Introduction
Acute respiratory distress syndrome (ARDS) is
characterized by acute respiratory failure resulting from acute
lung injury (ALI). The main characteristics of ARDS are diffuse
inflammation and increased microvascular permeability that cause
interstitial and alveolar edema and persistent refractory hypoxemia
(1). While ALI may be caused by
various insults, it has been suggested that a common pathway is
likely to result in lung damage (2–4).
In vivo alveolar fluid volume is determined
by alveolar fluid clearance (AFC), which is a function of
transepithelial Na+ transport (5). AFC has been reported to be impaired
in ARDS/ALI and has been associated with the function of epithelial
sodium channels (ENaCs) (6). ENaCs
are composed of three homologous subunits, α, β and γ (7). In a lipopolysaccharide (LPS)-induced
model of ALI in rats, all three ENaC subunits were observed to be
downregulated in lung epithelial tissue at the protein and mRNA
levels. This was concomitant with LPS-induced ALI (8).
It is well established that LPS is capable of
inducing inflammatory responses and that ALI involves a complex
series of inflammatory events that result in increased
alveolar-capillary membrane permeability (9–11).
Numerous cytokines are produced in the lung by resident cells,
including alveolar macrophages, lung epithelial cells and
fibroblasts, or by neutrophils, lymphocytes, monocytes and
platelets in response to local or systemic injury (12–16).
Inflammatory cytokines, including tumor necrosis factor α (TNF-α),
interleukin (IL)-6 and IL-8, have been reported to have a critical
role in the pathophysiology of septic shock, a condition that
frequently leads to ALI (17). It
has been hypothesized that ineffective lung repair following ALI is
a consequence of persistent inflammatory stimulation (18).
Panax notoginseng saponins (PNS) are extracts
of the Chinese herb, P. notoginseng. The major active
components of PNS are ginsenoside Rb1 (Rb1), ginsenoside Rg1 (Rg1)
and notoginsenoside R1 (R1), which have been identified to exhibit
a variety of pharmacological activities, including
anti-inflammatory and antioxidant effects, in addition to calcium
antagonist activities. It has been reported that PNS may protect
against cerebral ischemia and exert beneficial effects on the
cardiovascular system, as well as possess hemostatic, antioxidant
and estrogen-like activities (19–21).
We previously demonstrated that PNS (100 mg/kg) treatment was
capable of alleviating intestinal ischemia/reperfusion
(II/R)-induced ALI in rats and that it significantly reduced
oxidant enzyme activity, levels of malondialdehyde (MDA) and nitric
oxide, the activity of inducible nitric oxide synthase in lung
tissue and pro-inflammatory cytokine levels in the plasma (22).
The present study investigated the protective
effects of PNS on ALI induced in a rat model. ALI was induced using
oleic acid (OA) and LPS (23). OA
has been reported to inhibit alveolar fluid reabsorption, which is
a significant problem in ARDS (24). The endotoxin, LPS, has also been
reported to have adverse effects on lung tissue water homeostasis,
as it adversely affects ENaC-mediated Na+ transport
(25,26). This study analyzed the effects of
PNS on lung tissue histology, extravascular lung water (EVLW)
levels and lung tissue αENaC mRNA and protein expression. In order
to assess the anti-inflammatory properties of PNS, inflammatory
cytokines, including TNF-α, IL-6 and IL-10, were examined in the
serum and bronchoalveolar lavage fluid (BALF) of the rats. These
are the predominant cytokines associated with ALI in animal models
(23).
Materials and methods
Animals
Healthy male Wistar rats weighing 200–250g were
purchased from the Shanghai Laboratory Animal Centre (SLAC;
Shanghai, China). The rats were randomly assigned to experimental
groups and housed in groups of four under environmentally
controlled conditions in compliance with the Policy on Animal Care
and Use, Shanghai Jiaotong University (Shanghai, China). All
experiments were approved by the Ethics Committee of the Faculty of
Pharmacy, Shanghai Jiaotong University.
Rats used for the model of OA and LPS-induced ALI
were fasted and permitted solely water for 24 h prior to commencing
the experiment. Rats were anesthetized using 10% chloral hydrate
(0.35 ml/kg) and heparin (400 U/kg) administered intraperitoneally.
A 4-Fr double-lumen catheter (Arrow International, Reading, PA,
USA) was inserted through the jugular vein for fluid and drug
infusion. Anesthesia was maintained using ketamine at 1 mg/kg/h and
pancuronium at 0.3 mg/kg/h through the central venous line.
Lactated Ringer’s solution was infused (10 ml/kg/h) throughout the
procedure.
An arterial catheter was placed in the carotid
artery to monitor the arterial pressure and to sample the arterial
blood. A heating pad was used to maintain body temperature at
~37°C. Rats were placed in the supine position and a midline
cervical incision was performed followed by tracheostomy, whereby
the trachea was intubated with a 2.0-mm-diameter tracheal tube.
Mechanical ventilation was performed using an animal ventilator
(Model ALC-V8S; Shanghai Alcott Biotech Co. Ltd., Shanghai, China)
as follows: tidal volume=15 ml/kg; respiratory rate (RR)=40
breaths/min; inspiratory:expiratory ratio=1:1; fraction of
inspiratory oxygen (FiO2)=0.21.
Experimental protocols
A total of 28 rats were randomly assigned to four
groups of seven rats: a sham group (group 1), a sham + PNS group
(group 2), an ALI group (group 3) and an ALI + PNS group (group 4).
Rats in group 1 were anesthetized and received intravenous (IV)
saline (2 ml/kg), solely. Rats in group 2 were also administered IV
saline as well as PNS (100 mg/kg; Yunnan Phytopharmaceutical Co.
Ltd., Kunming, China) by bolus IV injection. PNS was prepared in a
5% glucose solution with sterile water. Rats in group 3 received IV
saline (2 ml/kg) and an IV injection of OA at 0.2 ml/kg
(Sigma-Aldrich, St. Louis, MO, USA) followed by 4 h of LPS
injection (5 mg/kg). Rats in group 4 were administered PNS (100
mg/kg) following ALI stabilization for 15 min. At 2 hr subsequent
to these treatments, rats were sacrificed by cardiac puncture under
anesthesia induced by intramuscular injection with ketamine and
xylazine at 8.7 and 1.3 mg/100 g body weight (BW), respectively.
Samples were taken for the analyses described below.
Lung histology
The right lower lobes of the lungs were harvested
and fixed in 10% formalin. After 48 h, the tissue samples were
embedded in paraffin. Paraffin-embedded, 4-mm-thick sections were
stained using hematoxylin and eosin (H&E) and examined using
light microscopy with an attached photo-documentation device (Carl
Zeiss Shanghai Co., Ltd., Shanghai, China).
Lung wet-to-dry (W/D) weight ratio and
EVLW
Pulmonary edema was determined by weighing both
lungs at necropsy and then drying the tissue at 70°C until the dry
weight remained unchanged for 24 h. The lung W/D weight ratio was
normalized to the initial body weight, as was EVLW, as described
previously (27). In brief, equal
quantities of lung tissue and distilled water were homogenized and
a total of 50 ml homogenate was centrifuged at 2,000 × g
(centrifuge radius=17.4 cm) for 10 min, prior to incubation at 5°C
for 1 h. The hemoglobin (Hb) concentration was measured in the
supernatant. Samples of arterial blood, tissue homogenates and
supernatants were dried at 80°C for 72 h and the water content
percentages of the samples were calculated. The calculations were
performed using the following formulae: Homogenate Hb concentration
= supernatant Hb × (homogenate water content %/supernatant water
content %); blood weight = homogenate weight × (homogenate Hb/blood
Hb); blood water weight = blood weight × blood water content %;
lung water content (TPW) = (homogenate water content % × homogenate
weight) - volume of distilled water added; EVLW = TPW - blood water
weight.
