Introduction
Psoriasis is a common chronic and complex autoimmune
inflammatory skin disorder, which requires long-term therapy.
Genetic background, environmental factors and immune system
disturbances with a strong cytokine component, determine the
disease epidemiology and clinical spectrum, which are heterogeneous
in different populations. However, the mechanisms involved in the
pathogenesis of psoriasis remain to be elucidated (1–4).
FOS-like antigen 1 (Fra-1) is a proto-oncogene,
located on chromosome 11q13, encoding a length of 1.7 kb mature
mRNA. Fra-1 was initially characterized as an immediate early
transcriptional response gene that is antigenically associated with
c-Fos and induced by serum (5,6).
While the basic-leucine zipper domain of Fra-1 is homologous to
that of other Fos family members, previous initial transcriptional
activation studies suggested that Fra-1 is a negative inhibitor of
activator protein-1 (AP-1) activity (7,8).
Subsequently, Bergers et al demonstrated that Fra-1 had
transforming activity (9).
Investigations into the molecular mechanisms responsible for skin
inflammation have revealed that Jun proteins control cytokine
expression, including granulocyte colony-stimulating factor,
interleukin-6 and tumor necrosis factor-α by transcriptional and
post-transcriptional pathways (10,11).
However, the effect of Fra-1 in psoriasis and its possible
underlying mechanisms remain to be elucidated (12–18).
A disturbance during cell division can lead to
abnormal cell proliferation and dysregulation in molecular
signaling is commonly associated with altered cell growth, cell
cycle progression and impaired apoptotic responses in diseases
(19–21). Apoptosis is the selective process
of physiological cell deletion that regulates the balance between
cell proliferation and cell death. The failure of apoptosis is
considered to contribute to the development of certain human
diseases, such as psoriasis, non-alcoholic fatty liver disease and
acute myelogenous leukemia (22,23).
In the present study, the reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
technique was used to identify differentially expressed Fra-1 in
psoriatic and in normal control tissues. Western blot analysis and
flow cytometry were then used to assess the protein levels of
Fra-1. Finally, Fra-1-stable expressing HaCaT/Fra-1 or control
HaCaT/vector cell lines were constructed in order to elucidate the
function of Fra-1 in the growth of HaCaT cells.
Materials and methods
Cell culture
The HaCaT cells were cultured in complete culture
medium composed of Dulbecco’s modified Eagle’s medium (DMEM;
Gibco-BRL, Grand Island, NY, USA), 10% fetal bovine serum (Gibco
BRL), 100 U/ml penicillin and 100 U/ml streptomycin (both Hyclone
Laboratories, South Logan, UT, USA). The cells were maintained in a
humidified atmosphere of 95% air and 5% CO2 at 37°C.
Patient samples
Tissue samples from 10 psoriatic tissues and 10
normal control tissues were obtained from Xiangya Hospital, Central
South University (Changsha, China). The patients were informed
about the sample collection and written informed consent was
obtained from each patient. The collection and use of tissues
samples was approved by the ethical review committees of Xiangya
Hospital. At the Xiangya Hospital, 10 cases of psoriasis were
confirmed histologically and 10 normal control tissue samples were
collected from individual patients with traumatism. All subjects
enrolled in the study were of the Chinese Han population and all
clinical and biological data were available for the samples
(Table I). No significant
differences in gender or age were identified between the psoriatic
and control groups (P>0.05). Samples were separated from the
surgical patient tissue samples, immediately snap-frozen in liquid
nitrogen (Zhen Kuuan Inc., Shenzhen, China) and stored until
use.
 | Table ICharacteristics of cases of psoriasis
and controls. |
Table I
Characteristics of cases of psoriasis
and controls.
