Introduction
According to 2011 statistics, acute myocardial
infarction (AMI) is one of the most prevalent causes of death and
morbidity (1,2). Adult cardiomyocytes lack the capacity
for regeneration, so scar tissue replaces lost myocardium following
myocardial infarction, and this leads to cardiac remodeling with
reduced left ventricular (LV) function (3). Cell therapy is a potential method for
the regeneration and repair of myocardium and the improvement of
myocardial function following ischemic injury (4). A number of cell types, including
foetal Flk1+CD34+CD31− bone marrow
stem cells (BMSCs) have demonstrated cardiac regenerative capacity
(5), however, the mechanism that
stem cell transplantation utilizes in order to improve cardiac
function following ischemic heart disease is unclear and there is
little information available regarding the medium- and long-term
effects. On the other hand, there exist a variety of
transplantation methods, including local intramyocardial injection,
intravenous injection, and intracoronary injection to the infarct
zone (6). Intramyocardial
injection of BMSCs has been demonstrated to be safe, and to lead to
reverse remodeling and improved contractile ability of scarred
areas (3,7). The mechanisms by which BMSCs reduce
infarct size and improve cardiac function in animal models are
complicated, involving engraftment, differentiation into functional
cardiomyocytes and paracrine signaling (8,9). In
the present study, fetal
Flk1+CD34+CD31− BMSCs were
transplanted via intramyocardial injection of the mini-swine, then
the myocardial function, stem cell migration and extent of survival
in the myocardium was evaluated. The current study was therefore
designed to determine the long-term (6-month) effect and mechanism
of BMSCs in AMI.
Materials and methods
Animals
Mini-swine weighing 25–30 kg were purchased from The
Experimental Animal Breeding Center of Beijing, China. All animals
received humane care in compliance with the Guide for the Care and
Use of Laboratory Animals published by the U.S. National Institute
of Health (NIH Publication no. 85-23, 1996). The present study was
approved by the Ethics Committee of Shandong University (Jinan,
China).
Isolation, culture and immunophenotype
analysis of BMSCs
BMSCs were isolated, cultured and immunophenotyped
using the methods of previous studies (10,11).
Under general anesthesia maintained by intramuscular injection of
ketamine (10 mg·kg−1) followed by an intravenous drip of
sodium pentobarbital (30 mg·kg−1), bone marrow (20 ml)
was aspirated from the anterior iliac crest with a syringe (Beijing
Nianyou Medical Instrument Co., Ltd., Beijing, China) containing
6,000 U heparin with a myeloid puncture needle. To separate BMSCs
from other cells, the Ficoll (1.077 g·ml−1) density
gradient centrifugation method was used. Following centrifugation
at 1,000 g for 10 min at 20°C, the white layer composed of
mononuclear cells from the upper section and interface was
carefully collected and washed three times with phosphate-buffered
saline (PBS) prior to final resuspension in 10 ml heparinized
saline. The cell pellet was then resuspended in Dulbecco’s modified
Eagle’s medium/F12 (1:1). When adherent cells were confluent
(defined as passage 0), they were continuously cultured until
passage 3–5 in trypsin (0.25%) and 1 mmol/l
ethylenediamine-tetraacetic acid (Sigma-Aldrich, St. Louis, MO,
USA) for 5 mins. All cultures were maintained at 37°C in a 95%
humidified incubator at 37°C and 5% CO2, and the
cultures were replenished with fresh medium every 3 days. For
immunophenotyping, cells were washed twice with PBS containing 0.5%
bovine serum albumin (Sigma-Aldrich) and then suspended in PBS and
incubated with primary antibodies raised against human CD34; Flk1
(Santa Cruz Biotechnology, Santa Cruz, CA, USA); CD44; CD31; CD29;
CD105; CD106; and HLA-ABC (BD Pharmingen, San Diego, CA, USA) for
30 min at 4°C. The secondary polyclonal antibody (ZSGB-Bio Co.,
Beijing, China) was added and incubated at 4°C for an additional 30
min in a dark room. An isotype control antibody from the same
species was used as a negative control. Following washing, cells
were resuspended in PBS for fluorescence activated cell sorting
(FACS) analysis.
Acute myocardial infarction model and
stem cell transplantation
A mini-swine model of AMI was generated by ligating
the left anterior descending artery (LAD) using the method of a
previous study (10): Under
general anesthesia, animals were intubated and positive pressure
ventilation was maintained. The middle third of the LAD was ligated
following three intermittent brief preconditioning occlusions, each
for 5 min. A bolus of lignocaine was given intravenously (1 mg
kg−1) and then maintained at 1 mg
min−1kg−1 with an intravenous drip.
Subsequently, the eligible animals were randomly divided into
non-treatment (control) and BMSC treatment groups (10 in each
group). Following coronary ligation, cells (1×107) in
100 μl saline were injected into animals of the BMSC group with a
sterile microinjection at 5 ischemic sites. The needle was advanced
5 mm into the myocardium and the cells were injected at 1.5 mm.
Hemostasis was performed and the chest was closed in layers.
Postoperatively, penicillin G benzathine (30,000 U/day; Qilu
Pharmaceutical Co., Ltd., Jinan, China) was administered
intravenously for 3 days.
Left ventricular function analysis
Via right external jugular access, a 7F Swan-Ganz
catheter (Edwards Lifesciences, Irvine, CA, USA) was advanced into
the pulmonary artery for the assessment of cardiac output,
pulmonary arterial wedge pressure and central venous pressure using
the MP150 system (Biopac Systems, Inc., Goleta, CA, USA) with
output to computer at a sampling rate of 1 kHz, using Biopac
AcqKnowledge software at the following time points: Pre-ligation,
30 min post-ligation, and 6 months post-treatment. Two pressure
catheters were placed in the aorta and LV to monitor pressure. The
maximum velocity of LV contraction (dP/dtmax) and the
maximum velocity of LV diastole (dP/dtmin) were used to
assess LV function.
