Introduction
Epstein-Barr virus (EBV) is a double-stranded DNA
virus of the herpes family, that targets lymphocytes and is closely
associated with multiple malignancies, including lymphoma,
nasopharyngeal cancer and gastric cancer (1). Epithelial cells, lymphocytes and
muscle cells are particularly vulnerable to EBV (2). Similar to other herpes viruses, EBV
has the capacity to induce lytic and latent infection. Latent EBV
infection results in the expression of only minimal numbers of
viral proteins, but it is able to bypass host immune surveillance
and thus carries a tumorigenic risk. Despite this, cytologists
often encounter EBV-associated malignancies in cytological
material, but in contrast to other herpes viruses, EBV does not
evoke viral cytopathic effects, such as cell merging and necrosis
(2). In vitro infection
with EBV induces activation and proliferation of human B
lymphocytes (3). EBV-specific
cytotoxic CD8+ T cells are responsible for the clearance
of EBV-infected lymphocytes by recognizing the virus-coded proteins
and therefore the majority of EBV carriers are asymptomatic
throughout life (3). Expression of
LMP-1 protein is associated with the proliferation of B
lymphocytes. By contrast, LMP-1 protein is absent in latent
infection with EBV type I and IIb, and infected cells have no
inherent proliferation capacity (4). Therefore, LMP-1 expression is used to
determine the proliferative ability of B lymphocytes.
Latent membrane proteins (LMPs) have three subtypes,
LMP-1, LMP-2A and LMP-2B. Expression of the three genes during
EBV-induced transformation of human B lymphocytes has not been
investigated. In the present study, the expression of LMP-1,
LMP-2A and LMP-2B genes in EBV-induced lymphoblasts
and paired normal lymphocytes was compared to elucidate its
significance during lymphocyte transformation.
Materials and methods
Blood samples
Peripheral blood was collected from seven healthy
volunteers. The study was approved by the Ethics Committee of
University of South China, Hengyang, China. Written informed
consent was obtained from the patients. EBV infection status was
detected with an ELISA kit (ADL Embedded Solutions Inc., San Diego,
CA, USA) using the anti-EBV-VCA IgG antibody (ADL Embedded
Solutions Inc.). DNA was extracted from the whole blood samples and
the LMP-1 gene sequence (82 bp, GI: 896226) was expanded
using polymerase chain reaction (PCR). The upstream primer of the
LMP-1 gene was: 5′-CTG CTC ATC GCT CTC TGG AA-3′ and the
downstream primer was: 5′-AGA CAA GTA AGC ACC CGA AGA TG-3′. The
PCR included 30 cycles of 94°C for 4 min, 94°C for 30 sec, 52°C for
30 sec and 72°C for 30 sec, and a final extension at 72°C for 5
min. The PCR products were separated on 2% agarose gels. The
results demonstrated EBV latency in the seven blood samples.
Isolation of lymphocytes
Peripheral blood (50 ml) was collected from seven
healthy volunteers. Normal PBLs were separated from fresh
peripheral blood samples using human lymphocyte separation medium
(catalogue no. LTS1077; Tian Jin Hao Yang Biological Manufacture
Co., Ltd., Tianjin, China) and washed twice with RPMI-1640.
Isolation of EBV
The B95-8 marmoset cell line was kindly provided by
the Cancer Research Institute, Central South University, Changsha,
China and used as a source of EBV. The culture medium of B95-8
cells was replenished for the final time. The cell density was
adjusted to 106–107/ml. The cells were
starved for 10 days at 37°C in 5% CO2 and were then
centrifuged at 3,700 × g at 4°C for 30 min. The supernatant was
passed through a 0.45-μm filter and stored at −80°C.