BALF collection and cell counts
The right main bronchus was clamped and BAL of the
left lung was performed three times using 2 ml saline solution. The
cell suspension was centrifuged at 100 × g for 10 min at 4°C,
following which cells were resuspended in 1 ml saline solution.
Glass slides were coated with 0.1 ml cell suspension, prior to
staining with Wright-Giemsa. A total of 400 nucleated cells were
examined using light microscopy to calculate total and differential
cell counts.
Cytokine ELISAs
Prior to sacrifice, blood samples were obtained from
the abdominal aorta and centrifuged to isolate the sera, which were
stored at −80°C. TNF-α, IL-6 and IL-10 levels were quantified using
a Bio-Plex Rat Serum Diluent Kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) according to a modified double-ligand method
described previously (28).
Flat-bottomed, 96-well microtiter plates were coated with 50
ml/well rat antibody against the various cytokines at a
concentration of 1 mg/ml in 0.6 M NaCl, 0.26 M
H3BO4 and 0.08 M NaOH (pH 9.6) for 16 h at
4°C. Samples were then washed with phosphate-buffered saline (PBS;
pH 7.5) containing 0.05% Tween 20 (wash buffer). Non-specific
binding sites were blocked using 2% bovine serum albumin (BSA) in
PBS and incubated for 90 min at 37°C. Plates were then rinsed four
times with wash buffer, and 50 μl neat and 1:10 diluted cell-free
supernatants were added to duplicate wells and incubated for 1 h at
37°C. Plates were washed four times, prior to the addition of 50
μl/well biotinylated rabbit antibodies against the specific
cytokines at a concentration of 3.5 mg/ml in wash buffer containing
2% FCS. Plates were then incubated for 30 min at 37°C, prior to
four washes and incubation with streptavidin-peroxidase conjugate
(Bio-Rad Laboratories, Inc.) for 30 min at 37°C. Plates were then
washed a further four times, followed by the addition of chromogen
substrate (Bio-Rad Laboratories, Inc.). Plates were incubated at
room temperature and the reaction was terminated with 50 μl/well 3
M H2SO4 solution. Plates were read at 490 nm
using an ELISA reader.
Standards were 1/2 log dilutions of recombinant
murine cytokines from 1 pg/ml to 100 ng/ml. This ELISA method
consistently detected murine cytokine concentrations >25 pg/ml.
These ELISAs were not detected to cross-react with IL-1, IL-2 or
IL-4, or with members of the murine chemokine family, including
murine JE/monocyte chemoattractant protein (MCP)-1, regulated on
activation, normal T cell expressed and secreted (RANTES),
keratinocyte-derived chemokine (KC), macrophage inflammatory
protein (MIP)-2, growth-related gene-a (GROa) or epithelial cell
dermid neutrophil-activating protein-78 (ENA-78).
Reverse transcription polymerase chain
reaction (RT-PCR) analysis of αENaC mRNA expression
A total of 100 mg fresh lung tissue homogenate was
prepared using TRIzol® reagent (Invitrogen Life
Technologies, Grand Island, NY, USA) according to the
manufacturer’s instructions. Purified RNA was extracted and RT-PCR
was performed using primer sequences targeting αENaC. The primer
sequences were as follows: 5′-CCATGAAGGGCAACCAAT-3′ (forward) and
5′-CGAACAGCAAGGCGAACT-3′ (reverse). β-actin was used as an internal
control with the primer sequences: 5′-AGCGGGAAATCGTGCGTGACATT-3′
(forward) and 5′-CAGGAAGGAAGGCTGGAAGAGTG-3′ (reverse).
Amplification was performed using the following conditions: 50°C
for 30 min, 95°C for 5 min, 95°C denaturation for 30 sec, 60°C
annealing for 30 sec and 72°C extension for 1 min, for 20 and 25
cycles. RT-PCR products were separated using agarose gel
electrophoresis. A gel imaging analysis system was used to
determine the integrated optical density values of αENaC and
β-actin and the quantity of α-ENaC mRNA was determined relative to
the integrated optical density value of β-actin mRNA.
Western blot analysis of αENaC protein
expression
Protein lysates were prepared according to standard
protocols. Lung tissues were washed thoroughly using PBS, prior to
homogenization at a dilution of 1:10 (v/v) in a lysis buffer
containing 50 mM Tris (pH 7.4), 150 mM NaCl, 0.5% NP-40, 50 mM NaF,
1 mM Na3VO4, 1 mM phenylmethylsulfonyl
fluoride, 25 mg/ml leupeptin and 25 mg/ml aprotinin. Lysates were
then centrifuged at 10,000 × g and the supernatants were collected.
Equal quantities of protein lysates were used for western blot
analyses with the indicated antibodies. Protein lysates (30 μg
protein/lane) were boiled for 5 min at 95°C, prior to being
separated on an 8% SDS-polyacrylamide gel and transferred to a
nitrocellulose membrane. Membranes were blocked using 5% milk in
tris-buffered saline (TBS) for 2 h and subsequently washed with
0.05% Tween-20-TBS and incubated overnight at 4°C with a 1:200
dilution of anti-αENaC antibody (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA). Membranes were then washed and incubated with
peroxidase-conjugated affinity-purified goat anti-mouse
immunoglobulin G (IgG) and goat anti-rabbit IgG secondary
antibodies at a 1:20,000 dilution (Jackson ImmunoResearch
Laboratories, Inc., West Grove, MA, USA) for 2 h at 37°C. Protein
bands were visualized using an enhanced chemiluminescence (ECL)
system (GE Healthcare, Waukesha, WI, USA) and quantified by
densitometry. β-actin (Santa Cruz Biotechnology, Inc.) was used as
a loading control at a 1:3,000 dilution. All experiments were
repeated at least three times.
Statistical analyses
Results are presented as the mean ± standard
deviation. Results for normally distributed continuous variables
were compared using one-way analysis of variance (ANOVA). When a
significant difference between groups was detected, multiple mean
comparisons were performed using the Bonferroni procedure with a
type-I error adjustment. All statistical analyses were two-sided
and performed using SPSS 15.0 statistics software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Lung histology
Histopathological examination of HE-stained lung
tissue sections from rats in the sham, sham + PNS, ALI and ALI +
PNS groups are shown in Fig. 1A–D,
respectively. ALI rats were observed to demonstrate the
characteristic features of pulmonary injury, whereas rats in the
sham and sham + PNS groups demonstrated normal alveolar
architecture. Specifically, rats in the ALI group exhibited an
increased thickness of the alveolar wall, pulmonary edema and
hemorrhage, as well as infiltration of inflammatory cells into the
alveolar spaces (Fig. 1C). PNS
treatment in ALI rats was observed to prevent pulmonary parenchymal
damage and the lung histology in these rats was identified to be
similar to that of the sham control rats, with numerous distended
alveoli with thin, flattened, delicate walls. Furthermore, in the
rats in the ALI + PNS group, alveoli were observed to be well
aerated and with few neutrophils within the interstitium (Fig. 1D).