Myocardial perfusion analysis
Following intravenous administration of
nitroglycerin (0.4 mg) (12), all
animals received 14.8MB q kg−1 99mTc-sestamibi
(Institute of Atomic Energy, Beijing, China) at 6° per frame. A
total of 180° was received by rotating a 64×64 matrix detector in a
20% energy window using electrocardiograph-gated single photon
emission computed tomography (SPECT; Millennium vG-5; GE
Healthcare, CT, USA), with reconstruction parameters as follows:
Prefilter, Butterworth filter; critical frequency, 0.52; and power,
5.0. Analysis of changes in mass defect percentage (MDP), and
determination of end diastolic volume (EDV), end systolic volume
(ESV) and ejection fraction (EF) were performed using Emory Cardiac
Toolbox software (ECTb; GE Healthcare).
Immunological and immunohistochemical
analysis
The animals were sacrificed by intracoronary
perfusion of 10% KCl solution 3 months postoperatively. The hearts
were quickly harvested and the tissues in the infarcted zone were
collected and fixed with 10% formaldehyde (Shanghai Sunshine
Reagent Co., Ltd., Shanghai, China). Serial sections (5-μm) were
stained with Masson’s stain (Baso Biotechnology, Shenzhen, China)
to detect the myofilament structure, and sections were captured as
digital images. Myocardial density (MD) was quantified with the
Image Pro Plus software package, version 6.0 (IPP, Media
Cybernetics, Rockville, MD, USA). To evaluate the effect of
combined therapy on angiogenesis, sections of each group were
stained with rabbit anti-human von Willebrand factor (vWF) antibody
(DakoCytomation, CPH, Denmark). The vascular density was measured
with the IPP software in five non-overlapping fields
(magnification, ×100). Positively stained areas were padded with a
single color and converted to pixels through optical density (OD)
calibration.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
The TUNEL method was used to detect levels of cell
apoptosis in the infarction zone according to the In Situ Cell
Death Detection kit (Roche Diagnostics, Mannheim, Germany).
Briefly, deparaffinized 5-μm sections were incubated at 37°C for 1
h with 50 μl TUNEL reaction mixture [5 μl terminal
desoxynucleotidyl-transferase (TdT) and 45 μl dUTP-biotin]
following proteinase K treatment. In the negative control, TdT was
omitted, and positive controls were pretreated with DNAse A.
Sections were visualized and photographed with a BX41 Microscope
(Olympus America Inc., Center Valley, PA, USA) equipped with a
digital camera. TUNEL-positive cells were observed under a
microscope (magnification, ×200).
Reverse transcription polymerase chain
reaction (RT-PCR)
Once the hearts were collected and total RNA was
extracted from cardiac tissue with TRIzol (Invitrogen, Life
Technologies, Carlsbad, CA, USA). RT-PCR analysis was performed to
detect the relative pulmonary expression levels of vascular
endothelial growth factor (VEGF), vWF, transforming growth
factor-β3 (TGF-β3) and interleukin-1β (IL-1β) as previously
described (10).
Glyceraldehyde-3-phosphate dehydrogenase was used as an internal
control for RNA input level. The sequences of primers and reaction
conditions are described in our previous study (10). Following RT-PCR, 1% agarose gel
electrophoresis was performed and the UVI pro gel documentation
system (Uvitec, Cambridge, UK) was used for semi-quantification
analysis of each PCR product.
Statistical analysis
Statistical analysis was conducted using SPSS,
version 13.0 (SPSS, Inc. Chicago, IL, USA). The paired student’s
t-test was used for self-comparison. The independent samples
student’s t-test was used to compare experimental measurements
between two groups. Data are presented as the mean ± standard
deviation, and P<0.05 was considered to indicate a statistically
significant difference.
Results
Characterization of cultured BMSCs
Following primary culture for 4 days, the mini-swine
BMSCs appeared as colonies of large flat cells or spindle-like
cells, and the cells were attached to the culture dish tightly and
proliferated rapidly in the culture medium (10). FACS analysis indicated that the
cells did not express the endothelial marker CD31. The proportion
of Flk1+ cells was about 60% (11,12).
After 48 h, the transfection rates reached 80%.
Left ventricular and myocardial perfusion
function
Following the ligation of the LAD, the left
ventricular EF was significantly reduced, while the EDV and ESV
were significantly increased, compared with pre-ligation
(P<0.05; Table I). No
significant differences were identified in the baseline data of the
two groups. This indicates the successful establishment of an AMI
model (Table I). Six months after
the operation, the hemodynamic parameters, including EF, EDV and
ESV in the BMSC group displayed a significant improvement compared
with the control group (P<0.01; Table II).
 | Table IHemodynamic parameters of left
ventricular function pre- and post-ligation. |
Table I
Hemodynamic parameters of left
ventricular function pre- and post-ligation.