Preparation of lymphoblasts
A total of 2×106–3×106
lymphocytes were suspended in 2 ml RPMI-1640 culture medium
supplemented with 25% fetal bovine serum (Gibco, Sydney, Australia)
and 2 μg/ml cyclosporine A (Sandoz, Basel, Switzerland). The cells
were transferred into a 24-well plate and incubated at 37°C with 5%
CO2 for one week. Subsequently, the lymphocytes were
induced into lymphoblasts that were enlarged and exhibited cell
clustering. The culture medium was replenished every 3–4 days and
~4 weeks later the cells were transferred into 25-ml flasks for
further culture.
Quantatitive (q)PCR
Total RNA was extracted from lymphoblasts, untreated
lymphocytes and B95-8 cells using TRIzol reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA). A total of 2×106 cells
were used for RNA extraction. The purity and integrity of the
extracted RNA were examined using electrophoresis. RNA was reverse
transcribed into cDNA (Promega Corporation, Madison, WI, USA)
according to the manufacturer’s instructions. The primers used in
the qPCR are listed in Table I.
The B95-8 cells that expressed the EBV genome were used as a
positive control to establish the standard curve for qPCR. Each
test was repeated twice.
 | Table IGene-specific PCR primer sequences
(5′-3′). |
Table I
Gene-specific PCR primer sequences
(5′-3′).
| Gene name | Forward primer | Reverse primer | Size (bp) |
|---|
| LMP-1 |
CTGCTCATCGCTCTCTGGAA |
AGACAAGTAAGCACCCGAAGATG | 82 |
| LMP-2A |
CGTCACTCGGACTATCAACCAC |
CTTCCTCTGCCCGCTTCTT | 149 |
| LMP-2B |
CGCCGTTTGACTGTTTGTG |
AGCAGCAGCGTCATGGAA | 125 |
| GAPDH |
GCACCGTCAAGGCTGAGAAC |
TGGTGAAGACGCCAGTGGA | 138 |
Western blotting
Total protein was extracted from the cells using SDS
lysis buffer (Beyotime, Shanghai, China) and quantified using an
Enhanced Bicinchoninic Acid Protein Assay kit (Beyotime). The
protein was denatured at 95°C for 10 min and then 50 μg was
separated in 8–12% SDS-PAGE and transferred onto a nitrocellulose
membrane (Beyotime). The membrane was blocked with 5% skimmed milk
in Tris buffer (25 mM Tris-HCl, 150 mM NaCl and 0.05% Tween-20, pH
7.5). Mouse anti-LMP-1 monoclonal antibody (1:200; DakoCytomation,
