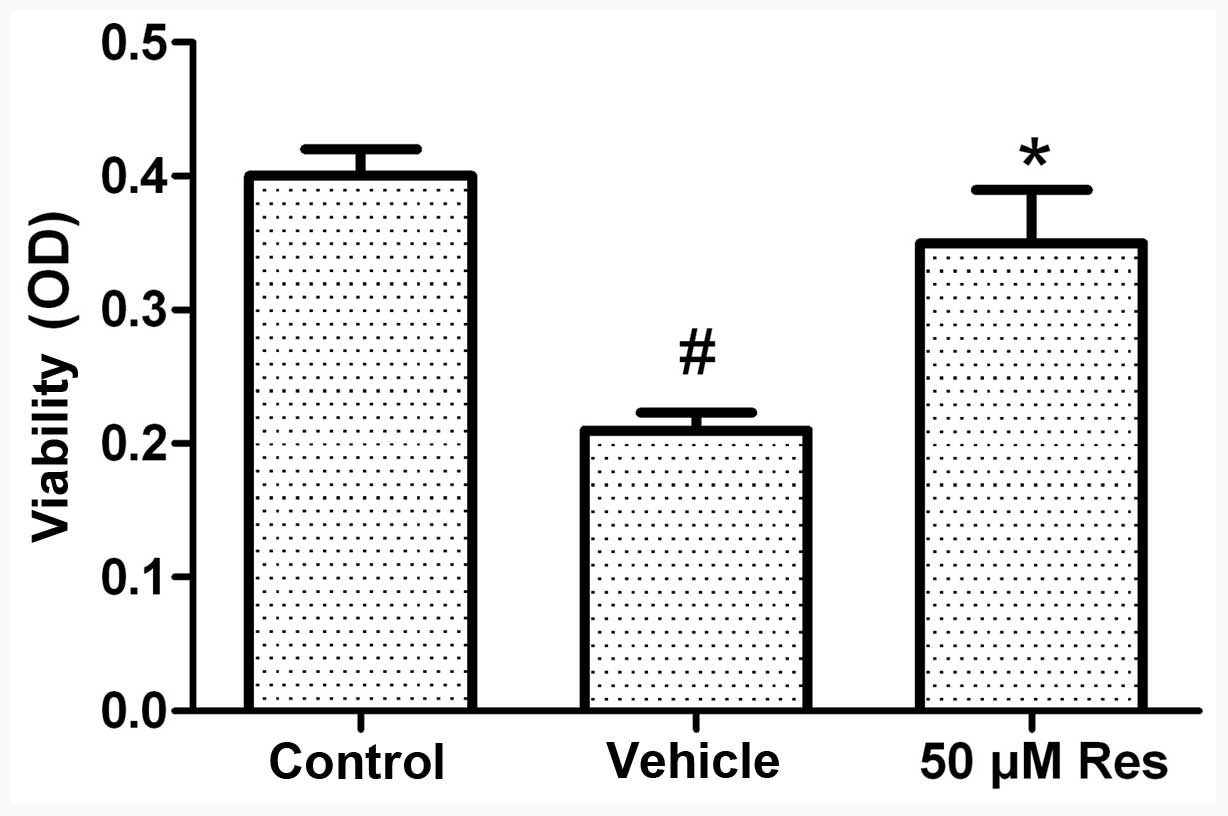
Cell viability assay
The MTT assay was used to analyze the viability of
cells. In this assay, the number of viable cells is directly
proportional to the level of the produced formazan product. Our
preliminary experiments demonstrated that treatment with 50 μM Res
exerted therapeutic effects. Exposure of the cells to OGD for 3 h
followed by 21 h of reoxygenation caused a reduction in cell
viability of ~45%. Under these conditions, pretreatment with Res
(50 μM) increased cell viability (as opposed to OGD-induced cell
death) by 75% (Fig. 1).
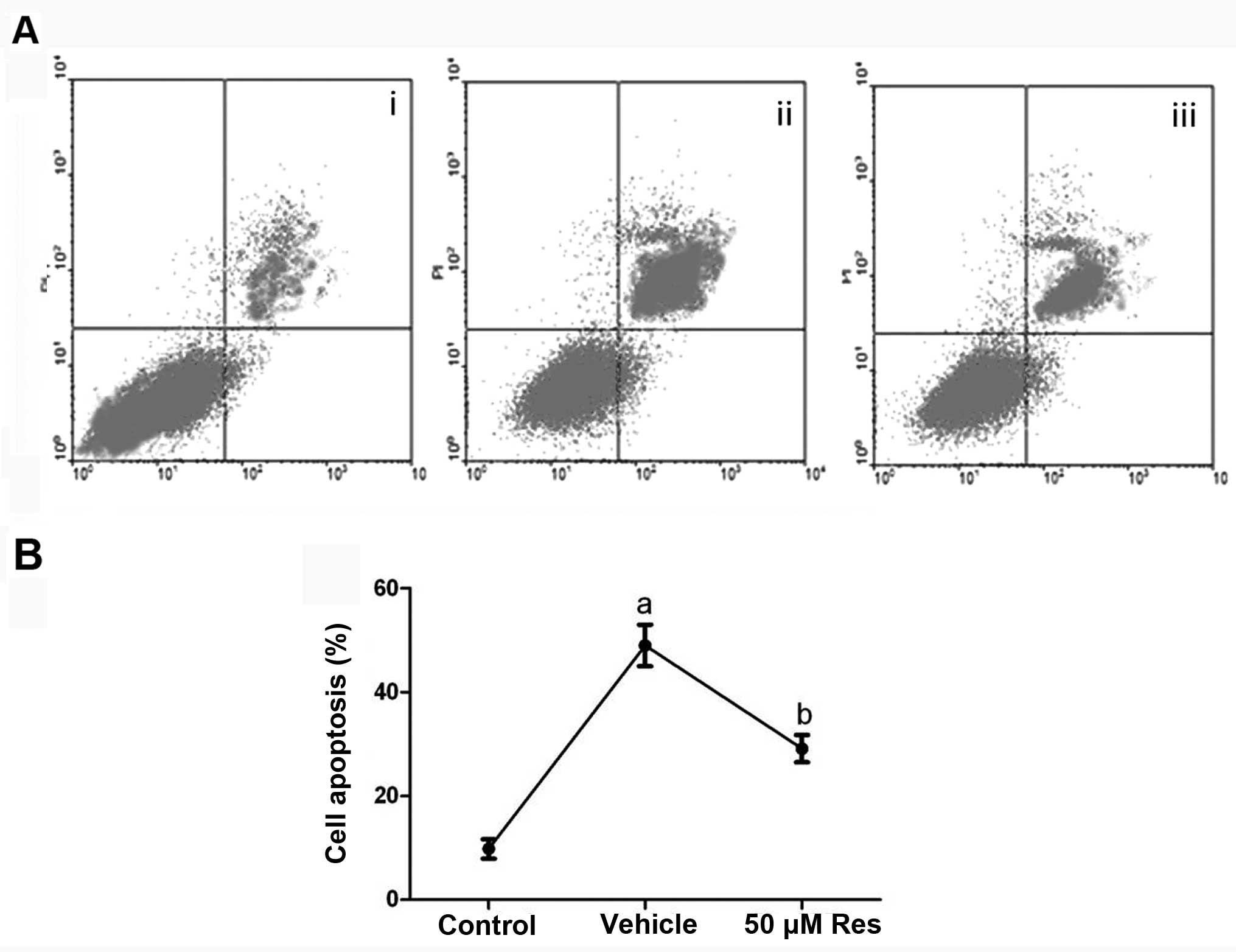
Res inhibits OGD-induced cell
apoptosis
Flow cytometry was used to quantify neuronal