| Parameter | Pre-ligation | Post-ligation | P-value |
|---|
| HR (beats/min) | 73.00±5.00 | 72.00±3.00 | 1.000 |
| CVP (mmHg) | 3.45±0.62 | 3.53±0.58 | 1.000 |
| MAP (mmHg) | 93.57±3.65 | 93.83±347 | 0.506 |
| PAWP (mmHg) | 6.48±0.76 | 6.51±0.69 | 0.804 |
| LVEDP (mmHg) | 6.39±0.78 | 6.72±0.85a | 0.033 |
| CI
(l/min/m2) | 5.38±0.13 | 5.06±0.18a | <0.001 |
| dP/dtmax
(mmHg/sec) | 3489±189 | 2988±186a | <0.001 |
| dP/dtmin
(mmHg/sec) | −1908±96 | −1475±97a | <0.001 |
| SvO2
(%) | 78.49±4.28 | 76.28±2.37 | 0.509 |
| LVEDV (ml) | 115.0±16.0 | 129±19a | <0.001 |
| LVESV (ml) | 29.0±2.3 | 45.0±3.6a | <0.001 |
| EF (%) | 68.2±8.0 | 50.3±6.0a | <0.001 |
 | Table IIHemodynamic parameters of left
ventricular function 6 months following surgery. |
Table II
Hemodynamic parameters of left
ventricular function 6 months following surgery.
| Parameter | Control | BMSCs |
|---|
| HR (beats/min) | 73±4 | 73±2 |
| CVP (mmHg) | 3.96±0.41 | 4.02±0.39 |
| MAP (mmHg) | 94.8±5.1 | 94.3±6.8 |
| PAWP (mmHg) | 7.33±1.03 | 7.07±0.77 |
| LVEDP (mmHg) | 9.24±0.18 | 7.4±0.15a |
| dP/dtmax
(mmHg/sec) | 1710±112 | 2530±174a |
| dP/dtmin
(mmHg/sec) | −648±27 | −1045±89a |
| LVEDV (ml) | 162.5±14.5 | 135.3±13.9a |
| LVESV (ml) | 83.2±6.3 | 61.2±5.1a |
| EF (%) | 50.6±5.3 | 62.7±6.2a |
| CI
(l/min/m2) | 4.80±0.28 | 4.82±0.38 |
| SvO2
(%) | 74.5±3.4 | 76.7±2.8 |
| Hb (g/dl) | 9.28±0.32 | 9.22±0.29 |
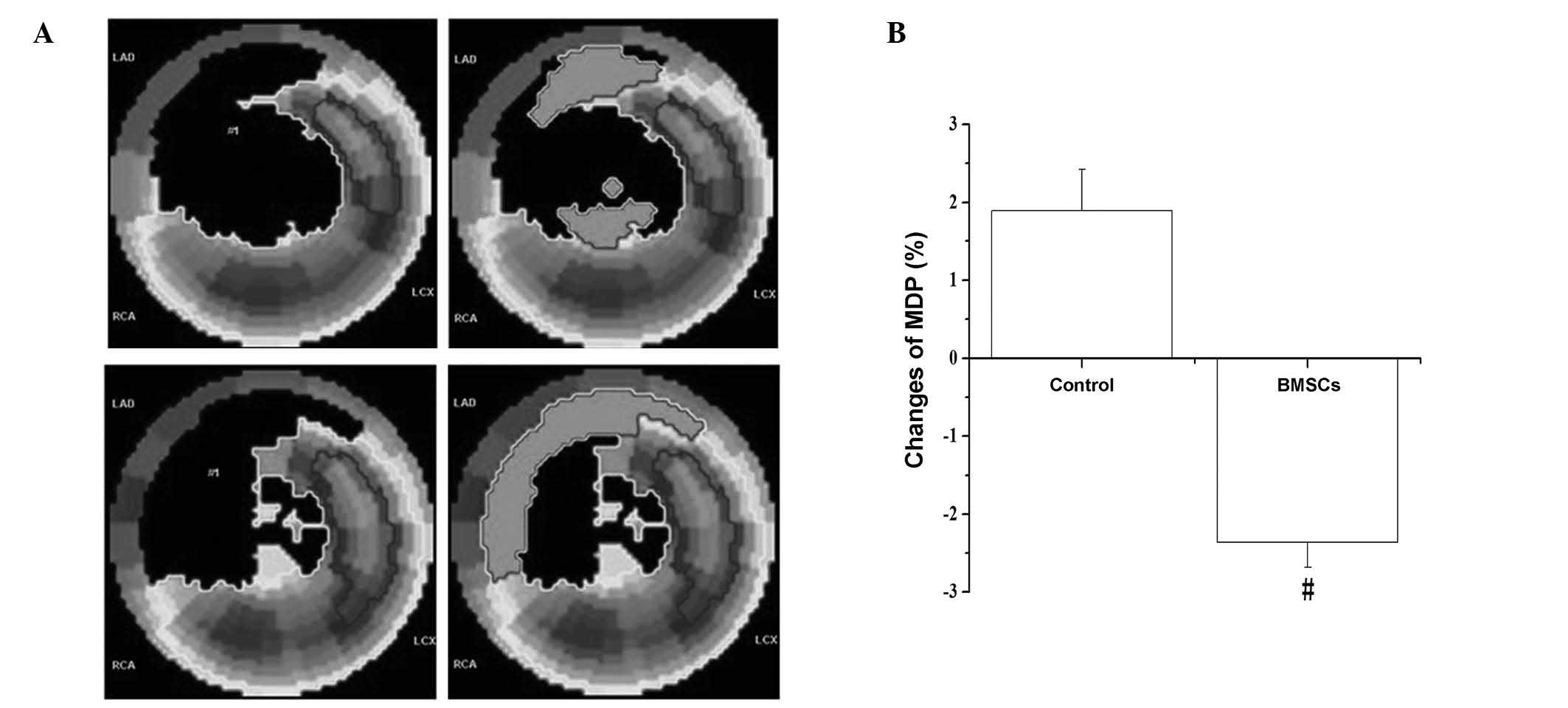
Myocardial perfusion was reflected by
99mTc-SPECT myocardial imaging
The image results revealed that the perfusion defect
was visibly enlarged in the control group, but significantly
reduced in the BMSC group (Fig.