Glostrup, Denmark) and mouse anti-β-actin monoclonal antibody
(1:1000; ComWin, Beijing, China) were added and the membranes were
incubated at 4°C overnight. Goat anti-mouse IgG (1:1000; ComWin)
was used as a secondary antibody, incubated at room temperature for
2 h. Each test was performed in triplicate.
Statistical analysis
All the data are expressed as the mean ± standard
deviation. Data were analyzed using one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
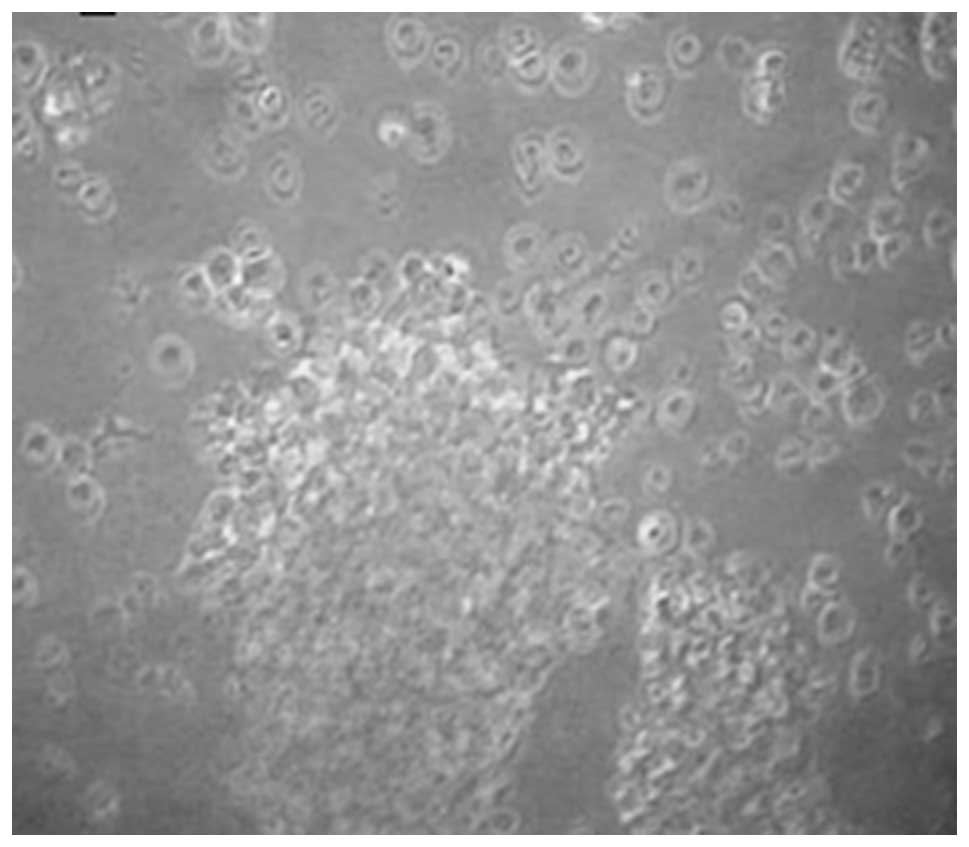
Lymphoblast morphology
EBV-transformed lymphoblasts (LCLs) were round or
oval and enlarged in size compared with the normal lymphocytes
(Fig. 1).
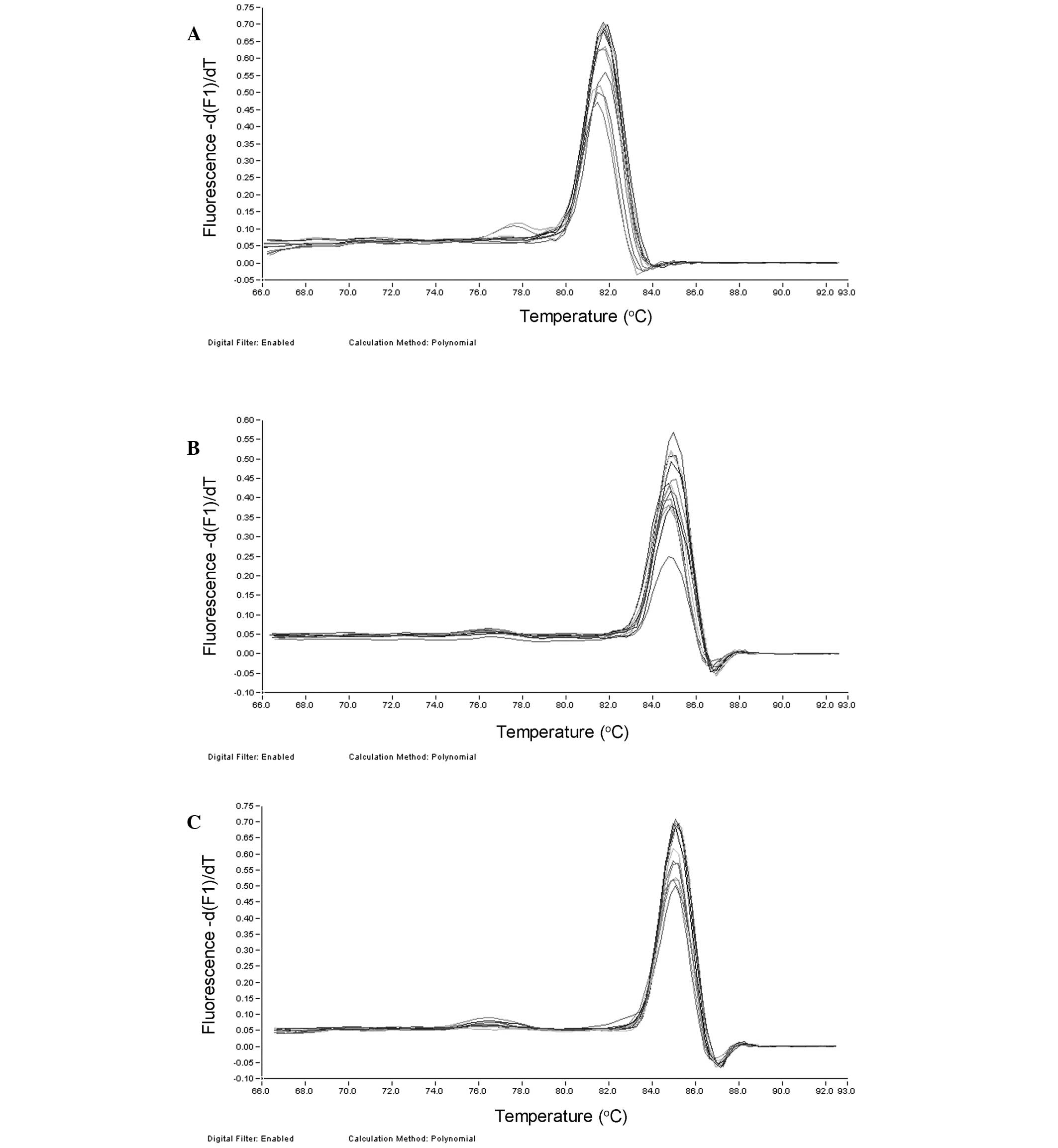
Expression of LMP genes
Melting curve analysis demonstrated only one
specific peak for LMP-1, LMP-2A and LMP-2B, indicating high
specificity of the qPCR (Fig.
2).
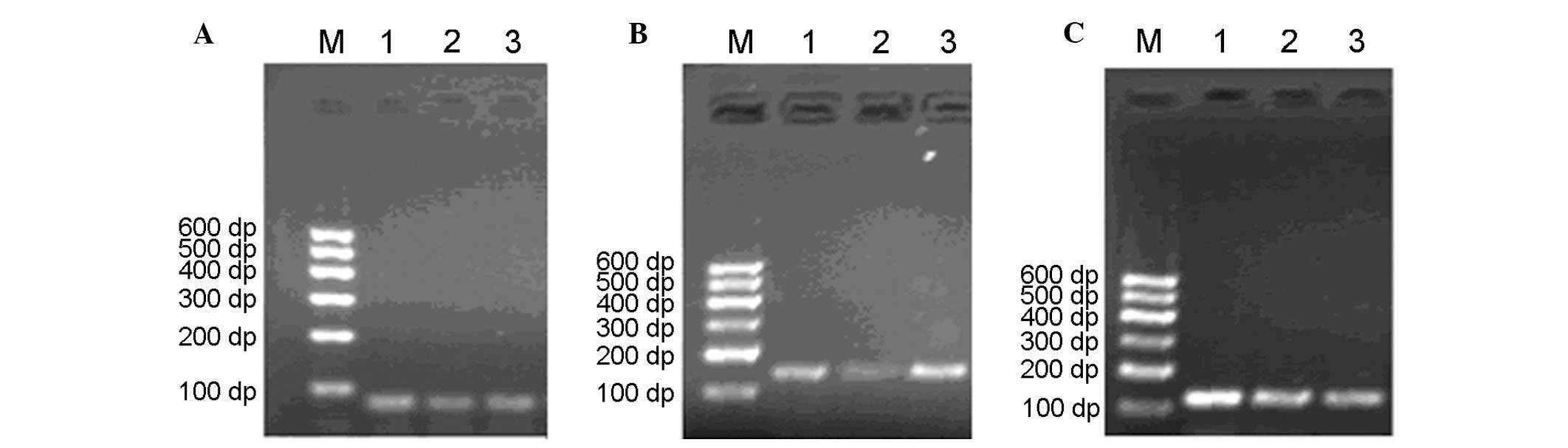
PCR products of LMP genes were separated on 2%
agarose gels, which revealed well-separated bands of the predicted
sizes (Fig. 3).