| Sample | Gender | Age (years) | Diagnosis |
|---|
| 1 | Male | 43 | Psoriasis |
| 2 | Male | 57 | Psoriasis |
| 3 | Female | 43 | Psoriasis |
| 4 | Male | 43 | Psoriasis |
| 5 | Male | 29 | Psoriasis |
| 6 | Male | 43 | Psoriasis |
| 7 | Female | 53 | Psoriasis |
| 8 | Female | 65 | Psoriasis |
| 9 | Male | 70 | Psoriasis |
| 10 | Female | 37 | Psoriasis |
| 11 | Female | 44 | Normal control
tissue |
| 12 | Female | 42 | Normal control
tissue |
| 13 | Female | 58 | Normal control
tissue |
| 14 | Male | 41 | Normal control
tissue |
| 15 | Female | 30 | Normal control
tissue |
| 16 | Female | 43 | Normal control
tissue |
| 17 | Male | 55 | Normal control
tissue |
| 18 | Male | 67 | Normal control
tissue |
| 19 | Male | 71 | Normal control
tissue |
| 20 | Female | 35 | Normal control
tissue |
RNA extraction and RT-qPCR analysis
Total RNA was extracted from the biopsy samples
using an RNeasy® kit (Qiagen, Carlsbad, CA, USA)
according to the manufacturer’s instructions. The total RNA samples
(1 μg) were used to generate cDNA. Following the reverse
transcription reaction, the PCR reaction was preceded. All RT-qPCR
reactions were repeated at least three times at different points of
the extension cycle to avoid false results from the PCR. GAPDH was
used as an endogenous control for normalization. The primer
sequences used for RT-qPCR were as follows: Fra-1, forward
5′-cgaaggccttgtgaacagat-3′ and reverse 5′-cttctgcttctgcagctcct-3′;
GAPDH, forward 5′-cgaccactttgtcaagctca-3′ and reverse
5′-actgagtgtggcagggactc-3′. The expression of mRNA was assessed
using evaluated threshold cycle (CT) values. The CT values were
normalized with the expression levels of GAPDH and the relative
quantity of mRNA specific to each of the target genes was
calculated using the 2−ΔΔCT method (24–26).
Western blot analysis
The proteins of the biopsy samples were prepared
using a lysis buffer (RIPA buffer; CWBio, Beijing, China) and the
protein concentrations were determined using the bicinchoninic acid
protein assay (Pierce Biotechnology, Inc., Rockford, IL, USA).
Extracts containing 50 μg protein were separated on 10% SDS-PAGE
gels and electroblotted onto nitrocellulose membranes (HyClone
Laboratories Inc.). The membranes were inhibited using
Tris-buffered saline/Tween 20 (25 mM Tris-hydrochloride, 150 mM
sodium chloride, pH 7.5 and 0.05% Tween 20) containing 5% non-fat
milk. This was followed by overnight incubation at 4°C with primary
antibodies (1:500 polyclonal rabbit anti-Fra-1 antibody; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). Following washing three
times, secondary antibodies (1:2,000 horseradish
peroxidase-conjugated monoclonal mouse anti-rabbit antibodies;
Santa Cruz Biotechnology Inc.) were added and incubated for 1 h.
Anti-β-actin antibody (1:3,000; Santa Cruz Biotechnology, Inc.) was
used as a loading control.
Cell transfection
To establish a stable Fra-1-expressing cell line, a
plasmid (pEGFP-N1/Fra-1) was constructed by inserting the
full-length sequence of human Fra-1 cDNA upstream of enhanced green
fluorescent protein (EGFP) in the plasmid pEGFP-N1. Subsequently,
the plasmid pEGFP-N1/Fra-1 or empty control vector pEGFP-N1 were
transfected into HaCaT cells, respectively, using Lipofectin
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions, followed by G418 selection. The stable
transfectants, HaCaT/Fra-1 and HaCaT/vector, were isolated and the
transcription of Fra-1 mRNA was determined using RT-qPCR with
specific primers (forward 5′-cgaaggccttgtgaacagat-3′ and reverse
5′-cttctgcttctgcagctcct-3′).
Cell proliferation assay
The impact of Fra-1 on HaCaT cell proliferation was
measured using an MTT assay, as described previously (14). Briefly, the HaCaT, HaCaT/vector and
HaCaT/Fra-1 cells (104 cells/well) were cultured in
triplicate with 10% fetal calf serum (FCS) DMEM in 96-well plates.
The cells were then exposed to 5 mg/ml MTT for 4 h. The generated
formazan was dissolved with dimethyl sulfoxide and measured at 570
nm using an ELX-800 microplate reader (Bio-Tek Instruments, Inc.,
Winooski, VT, USA).
Flow cytometric analysis of the cell
cycle
The HaCaT, HaCaT/vector and HaCaT/Fra-1 cells were
cultured in 10% FCS DMEM up to ~70% confluence. The adherent cells
were trypsinized, harvested and fixed in 70% ethanol (Huihong Inc.,
Changsha, China) at 4°C. Subsequently, the cells were washed with
cold phosphate-buffered saline (PBS; Solarbio Inc., Beijing, China)
and stained with propidium iodide (PI; Biotium Inc, Hayward, CA,
USA) in working solution (0.5 mg/ml RNase and 0.1 mg/ml PI in PBS).
The cell cycle was characterized by flow cytometric analysis using
a MoFlo™ XDP High-Performance Cell Sorter (Beckman Coulter, Miami,
FL, USA) and the data were analyzed by the CellQuest software
version 3.0 (Becton Dickinson, San Jose, CA, USA).
