Introduction
Doxorubicin (DOX) was introduced in cancer therapy
in the late 1960s. It has emerged as one of the most potent
broad-spectrum antitumor anthracycline antibiotics. DOX can be
administered as a single agent or in combination with other
chemotherapeutic agents. It is widely used in the treatment of a
variety of cancer types, including leukemia, lymphoma, soft-tissue
sarcoma and solid tumors. Its cytotoxic effects on malignant cells,
however, are complicated by an increase in the risk of
cardiotoxicity (1,2).
It is notable that DOX directly generates free
radicals. Moreover, through redox cycling, DOX is a strong chemical
catalyst for the production of oxygen radicals (3,4).
Furthermore, a reduction in the quantity of endogenous antioxidants
has been demonstrated following DOX treatment (5). The oxidative damage induced by DOX
affects the lysosomes, microfibrils, mitochondria and the
sarcoplasmic reticulum (6–9). Eventually, these intracellular
modifications result in increased apoptosis in the cardiac
myocytes.
In an attempt to minimize the effective
chemotherapeutic dose of DOX and thereby, its side-effects, a
variety of approaches have been adopted. One of these approaches is
the screening of natural compounds with chemopreventive or
anticancer properties that can be used in combination with DOX.
Resveratrol (RSVL) is a naturally occurring polyphenolic compound
(trans-3,5,4-trihydroxystilbene) found primarily in root extracts
of the oriental plant Polygonum cuspidatum and of numerous
plant species (10). It is highly
abundant in the skin of red grapes and moderately abundant in
peanuts and blueberries (10).
A previous study by our group using a model of
DOX-induced heart damage in rats showed that pretreatment with aged
garlic extract, a strong antioxidant, provides protection from
DOX-induced myocardial cell damage (11). Therefore, the present study was
undertaken to test whether RSVL, a compound with known antioxidant
properties, can also protect cells from DOX-induced cardiotoxicity.
Using electron microscopy, we studied the subcellular effects of
DOX in the heart and the underlying mechanisms of these
effects.
Materials and methods
Reagents
DOX hydrochloride and RSVL were purchased from
Sigma-Aldrich (St. Louis, MO, USA). The stock solution of both
drugs was dissolved in normal saline and preserved at −20°C. The
solutions were diluted in normal saline to reach the desired final
concentration immediately prior to each experiment.
Animals
Male Wistar albino rats (8–10 weeks of age, 180–200
g body weight) were obtained from the King Fahd Medical Research
Center, King Abdulaziz University, Jeddah, Saudi Arabia. The
animals were acclimatized for one week. Rats had access to a
commercial balanced diet and water ad libitum throughout the
experiment. This study was approved by the Ethical Committee of the
King Abdulaziz Hospital.
Experimental protocol
A total of 24 male Wistar rats were divided into 4
equal groups consisting of 6 animals each and housed in a room with
regular 12-h light/dark cycle with free access to food and water.
Two groups (I and II) were used as controls, and rats in these
groups received an intraperitoneal (i.p.) injection of normal
saline (0.9%) and RSVL (10 mg/kg, i.p.), respectively. Group III
received DOX (20 mg/kg), while Group IV received DOX (20 mg/kg)
simultaneously with RSVL (10 mg/kg). At the end of the experiment,
i.e. 48 h after the DOX injection, rats were anesthetized and blood
samples were collected from the ophthalmic artery in the orbital
rim prior to sacrifice. The serum was isolated from these samples
and the heart specimens were fixed in 2.5% formaldehyde and 2.5%
glutaraldehyde for electron microscopy.
Assessment of cardiac enzyme
activities
Plasma total lactate dehydrogenase (LDH) and total
creatine phosphokinase (CPK) activities were determined using
commercial kits from Randox Laboratories (Crumlin, UK) and
Spinreact (Girona, Spain), respectively.
Determination of lipid peroxides
Frozen heart samples were thawed, rinsed
successively with 0.9% NaCl and with cold (4°C) 20 mM Tris-HCl,
followed by homogenization in a Branson sonifier (250; VWR
International, Danbury, CT, USA). The homogenates were diluted with
cold 20 mM Tris-HCl and centrifuged (10 min at 4°C; 3,000 × g). The
levels of malondialdehyde (MDA) and reduced glutathione were
assayed spectrophotometrically at 534 nm using commercial kits from
Randox Laboratories in accordance with the instructions of the
manufacturer.
Determination of total antioxidant
capacity (TAC) in the serum
TAC was determined using a previously described
method (12) based on the
quenching of the radical ABRS+ (2,2-azino-di(3-ethyl
benzthiazolin sulphonate cation) by antioxidants. The Total
Antioxidant Assay kit (NX2332; Randox Laboratories) was used for
this purpose.
Examination of heart sections by electron
microscopy
Preparation of samples for electron microscopy was
performed as follows: biopsies were placed into fixative buffer
containing 2.5% formaldehyde and 2.5% glutaraldehyde for ≥1 h.
Tissue samples were rinsed three times for 15 min each with 0.075 M
sodium phosphate buffer, after which the samples were placed in a
1% osmium tetraoxide (OsO4) secondary fixative buffer
for 1 h. Samples were finally embedded with Quetol epoxy resin
(Polysciences Europe GmbH, Eppelheim, Germany) in rubber moulds and
allowed to polymerize in an oven at 60°C for ~39 h prior to
ultramicrotomy. Samples were subsequently cut at a ~70–100 nm
thickness, placed onto copper grids and stained. Transmission
electron microscopy analysis was then performed using a JEM 2100F
transmission electron microscope (JEOL, Peabody, MA, USA).
Statistical analysis
Results were expressed as the mean ± standard error
of the mean. Comparisons between different groups were carried out
by one way analysis of variance tests. P≤0.05 was considered to
indicate a statistically significant difference.