Expression levels of LMP-1, LMP-2A and LMP-2B in
EBV-transformed lymphoblasts were 863-fold (P<0.01), 1,763-fold
(P<0.05) and 90,078-fold (P<0.05) of that in untreated
lymphocytes, respectively (Table
II).
 | Table IIExpression levels of LMP genes as
determined by qPCR |
Table II
Expression levels of LMP genes as
determined by qPCR
| Cell type | LMP1 ± SD | LMP-2A ± SD | LMP-2B ± SD |
|---|
| Normal
lymphocytes |
2.414×10−4±1.080×10−4 |
2.97×10−4±1.64×10−4 |
6.40×10−5±3.04×10−5 |
| Induced
lymphoblasts |
2.082×10−1±6.120×10−2 |
5.235×10−1±2.37×10−1 | 5.765±2.914 |
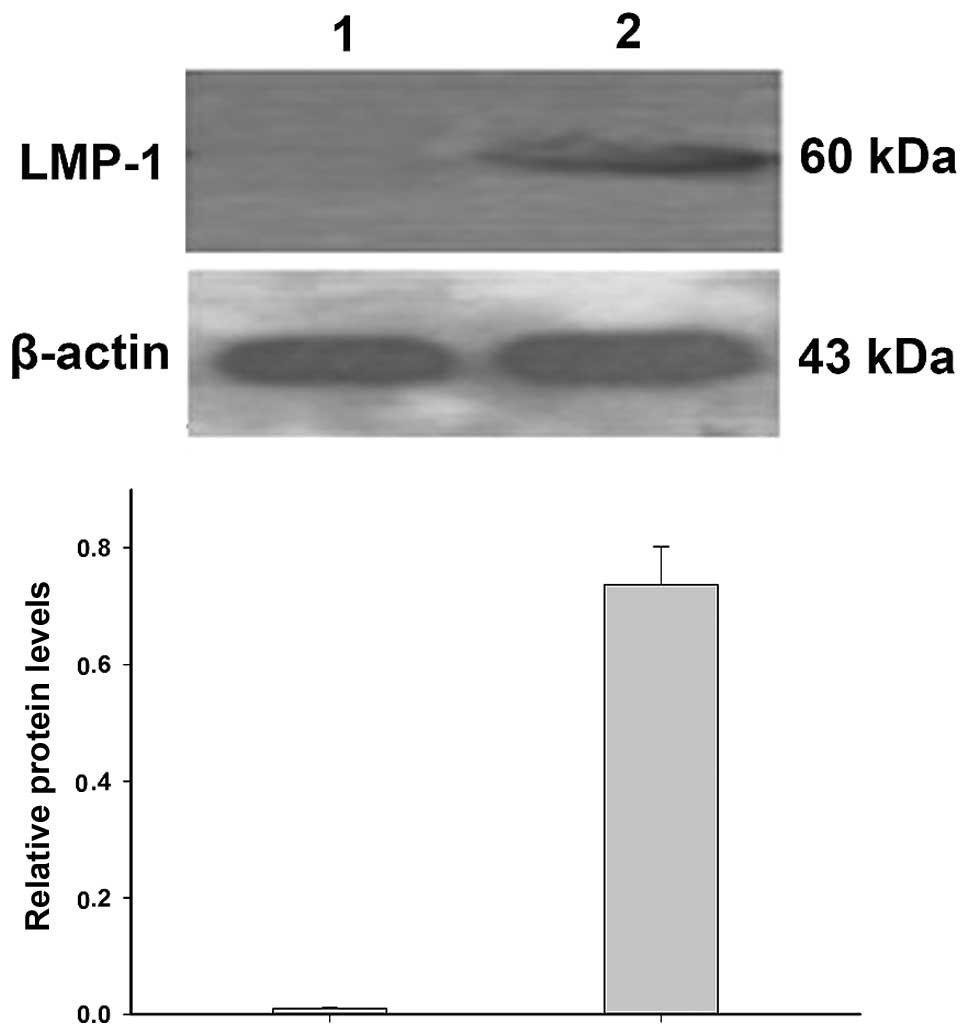
Detection of LMP-1 protein levels
Western blotting demonstrated that LMP-1 protein
levels were significantly increased in EBV-transformed lymphoblasts
compared with that in the normal lymphocytes (P<0.05; Fig. 4), which is consistent with the
results of qPCR.