Effect of Fra-1 on HaCaT cell
apoptosis
Cell apoptosis was analyzed by flow cytometric
analysis using a MoFlo™ XDP High-Performance Cell Sorter (Beckman
Coulter) and PI + annexin V-fluorescein isothiocyanate (FITC)
double staining (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China).
Briefly, the HaCaT, HaCaT/Fra-1 and HaCaT/vector cells were seeded
at a density of 3×105 cells per well in 24-well culture
plates. Cells were collected in an Eppendorf tube at 24 h and
washed twice with PBS by centrifugation at 500 × g for 10 min. The
supernatants were discarded. To detect apoptosis, 500 μl PBS, 5 μl
Annexin V-FITC and 5 μl PI were added to each tube and the contents
of the tube were mixed in the dark at room temperature for 15 min,
followed by flow cytometric analysis. Data were acquired and
analyzed with Summit v5.2 software (Becton-Dickinson, Franklin
Lakes, NJ, USA).
Statistical analysis
Differences between nonparametric variables were
analyzed by Fisher’s exact test using EPI software (EPI Info,
version 3.2.2, www.cdc.gov/epiinfo). Differences in
the quantitative variables between groups were analyzed by
Student’s t-test using SPSS 11.0 program (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Detection of mRNA expression levels of
the Fra-1 gene in psoriasis
To detect the mRNA expression levels of the Fra-1
gene in psoriasis and in controls, 10 psoriatic and 10 control
tissue samples were obtained to perform RT-qPCR on the Fra-1 genes.
Sample spreadsheet of data analysis using the 2−ΔΔCT
method. The fold change in the expression of the Fra-1 gene
relative to the internal control gene (GAPDH) was investigated.
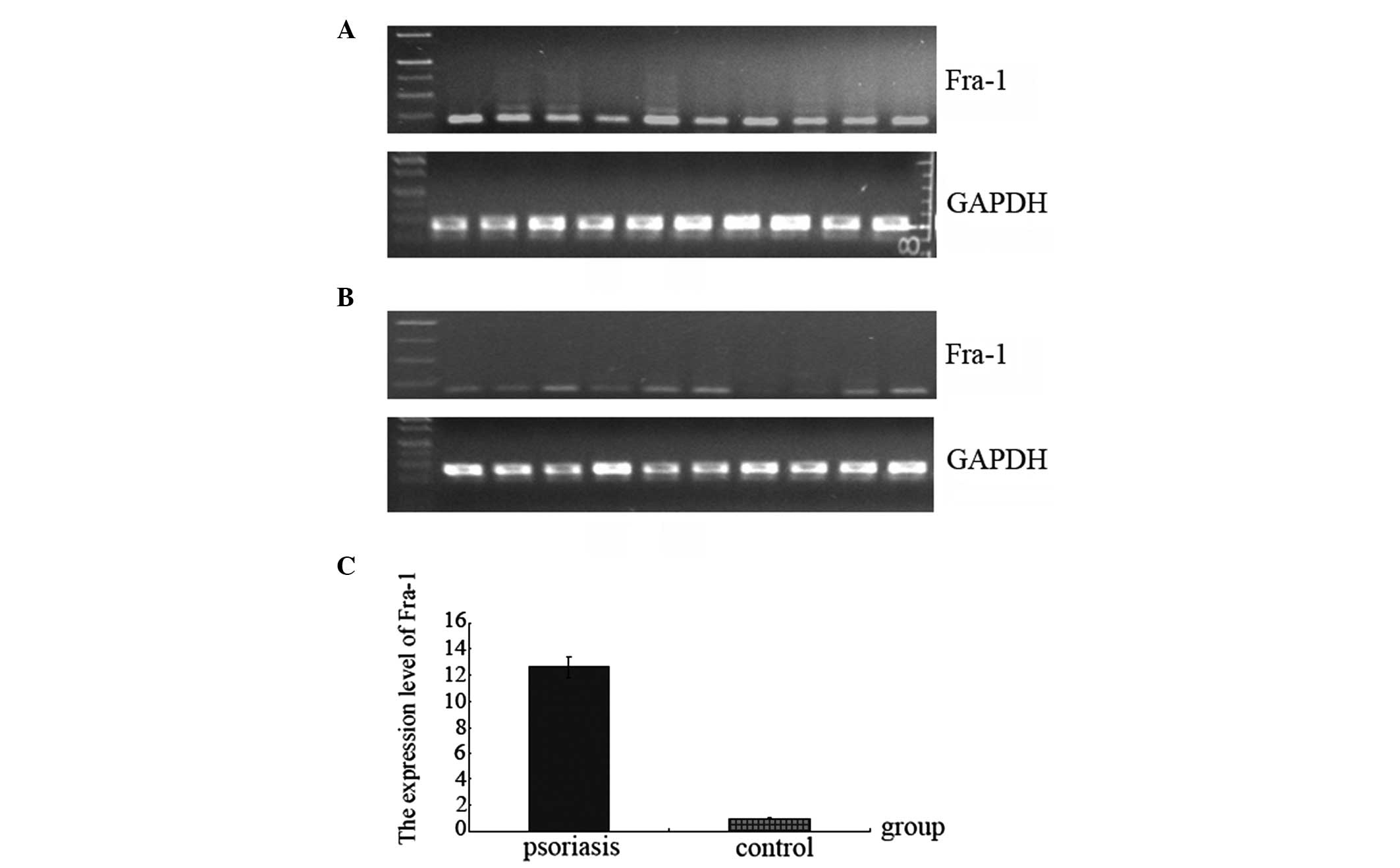
Expression of the Fra-1 gene was high in psoriasis (Table II; Fig. 1) and, compared with the control
samples, the normalized gene expression of Fra-1 in psoriasis was
12.6 times higher (Fig. 1C).