Results
Effect of RSVL on DOX-induced
cardiotoxicity
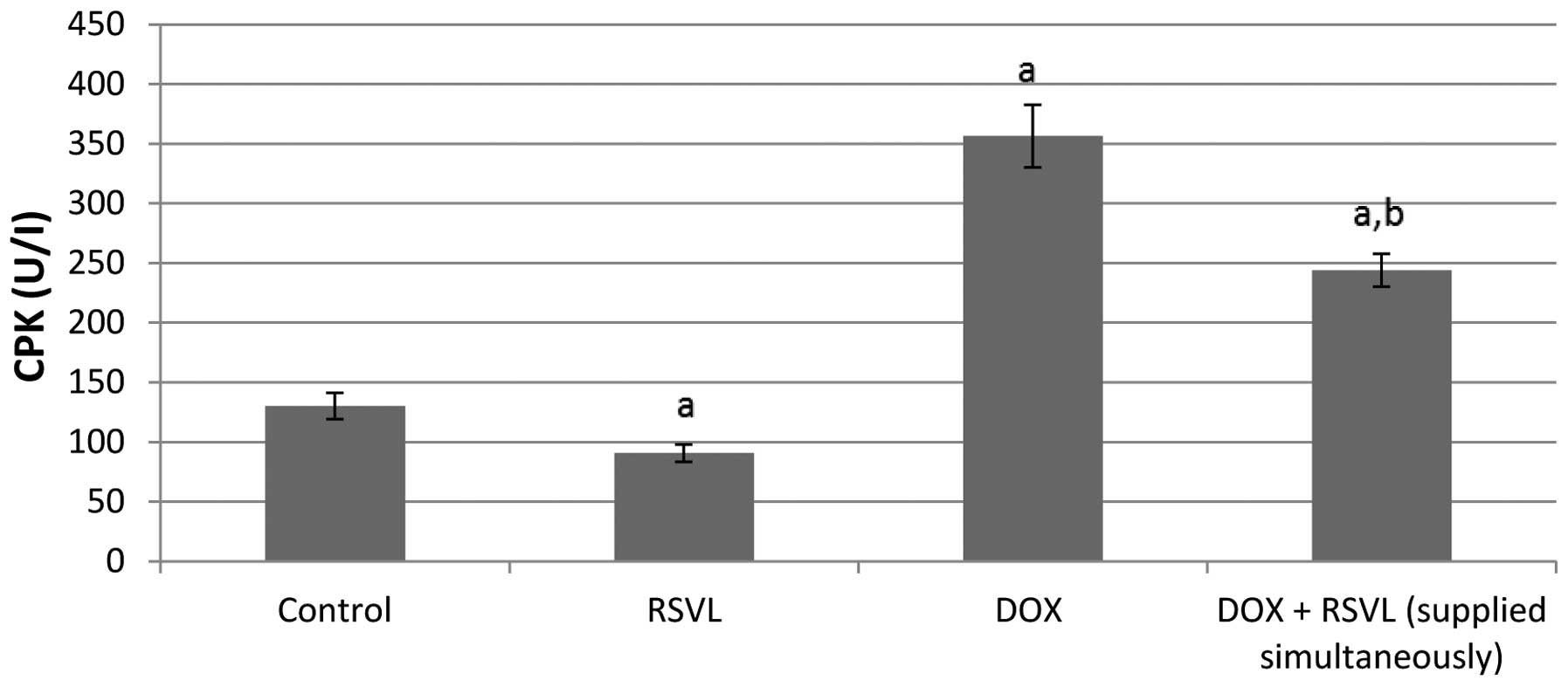
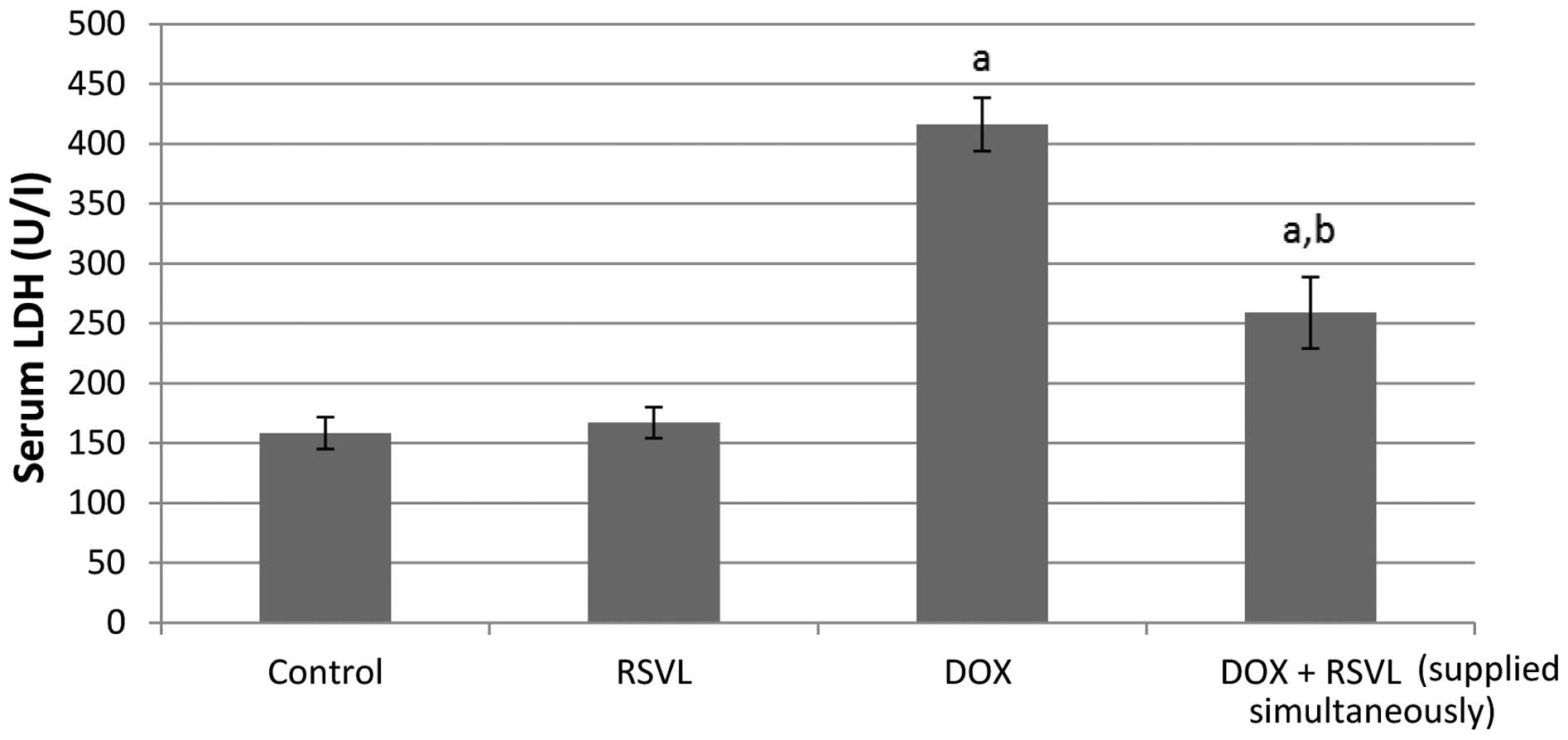
Treatment of rats with DOX (20 mg/kg, i.p.) caused a
significant 2.7-fold increase in the activity of both serum CPK and
LDH enzymes (Figs. 1 and 2). Simultaneous treatment with DOX and
RSVL reduced the effect of DOX by 1.9- and 1.6-fold,
respectively.