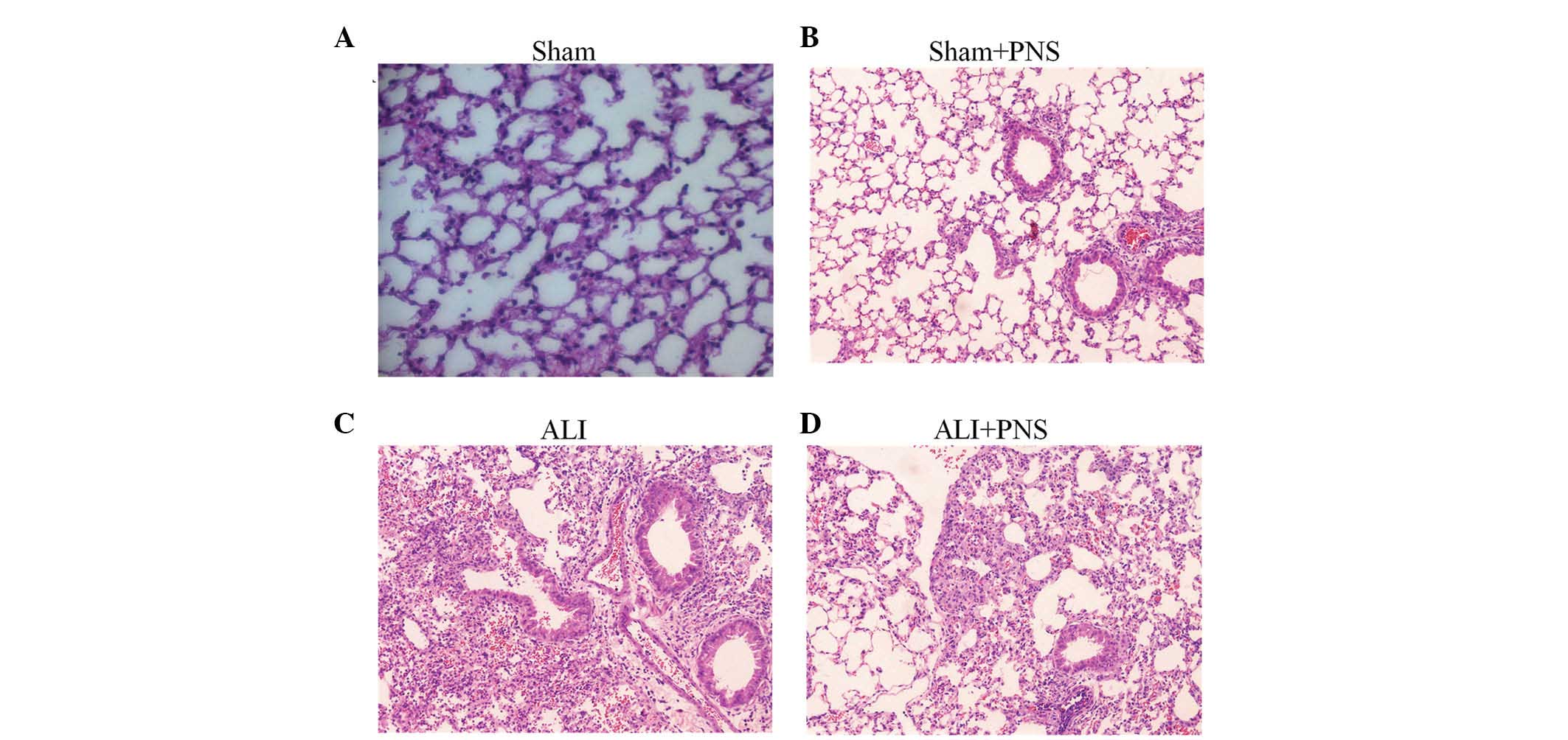 | Figure 1Histopathological examinations of
H&E-stained lung tissue sections. Lung histology of rats
subjected to sham injection with or without treatment with PNS and
injection of OA followed by 4 h of LPS injection, with or without
treatment with PNS. (A) Sham group (magnification, ×100). (B) Sham
with PNS treatment group (magnification, ×100). (C) OA-LPS-induced
ALI group (magnification, ×400). Thickened interstitial walls and a
dense inflammatory cell infiltrate were observed. (D) ALI with PNS
treatment group (magnification, ×400). Relatively normal histology
with thin alveolar walls and few infiltrating inflammatory cells.
PNS, Panax notoginseng saponins; ALI, acute lung injury;
H&E, hematoxylin and eosin; LPS, lipopolysaccharide; OA, oleic
acid. |
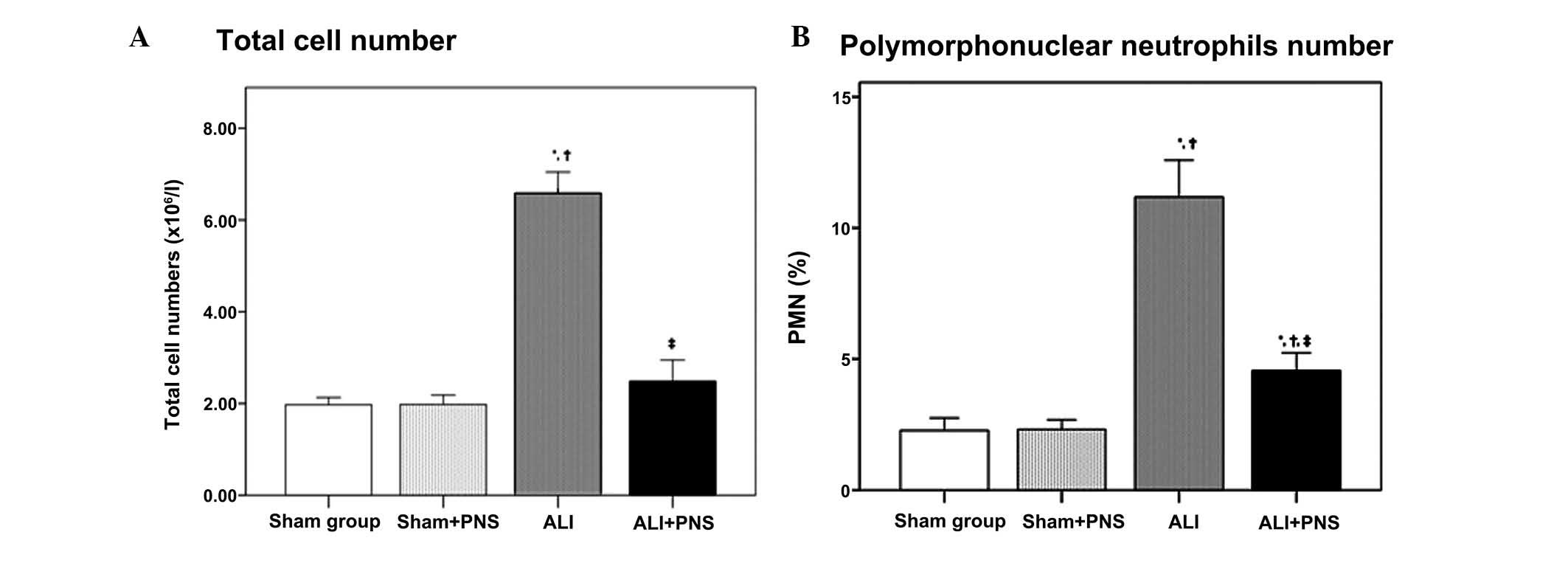
BALF collection and cell counts
As shown in Fig. 2,
at 2 h subsequent to treatment, no significant differences were
observed in the total leukocyte count or the percentage of
polymorphonuclear neutrophils (PMN) in the BALF between the sham
and sham + PNS groups (both P=1.000). However, the ALI group, in
which OA-LPS injury had been induced, exhibited significantly
higher total leukocyte counts and PMN percentages in the BALF,
compared with those of the sham groups (all P<0.001). Following
PNS administration (ALI + PNS group), ALI rats demonstrated
significantly reduced total leukocyte counts and PMN percentages in
the BALF compared with the ALI group (both P<0.001).