apoptosis induced by OGD. As shown in Fig. 2, in the control (no OGD) cells,
there was a very low level (9.8%) of neuronal apoptosis, but the
percentage of apoptosis was significantly increased to 49%
(P<0.01) upon OGD, and was reversed to 29.1% when 50 μM of Res
were applied during OGD (P<0.05). The vehicle solution (DMSO)
had no effect on cell apoptosis induced by OGD (P>0.05).
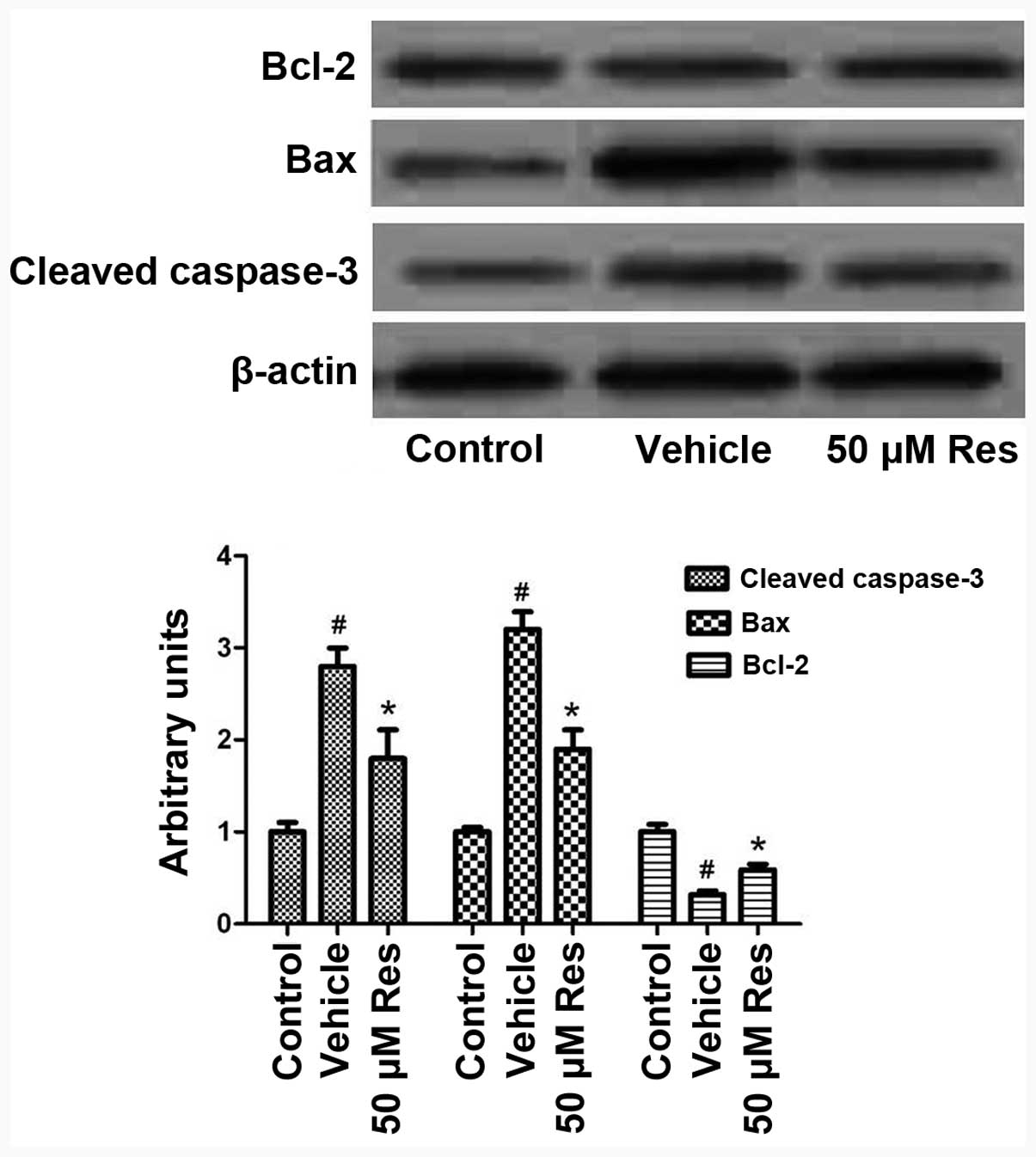
Res induces the expression of
anti-apoptotic and inhibits the expression of pro-apoptotic
proteins
To gain insights into the mechanisms by which Res
attenuates OGD-induced cell apoptosis, we studied the expression of
pro- and anti-apoptotic proteins following OGD and Res treatment.