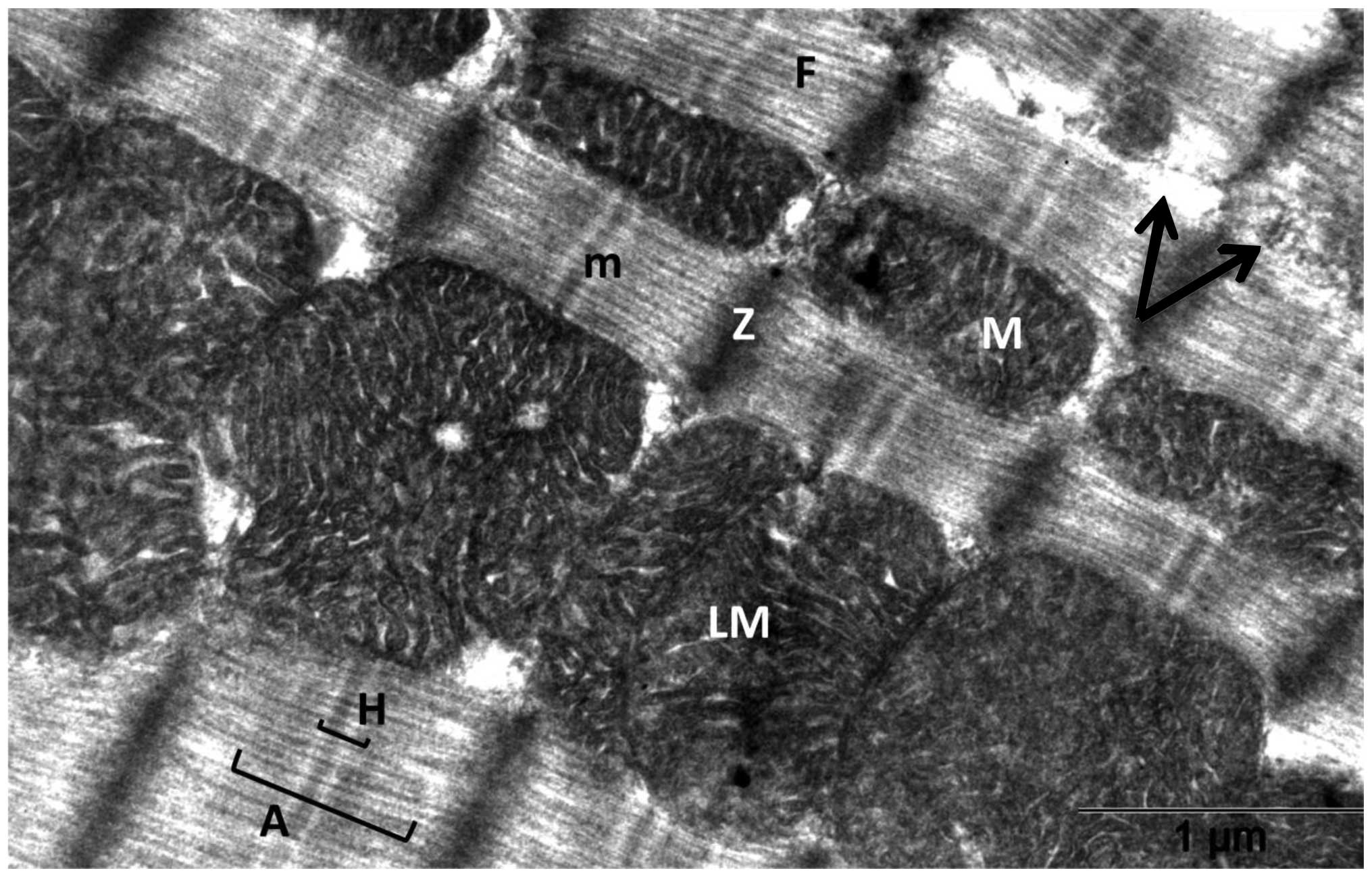
The comparison of heart tissues of control rats
(Fig. 3) and of rats treated with
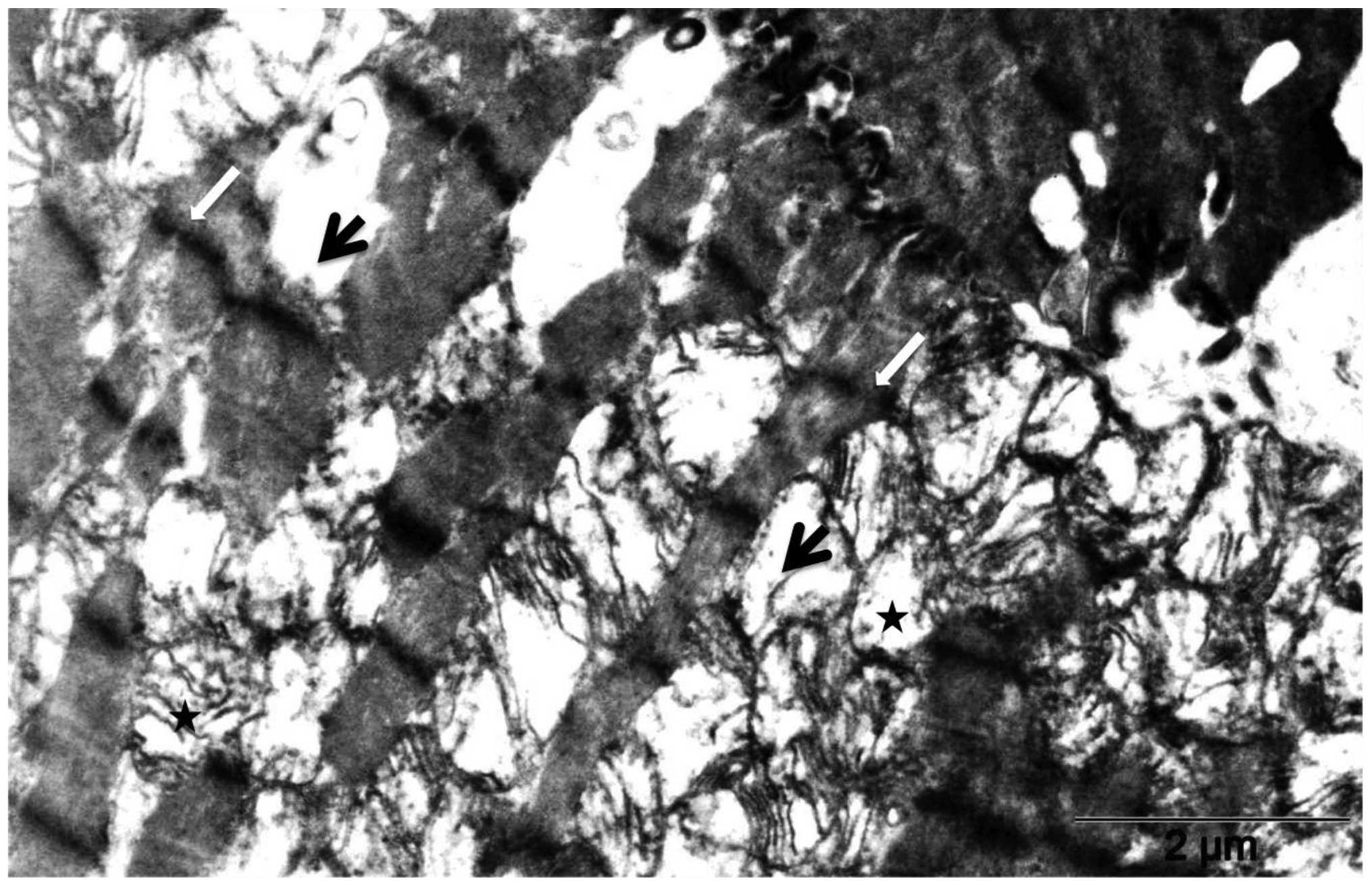