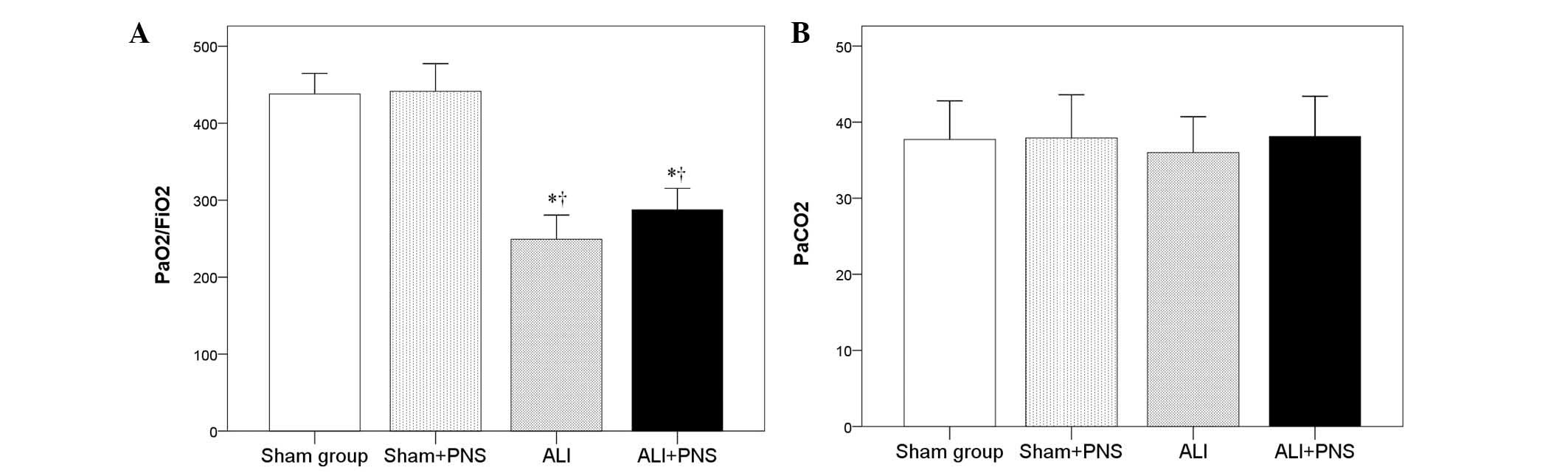
Partial pressure
(Pa)O2/FiO2 ratio and PaCO2
As shown in Fig. 3
no significant difference was observed in the
PaO2/FiO2 ratio in the sham + PNS group
compared with the sham group. By contrast, rats in the ALI and ALI
+ PNS groups exhibited significantly lower
PaO2/FiO2 ratios (P<0.001; Fig. 3A). However, PNS administration was
not observed to significantly improve the
PaO2/FiO2 ratio in ALI rats (P=0.172). No
significant differences were observed in the PaCO2
values among the rats in the four groups (P=0.865; Fig. 3B)
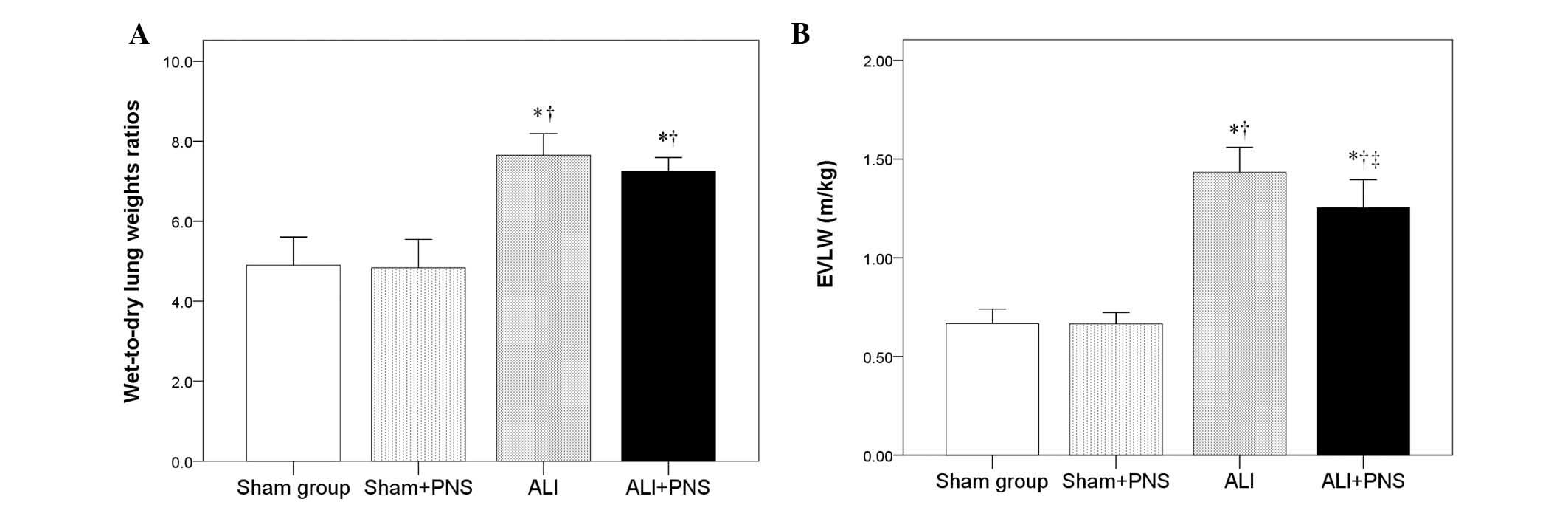
Lung W/D weight ratio and EVLW
A significantly increased lung W/D weight ratio was
observed in the rats in the ALI and ALI + PNS groups compared with
those in the sham and sham + PNS groups (P<0.001; Fig. 4A). Moreover, the EVLW level was
identified to be significantly higher in the rats in the ALI and
ALI + PNS groups compared with those in the sham and sham + PNS
groups (P<0.001; Fig. 4B).
However, following administration of PNS in ALI rats, the EVLW
level in lung tissues was observed to significantly decrease
compared with the ALI rats that had not received PNS treatment
(P=0.026; Fig. 4B).