Discussion
EBV is considered to be associated with several
malignancies, including Hodgkin’s lymphoma, NK-T cell lymphoma,
Burkitt’s lymphoma and nasopharyngeal cancer (5). Latent EBV infection induces the
expression of three LMP proteins, LMP-1, LMP-2A and LMP-2B
(6). LMP-1 is the major factor
that induces transformation and tumorigenesis of B cells (7) and mimics the constitutionally
activated receptor of tumor necrosis factor (TNF), CD40. LMP-1, via
this TNF receptor, regulates cell proliferation and death (8) and is thus important in cell growth,
differentiation and apoptosis. LMP-2A and LMP-2B are two isoforms
of the LMP-2 protein expressed in B cells with latent EBV infection
(9). LMP-2 proteins promote the
development and progression of tumors (10). LMP-2A in particular, has been
demonstrated to protect B cells from various proapoptotic
mechanisms (11).
EBV latency in healthy carriers is usually
asymptomatic. In the present study, it was identified that
expression of LMP mRNA in normal lymphocytes was low at
10−4–10−5, which was significantly
upregulated in the EBV-induced lymphoblasts at 2×10−2.
The expression levels of LMP mRNA were determined using qPCR.
The expression of the LMP-1 gene was
significantly increased in the EBV-transformed lymphoblasts at the
mRNA (863-fold; P<0.01) and protein levels compared with that in
normal lymphocytes. Rasul et al (3) cultured mononuclear cells with the
supernatant of B95-8 cells for 1.5 h to infect B cells with EBV. By
using immunostaining and western blotting following seven days in
culture, it was identified that the B cells expressing LMP-1
protein were mostly positive for Ki-67, while those not expressing
LMP-1 protein demonstrated a weak Ki-67 expression and were
non-proliferative. Only EBV nuclear antigen (EBNA)-1 was expressed
in cells with type I EBV latency, and these cells were
nonproliferative and revealed a resting B cell phenotype, which was
also observed in the control memory B cells in healthy individuals.
B cells with type IIb EBV latency expressed all the EBNAs but not
LMP-1, and these cells were also non-proliferative (4). Therefore, it was considered that the
expression of LMP-1 is critical in the proliferation and
transformation of B cells (12).
EBV-induced immortal lymphoblasts promoted lymphoma genesis
(13). Increased expression of
LMP-1 mRNA and protein promoted the proliferation of NK/T lymphoma
cells (14). LMP-1 expression is
also a typical feature of R/S cells in Hodgkin’s lymphoma and
improves the survival and proliferation of R/S cells by altering
the cellular phenotype and interacting with the surrounding
microenvironment (3).
The mechanisms underlying the proliferation and
transformation of B cells promoted by LMP-1 may include the
activation of cell signaling pathways and the increase in cell
cycle activators. It has been demonstrated that LMP-1 activates
β-catenin via the phosphatidylinositol 3-kinase/AKT pathway, thus
promoting the proliferation of EBV-infected B cells (7). LMP-1 regulates the expression of
death-associated protein kinase 1 and activates nuclear factor
(NF)-κB signaling in LCL cells, thus upregulating the MHC class I
antigen processing pathway (8).