As shown in Fig. 3, OGD induced
the cleavage of the pro-apoptotic protein caspase-3, and this
effect was reversed by treatment with 50 μM Res (P<0.05);
similarly, OGD-induced Bax expression was also reduced by Res. By
contrast, the expression of the anti-apoptotic protein Bcl-2 was
reduced by OGD, and this effect was reversed by Res treatment.
These results suggest that Res achieves its anti-apoptotic effects
through canonical apoptosis signaling pathways.
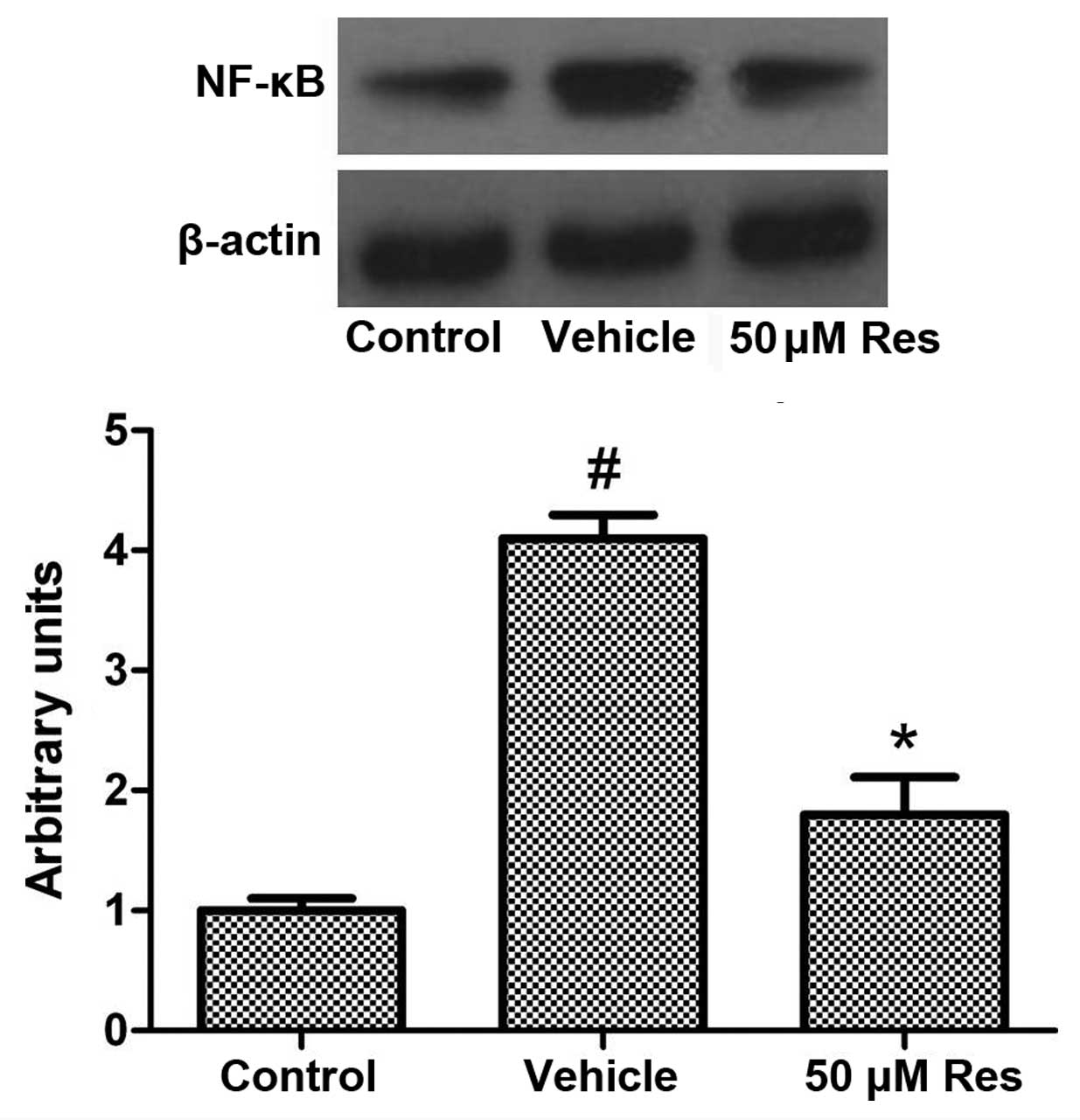
Res inhibits OGD-induced NF-κB
expression
The expression of MMP-3 is regulated by the
transcription factor NF-κB. NF-κB is an ubiquitous transcription
factor that resides in the cytoplasm but, when activated, is
translocated to the nucleus, where it induces gene transcription.