Fig. 1A and B show the results of
the agarose gel electrophoresis following RT-qPCR for the Fra-1 and
GAPDH genes in psoriasis and in the control. Fra-1 was highly
expressed in 10 psoriasis samples compared with the control samples
(Fig. 1A and B). In two of the 10
control samples, the expression of Fra-1 gene was not deleted
(Fig. 1B).
 | Table IIIdentification of mRNA expression
level of the Fra-1 gene in psoriasis by RT-qPCR. |
Table II
Identification of mRNA expression
level of the Fra-1 gene in psoriasis by RT-qPCR.
| Gene | Sample | N | GAPDH CT
(mean ± SD) | Fra-1 CT
(mean ± SD) | ΔCT
(mean ± SD) | ΔΔCT
(mean ± SD) | Fold |
|---|
| Fra-1 | Psoriasis | 10 | 17.41±1.51 | 29.32±1.41 | 11.91±0.91 | −3.66±0.74 | 12.60 |
| Control | 10 | 18.23±1.75 | 33.79±1.63 | 15.56±1.12 | | |
Analysis of protein expression levels of
the Fra-1 gene in psoriasis by western blot analysis
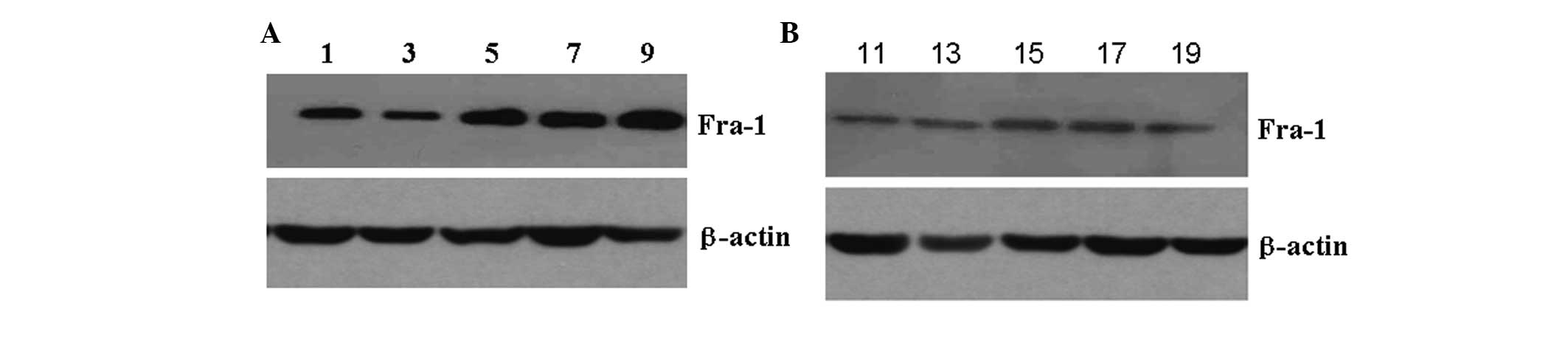
To determine whether Fra-1 had a higher level of
expression in psoriasis than in the control, the levels of Fra-1
protein expression were examined in psoriasis and in controls using
western blot analysis. In comparison with the control, the protein
expression of Fra-1 was highly expressed in psoriatic compared with
the control tissues (Fig. 2),
particularly in the specimen 5, 7 and 9. This corresponded with the
results of RT-qPCR and again confirmed that Fra-1 was highly
expressed in psoriatic tissues.