LMP-1 also induces CD8+ T cell reaction and bypasses
immune surveillance (15). The
expression of LMP1-induced protein (LMPIP) is increased in
EBV-infected peripheral lymphocytes and LMP-1-transfected 293
cells. Nasopharyngeal carcinoma cells overexpressing LMPIP
demonstrated a decrease in G1 phase cells and an increase in sub-G1
phase cells, accompanied by an increase in cell cycle activators
cyclin D1 and cyclin-dependent kinase 4 (16). It has also been revealed that EBV
promotes epithelial tumorigenesis by downregulating microRNA-203
via LMP-1 (17).
In the present study, it was identified that
expression of LMP-2A in EBV-transformed lymphoblasts was 1,763-fold
(P<0.05) of that in untreated lymphocytes, suggesting that
LMP-2A is important in B cell transformation. LMP-2A maintains the
persistence of EBV infection by inhibiting the activation of B
cells. LMP-2A mRNA is consistently expressed in primary and
metastasized nasopharyngeal cancer, suggesting that LMP-2A has an
initiating role in EBV-associated malignancy (5). LMP-2A regulates the expression of
tumor necrosis receptor-associated factor 2 and thus modulates
LMP-1-induced activation of the NF-κB pathway, finally preventing
the apoptosis of lymphoma cells (18,19).
LMP-2A induces expression of ΔNp63α and regulates the
proliferation, transformation and differentiation of epithelial
cells, which may promote the growth of malignant tumors (20). It has also been identified that
LMP-2A promotes malignant transformation by enhancing the cell
cycle, inhibiting apoptosis and regulating LMP-1 expression
(11). These results suggest that
LMP-2A is important in the processes of transformation and
tumorigenesis.
It was also identified that the expression of LMP-2B
in EBV-transformed lymphoblasts was 90,078-fold (P<0.05) greater
than that in the untreated lymphocytes. LMP-2B modulates the
activity of LMP-2A during the transformation of B cells and
maintains persistent EBV latency together with LMP-2A (21). LMP-2B inhibits LMP-2A and prevents
the potential lytic viral replication of EBV. In addition,
upregulated expression of LMP-2B promotes the progression from EBV
latency to replicative infection (9).
In conclusion, LMP-2A and LMP-1 promote the
proliferation, survival and transformation of B cells. LMP-1 and
LMP-2 are frequently expressed in EBV-associated lymphoma and
epithelial carcinoma, and therefore may promote tumor
progression.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81272182 and 81372134) and
the Construct Program of the Key Discipline in Hunan Province
(grant no. 2011-76).
References
|
1
|
Lan K, Verma SC, Murakami M, Bajaj B and
Robertson ES: Epstein-Barr Virus (EBV): infection, propagation,
quantitation, and storage. Curr Protoc Microbiol. Chapter 14(Unit
14E): 122007.PubMed/NCBI
|
|
2
|
Michelow P, Wright C and Pantanowitz L: A
review of the cytomorphology of Epstein-Barr virus-associated
malignancies. Acta Cytol. 56:1–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rasul AE, Nagy N, Sohlberg E, Ádori M,
Claesson HE, Klein G and Klein E: Simultaneous detection of the two
main proliferation driving EBV encoded proteins, EBNA-2 and LMP-1
in single B cells. J Immunol Methods. 385:60–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klein E, Kis LL and Klein G: Epstein-Barr
virus infection in humans: from harmless to life endangering
virus-lymphocyte interactions. Oncogene. 26:1297–1305. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pang MF, Lin KW and Peh SC: The signaling
pathways of Epstein-Barr virus-encoded latent membrane protein 2A
(LMP2A) in latency and cancer. Cell Mol Biol Lett. 14:222–247.