The activated NF-κB induces the expression of >400 genes, some
of which are intimately involved in regulation of apoptosis,
proliferation and inflammation. Hence, we examined whether Res can
modulate OGD-induced expression of NF-κB. As shown in Fig. 4, OGD induced the expression of
NF-κB, while Res significantly inhibited its expression.
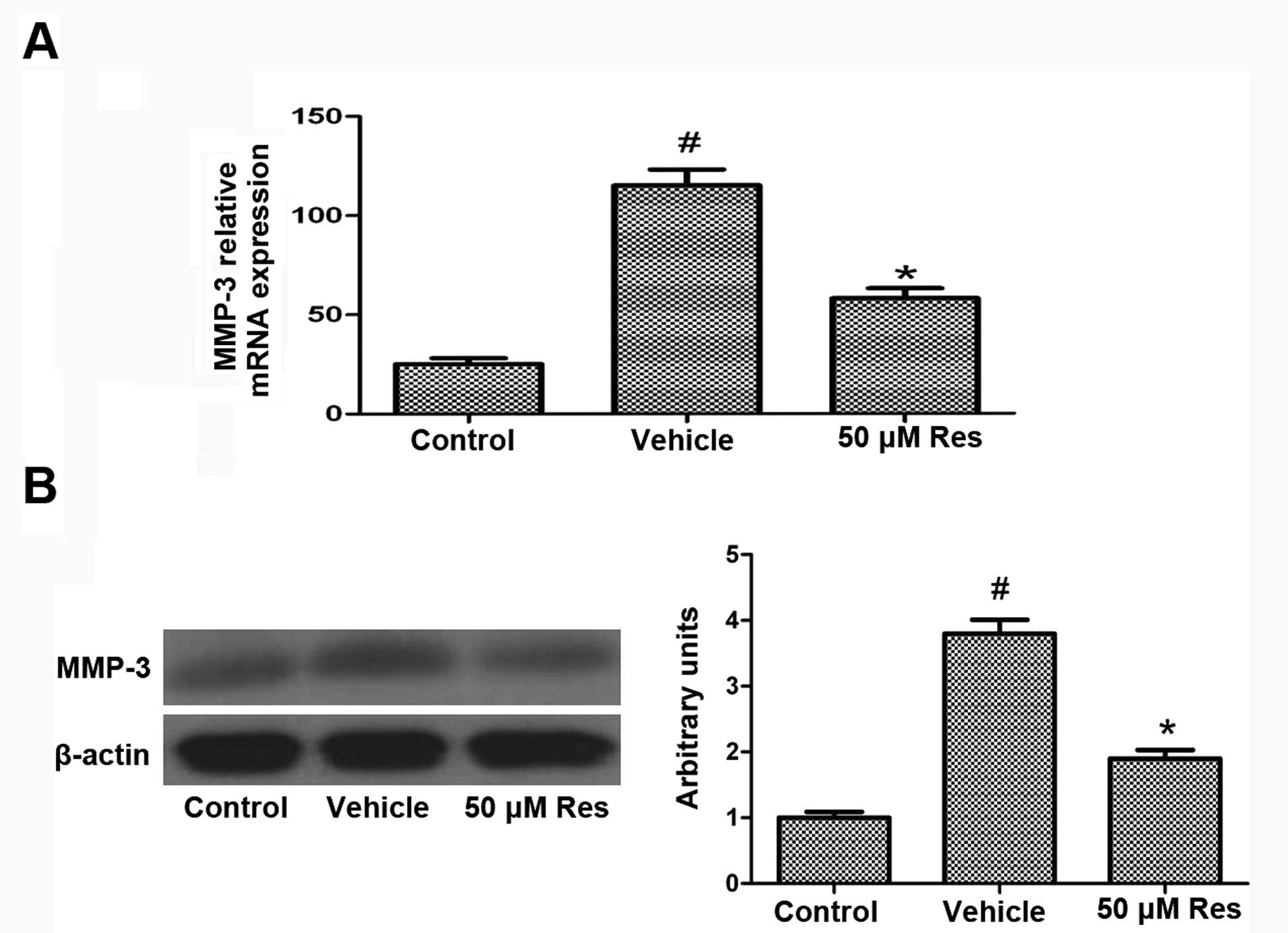
Effect of Res on the expression of
MMP-3
To further investigate the mechanisms of
Res-mediated neuroprotection, we studied its effect on MMP-3
expression. In both RT-PCR and western blot analyses, the exposure
of cells to OGD significantly induced the expression of MMP-3
compared to the control cells, while Res treatment inhibited the
OGD-induced expression of MMP-3 (Fig.
5). The level of the housekeeping protein β-actin remained
unaffected.