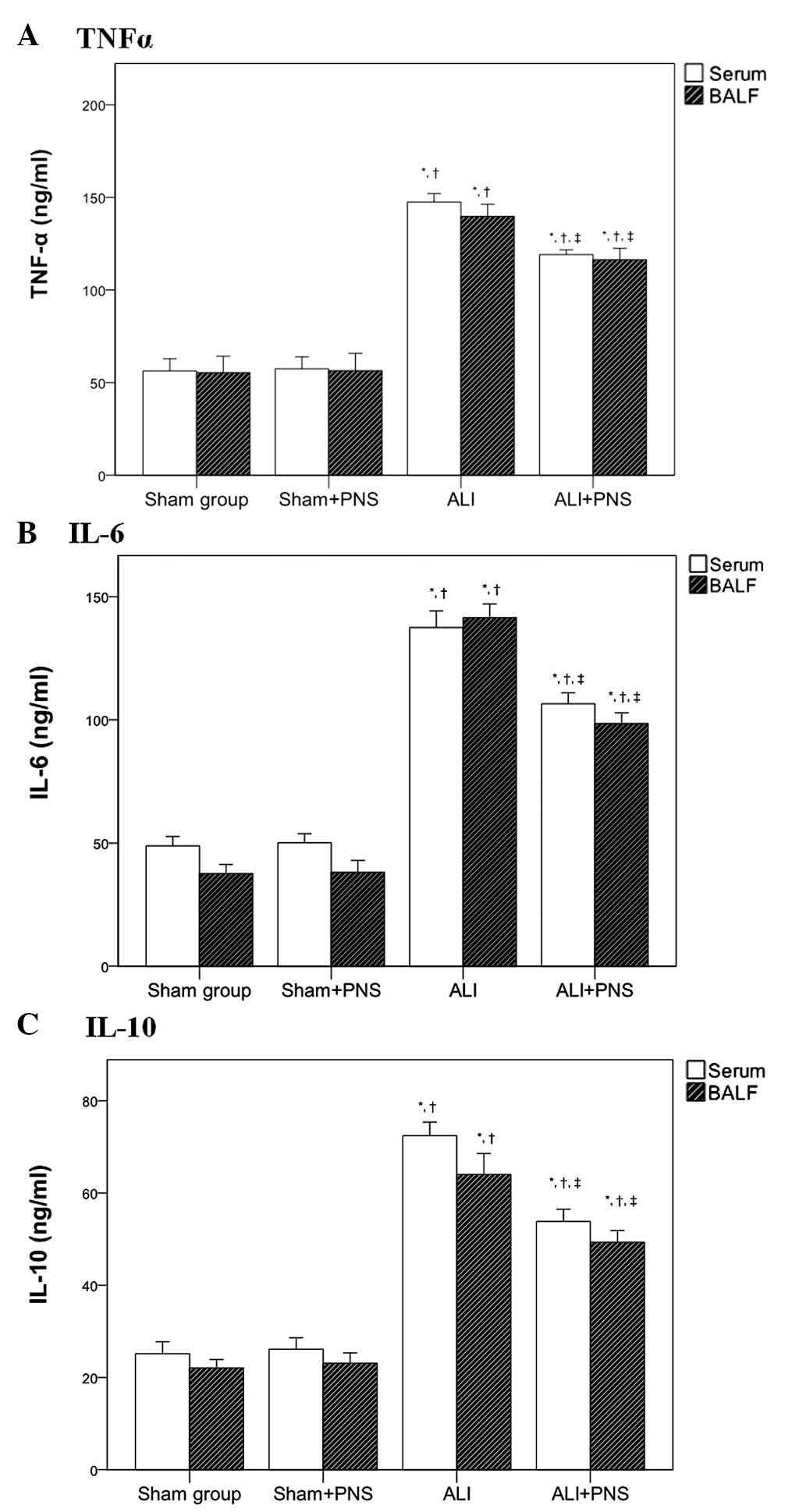
Serum and BALF cytokine levels
Serum levels of TNF-α, IL-6 and IL-10 (Fig. 5A–C, respectively) were observed to
be significantly increased in rats in the ALI and ALI + PNS groups
compared with those in the sham and sham + PNS groups (all
P<0.001). However, no significant differences were detected in
the levels of TNF-α, IL-6 and IL-10 in the sera of rats between the
sham and the sham + PNS group. A similar pattern of cytokine
concentration was observed in the BALF of the four groups of rats.
However, compared with the ALI group, the levels of TNF-α, IL-6 and
IL-10 were significantly decreased in the sera and BALF of ALI rats
following PNS administration (all P<0.001).
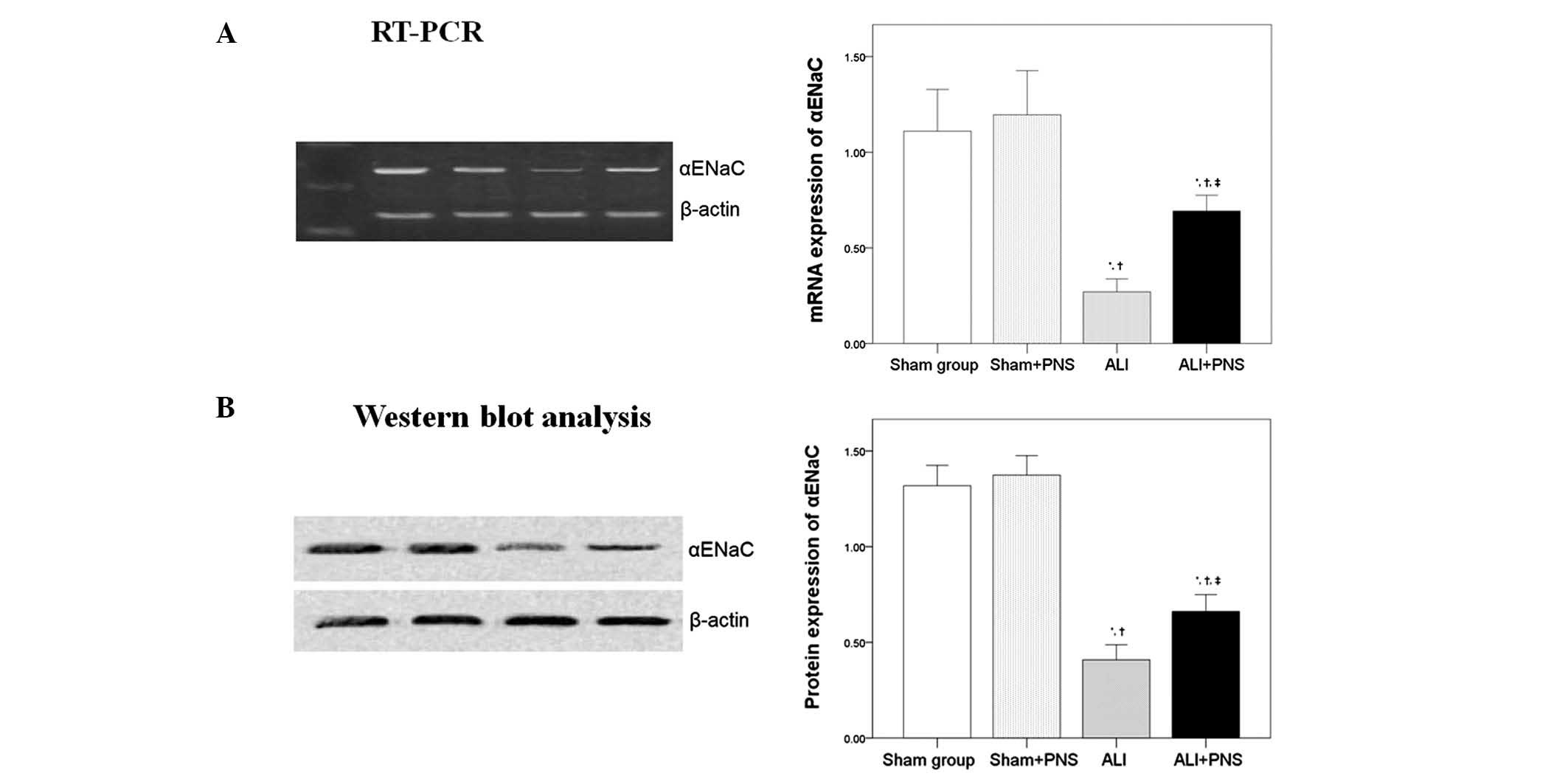
αENaC mRNA and protein expression
As shown in Fig. 6,
no significant differences were observed in the levels of αENaC
mRNA (Fig. 6A) and protein
(Fig. 6B) expression in left lung
tissues between the rats in the sham and the sham + PNS groups.