1A); MDP underwent a significant reduction in the BMSC group
(−2.36±0.32) compared with the level in the control group
(1.89±0.53) (P<0.05; Fig. 1B).
This indicates that the mass of infarct-related defect myocardium
was reduced and the regional myocardial blood flow was
increased.
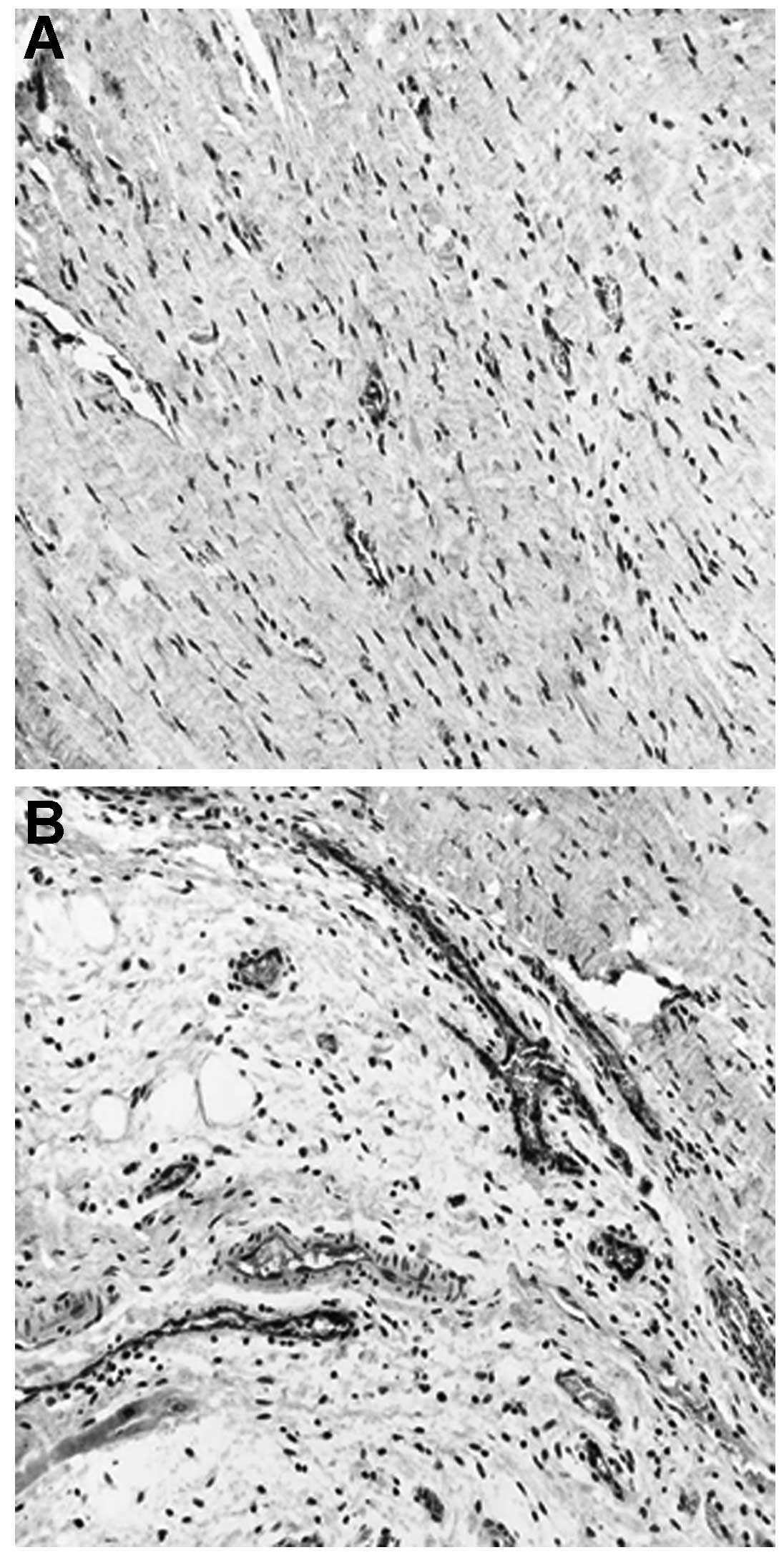
Vessel and myocardial density
analysis
The vessel density was determined by histological
staining (vWF) of the infarcted area (Fig. 2). A significant increase in the
BMSC group OD (OD=5,327+401 pixels/hpf) was observed, compared with
that of the control group (OD=2,511+308 pixels/hpf)
(P<0.05).