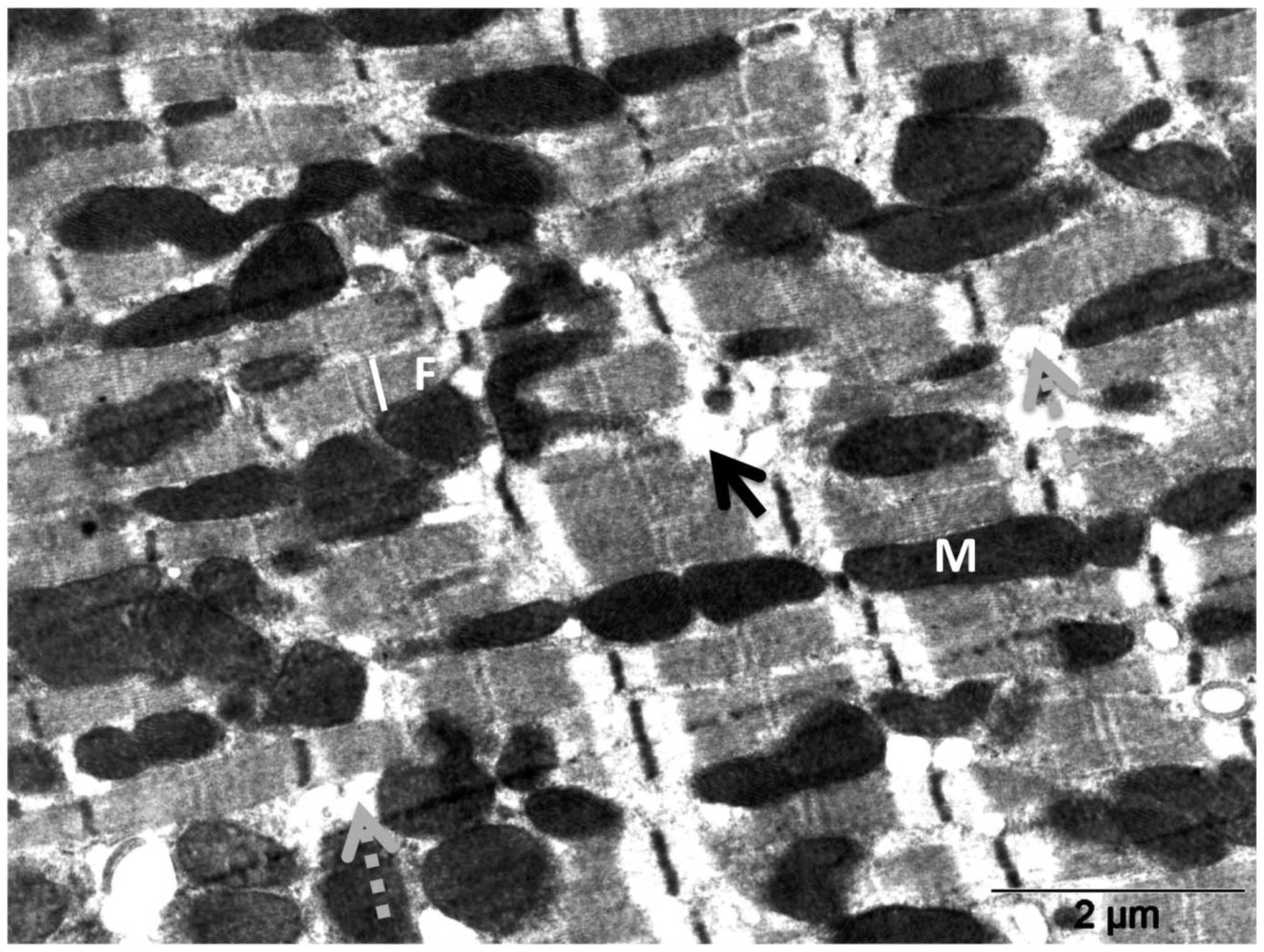
20 mg/kg DOX (Fig. 4) by electron
microscopy revealed massive fragmentation and lysis of the
myofibrils upon DOX treatment (Fig.