However, it was observed that OA-LPS-induced ALI significantly
decreased the mRNA and protein expression of αENaC in rat left lung
tissues. Moreover, following PNS administration, a significant
increase in αENaC mRNA and protein expression was observed in the
left lung tissue of ALI rats compared with ALI rats without PNS
treatment (P=0.017).
Discussion
This study has demonstrated that OA injection
followed by LPS administration is capable of inducing severe
pulmonary parenchymal damage in rats, consistent with various
adverse effects on lung water homeostasis that are associated with
ALI in animal models (8,23). OA has been reported to inhibit
alveolar fluid reabsorption (24)
and the endotoxin, LPS, has been indicated to adversely affect
ENaC-mediated Na+ transport (25,26).
In the present study, PNS treatment was observed to
ameliorate some of the ALI-induced effects within a clinically
relevant time frame. PNS treatment was observed to decrease the
lung EVLW level in ALI rats and partially restore the reduced αENaC
mRNA and protein levels detected in ALI lung tissue. Thus, PNS
treatment may ameliorate some of the adverse characteristics
associated with lung water homeostasis in ALI through improving
AFC, which is a function of transepithelial Na+
transport (5). Furthermore, the
results of this study identified that PNS may attenuate the
inflammation associated with OA-LPS injury in rats.
Neutrophil infiltration has been reported to have a
significant role in the development of pulmonary edema and
microvascular leakage in animal models. Moreover, activated
neutrophils are suggested to be the primary mediators of local and
remote tissue damage induced by II/R (29,30).
The results of this study show that PNS treatment is capable of
significantly reducing the total leukocyte and PMN counts in the
lungs of ALI rats (Fig. 2).
ALI and its more severe form, ARDS, are
characterized by an excessive inflammatory response in the lung,
which is associated with, and may be promoted by, an increase in
inflammatory cytokines in the plasma (5,31).
Previous clinical investigations have revealed a positive
correlation between mortality and levels of circulating TNF-α,
IL-1β, IL-6 and IL-8 in ALI (22–35).
In the present study, the reduction in inflammatory cytokine levels
observed in the plasma of ALI rats following PNS treatment may have
contributed to a reduction in leukocyte adhesion and infiltration.
In ALI rats, PNS treatment was observed to reduce neutrophil
infiltration into the lungs; however, infiltration levels were
still greater than those observed in the sham and sham + PNS groups
of rats.
In a study by Sun et al (36), PNS treatment was reported to reduce
LPS-induced leukocyte adhesion in rat mesenteric venules by
inhibiting the expression of the adhesion molecules cluster of
differentiation molecule 11B (CD11b) and integrin β-2 (CD18) in
neutrophils. In a previous study, we showed that PNS administration
decreased bacterial translocation and reduced pulmonary parenchymal
damage, following II/R in rats. Furthermore, PNS was observed to
reduce the activity of oxidative enzymes, the levels of MDA and
nitric oxide, the activity of inducible nitric oxide synthase in
the lung tissue and the levels of pro-inflammatory cytokines in the
plasma (22).
In addition to its effects on lung water
homeostasis, the OA-LPS model may also induce severe ALI through
its effects on lung inflammatory responses (23). These effects may be associated with
abnormally increased levels of pro-inflammatory cytokines.
Experimental studies suggest that cytokine responses are normally
compartmentalized within the lungs; therefore, the study of blood
samples may provide an incomplete representation of the
inflammatory events within the lungs (37). However, it has been suggested that
compartmentalization is lost to some extent during severe
inflammatory responses (38),
although measuring cytokine levels within the lungs is likely to be
more accurate than measuring those in plasma or serum samples.
Cytokines are active in the alveolar compartment, where they may
exist as soluble constituents of alveolar fluids, as well as in the
tissue compartment; therefore, sampling cytokines in the lungs may
be problematic. Sampling alveolar fluids using BAL may represent an
alternative technique for measuring the concentration and function
of specific cytokines in the lungs (39).
Of note, TNF-α has been identified to stimulate
cytokine production by lung epithelial and mesenchymal cells that
are unresponsive to bacteria and their products directly (40). Suter et al (41) found significant levels of TNF-α in
the lung fluid of patients at the onset of ARDS. TNF-α mediates its
effects through two TNF-α receptors: TNF-receptor I (RI) and
TNF-RII. Park et al (16)
demonstrated that the concentrations of TNFR-I and TNFR-II exceeded
the concentration of immunoreactive TNF-α in BALF during the course
of ARDS. Furthermore, they observed that the activity of TNF-α was
effectively inhibited in the aqueous phase of lung fluids of
patients with ARDS. TNF-α has also been suggested to induce the
release of IL-6 by endothelial cells and macrophages (42).
IL-6 was originally identified as a B-cell growth
factor (43). IL-6 is produced by
activated macrophages and stimulates acute-phase responses in the
liver. IL-6 production is induced, in part, by TNF-α and IL-1β and
it has been proposed that IL-6 integrates signals produced in the
early stages of the inflammatory response (16). Experimental studies have identified
that, in the BALF of patients at risk for ARDS, IL-6 concentrations
are high and remain elevated throughout the course of established
ARDS. It has been reported that the IL-6 receptor, IL-6R, is
released from cell membranes and enters the circulation. Martin
(14) observed that the
concentration of soluble IL-6R was elevated in the BALF of patients
at risk of ARDS and throughout the course of the condition.
Therefore, cellular reactions mediated by IL-6 may be of
significance during ARDS (14).
In the present study the levels of TNF-α and IL-6
were observed to be significantly higher in the BALF of rats in the
ALI groups compared with those in the sham groups (P<0.05;
Fig. 5). Of note, TNF-α and IL-6
levels were found to be significantly lower in the BALF and serum
of ALI rats following PNS administration, which may have
contributed in alleviating subsequent systemic inflammatory
responses.
IL-10 is a counter-regulatory cytokine that inhibits
cytokine production by stimulated macrophages (44,45).
IL-10 is detectable in ARDS BALF and it has been reported that
IL-10 treatment is capable of life extension in animals (46,47).
Donnelly et al (48) found
that patients who died with ARDS had low concentrations of IL-10 in
the BALF at the onset of ARDS, suggesting inadequate attenuation of
lung inflammatory responses. Lo et al (49) observed that IL-10 inhibited the
production of TNF by LPS-stimulated macrophages. Furthermore, IL-10
has been reported to reduce TNF mRNA expression (49). In the present study, PNS treatment
was also observed to decrease IL-10 levels in the BALF and serum,
in addition to those of TNF-α and IL-6. Therefore, PNS
administration may decrease the subsequent systemic inflammatory
response and may aid in preventing ALI from developing to ARDS. To
summarize, following OA-LPS treatment, the levels of IL-10 in the
serum and BALF of ALI rats were markedly higher than those in rats
in the PNS-treated group. These findings suggest that ALI may be
associated with pro- and anti-inflammatory responses and that an
imbalance between these responses may be a major mechanism
underlying the deterioration associated with ALI and the
progression to ARDS. The anti-inflammatory effects of PNS are well
established, and in the present study, PNS was not only found to
reduce TNF-α and IL-6 levels in the lungs of rats with
OA-LPS-induced ALI, but also significantly decrease IL-10 levels in
the lungs. These findings suggest that PNS may not only improve
lung inflammation, but also aid in balancing pro- and
anti-inflammatory responses, which may limit progression from ALI
to ARDS.