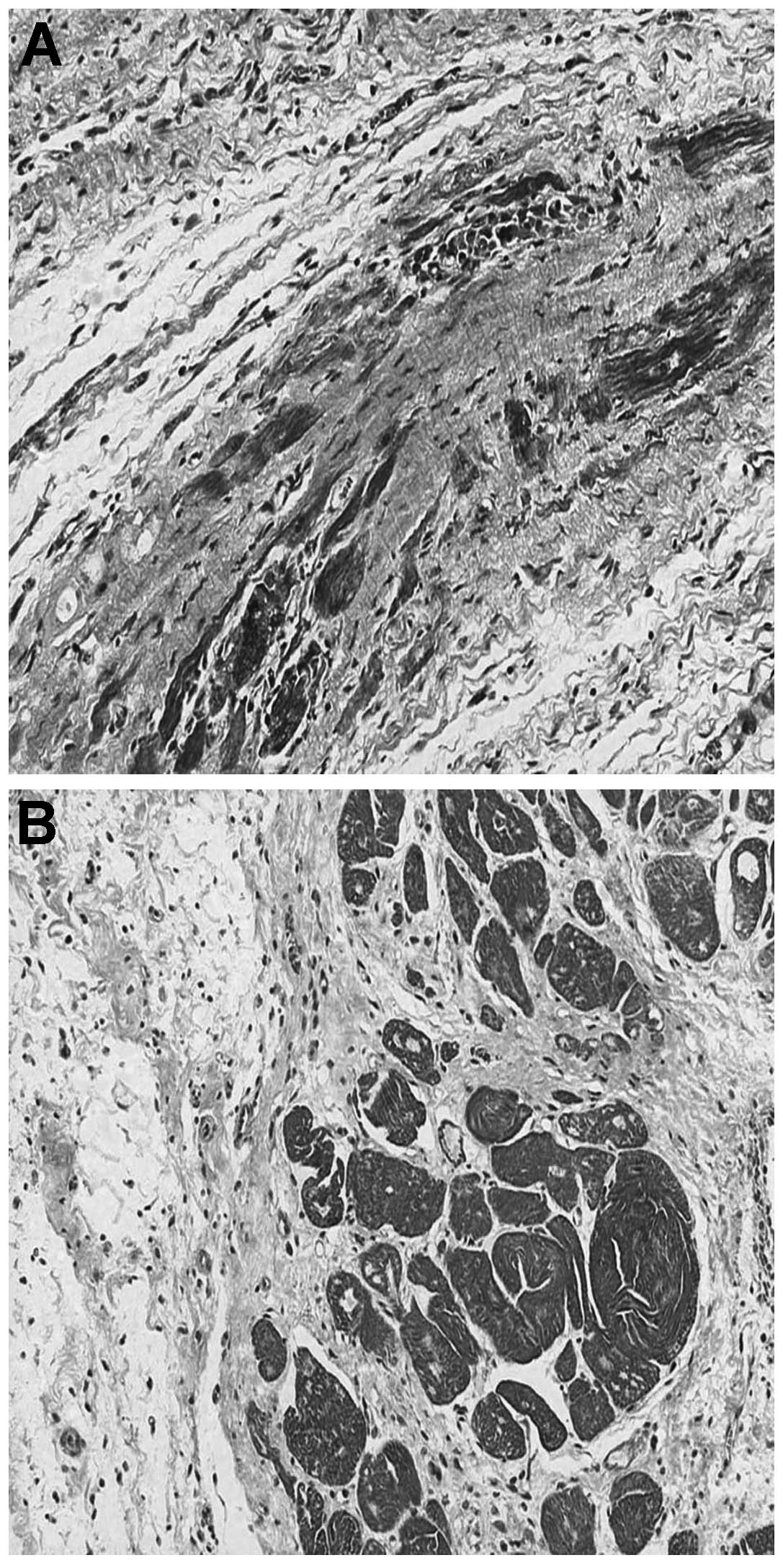
Masson’s trichrome staining
Survived myocardial tissue in the infarcted area was
significantly greater in the BMSC group compared with that of the
control group (Fig. 3). Following
calculations, MD demonstrated significant increases in myocardial
viability in the BMSC group (OD=80,402±3,015 pixels/hpf) compared
with that in control group (OD=25,340±2,918 pixels/hpf,
P<0.05).
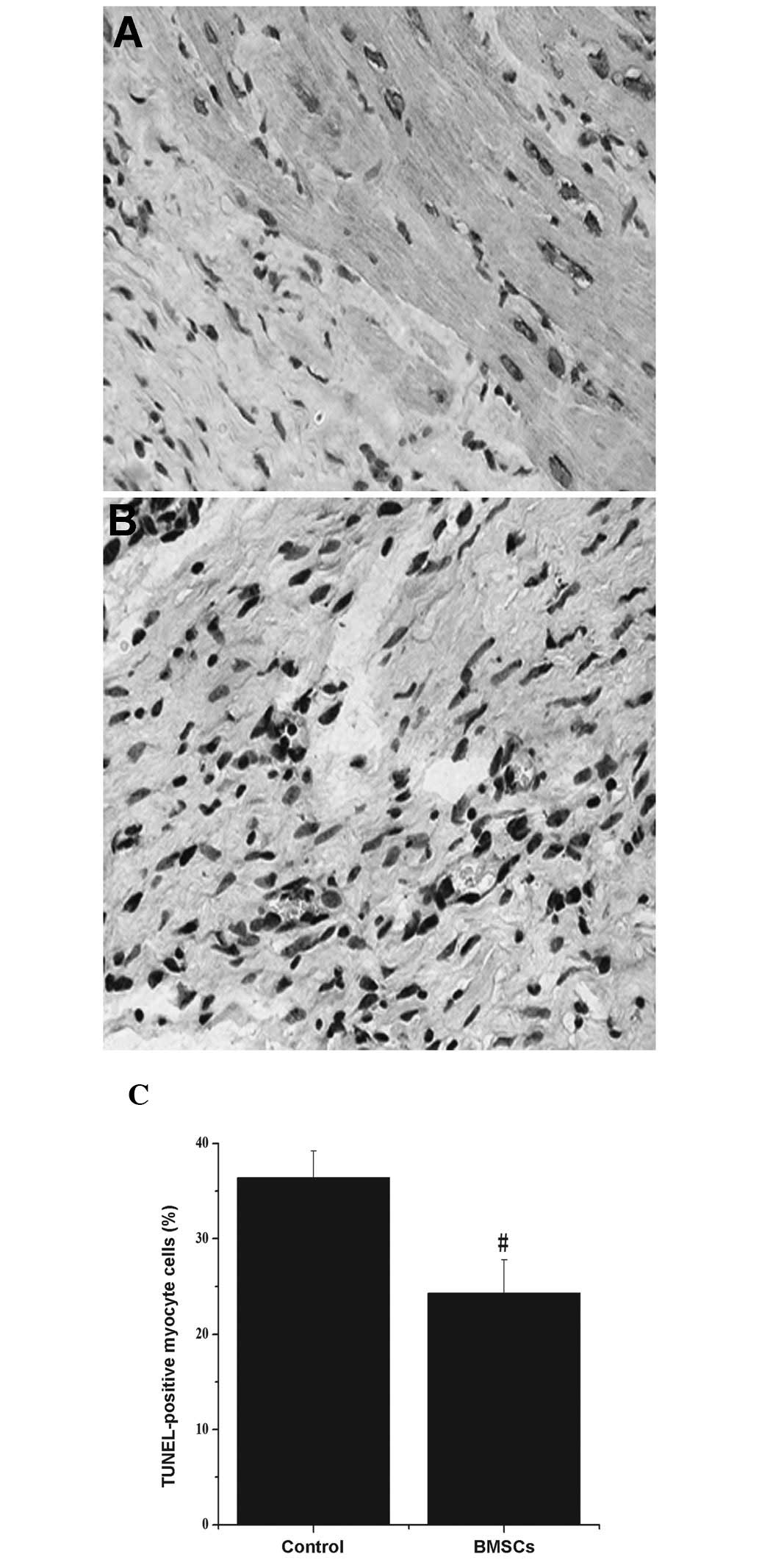
Apoptosis measurement