Fra-1 promotes the growth of HaCaT cells
in vitro
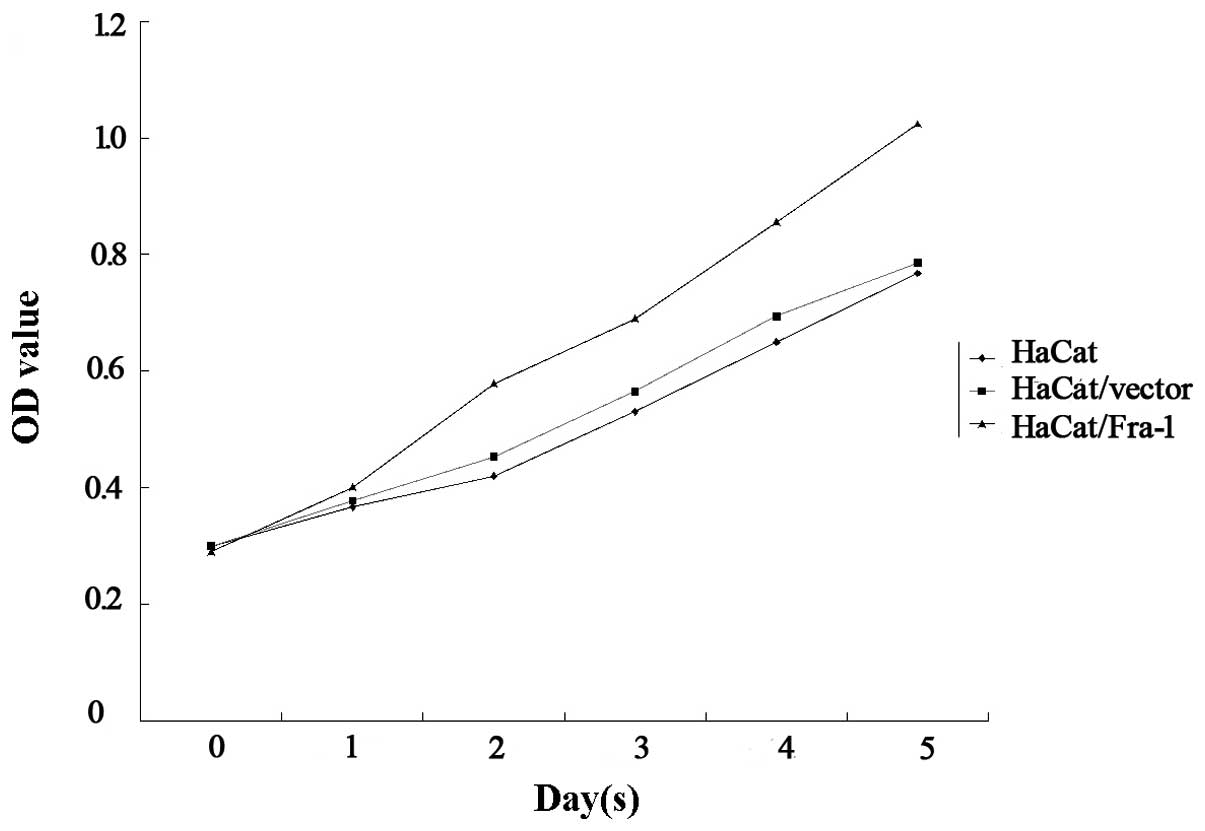
In order to elucidate the function of Fra-1 in the
growth of HaCaT cells, the HaCaT cells were transfected with either
the plasmid pEGFP-N1/Fra-1 or a control vector to generate
Fra-1-stable expressing HaCaT/Fra-1 or control HaCaT/vector cell
lines. Following demonstrating Fra-1 mRNA transcription by RT-qPCR,
the spontaneous proliferation of HaCaT, HaCaT/vector and
HaCaT/Fra-1 cells was determined using MTT assays. Notably, Fra-1
significantly promoted the proliferation of HaCaT cells (Fig. 3). Therefore, endogenous Fra-1
overexpression promoted the proliferation of HaCaT cells in
vitro.