It has been shown that PNS may exert protective
effects against cerebral ischemia, be beneficial for the
cardiovascular system and possess hemostatic, antioxidant and
estrogen-like activities (19–21).
We previously reported that PNS treatment (100 mg/kg) was capable
of alleviating II/R-induced ALI in rats and significantly reduced
the activity of oxidant enzymes and inducible nitric oxide synthase
in the lung, as well as the levels of MDA and nitric oxide, and
pro-inflammatory cytokines in the plasma (22).
It has previously been demonstrated that extracts
from certain naturally derived substances may be capable of
attenuating diseases associated with adverse inflammatory
responses, including models of ALI. Chen et al (29) reported that pre-treatment with 30
mg/kg Radix Paeoniae Rubra (RPR), injected into the femoral
vein for 2 h, 4 h prior to reperfusion, was capable of attenuating
ALI induced by 1 h of intestinal ischemia followed by 2 h of
reperfusion in rats. The RPR treatment was observed to decrease MDA
levels and prevent the decrease in superoxide dismutase (SOD)
activity in the lungs (29).
Furthermore, a study by Peng et al (50) reported that PNS treatment had
anti-fibrotic effects in a rat model of hepatic fibrosis as a
consequence of altered expression of the same cytokines assessed in
the present study, namely TNF-α, IL-6 and IL-10. In accordance with
this finding, Lee et al (51) found that Korean red ginseng
ameliorated focal cerebral ischemia/reperfusion injury in rats and
that these neuroprotective effects were a result of altered
inflammatory cytokine levels. The present study showed that in a
model of ALI, PNS treatment demonstrated anti-inflammatory
properties with a similar cytokine profile to that reported
previously, and prevented the reduction in αENaC mRNA and protein
levels in the lung tissue. As this was an exploratory
investigation, only the α subunit of ENaC was investigated;
however, of note, it has been reported that the β and γ subunits
may be involved in rat models of ALI (8). Further in-depth investigations are
required to elucidate the complex interrelation between cytokines,
ENaCs and PNS in the damage associated with ALI.
In conclusion, this study showed that PNS
administration was capable of ameliorating OA-LPS-induced ALI in
Wistar rats. These effects were primarily a consequence of the
anti-inflammatory properties of PNS. The capacity of PNS to
alleviate ALI if administered following OA-LPS injection in this
model has yet to be investigated. The present study has highlighted
the requirement for further investigation into the protective
effects of PNS, as well as its clinical use in OA-LPS-induced ALI.
This may lead to the development of PNS as a therapeutic agent for
ALI and, potentially, its more severe manifestation, ARDS.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81170028).
References
|
1
|
Bernard GR, Artigas A, Brigham KL, et al:
The American-European Consensus Conference on ARDS. Definitions,
mechanisms, relevant outcomes, and clinical trial coordination. Am
J Respir Crit Care Med. 149:818–824. 1994. View Article : Google Scholar
|
|
2
|
Fowler AA, Hamman RF, Good JT, et al:
Adult respiratory distress syndrome: risk with common
predispositions. Ann Intern Med. 98:593–597. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pepe PE, Potkin RT, Reus DH, Hudson LD and
Carrico CJ: Clinical predictors of the adult respiratory distress
syndrome. Am J Surg. 144:124–130. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rinaldo JE and Christman JW: Mechanisms
and mediators of the adult respiratory distress syndrome. Clin
Chest Med. 11:621–632. 1990.PubMed/NCBI
|
|
5
|
Ware LB and Matthay MA: Alveolar fluid
clearance is impaired in the majority of patients with acute lung
injury and the acute respiratory distress syndrome. Am J Respir
Crit Care Med. 163:1376–1383. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matthay MA, Robriquet L and Fang X:
Alveolar epithelium: role in lung fluid balance and acute lung
injury. Proc Am Thorac Soc. 2:206–213. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Canessa CM, Schild L, Buell G, et al:
Amiloride-sensitive epithelial Na+ channel is made of
three homologous subunits. Nature. 367:463–467. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deng W, Li CY, Tong J, Zhang W and Wang
DX: Regulation of ENaC-mediated alveolar fluid clearance by insulin
via PI3K/Akt pathway in LPS-induced acute lung injury. Respir Res.
13:292012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bellingan GJ: The pulmonary physician in
critical care * 6: The pathogenesis of ALI/ARDS. Thorax.
57:540–546. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goodman RB, Strieter RM, Martin DP, et al:
Inflammatory cytokines in patients with persistence of the acute
respiratory distress syndrome. Am J Respir Crit Care Med.
154:602–611. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tomashefski JF Jr: Pulmonary pathology of
acute respiratory distress syndrome. Clin Chest Med. 21:435–466.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johnson JL, Moore EE, Tamura DY, Zallen G,
Biffl WL and Silliman CC: Interleukin-6 augments neutrophil
cytotoxic potential via selective enhancement of elastase release.
J Surg Res. 76:91–94. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kiehl MG, Ostermann H, Thomas M, Müller C,
Cassens U and Kienast J: Inflammatory mediators in bronchoalveolar
lavage fluid and plasma in leukocytopenic patients with septic
shock-induced acute respiratory distress syndrome. Crit Care Med.
26:1194–1199. 1998. View Article : Google Scholar
|
|
14
|
Martin TR: Lung cytokines and ARDS: Roger
S. Mitchell Lecture. Chest. 116(Suppl 1): 2S–8S. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meduri GU, Kanangat S, Stefan J, Tolley E
and Schaberg D: Cytokines IL-1beta, IL-6, and TNF-alpha enhance in
vitro growth of bacteria. Am J Respir Crit Care Med. 160:961–967.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park WY, Goodman RB, Steinberg KP, et al:
Cytokine balance in the lungs of patients with acute respiratory
distress syndrome. Am J Respir Crit Care Med. 164:1896–1903. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Headley AS, Tolley E and Meduri GU:
Infections and the inflammatory response in acute respiratory
distress syndrome. Chest. 111:1306–1321. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meduri GU, Kohler G, Headley S, Tolley E,
Stentz F and Postlethwaite A: Inflammatory cytokines in the BAL of
patients with ARDS. Persistent elevation over time predicts poor
outcome. Chest. 108:1303–1314. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li SH and Chu Y: Anti-inflammatory effects
of total saponins of Panax notoginseng. Zhongguo Yao Li Xue
Bao. 20:551–554. 1999.PubMed/NCBI
|
|
20
|
Ng TB: Pharmacological activity of sanchi
ginseng (Panax notoginseng). J Pharm Pharmacol.
58:1007–1019. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun K, Wang CS, Guo J, et al: Protective
effects of ginsenoside Rb1, ginsenoside Rg1, and notoginsenoside R1
on lipopolysaccharide-induced microcirculatory disturbance in rat
mesentery. Life Sci. 81:509–518. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rong L, Chen Y, He M and Zhou X: Panax
notoginseng saponins attenuate acute lung injury induced by
intestinal ischaemia/reperfusion in rats. Respirology. 14:890–898.