Myocardial apoptosis was detected by TUNEL staining
(Fig. 4A and B). Following
calculations, the number of TUNEL-positive myocytes was
significantly reduced in the BMSC group (24.3±3.5) compared with
levels in the control group (36.4±2.8) (Fig. 4C; P<0.05).
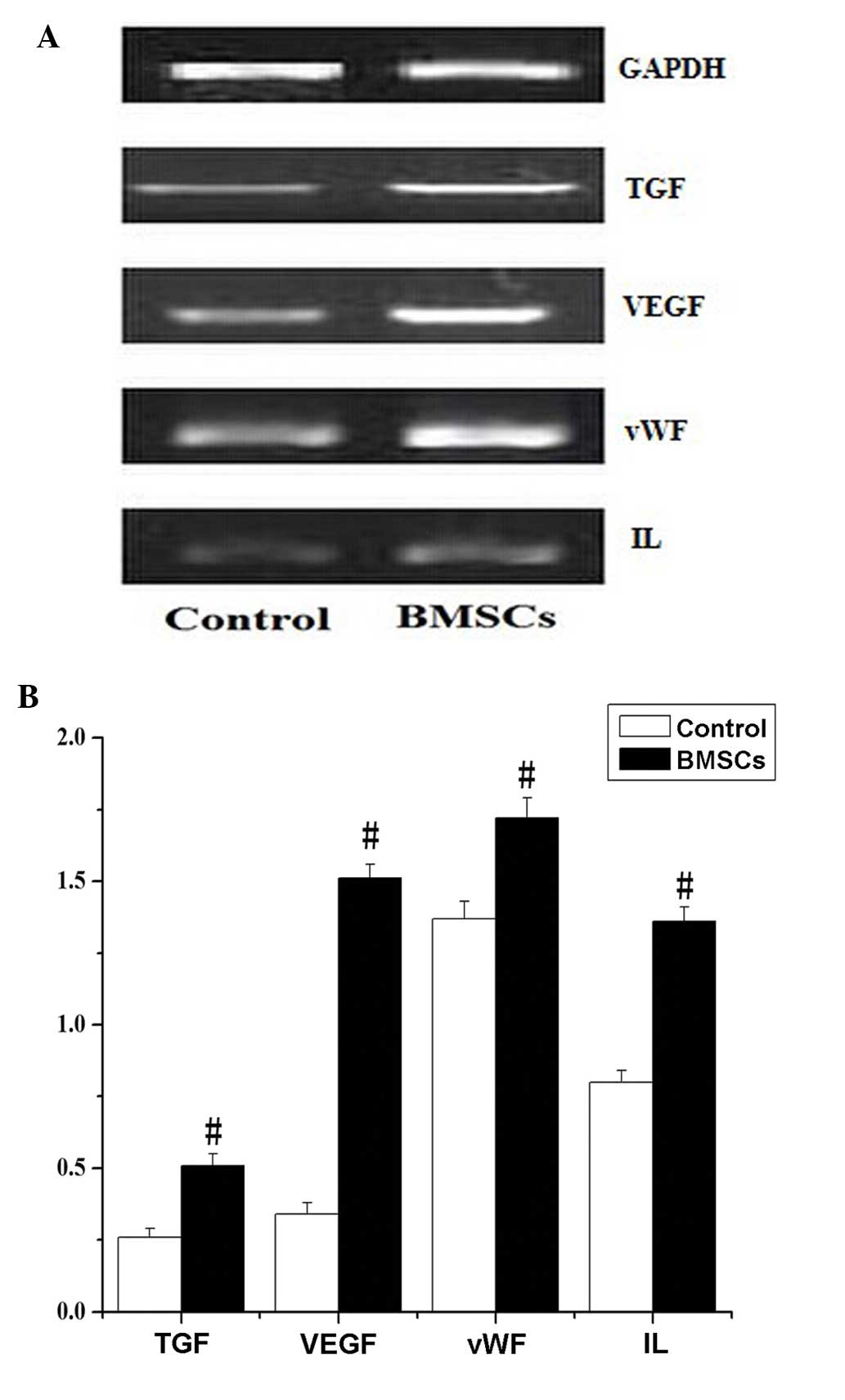
RT-PCR analysis
RT-PCR results demonstrated that the expression
levels of VEGF, vWF, TGF-3β and IL-1β in the infarction zone were
significantly increased in the BMSC group compared with levels in
the control group (P<0.05; Fig.