Fra-1 induces HaCaT cell cycle arrest and
inhibits cell apoptosis
The promotion of cell proliferation is usually
mediated by inducing cell cycle arrest and/or inhibiting cell
apoptosis. To assess this possibility, the cell cycle of HaCaT, the
HaCaT/vector and HaCaT/Fra-1 cells was examined using FACS
analysis. Compared with the HaCaT and HaCaT/vector cells, the
percentage of HaCaT/Fra-1 cells that remained at the S phase of the
cell cycle was increased (27.9, 28.9 and 35.6%, respectively),
accompanied by a reduction in the percentage of the cells in the
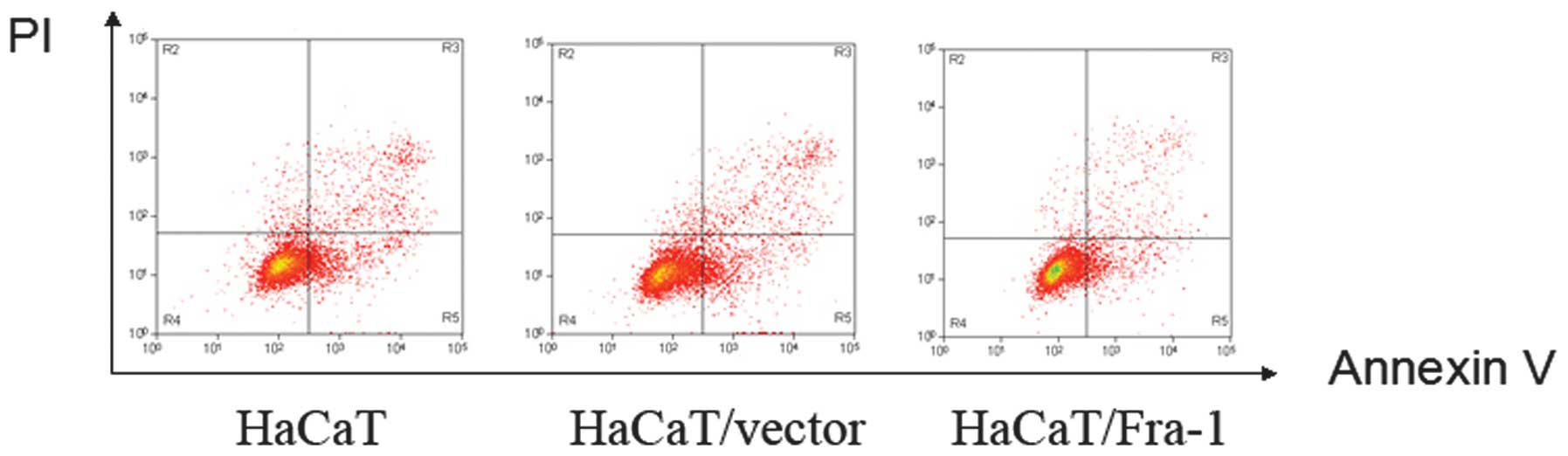
G0/G1-phase (61.2, 60.5 and 51.4%, respectively; Table III). To determine whether
apoptosis mediated cell growth in HaCaT, HaCaT/vector and
HaCaT/Fra-1 cells, an annexin V-FITC/PI double-staining experiment
was performed. As shown in Fig. 4,
a considerable decrease in the percentage of apoptotic cells was
observed in HaCaT/Fra-1 cells (2.36±0.42%) compared with HaCaT
cells (11.07±0.82%) and HaCaT/vector cells (10.83±0.79%).
 | Table IIICell cycle analysis of the HaCaT,
HaCaT/vector and HaCaT/Fra-1 cells by flow cytometry. |
Table III
Cell cycle analysis of the HaCaT,
HaCaT/vector and HaCaT/Fra-1 cells by flow cytometry.
| Phase of cell
cyclea |
|---|
|
|
|---|
| Cell line |
%G0-G1 | %S |
%G2-M |
|---|
| HaCaT | 61.2±5.4 | 27.9±2.9 | 10.9±0.9 |
| HaCaT/vector | 60.5±5.1 | 28.9±3.1 | 10.6±0.8 |
| HaCaT/Fra-1 | 51.4±4.6 | 35.6±3.5 | 13.0±1.1 |
Discussion
Psoriasis is a cutaneous and articular disease, the
incidence of which ranges between 1 and 3%. It is a chronic
recurrent inflammatory skin disorder with a multifactorial
etiology, including genetic background, environmental factors and
immune system disturbances with a strong cytokine component
(1–4). A study by Gunduz et al
demonstrated that there are similar expression levels of NF-κB and
survivin in normal and psoriatic epidermis, and that survivin and
NF-κB levels cannot be attributed to the epidermal proliferation
and thickness observed in psoriasis (27). A study by Han et al
supported observations that apolipoprotein E polymorphisms are
associated with the risk of psoriasis, particularly the ɛ2 and ɛ3
alleles (28). However, the effect
and possible mechanisms of Fra-1 in psoriasis remain to be
elucidated.