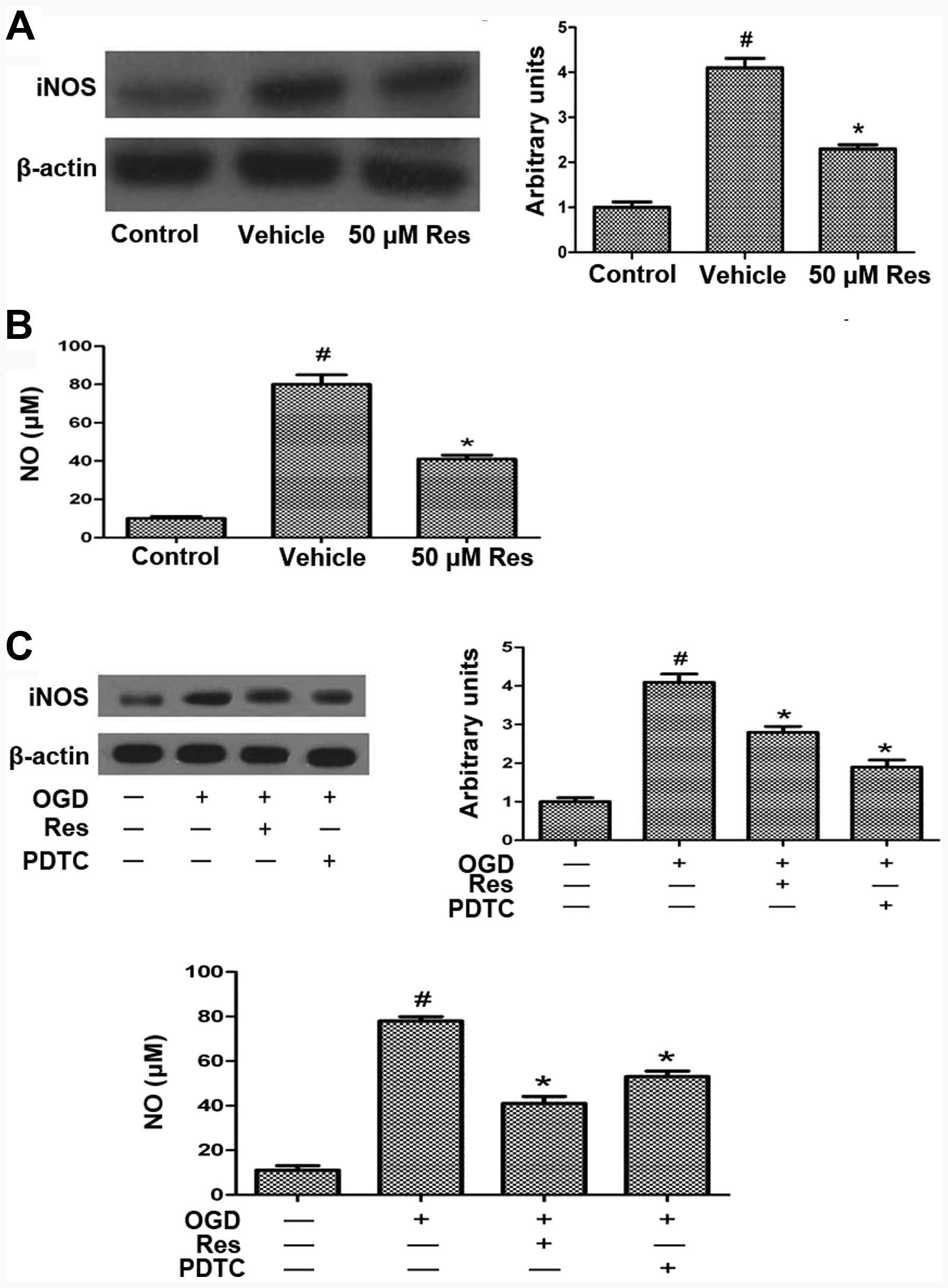
Res attenuates OGD-induced iNOS and NO
production via NF-κB
Excessive ROS production leads to activation of the
transcription factor NF-κB, which is associated with cell death
during cerebral ischemia (28).
OGD, excessive ROS production or glutamate toxicity induce iNOS
expression (29,30). We evaluated the expression of iNOS
by western blot analysis, which showed that OGD increases the iNOS
level compared to the control, and that this effect is reversed by
treatment with 50 μM Res (Fig.
6A). NO is a signaling molecule that regulates numerous
biological processes in the nervous system, including
neurotransmitter release, plasticity, and apoptosis, and can
modulate the biological activity of numerous proteins, including
MMPs. Cerebral ischemia and reperfusion injury result in
nitrosative stress and hence the production of NO (11). OGD increased the NO level compared
to the control, and Res treatment reversed this effect (Fig. 6B). To investigate whether Res
attenuates OGD-induced iNOS and NO production via affecting the
expression of NF-κB, cells were pretreated with the NF-κB inhibitor
PDTC 15 min prior to Res treatment. PDTC treatment reduced
OGD-induced iNOS and NO expression, similar to the effect of Res
(Fig. 6C). This result suggests
that Res inhibits iNOS and NO expression via inhibiting the NF-κB
expression.