4, black arrows). Mitochondria showed either vacuolization or
complete loss of the cristae. Interruption of Z lines (Fig. 4, white arrows) was also evident.
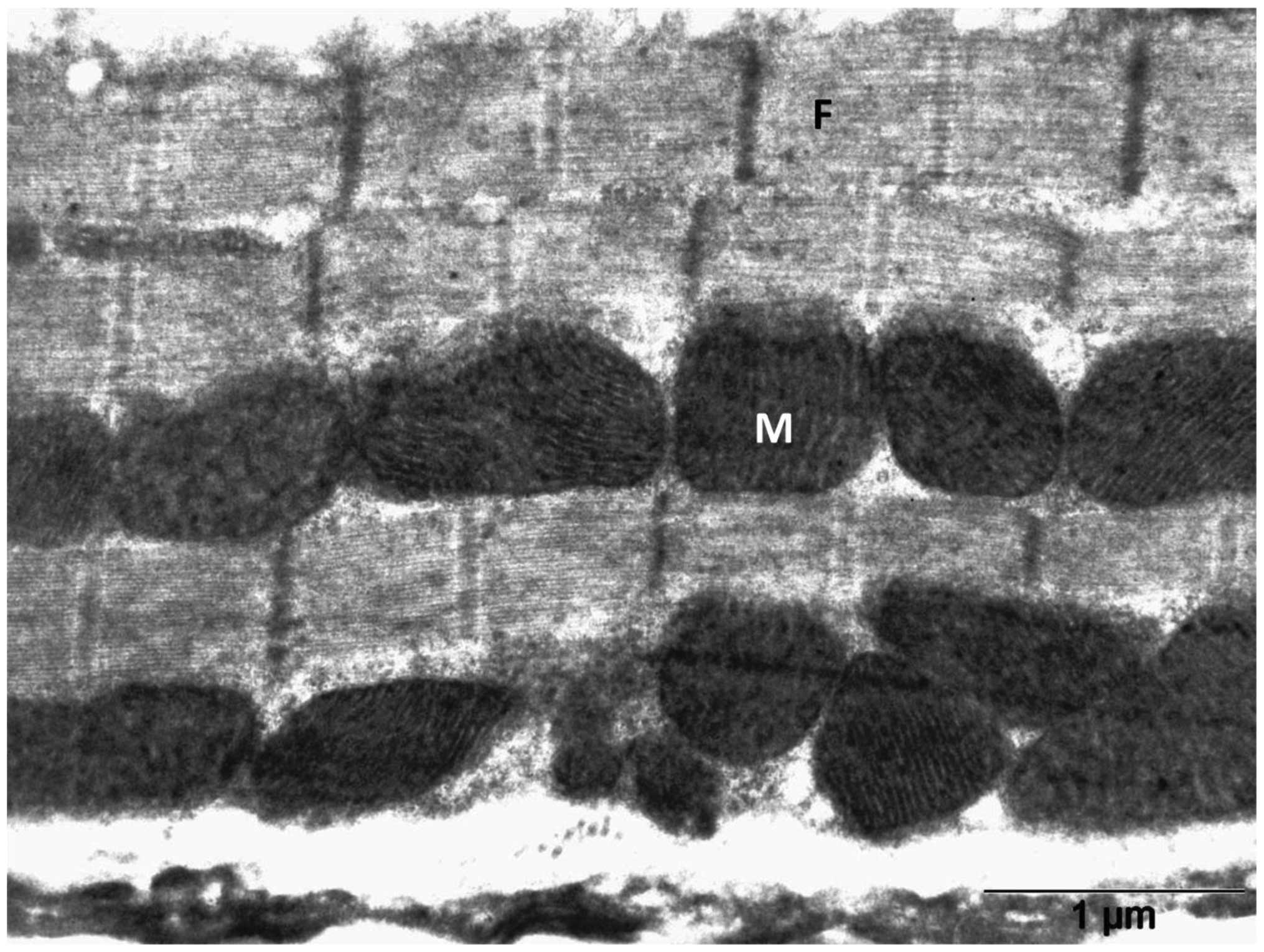
The heart tissues showed organized myofibrils with mitochondria in
between upon simultaneous treatment with DOX with RSVL ((Fig. 5). The mitochondria retained a
normal structure similarly to those of control rats (Fig. 3). Focal areas of myofibrilar loss
(Fig. 5, black arrow) and dilated
sarcoplasmic reticulum (Fig. 5,
grey dashed arrow) were observed. Rats treated with RSVL alone
(Fig. 6) showed a generally
organized myofibril architecture. The mitochondria in these rats
showed a regular cristae pattern.