2009.PubMed/NCBI
|
|
6
|
Kanegane H, Yachie A, Miyawaki T and
Tosato G: EBV-NK cells interactions and lymphoproliferative
disorders. Leuk Lymphoma. 29:491–498. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tomita M, Dewan MZ, Yamamoto N, Kikuchi A
and Mori N: Epstein-Barr virus-encoded latent membrane protein 1
activates beta-catenin signaling in B lymphocytes. Cancer Sci.
100:807–812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee CW, Leu SJ, Tzeng RY, et al: Latent
membrane protein 1 of Epstein-Barr virus regulates death-associated
protein kinase 1 in lymphoblastoid cell line. Virology. 413:19–25.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rechsteiner MP, Berger C, Zauner L, et al:
Latent membrane protein 2B regulates susceptibility to induction of
lytic Epstein-Barr virus infection. J Virol. 82:1739–1747. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shair KH, Bendt KM, Edwards RH, Nielsen
JN, Moore DT and Raab-Traub N: Epstein-Barr virus-encoded latent
membrane protein 1 (LMP1) and LMP2A function cooperatively to
promote carcinoma development in a mouse carcinogenesis model. J
Virol. 86:5352–5365. 2012. View Article : Google Scholar
|
|
11
|
Bultema R, Longnecker R and
Swanson-Mungerson M: Epstein-Barr virus LMP2A accelerates
MYC-induced lymphomagenesis. Oncogene. 28:1471–1476. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang B, Kracker S, Yasuda T, et al:
Immune surveillance and therapy of lymphomas driven by Epstein-Barr
virus protein LMP1 in a mouse model. Cell. 148:739–751. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dellis O, Arbabian A, Papp B, Rowe M, Joab
I and Chomienne C: Epstein-Barr virus latent membrane protein 1
increases calcium influx through store-operated channels in B
lymphoid cells. J Biol Chem. 286:18583–18592. 2011. View Article : Google Scholar
|
|
14
|
Ramakrishnan R, Donahue H, Garcia D, Tan
J, Shimizu N, Rice AP and Ling PD: Epstein-Barr virus BART9 miRNA
modulates LMP1 levels and affects growth rate of nasal NK T cell
lymphomas. PLoS One. 6:e272712011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoshizaki T: A novel immune evasion
mechanism of LMP-1, an EBV-primary oncogene, in nasopharyngeal
carcinoma. Adv Otorhinolaryngol. 72:157–159. 2011.PubMed/NCBI
|
|
16
|
Wang LT, Lin CS, Chai CY, Liu KY, Chen JY
and Hsu SH: Functional interaction of Ugene and EBV infection
mediates tumorigenic effects. Oncogene. 30:2921–2932. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu H, Lu J, Zuo L, et al: Epstein-Barr
virus downregulates microRNA 203 through the oncoprotein latent
membrane protein 1: a contribution to increased tumor incidence in
epithelial cells. J Virol. 86:3088–3099. 2012. View Article : Google Scholar
|
|
18
|
Guasparri I, Bubman D and Cesarman E: EBV
LMP2A affects LMP1-mediated NF-kappaB signaling and survival of
lymphoma cells by regulating TRAF2 expression. Blood.
111:3813–3820. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vrazo AC, Chauchard M, Raab-Traub N and
Longnecker R: Epstein-Barr virus LMP2A reduces hyperactivation
induced by LMP1 to restore normal B cell phenotype in transgenic
mice. PLoS Pathog. 8:e10026622012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fotheringham JA, Mazzucca S and Raab-Traub
N: Epstein-Barr virus latent membrane protein-2A-induced
DeltaNp63alpha expression is associated with impaired
epithelial-cell differentiation. Oncogene. 29:4287–4296. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rechsteiner MP, Bernasconi M, Berger C and
Nadal D: Role of latent membrane protein 2 isoforms in Epstein-Barr
virus latency. Trends Microbiol. 16:520–527. 2008. View Article : Google Scholar : PubMed/NCBI
|