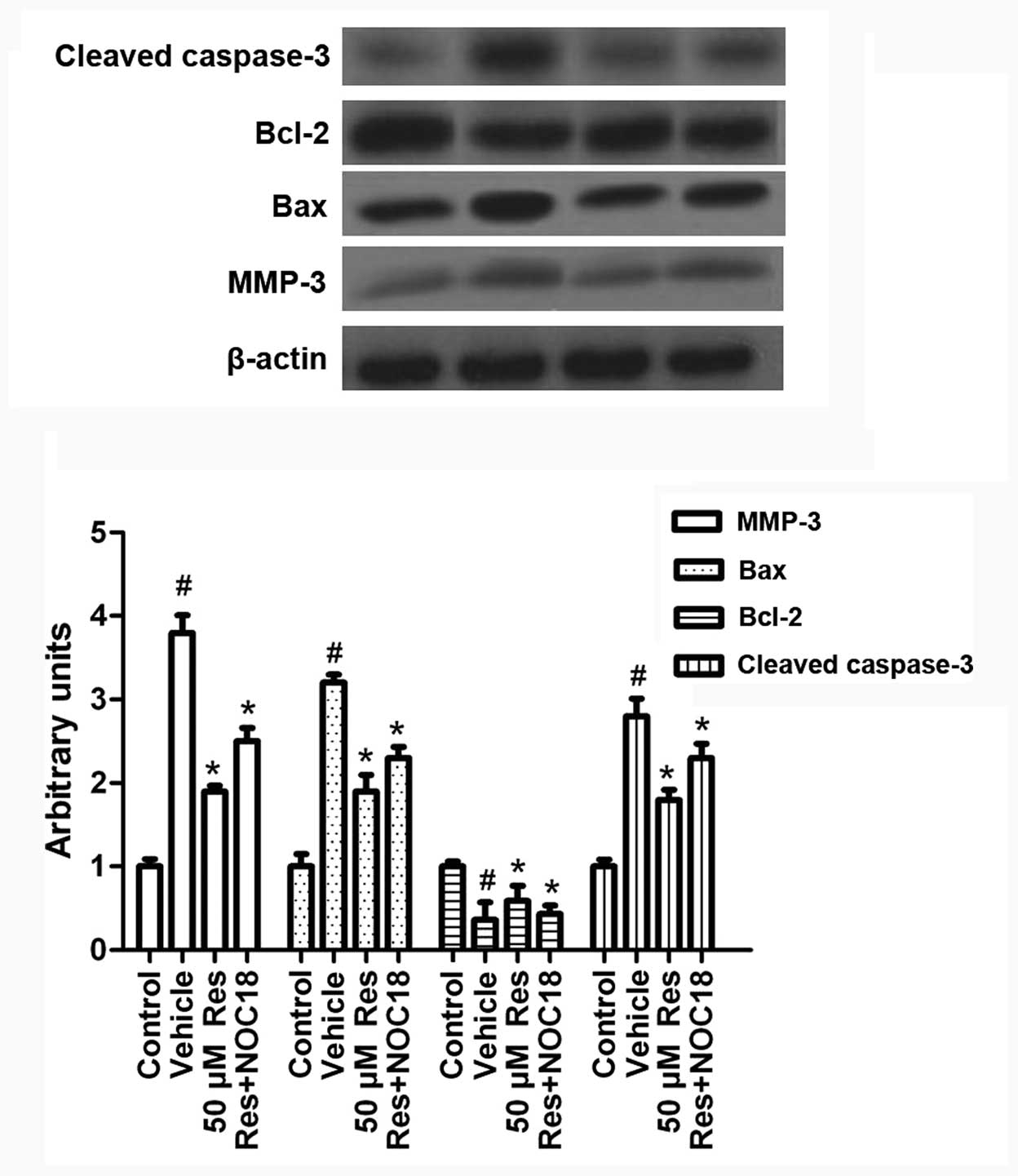
Res inhibits OGD-induced MMP-3 expression
and cell apoptosis via iNOS/NO
To investigate the mechanism by which Res regulates
OGD-induced cell apoptosis, we studied the exprerssion of
apoptosis-related molecules in the presence of Res and NOC-18.
Cells were treated with NOC-18 (500 μM) during Res treatment (50
μM). As shown in Fig. 7,
supplementing NO via NOC-18 addition partly reversed the effect of
Res on MMP-3, Bax, Bcl-2 and activated caspase-3 expression. This
suggests that Res inhibits MMP-3 expression and cell apoptosis via
iNOS/NO.
Discussion
This study shows for the first time that Res can
protect neurons from cell apoptosis induced by OGD via a mechanism
that involves NF-κB-iNOS/NO and the modulation of the expression of
MMP-3.
Stroke causes brain injury in millions of people
worldwide each year. Although it is well known that ischemia causes
cellular damage, the underlying mechanism is not fully understood,
and there is currently no approved therapy that can reduce
infarction size or neurological disability (31,32).
Cerebral ischemia-reperfusion injury results in cell
destruction, with MMPs being the major proteases involved in cell
damage in the ischemic tissue (14). MMPs are upregulated in permanent
and transient ischemia. These proteins attack the extracellular
matrix around the blood vessels, and facilitate cell death by
attacking the matrix enveloping the neurons. A number of studies
have provided evidence that the activation of MMPs leads to the
proteolytic breakdown of the BBB during cerebral ischemia and
reperfusion injury (33–35). A role for MMPs has also been
suggested in the pathogenesis of both acute and chronic
neurodegenerative disorders such as stroke (11). Immunohistochemical examinations of
ischemic tissues in the rat brain revealed the expression of MMP-3
in microglia and neurons (36,37).
A key approach in the development of therapy for stroke may be the
interference with apoptotic signaling. In this context, a potential
target is MMP-3, which participates in apoptotic signaling and the
expression of which is specifically increased under cell stress
conditions (17,18). It has been reported that an
antioxidant system that has a neuroprotective effect in ischemic
brain injury is involved in neuronal apoptosis of vulnerable
neurons in the cortex and the hippocampus during early reperfusion
(38,39).
In the present study, we showed that OGD insult
causes a marked increase in the expression of MMP-3 at both the
mRNA and the protein level, and in the percentage of apoptotic
cells. Res, a natural phytoalexin that has anticancer,
neuroprotective and anti-inflammatory effects, can reverse
OGD-induced MMP-3 expression and cell apoptosis.
The transcriptional factor NF-κB is one of the most
critical intracellular signaling molecules that regulates the
expression of genes encoding, among others, MMPs and iNOS (40). Res can inhibit the activation of
NF-κB and thus downregulate NF-κB-regulated pro-inflammatory
proteins such as MMP-9 and MMP-3 in osteoarthritis (19,41).
Res is a polyphenol with pleiotropic effects, which include the
reduction of oxidative stress and increased vascular NO production
(42). Whether Res can inhibit the
expression of NF-κB and regulate downstream genes such as MMP-3 and
iNOS has not been clearly shown to date. In this study, we found
that Res can inhibit the OGD-induced expression of NF-κB, iNOS and
MMP-3.