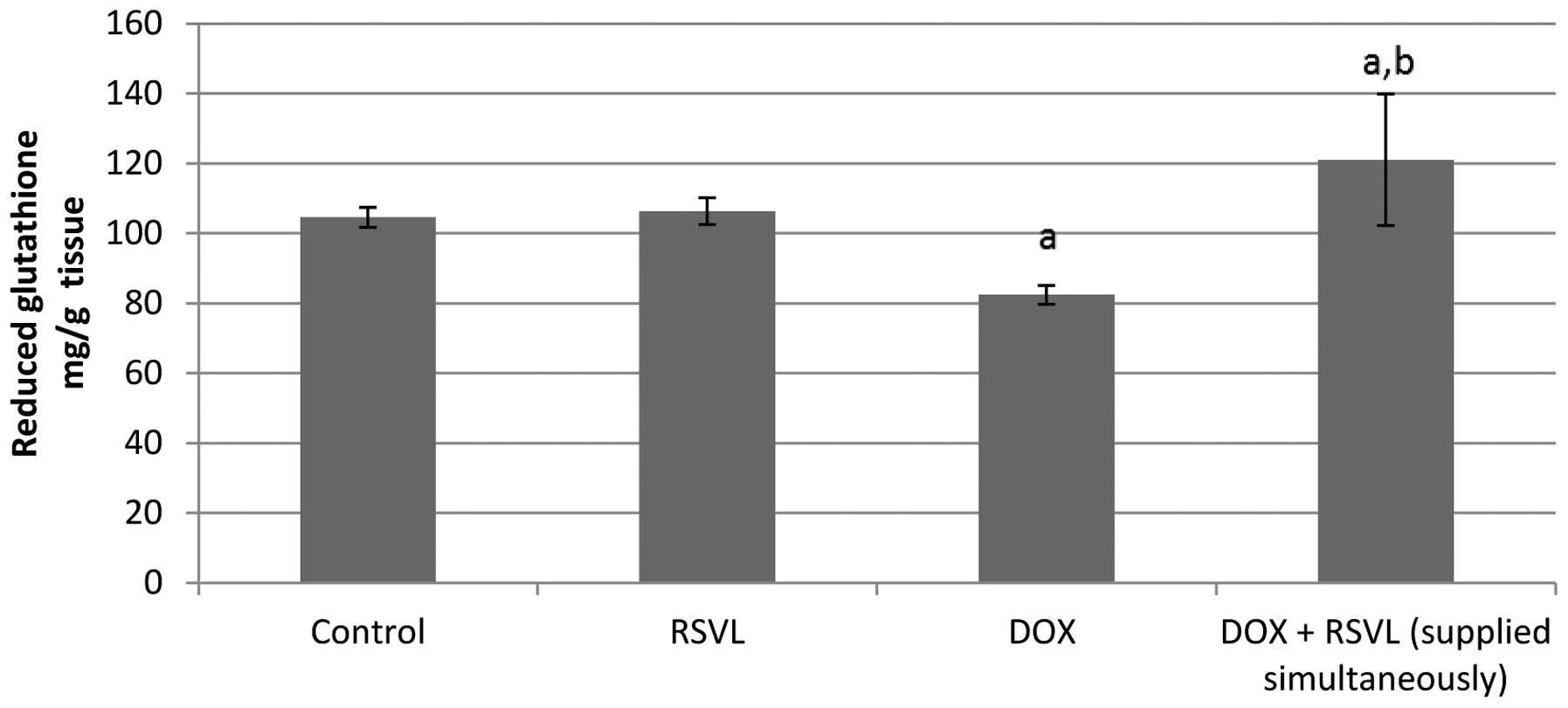
Effect of RSVL on DOX-induced changes in
the levels of MDA and reduced glutathione
Table I and
Fig. 7 show the effect of combined
RSVL and DOX treatment on the levels of MDA and reduced glutathione
in the heart tissues. DOX treatment caused a 12% increase and a 21%
decrease in the MDA and reduced glutathione level,
respectively.
 | Table IEffect of doxorubicin (DOX) and/or
resveratrol (RSVL) on the malondialdehyde (MDA) level in the heart
homogenate of rats. |
Table I
Effect of doxorubicin (DOX) and/or
resveratrol (RSVL) on the malondialdehyde (MDA) level in the heart
homogenate of rats.
| Treatment | MDA level |
|---|
| Control (normal
saline) | 1,164.10±31.81 |
| RSVL | 1,108.29±44.60 |
| DOX | 1,345.84a±62.26 |
| DOX + RSVL
(simultaneously applied) | 1,096.25b±69.44 |
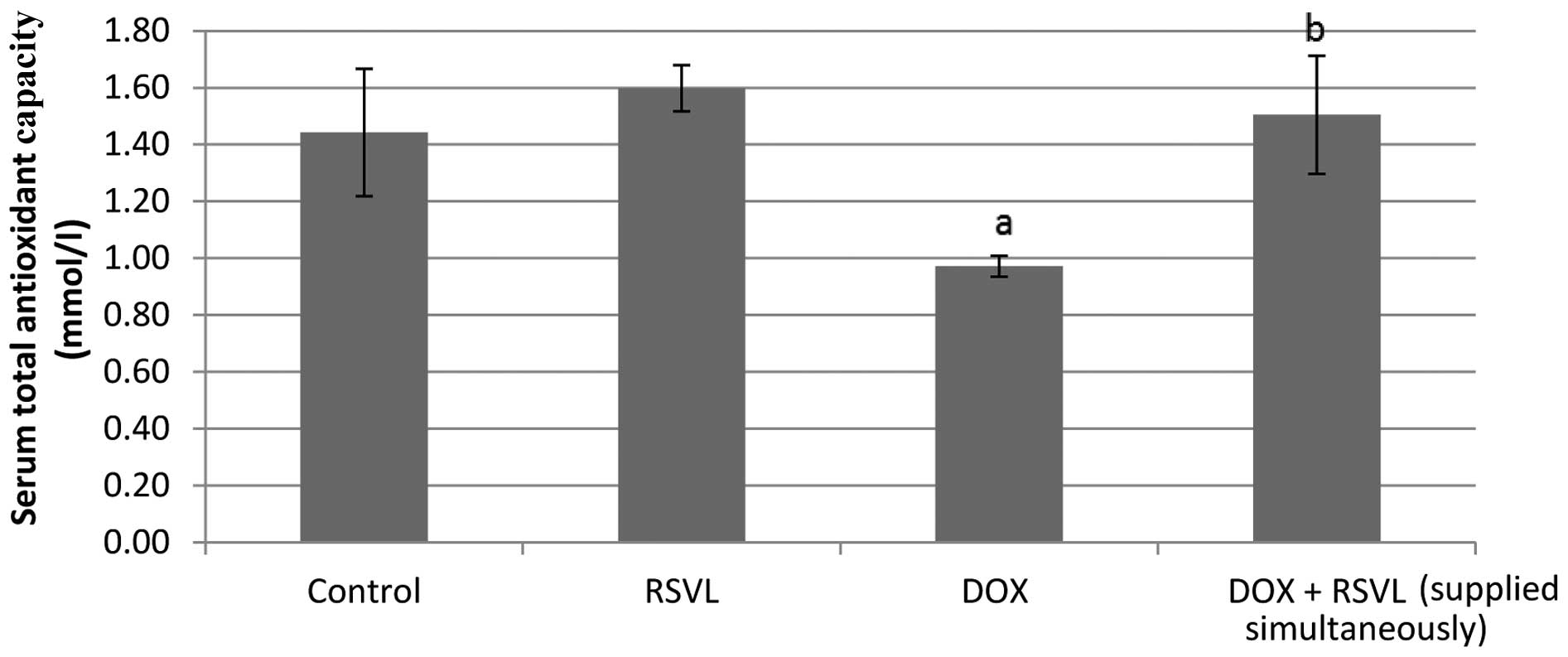
Evaluation of serum TAC
Fig. 8 shows the
effect of DOX (20 mg/kg, i.p.) and/or RSVL (10 mg/kg, i.p.) on the
TAC, meaured in the serum of treated rats. An important and
significant decrease (32%) in TAC was observed in DOX-treated rats
compared to controls, while combined treatment with RSVL and DOX
did not significantly affect TAC compared to control treatment.
Discussion
Anthracyclines are used to treat a variety of cancer
types, but are widely associated with irreversible cardiomyopathy.
The mechanism of DOX-induced oxidative stress is the formation of
an anthracycline-iron (Fe2+) free radical complex. The
latter reacts with hydrogen peroxide to produce the hydroxyl
(OH•) radical (13).
Iron chelators and free radical scavengers may provide cardiac
protection by preventing the formation of the reactive hydroxyl
radical and by scavenging radicals that have been formed. The iron
chelator ICRF-187 has been shown to protect against DOX-induced
cardiotoxicity. However, its clinical success is limited because it
increases hematotoxicity in cancer patients (14,15).
This study investigated the potential cardioprotective effects of
RSVL against DOX-induced cardiotoxicity. In animal models of
cardiovascular disease, RSVL has been shown to protect the heart
from ischemia reperfusion injury, reduce blood pressure and cardiac
hypertrophy on hypertensive animals, and inhibit the progression of
atherosclerosis (16). The exact
mechanism underlying the cardioprotective effect of RSVL is not
known, but previous studies reporting that RSVL can inhibit the
DOX-induced rapid increase in reactive oxygen species (ROS) in the
mitochondria of cardiac cells (17) by increasing superoxide dismutase
activity (18), suggest that the
antioxidant properties of RSVL may play a role in its
cardioprotective effects. In this context, the present study was
designed to investigate the effect of RSVL treatment on oxidative