In order to identify the levels of Fra-1 mRNA
expression in psoriasis, the RT-qPCR technique was used for
analysis. Compared with the control samples, the normalized Fra-1
gene expression in psoriasis was 12.6 times higher. Western blot
analysis and flow cytometry were then used to assess the protein
levels of Fra-1. The results of the western blotting demonstrated
that the level of Fra-1 protein expression was high in the
psoriatic tissue samples. This corresponded with the results of the
RT-qPCR. The mRNA and protein levels observed in the present study
confirmed that the level of Fra-1 expression was high in psoriasis.
A previous study revealed that the Fos-related proteins Fra-1 and
Fra-2 were possibly causally involved in inflammatory skin
diseases, including psoriasis (12). Our data are consistent with
previous observations and suggest that Fra-1 may be important in
psoriasis.
Furthermore, the results of the present study
demonstrated that Fra-1 promotes the growth of HaCaT cells in
vitro by arresting the cell cycle and inhibiting cell
apoptosis. Mitogen-activated protein kinase cascades were activated
by a variety of environmental stresses, such as hormones and growth
factors. In addition, they promoted Jun/AP1 activity and regulated
cell proliferation, differentiation, transformation and/or
apoptosis. During development and in skin cancer, Jun is known to
be a regulator of keratinocyte proliferation and differentiation by
its direct transcriptional effect on epidermal growth factor
receptor expression (13).
Johansen et al revealed that the protein and mRNA expression
of the AP-1 subunits c-Fos, Fra-1 and c-Jun were reduced in
lesional psoriatic skin compared with non-lesional psoriatic skin
(14). In this study, we found
that Fra-1 was highly expressed in psoriasis tissues and may be
important in psoriasis. Thus the effect of Fra-1 on growth, the
cell cycle and apoptosis of HaCaT cells was investigated in
vitro. The results showed that Fra-1 could inhibit apoptosis of
HaCaT cells and promote cell growth. Our results offer novel
evidence of the association between Fra-1 and psoriasis. However,
the detailed mechanism underlying the effect of Fra-1 in psoriasis
requires further investigation.
Acknowledgements
This study was supported by the National Natural
Sciences Foundation of China (no. 81272975), the Planned Science
and Technology Project of Hunan Province (nos. 2013FJ6004 and
2012TT2002), the Key Project of Hunan Provincial Natural Science
Foundation (no. 12JJ2044) and the Planned Project of Department of
Health of Hunan Province (no. B-2009-002).
Abbreviations:
|
Fra-1
|
FOS-like antigen 1
|
|
AP-1
|
activator protein-1
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
References
|
1
|
Lee YS, Cheon IS, Kim BH, Kwon MJ, Lee HW
and Kim TY: Loss of extracellular superoxide dismutase induces
severe IL-23-mediated skin inflammation in mice. J Invest Dermatol.
133:732–741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grayson M: Psoriasis. Nature. 492:S492012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balato N, Megna M, Di Costanzo L, Balato A
and Ayala F: Educational and motivational support service: a pilot
study for mobile-phone-based interventions in patients with
psoriasis. Br J Dermatol. 168:201–205. 2013. View Article : Google Scholar
|
|
4
|
Hirotsu C, Rydlewski M, Araújo MS, Tufik S
and Andersen ML: Sleep loss and cytokines levels in an experimental
model of psoriasis. PLoS One. 7:e511832012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Young MR and Colburn NH: Fra-1 a target
for cancer prevention or intervention. Gene. 379:1–11. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rauscher FJ III, Cohen DR, Curran T, Bos
TJ, Vogt PK, Bohmann D, Tjian R and Franza BR Jr: Fos-associated
protein p39 is the product of the jun proto-oncogene. Science.
240:1010–1016. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suzuki T, Okuno H, Yoshida T, Endo T,
Nishina H and Iba H: Difference in transcriptional regulatory
function between c-Fos and Fra-2. Nucleic Acids Res. 19:5537–5542.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshioka K, Deng T, Cavigelli M and Karin
M: Antitumor promotion by phenolic antioxidants: inhibition of AP-1
activity through induction of Fra expression. Proc Natl Acad Sci
USA. 92:4972–4976. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bergers G, Graninger P, Braselmann S,
Wrighton C and Busslinger M: Transcriptional activation of the
fra-1 gene by AP-1 is mediated by regulatory sequences in the first
intron. Mol Cell Biol. 15:3748–3758. 1995.PubMed/NCBI
|
|
10
|
Schonthaler HB, Guinea-Viniegra J and
Wagner EF: Targeting inflammation by modulating the Jun/AP-1
pathway. Ann Rheum Dis. 70(Suppl 1): i109–i112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guinea-Viniegra J, Zenz R, Scheuch H,
Hnisz D, Holcmann M, Bakiri L, Schonthaler HB, Sibilia M and Wagner
EF: TNFalpha shedding and epidermal inflammation are controlled by
Jun proteins. Genes Dev. 23:2663–2674. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wagner EF: Bone development and
inflammatory disease is regulated by AP-1 (Fos/Jun). Ann Rheum Dis.