In conclusion, OGD induces apoptosis through
canonical apoptosis signaling and by regulating the expression of
MMP-3; Res can reverse OGD-induced MMP-3 expression and cell
apoptosis via the NF-κB-iNOS/NO pathway. The results reported here
further support the idea that Res, which is naturally found in red
wine and other products, exerts neuroprotective effects in
experimental models of cerebral ischemia. Thus, Res may be
considered a candidate agent for the treatment of stroke.
Acknowledgements
The current study was supported by a grant from the
National Natural Science Fund of China (no. 30901553).
References
|
1
|
Hallenbeck JM and Dutka AJ: Background
review and current concepts of reperfusion injury. Arch Neurol.
47:1245–1254. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Magnoni S, Baker A, George SJ, Duncan WC,
Kerr LE, McCulloch J and Horsburgh K: Differential alterations in
the expression and activity of matrix metalloproteinases 2 and 9
after transient cerebral ischemia in mice. Neurobiol Dis.
17:188–197. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oguro K, Jover T, Tanaka H, Lin Y, Kojima
T, Oguro N, Grooms SY, Bennett MV and Zukin RS: Global
ischemia-induced increases in the gap junctional proteins connexin
32 (Cx32) and Cx36 in hippocampus and enhanced vulnerability of
Cx32 knock-out mice. J Neurosci. 21:7534–7542. 2001.PubMed/NCBI
|
|
4
|
Amantea D, Nappi G, Bernardi G, Bagetta G
and Corasaniti MT: Post-ischemic brain damage: pathophysiology and
role of inflammatory mediators. FEBS J. 276:13–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choi JS, Kim SJ, Shin JA, Lee KE and Park
EM: Effects of estrogen on temporal expressions of IL-1beta and
IL-1ra in rat organotypic hippocampal slices exposed to
oxygen-glucose deprivation. Neurosci Lett. 438:233–237. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fekete A, Vizi ES, Kovács KJ, Lendvai B
and Zelles T: Layer-specific differences in reactive oxygen species
levels after oxygen-glucose deprivation in acute hippocampal
slices. Free Radic Biol Med. 44:1010–1022. 2008. View Article : Google Scholar
|
|
7
|
Kiewert C, Kumar V, Hildmann O, Hartmann
J, Hillert M and Klein J: Role of glycine receptors and glycine
release for the neuroprotective activity of bilobalide. Brain Res.
1201:143–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosenberg GA: Matrix metalloproteinases in
neuroinflammation. Glia. 39:279–291. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gurney KJ, Estrada EY and Rosenberg GA:
Blood-brain barrier disruption by stromelysin-1 facilitates
neutrophil infiltration in neuroinflammation. Neurobiol Dis.
23:87–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garcia AJ, Tom C, Guemes M, Polanco G,
Mayorga ME, Wend K, Miranda-Carboni GA and Krum SA: ERα signaling
regulates MMP3 expression to induce FasL cleavage and osteoclast
apoptosis. J Bone Miner Res. 28:283–290. 2013.
|
|
11
|
Gu Z, Kaul M, Yan B, Kridel SJ, Cui J,
Strongin A, Smith JW, Liddington RC and Lipton SA: S-nitrosylation
of matrix metalloproteinases: signaling pathway to neuronal cell
death. Science. 297:1186–1190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rosenberg GA, Cunningham LA, Wallace J,
Alexander S, Estrada EY, Grossetete M, Razhagi A, Miller K and
Gearing A: Immunohistochemistry of matrix metalloproteinases in
reperfusion injury to rat brain: activation of MMP-9 linked to
stromelysin-1 and microglia in cell cultures. Brain Res.
893:104–112. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wetzel M, Rosenberg GA and Cunningham LA:
Tissue inhibitor of metalloproteinases-3 and matrix
metalloproteinase-3 regulate neuronal sensitivity to
doxorubicin-induced apoptosis. Eur J Neurosci. 18:1050–1060. 2003.
View Article : Google Scholar
|
|
14
|
Cheng Z, He W, Zhou X, Lv Q, Xu X, Yang S,
Zhao C and Guo L: Cordycepin protects against cerebral
ischemia/reperfusion injury in vivo and in vitro. Eur J Pharmacol.
664:20–28. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walker EJ and Rosenberg GA: TIMP-3 and
MMP-3 contribute to delayed inflammation and hippocampal neuronal
death following global ischemia. Exp Neurol. 216:122–131. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim YS, Kim SS, Cho JJ, Choi DH, Hwang O,
Shin DH, Chun HS, Beal MF and Joh TH: Matrix metalloproteinase-3: a
novel signaling proteinase from apoptotic neuronal cells that
activates microglia. J Neurosci. 25:3701–3711. 2005. View Article : Google Scholar
|
|
17
|
Choi DH, Kim EM, Son HJ, Joh TH, Kim YS,
Kim D, Flint Beal M and Hwang O: A novel intracellular role of
matrix metalloproteinase-3 during apoptosis of dopaminergic cells.
J Neurochem. 106:405–415. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim EM, Shin EJ, Choi JH, Son HJ, Park IS,
Joh TH and Hwang O: Matrix metalloproteinase-3 is increased and
participates in neuronal apoptotic signaling downstream of
caspase-12 during endoplasmic reticulum stress. J Biol Chem.
285:16444–16452. 2010. View Article : Google Scholar
|
|
19
|
Csaki C, Mobasheri A and Shakibaei M:
Synergistic chondroprotective effects of curcumin and resveratrol
in human articular chondrocytes: inhibition of IL-1β-induced
NF-κB-mediated inflammation and apoptosis. Arthritis Res Ther.
11:R1652009.PubMed/NCBI
|
|
20
|
Celotti E, Ferrarini R, Zironi R and Conte
LS: Resveratrol content of some wines obtained from dried
Valpolicella grapes: Recioto and Amarone. J Chromatogr A.