stress and examine the subcellular effect of DOX in the heart,
along with the underlying mechanisms. The tested dose of DOX
induced marked and acute cardiotoxicity in rats, manifested by an
increase in the plasma CPK and LDH activities and confirmed by
electron microscopy, which revealed changes in the heart tissue,
such as massive fragmentation and lysis of the myofibrils and
vacuolization or complete loss of the cristae in the mitochondria.
It is well known that certain enzymes (CPK, LDH) are released from
the heart muscle cells when it is injured, and the magnitude of CPK
and LDH activities in the blood following myocardial injury
reflects the extent of damage in its musculature (19).
The mechanism of DOX-induced cardiotoxicity has been
reported to involve formation of superoxide anions and their
derivatives, particularly highly reactive and damaging hydroxyl
radicals, which cause peroxidation of the cell membrane lipids
(20,21). Our results are in agreement with
other studies that reported cardiac toxicity following DOX
treatment (22–25). The mechanism of DOX-induced
cardiotoxicity has been investigated by numerous research groups.
In terms of specific organ toxicity, lipid peroxidation has been
highlighted as the primary mechanism underlying DOX-induced cardiac
toxicity (7,26). A significant increase in lipid
peroxidation was also observed in our study, as manifested by the
increased plasma MDA level in the DOX-treated group (Table I).
The increase in CK activity following DOX treatment
was prevented by simultaneous treatment with RSVL. In line with the
hypothesis that RSVL acts through inhibition of the DOX-induced
rapid accumulation of ROS in the mitochondria of cardiac cells, we
found a significantly reduced level of MDA in cardiac tissue
(Table I), normal mitochondrial
structures (Fig. 5), and increased
TAC (Fig. 8) in DOX + RSVL-treated
rats, suggesting that the antioxidant properties of RSVL may play a
role in its cardioprotective effects. However, antioxidant
therapies have failed to provide satisfactory results in clinical
trials (27), casting doubt on the
notion that the inhibition of oxidative stress is the sole
mechanism underlying the cardioprotective effects of RSVL.
Recently, Osman et al (25)
showed that RSVL increases the DOX uptake into Ehrlich ascite
cells, allowing to use a reduced dose of DOX with reduced
side-effects. In conclusion, RSVL can protect cardiac cells from
the deleterious effects of DOX via its antioxidant properties.
Additional clinical trials with RSVL may allow to further elucidate
its protective role against agents that induce tissue-damaging
effects, while further studies are necessary to reveal the
molecular basis of such effects.