5).
Discussion
In the current study,
Flk1+CD34+CD3− BMSCs were
intramyocardially injected into a mini-swine model of AMI. Six
months later, the results indicated that: i) Myocardial filling
defect was reduced and left ventricular ejection fraction was
increased; ii) the percentage of apoptosis was decreased and the
survived myocardial tissue was increased; iii) vessel density was
augmented and a number of inflammatory cytokines were upregulated;
and iv) the transplantation cells were able to survive in
vivo and were concentrated around blood vessels. To the best of
our knowledge, the present study demonstrated for the first time,
the mid-term effect of BMSCs in an AMI animal model, and revealed a
possible mechanism.
BMSCs are multipotent progenitor cells derived from
the fetal bone marrow, and have the characteristics of self-renewal
and multiple differentiation potential. Following direct injection
into an infarcted heart, the cells have been demonstrated to
improve cardiac function (13,14),
decrease fibrous tissue accumulation (15), and repair cardiac infarcts
(16). BMSCs are easy to obtain
and have a high transfection efficiency, and therefore are ideal
for genetic engineering and clinical applications (17). Although BMSC transplantation has
provided a promising therapeutic option, the protective effect in
clinical trials remains controversial (18). In fact, many questions remain
unsolved (19), including the
choice of animal for the model, and the transplantation method. On
the other hand, most studies have focused on the short-term effects
of BMSC transplantation (14–20),
and studies on medium and long-term effects are few (21). Intramyocardial injection of MSCs
has been demonstrated to be safe in large animal models (5,11),
and in the present study, 6 months following intramyocardial
injection of Flk1+CD34+CD3− BMSCs,
SPECT detection indicated that the myocardial filling defect was
reduced and LV ejection fraction was significantly improved in AMI
models injected with BMSCs compared with uninjected models.
Histopathological examination indicated that the area covered by
the myocardial infarction and the percentage of cells undergoing
apoptosis were reduced; and the percentage of survived myocardial
tissue and the vessel densities were augmented, following BMSC
injection (P<0.05, compared with controls).
As reported previously, the effect of paracrine
signaling may further improve angiogenesis (20,22–26).
It has been demonstrated that BMSCs secrete angiogenic and
anti-apoptotic factors that stimulate proliferation of endothelial
cells and smooth muscle cells (26–29).
In the present study, RT-PCR results indicated that the expression
levels of VEGF, vWF, TGF-3β and IL-1β in the infarction zone were
significantly increased in the BMSC group compared with the control
group. BMSCs have been demonstrated to survive in infarcted
myocardium for at least 6 months and the cells expressed muscle
markers (21). In our previous
study, DiI-labeled BMSCs survived in infarcted myocardium and
expressed the cardiac marker, cTnT, vWF and smooth muscle actin at
3 months following transplantation.
In conclusion, in the present study,
Flk1+CD34+CD3− BMSC
transplantation into a mini-swine model of AMI was demonstrated to
greatly increase LV function, cardiac blood flow, and vascular
density; and decrease cell apoptosis in the long-term postoperative
period. The present study provides useful information for the
development of potential BMSC-based therapies for myocardial
infarction.
Acknowledgements
This study was supported by a grant from the Youth
Foundation of the Second Hospital of Shandong University (grant no.
Y2013010068) and the Natural Science Foundation of Shandong
Province (grant no. ZR2010HM125).
Abbreviations:
|
AMI
|
acute myocardial infarction
|
|
BMSCs
|
bone marrow-derived stem cells
|
|
CI
|
cardiac index
|
|
CVP
|
central venous pressure
|
|
dP/dtmax
|
velocity of LV contraction
|
|
dP/dtmin
|
velocity of LV diastole
|
|
EF
|
ejection fraction
|
|
HR
|
heart rate
|
|
LVEDP
|
left ventricular end-diastolic
perfusion
|
|
LVEDV
|
left ventricular end-diastolic
volume
|
|
LVESV
|
left ventricular end-systolic
volume
|
|
MAP
|
mean arterial pressure
|
|
MD
|
myocardial density
|
|
MDP
|
mass defect percentage
|
|
PAWP
|
pulmonary arterial wedge pressure
|
|
SvO2
|
percentage oxygen saturation
|
References
|
1
|
Roger VL, Go AS, Lloyd-Jones DM, et al;
American Heart Association Statistics Committee and Stroke
Statistics Subcommittee. Heart disease and stroke statistics - 2011
update: a report from the American Heart Association. Circulation.
123:e18–e209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karapetyan AV, Klyachkin YM, Selim S, et
al: Bioactive lipids and cationic antimicrobial peptides as new
potential regulators for trafficking of bone marrow-derived stem
cells in patients with acute myocardial infarction. Stem Cells Dev.
22:1645–1656. 2013. View Article : Google Scholar
|
|
3
|
Williams AR, Hatzistergos KE, Addicott B,
et al: Enhanced effect of combining human cardiac stem cells and
bone marrow mesenchymal stem cells to reduce infarct size and to
restore cardiac function after myocardial infarction. Circulation.
127:213–223. 2013. View Article : Google Scholar
|
|
4
|
Welt FG, Gallegos R, Connell J, et al:
Effect of cardiac stem cells on left-ventricular remodeling in a
canine model of chronic myocardial infarction. Circ Heart Fail.