730:47–52. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pany S, Majhi A and Das J: PKC activation
by resveratrol derivatives with unsaturated aliphatic chain. PLoS
One. 7:e528882012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Das J, Pany S and Majhi A: Chemical
modifications of resveratrol for improved protein kinase C alpha
activity. Bioorg Med Chem. 19:5321–5333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carrizzo A, Puca A, Damato A, Marino M,
Franco E, Pompeo F, Traficante A, Civitillo F, Santini L, Trimarco
V and Vecchione C: Resveratrol improves vascular function in
patients with hypertension and dyslipidemia by modulating NO
metabolism. Hypertension. 62:359–366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kesherwani V, Atif F, Yousuf S and Agrawal
SK: Resveratrol protects spinal cord dorsal column from hypoxic
injury by activating Nrf-2. Neuroscience. 241:80–88. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Q, Xu J, Rottinghaus GE, Simonyi A,
Lubahn D, Sun GY and Sun AY: Resveratrol protects against global
cerebral ischemic injury in gerbils. Brain Res. 958:439–447. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao D, Zhang X, Jiang X, Peng Y, Huang W,
Cheng G and Song L: Resveratrol reduces the elevated level of MMP-9
induced by cerebral ischemia-reperfusion in mice. Life Sci.
78:2564–2570. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tauskela JS, Comas T, Hewitt K, Monette R,
Paris J, Hogan M and Morley P: Cross-tolerance to otherwise lethal
N-methyl-D-aspartate and oxygen-glucose deprivation in
preconditioned cortical cultures. Neuroscience. 107:571–584. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dal-Cim T, Ludka FK, Martins WC, Reginato
C, Parada E, Egea J, López MG and Tasca CI: Guanosine controls
inflammatory pathways to afford neuroprotection of hippocampal
slices under oxygen and glucose deprivation conditions. J
Neurochem. 126:437–450. 2013. View Article : Google Scholar
|
|
29
|
Martín-de-Saavedra MD, del Barrio L, Cañas
N, Egea J, Lorrio S, Montell E, Vergés J, García AG and López MG:
Chondroitin sulfate reduces cell death of rat hippocampal slices
subjected to oxygen and glucose deprivation by inhibiting p38, NFκB
and iNOS. Neurochem Int. 58:676–683. 2011.PubMed/NCBI
|
|
30
|
Molz S, Dal-Cim T, Budni J,
Martín-de-Saavedra MD, Egea J, Romero A, del Barrio L, Rodrigues
AL, López MG and Tasca CI: Neuroprotective effect of guanosine
against glutamate-induced cell death in rat hippocampal slices is
mediated by the phosphatidylinositol-3 kinase/Akt/glycogen synthase
kinase 3β pathway activation and inducible nitric oxide synthase
inhibition. J Neurosci Res. 89:1400–1408. 2011.PubMed/NCBI
|
|
31
|
Schaller B and Graf R: Cerebral ischemia
and reperfusion: the pathophysiologic concept as a basis for
clinical therapy. J Cereb Blood Flow Metab. 24:351–371. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moskowitz MA, Lo EH and Iadecola C: The
science of stroke: mechanisms in search of treatments. Neuron.
67:181–198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aoki T, Sumii T, Mori T, Wang X and Lo EH:
Blood-brain barrier disruption and matrix metalloproteinase-9
expression during reperfusion injury: mechanical versus embolic
focal ischemia in spontaneously hypertensive rats. Stroke.
33:2711–2717. 2002. View Article : Google Scholar
|
|
34
|
Maier CM, Hsieh L, Yu F, Bracci P and Chan
PH: Matrix metalloproteinase-9 and myeloperoxidase expression:
quantitative analysis by antigen immunohistochemistry in a model of
transient focal cerebral ischemia. Stroke. 35:1169–1174. 2004.
View Article : Google Scholar
|
|
35
|
Pfefferkorn T and Rosenberg GA: Closure of
the blood-brain barrier by matrix metalloproteinase inhibition
reduces rtPA-mediated mortality in cerebral ischemia with delayed
reperfusion. Stroke. 34:2025–2030. 2003. View Article : Google Scholar
|
|
36
|
Cunningham LA, Wetzel M and Rosenberg GA:
Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia.
50:329–339. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dzwonek J, Rylski M and Kaczmarek L:
Matrix metalloproteinases and their endogenous inhibitors in
neuronal physiology of the adult brain. FEBS Lett. 567:129–135.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fujimura M, Tominaga T and Chan PH:
Neuroprotective effect of an antioxidant in ischemic brain injury:
involvement of neuronal apoptosis. Neurocrit Care. 2:59–66. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Krause GS, White BC, Aust SD, Nayini NR
and Kumar K: Brain cell death following ischemia and reperfusion: a
proposed biochemical sequence. Crit Care Med. 16:714–726. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Marcu KB, Otero M, Olivotto E, Borzi RM
and Goldring MB: NF-kappaB signaling: multiple angles to target OA.
Curr Drug Targets. 11:599–613. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shakibaei M, Csaki C, Nebrich S and
Mobasheri A: Resveratrol suppresses interleukin-1β-induced
inflammatory signaling and apoptosis in human articular
chondrocytes: potential for use as a novel nutraceutical for the
treatment of osteoarthritis. Biochem Pharmacol. 76:1426–1439.
2008.
|
|
42
|
Dolinsky VW, Chakrabarti S, Pereira TJ, et
al: Resveratrol prevents hypertension and cardiac hypertrophy in
hypertensive rats and mice. Biochim Biophys Acta. 1832:1723–1733.
2013. View Article : Google Scholar : PubMed/NCBI
|