69:i86–i88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zenz R and Wagner EF: Jun signalling in
the epidermis: From developmental defects to psoriasis and skin
tumors. Int J Biochem Cell Biol. 38:1043–1049. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Johansen C, Kragballe K, Rasmussen M, Dam
TN and Iversen L: Activator protein 1 DNA binding activity is
decreased in lesional psoriatic skin compared with nonlesional
psoriatic skin. Br J Dermatol. 151:600–607. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oberprieler NG and Taskén K: Analysing
phosphorylation-based signalling networks by phospho flow
cytometry. Cell Signal. 23:14–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou Y, Zeng Z, Zhang W, Xiong W, Wu M,
Tan Y, Yi W, Xiao L, Li X, Huang C, Cao L, Tang K, Li X, Shen S and
Li G: Lactotransferrin: a candidate tumor suppressor, deficient
expression in human nasopharyngeal carcinoma and inhibits NPC cell
proliferation by modulating the mitogen-activated protein kinase
pathway. Int J Cancer. 123:2065–2072. 2008. View Article : Google Scholar
|
|
17
|
Krutzik PO, Irish JM, Nolan GP and Perez
OD: Analysis of protein phosphorylation and cellular signaling
events by flow cytometry: techniques and clinical applications.
Clin Immunol. 110:206–221. 2004. View Article : Google Scholar
|
|
18
|
Zhang L, Tang A, Zhou Y, Tang J, Luo Z,
Jiang C, Li X, Xiang J and Li G: Tumor-conditioned mesenchymal stem
cells display hematopoietic differentiation and diminished influx
of Ca2+. Stem Cells Dev. 21:1418–1428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li FL, Xu R, Zeng QC, Li X, Chen J, Wang
YF, Fan B, Geng L and Li B: Tanshinone IIA inhibits growth of
keratinocytes through cell cycle arrest and apoptosis: underlying
treatment mechanism of psoriasis. Evid Based Complement Alternat
Med. 2012:9276582012.PubMed/NCBI
|
|
20
|
Abou EL-Ela M, Nagui N, Mahgoub D,
El-Eishi N, Fawzy M, El-Tawdy A, Abdel Hay R and Rashed L:
Expression of cyclin D1 and p16 in psoriasis before and after
phototherapy. Clin Exp Dermatol. 35:781–785. 2010.PubMed/NCBI
|
|
21
|
Wittayarat M, Fujiwara A, Chatdarong K,
Techakumphu M, Sato Y, Tanihara F, Morita Y, Taniguchi M and Otoi
T: Cell cycle analysis and interspecies nuclear transfer of cat
cells treated with chemical inhibitors. Acta Vet Hung. 62:233–242.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mok CF, Xie CM, Sham KW, Lin ZX and Cheng
CH: 1,4-dihydroxy-2-naphthoic acid induces apoptosis in human
keratinocyte: potential application for psoriasis treatment. Evid
Based Complement Alternat Med. 2013:7928402013.PubMed/NCBI
|
|
23
|
Broshtilova V, Lozanov V and Miteva L:
Polyamine metabolism changes in psoriasis. Indian J Dermatol.
58:306–309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
|
|
25
|
Zhou Y, Wang W, Zheng D, Peng S, Xiong W,
Ma J, Zeng Z, Wu M, Zhou M, Xiang J, Xiang B, Li X, Li X and Li G:
Risk of nasopharyngeal carcinoma associated with polymorphic
lactotransferrin haplotypes. Med Oncol. 29:1452–1462. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ruan L, Wang GL, Chen Y, Yi H, Tang CE,
Zhang PF, Li MY, Li C, Peng F, Li JL, Chen ZC and Xiao ZQ:
Identification of tyrosine phosphoproteins in signaling pathway
triggered TGF-α by using functional proteomics technology. Med
Oncol. 27:1407–1414. 2010.PubMed/NCBI
|
|
27
|
Gunduz K, Temiz P, Gencoglan G, Inanir I
and Catalkaya A: Expression of nuclear factor kappa B and survivin
in psoriasis. ISRN Dermatol. 2012:2570592012.PubMed/NCBI
|
|
28
|
Han Y, Liu T and Lu L: Apolipoprotein E
gene polymorphism in psoriasis: a meta-analysis. Arch Med Res.
44:46–53. 2013. View Article : Google Scholar : PubMed/NCBI
|