6:99–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang GW, Liu XC, Li-Ling J, et al:
Mechanisms of the protective effects of BMSCs promoted by TMDR with
heparinized bFGF-incorporated stent in pig model of acute
myocardial ischemia. J Cell Mol Med. 15:1075–1086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peng C, Yang K, Xiang P, et al: Effect of
transplantation with autologous bone marrow stem cells on acute
myocardial infarction. Int J Cardiol. 162:158–165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hatzistergos KE, Quevedo H, Oskouei BN, et
al: Bone marrow mesenchymal stem cells stimulate cardiac stem cell
proliferation and differentiation. Circ Res. 107:913–922. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Quevedo HC, Hatzistergos KE, Oskouei BN,
et al: Allogeneic mesenchymal stem cells restore cardiac function
in chronic ischemic cardiomyopathy via trilineage differentiating
capacity. Proc Natl Acad Sci USA. 106:14022–14027. 2009. View Article : Google Scholar
|
|
9
|
Gnecchi M, Zhang Z, Ni A and Dzau VJ:
Paracrine mechanisms in adult stem cell signaling and therapy. Circ
Res. 103:1204–1219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luan Y, Liu XC, Zhang GW, et al: Mid-term
effect of stem cells combined with transmyocardial degradable stent
on swine model of acute myocardial infarction. Coron Artery Dis.
21:233–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Liu XC, Zhang GW, et al: A new
transmyocardial degradable stent combined with growth factor,
heparin, and stem cells in acute myocardial infarction. Cardiovasc
Res. 84:461–469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spadafora M, Varrella P, Acampa W, et al:
Direct imaging of viable myocardium by gated SPECT in patients with
ischaemic left ventricular dysfunction. Eur J Nucl Med Mol Imaging.
37:1730–1735. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagaya N, Kangawa K, Itoh T, et al:
Transplantation of mesenchymal stem cells improves cardiac function
in a rat model of dilated cardiomyopathy. Circulation.
112:1128–1135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hou M, Yang KM, Zhang H, et al:
Transplantation of mesenchymal stem cells from human bone marrow
improves damaged heart function in rats. Int J Cardiol.
115:220–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyahara Y, Nagaya N, Kataoka M, et al:
Monolayered mesenchymal stem cells repair scarred myocardium after
myocardial infarction. Nat Med. 12:459–465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Imanishi Y, Saito A, Komoda H, et al:
Allogenic mesenchymal stem cell transplantation has a therapeutic
effect in acute myocardial infarction in rats. J Mol Cell Cardiol.
44:662–671. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pittenger MF and Martin BJ: Mesenchymal
stem cells and their potential as cardiac therapeutics. Circ Res.
95:9–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi SC, Kim SJ, Choi JH, et al:
Fibroblast growth factor-2 and -4 promote the proliferation of bone
marrow mesenchymal stem cells by the activation of the PI3K-Akt and
ERK1/2 signaling pathways. Stem Cells Dev. 17:725–736. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moelker AD, Baks T, van den Bos EJ, et al:
Reduction in infarct size, but no functional improvement after bone
marrow cell administration in a porcine model of reperfused
myocardial infarction. Eur Heart J. 27:3057–3064. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nanjundappa A, Raza JA, Dieter RS, et al:
Cell transplantation for treatment of left-ventricular dysfunction
due to ischemic heart failure: from bench to bedside. Expert Rev
Cardiovasc Ther. 5:125–131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dai W, Hale SL, Martin BJ, et al:
Allogeneic mesenchymal stem cell transplantation in postinfarcted
rat myocardium: short- and long-term effects. Circulation.
112:214–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang D, Wang W, Li L, et al: The relative
contribution of paracine effect versus direct differentiation on
adipose-derived stem cell transplantation mediated cardiac repair.
PLoS One. 8:e590202013. View Article : Google Scholar
|
|
23
|
Kinnaird T, Stabile E, Burnett MS, et al:
Local delivery of marrow-derived stromal cells augments collateral
perfusion through paracrine mechanisms. Circulation. 109:1543–1549.
2004. View Article : Google Scholar
|
|
24
|
Rahbarghazi R, Nassiri SM, Khazraiinia P,
et al: Juxtacrine and paracrine interactions of rat marrow-derived
mesenchymal stem cells, muscle-derived satellite cells, and
neonatal cardiomyocytes with endothelial cells in angiogenesis
dynamics. Stem Cells Dev. 22:855–865. 2013. View Article : Google Scholar
|
|
25
|
Wang Y, Tang H, Wang D, et al:
Pretreatment with transmyocardial revascularization might improve
ischemic myocardial function performed with cell transplantation.
Circ J. 70:625–630. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu MH, Jin H, Floten HS, et al: Vascular
endothelial growth factor-mediated, endothelium-dependent
relaxation in human internal mammary artery. Ann Thorac Surg.
73:819–824. 2002. View Article : Google Scholar
|
|
27
|
Muraoka N, Shum L, Fukumoto S, et al:
Transforming growth factor-beta3 promotes mesenchymal cell
proliferation and angiogenesis mediated by the enhancement of
cyclin D1, Flk-1, and CD31 gene expression during CL/Fr mouse lip
fusion. Birth Defects Res A Clin Mol Teratol. 73:956–965. 2005.
View Article : Google Scholar
|
|
28
|
Li H, Zuo S, He Z, et al: Paracrine
factors released by GATA-4 overexpressed mesenchymal stem cells
increase angiogenesis and cell survival. Am J Physiol Heart Circ
Physiol. 299:H1772–H1781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takehara N, Tsutsumi Y, Tateishi K, et al:
Controlled delivery of basic fibroblast growth factor promotes
human cardiosphere-derived cell engraftment to enhance cardiac
repair for chronic myocardial infarction. J Am Coll Cardiol.
52:1858–1865. 2008. View Article : Google Scholar
|