References
|
1
|
Lefrak EA, Pitha J, Rosenheim S and
Gofottiebm JA: A clinicopathological analysis of adriamycin
cardiotoxicity. Cancer. 32:302–314. 1973. View Article : Google Scholar
|
|
2
|
Singal PK and Iliskovic N:
Doxorubicin-induced cardiomyopathy. N Engl J Med. 339:900–905.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Doroshow JH: Effect of anthracycline
antibiotics on oxygen radical formation in rat heart. Cancer Res.
43:460–472. 1983.PubMed/NCBI
|
|
4
|
Powis G: Free radical formation by
antitumor quinones. Free Radic Biol Med. 6:63–101. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Olson RD and Mushlin PS: Doxorubicin
cardiotoxicity: analysis of prevailing hypotheses. FASEB J.
4:3076–3086. 1990.PubMed/NCBI
|
|
6
|
Ogura R, Sugiyama M, Haramaki N and Hidaka
T: Electron spin resonance studies on the mechanism of
adriamycin-induced heart mitochondrial damages. Cancer Res.
51:3555–3558. 1991.PubMed/NCBI
|
|
7
|
Myers CE, McGuire WP, Liss RH, Ifrim I,
Grotzinger K and Young RC: Adriamycin: the role of lipid
peroxidation in cardiac toxicity and tumor response. Science.
197:165–167. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mimnaugh EG, Trush MA, Bhatnagar M and
Gram TE: Enhancement of reactive oxygen-dependent mitochondrial
membrane lipid peroxidation by the anticancer drug adriamycin.
Biochem Pharmacol. 34:847–856. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singal PK, Deally CM and Weinberg LE:
Subcellular effects of adriamycin in the heart: a concise review. J
Mol Cell Cardiol. 19:817–828. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goswami SK and Das DK: Resveratrol and
chemoprevention. Cancer Lett. 284:1–6. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alkreathy H, Damanhouri ZA, Ahmed N,
Slevin M, Ali SS and Osman AM: Aged garlic extract protects against
doxorubicin-induced cardiotoxicity in rats. Food Chem Toxicol.
48:951–956. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miller NJ, Rice-Evans C, Davies MJ,
Gopinathan V and Milner A: A novel method for measuring antioxidant
capacity and its application to monitoring the antioxidant status
in premature neonates. Clin Sci (Lond). 84:407–412. 1993.PubMed/NCBI
|
|
13
|
Sugioka K and Nakano M: Mechanism of
phospholipid peroxidation induced by ferric
ion-ADP-adriamycin-co-ordination complex. Biochim Biophys Acta.
713:333–343. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sparano JA: Use of dexrazoxane and other
strategies to prevent cardiomyopathy associated with
doxorubicin-taxane combinations. Semin Oncol. 25(Suppl 10): 66–71.
1998.PubMed/NCBI
|
|
15
|
Speyer JL, Green MD, Zeleniuch-Jacquotte
A, Wernz JC, Rey M, Sanger J, Kramer E, Ferrans V, Hochster H,
Meyers M, et al: ICRF-187 permits longer treatment with doxorubicin
in women with breast cancer. J Clin Oncol. 10:117–127.
1992.PubMed/NCBI
|
|
16
|
Li H, Xia N and Förstermann U:
Cardiovascular effects and molecular targets of resveratrol. Nitric
Oxide. 26:102–110. 2012. View Article : Google Scholar
|
|
17
|
Danz ED, Skramsted J, Henry N, Bennett JA
and Keller RS: Resveratrol prevents doxorubicin cardiotoxicity
through mitochondrial stabilization and the Sirt1 pathway. Free
Radic Biol Med. 46:1589–1597. 2009. View Article : Google Scholar
|
|
18
|
Tatlidede E, Sehirli O, Velioğlu-Oğünc A,
Cetinel S, Yeğen BC, Yarat A, Süleymanoğlu S and Sener G:
Resveratrol treatment protects against doxorubicin-induced
cardiotoxicity by alleviating oxidative damage. Free Radic Res.
43:195–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Preus M, Bhargava AS, Khater AE and Günzel
P: Diagnostic value of serum creatine kinase and lactate
dehydrogenase isoenzyme determinations for monitoring early cardiac
damage in rats. Toxicol Lett. 42:225–233. 1988. View Article : Google Scholar
|
|
20
|
Osman AM, Al-Shabanah OA and Al-Harbi MM:
Effect of desferrioxamine on doxorubicin-induced cardiotoxicity and
haematotoxicity in mice. Med Sci Res. 21:193–194. 1993.
|
|
21
|
Hemnani T and Parihar MS: Reactive oxygen
species and oxidative DNA damage. Indian J Physiol Pharmacol.
42:440–452. 1998.PubMed/NCBI
|
|
22
|
Van Vleet JF, Ferrans VJ and Weirich WE:
Cardiac disease induced by chronic adriamycin administration in
dogs and an evaluation of vitamin E and selenium as
cardioprotectants. Am J Pathol. 99:13–42. 1980.PubMed/NCBI
|
|
23
|
Nagi MN and Mansour MA: Protective effect
of thymoquinone against doxorubicin-induced cardiotoxicity in rats:
a possible mechanism of protection. Pharmacol Res. 41:283–289.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Al-Majed AA, Gado AM, Al-Shabanah OA and
Mansour MA: Alpha-lipoic acid ameliorates myocardial toxicity
induced by doxorubicin. Pharmacol Res. 46:499–503. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Osman AM, Al-Harthi SE, Alarabi OM, Elshal
MF, Ramadan WS, Alaama MN, Al-Kreathy HM, Damanhouri ZA and Osman
OH: Chemosensetizing and cardioprotective effects of resveratrol in
doxorubicin-treated animals. Cancer Cell Int. 13:522013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Singal PK and Pierce GN: Adriamycin
stimulates low-affinity Ca2+ binding and lipid
peroxidation but depresses myocardial function. Am J Physiol.
250:H419–H425. 1986.PubMed/NCBI
|
|
27
|
Gianni L, Herman EH, Lipshultz SE, Minotti
G, Sarvazyan N and Sawyer DB: Anthracycline cardiotoxicity: from
bench to bedside. J Clin Oncol. 26:3777–3784. 2008. View Article : Google Scholar : PubMed/NCBI
|