2009. View Article : Google Scholar
|
|
23
|
Wang HM, Bodenstein M and Markstaller K:
Overview of the pathology of three widely used animal models of
acute lung injury. Eur Surg Res. 40:305–316. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vadász I, Morty RE, Kohstall MG, et al:
Oleic acid inhibits alveolar fluid reabsorption: a role in acute
respiratory distress syndrome? Am J Respir Crit Care Med.
171:469–479. 2005.PubMed/NCBI
|
|
25
|
Adebamiro A, Cheng Y, Johnson JP and
Bridges RJ: Endogenous protease activation of ENaC: effect of
serine protease inhibition on ENaC single channel properties. J Gen
Physiol. 126:339–352. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baines DL, Albert AP, Hazell MJ, Gambling
L, Woollhead AM and Dockrell ME: Lipopolysaccharide modifies
amiloride-sensitive Na+ transport processes across human
airway cells: role of mitogen-activated protein kinases ERK 1/2 and
5. Pflugers Arch. 459:451–463. 2010.PubMed/NCBI
|
|
27
|
Shen JF, Qiu HB, Yang Y, et al: Comparison
of single-indicator thermodilution versus gravimetric measurement
in determination of extra-vascular lung water in dogs with acute
respiratory distress syndrome. Zhongguo Wei Zhong Bing Ji Jiu Yi
Xue. 18:327–330. 2006.(In Chinese).
|
|
28
|
Walley KR, Lukacs NW, Standiford TJ,
Strieter RM and Kunkel SL: Balance of inflammatory cytokines
related to severity and mortality of murine sepsis. Infect Immun.
64:4733–4738. 1996.PubMed/NCBI
|
|
29
|
Chen C, Zhang F, Xia ZY, Lin H and Mo AS:
Protective effects of pretreatment with Radix Paeoniae Rubra
on acute lung injury induced by intestinal ischemia/reperfusion in
rats. Chin J Traumatol. 11:37–41. 2008.
|
|
30
|
Türüt H, Kurutas EB, Bulbuloglu E, et al:
Zinc aspartate alleviates lung injury induced by intestinal
ischemia-reperfusion in rats. J Surg Res. 151:62–67.
2009.PubMed/NCBI
|
|
31
|
Ware LB and Matthay MA: The acute
respiratory distress syndrome. N Engl J Med. 342:1334–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Casey LC, Balk RA and Bone RC: Plasma
cytokine and endotoxin levels correlate with survival in patients
with the sepsis syndrome. Ann Intern Med. 119:771–778. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hack CE, Hart M, van Schijndel RJ, et al:
Interleukin-8 in sepsis: relation to shock and inflammatory
mediators. Infect Immun. 60:2835–2842. 1992.PubMed/NCBI
|
|
34
|
Meduri GU, Headley S, Kohler G, et al:
Persistent elevation of inflammatory cytokines predicts a poor
outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent
and efficient predictors of outcome over time. Chest.
107:1062–1073. 1995. View Article : Google Scholar
|
|
35
|
Pinsky MR, Vincent JL, Deviere J, Alegre
M, Kahn RJ and Dupont E: Serum cytokine levels in human septic
shock. Relation to multiple-system organ failure and mortality.
Chest. 103:565–575. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun K, Wang CS, Guo J, et al: Effect of
Panax notoginseng saponins on lipopolysaccharide-induced
adhesion of leukocytes in rat mesenteric venules. Clin Hemorheol
Microcirc. 34:103–108. 2006.
|
|
37
|
Nelson S, Bagby GJ, Bainton BG, Wilson LA,
Thompson JJ and Summer WR: Compartmentalization of intraalveolar
and systemic lipopolysaccharide-induced tumor necrosis factor and
the pulmonary inflammatory response. J Infect Dis. 159:189–194.
1989. View Article : Google Scholar
|
|
38
|
Tutor JD, Mason CM, Dobard E, Beckerman
RC, Summer WR and Nelson S: Loss of compartmentalization of
alveolar tumor necrosis factor after lung injury. Am J Respir Crit
Care Med. 149:1107–1111. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Steinberg KP, Mitchell DR, Maunder RJ,
Milberg JA, Whitcomb ME and Hudson LD: Safety of bronchoalveolar
lavage in patients with adult respiratory distress syndrome. Am Rev
Respir Dis. 148:556–561. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Standiford TJ, Kunkel SL, Phan SH, Rollins
BJ and Strieter RM: Alveolar macrophage-derived cytokines induce
monocyte chemoattractant protein-1 expression from human pulmonary
type II-like epithelial cells. J Biol Chem. 266:9912–9918.
1991.
|
|
41
|
Suter PM, Suter S, Girardin E,
Roux-Lombard P, Grau GE and Dayer JM: High bronchoalveolar levels
of tumor necrosis factor and its inhibitors, interleukin-1,
interferon, and elastase, in patients with adult respiratory
distress syndrome after trauma, shock, or sepsis. Am Rev Respir
Dis. 145:1016–1022. 1992. View Article : Google Scholar
|
|
42
|
Moriceau G, Ory B, Gobin B, et al:
Therapeutic approach of primary bone tumours by bisphosphonates.
Curr Pharm Des. 16:2981–2987. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Papanicolaou DA, Wilder RL, Manolagas SC
and Chrousos GP: The pathophysiologic roles of interleukin-6 in
human disease. Ann Intern Med. 128:127–137. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fiorentino DF, Zlotnik A, Mosmann TR,
Howard M and O’Garra A: IL-10 inhibits cytokine production by
activated macrophages. J Immunol. 147:3815–3822. 1991.PubMed/NCBI
|
|
45
|
Ramani M, Ollivier V, Khechai F, et al:
Interleukin-10 inhibits endotoxin-induced tissue factor mRNA
production by human monocytes. FEBS Lett. 334:114–116. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Standiford TJ, Strieter RM, Lukacs NW and
Kunkel SL: Neutralization of IL-10 increases lethality in
endotoxemia. Cooperative effects of macrophage inflammatory
protein-2 and tumor necrosis factor. J Immunol. 155:2222–2229.
1995.
|
|
47
|
van der Poll T, Marchant A, Buurman WA, et
al: Endogenous IL-10 protects mice from death during septic
peritonitis. J Immunol. 155:5397–5401. 1995.PubMed/NCBI
|
|
48
|
Donnelly SC, Strieter RM, Reid PT, et al:
The association between mortality rates and decreased
concentrations of interleukin-10 and interleukin-1 receptor
antagonist in the lung fluids of patients with the adult
respiratory distress syndrome. Ann Intern Med. 125:191–196. 1996.
View Article : Google Scholar
|
|
49
|
Lo CJ, Fu M and Cryer HG: Interleukin 10
inhibits alveolar macrophage production of inflammatory mediators
involved in adult respiratory distress syndrome. J Surg Res.
79:179–184. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Peng XD, Dai LL, Huang CQ, He CM, Yang B
and Chen LJ: Relationship between anti-fibrotic effect of Panax
notoginseng saponins and serum cytokines in rat hepatic
fibrosis. Biochem Biophys Res Commun. 388:31–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee JS, Choi HS, Kang SW, et al:
Therapeutic effect of Korean red ginseng on inflammatory cytokines
in rats with focal cerebral ischemia/reperfusion injury. Am J Chin
Med. 39:83–94. 2011. View Article : Google Scholar : PubMed/NCBI
|