Introduction
Acute myocardial infarction (AMI) is a common type
of cardiovascular disease with high mortality and morbidity, and
epidemiological evidence indicated that ~27% of 1 million patients
have died of AMI in the US (1).
One of the most significant factors responsible for the high
mortality and poor recovery rate of AMI is the myocardial
ischemia/reperfusion injury (MIRI), which occurs during
revascularization therapy (2).
MIRI leads to the apoptosis of a large number of cardiomyocytes by
various mechanisms, including intracellular Ca2+
overload (3). Intracellular
Ca2+ overload results in the opening of the
mitochondrial permeability transition pores and depolarization of
the inner mitochondrial membrane, all of which lead to
cardiomyocyte apoptosis (4).
Therefore, novel methods to prevent and attenuate the intracellular
Ca2+ overload during MIRI are urgently required for the
treatment of AMI.
Resveratrol is an edible polyphenolic phytoalexin
found in grapes and red wine and has been identified to exhibit a
wide range of biological and pharmacological properties, including
anti-ageing, anti-inflammation and anti-cancer effects (5–7). In
addition, resveratrol has aroused attention due to its various
cardioprotective effects (8).
Studies have reported that resveratrol is able to attenuate
ischemia-reperfusion injury, inhibit low-density lipoprotein
oxidation and promote vasorelaxation, which clearly support its
potential role for the prevention and therapy of MIRI (9,10).
Additionally, Shen et al (11) identified that resveratrol
alleviates the cardiomyocyte Ca2+ overload induced by
H2O2. However, the mechanisms underlying its
effects against MIRI and Ca2+ overload are not well
established.
The Wnt5a/Frizzled-2 signaling pathway is a
non-canonical Wnt pathway responsible for the intracellular
Ca2+ release (12).
Upon binding to Wnt5a, the Frizzled-2 receptor induces
Ca2+ release from intracellular stores and activates two
kinases, Ca2+/calmodulin-dependent protein kinase II and
protein kinase C, in a G-protein dependent manner (13,14).
It has been identified that Frizzled-2 overexpression activates the
Wnt5a/Frizzled-2 pathway and thereby induces Ca2+
accumulation and cardiomyocyte apoptosis and that this effect can
be reversed through the inhibition of Frizzled-2 (15). Therefore, resveratrol may protect
cardiomyocytes from MIRI injury through the inhibition of
Wnt5a/Frizzled-2.
In the present study, an H/R cardiomyocyte model was
established in order to study the effects of resveratrol on the
expression of Wnt5a/Frizzled-2 and intracellular Ca2+
overload. In addition, the cardiomyocyte activity and apoptosis
were detected in order to determine the cardioprotection effects of
resveratrol. The aim of the present study was to elucidate the
mechanisms through which resveratrol protects cardiomyocytes in
vitro.
Materials and methods
Hypoxia/reoxygenation model and
experimental groups
H9c2 cells (American Type Culture Collection,
Manassas, VA, USA) were maintained in Dulbecco’s modified Eagle’s
medium (DMEM) supplemented with 4.5 g/l glucose, 10% (v/v) fetal
bovine serum and 1% penicillin/streptomycin (v/v) at a density of
~1×106 cells/ml. The medium was replaced with fresh DMEM
and the cells incubated in fresh medium for 24 h prior to H/R
treatment. The H/R injury model was achieved according to
previously described methods (16,17).
The culture medium was first changed to pH 7.4 at 37°C without
glucose or Ca2+ and then the cardiomyocytes were exposed
to hypoxia by transferring the culture plates to a humidified
incubation chamber thermoregulated at 37°C with a gas mixture of
95% N2 and 5% CO2. Following three hours, the
cardiomyocytes were transferred to another chamber containing 95%
air and 5% CO2 for reoxygenation. The cardiomyocytes
were randomly divided into three groups as follows: i) The control
group, in which the cardiomyocytes were incubated in normal Hank’s
solution throughout the experimental period; ii) the H/R group, in
which the cardiomyocytes were treated under anoxic conditions for 3
h and under reoxygenation conditions for 3 h; and iii) the
resveratrol group, in which the cardiomyocytes were treated as in
the H/R group with the addition of resveratrol (Sigma Aldrich, St.
Louis, MO, USA) at a final concentration of 5, 15 or 30 μmol to the
culture medium 10 min after reoxygenation. The cell viability and
the lactate dehydrogenase (LDH) levels were detected at the end of
the reoxygenation treatment, and all other determinations were
performed following incubation of the cardiomyocytes with
resveratrol for an additional 24 h (18).
Cell viability
A cell counting kit (CCK)-8 (Dojindo, Tokyo, Japan)
was used to measure the cell viability according to the
manufacturer’s instructions. The cardiomyocytes were seeded in
96-well plates at 1×104 cells/well and pretreated with
different concentrations of resveratrol (0, 5, 15 or 30 μM) for 48
h or treated with resveratrol 10 min after the initiation of
reoxygenation. The cardiomyocytes were treated under anoxic
conditions for 3 h and under reoxygenation conditions for 3 h. At
the end of the treatment period, 10 μl WST-8 solution was added to
the cardiomyocytes and the cells were incubated for 2 h at 37°C.
The absorbance of each well at 450 nm related to the reference
absorbance at 630 nm was measured using a microplate reader
(Bio-Rad, Hercules, CA, USA). The cell viability percentage was
calculated using the following formula: % Cell viability = (mean
absorbance in the test wells)/(mean absorbance in the control well)
× 100 (18).
Lactate dehydrogenase (LDH) activity
The membrane damage was monitored by measuring the
LDH leakage. In total, 100 μl culture medium was added to assess
the amount of LDH by measuring the levels of pyruvic acid with a
spectrophotometer, (Shimadzu UV-2201, Shimadzu Corporation, Kyoto,
Japan) and the absorbance was detected at a wavelength of 440 nm
(15).
Flow cytometric analysis
The apoptotic cells were measured using a
Fluorescein FragELTM DNA Fragmentation Detection kit (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
Briefly, the cardiomyocytes were collected 24 h after
reoxygenation. Next, 100 μl binding buffer and 10 μl fluorescein
isothiocyanate-labeled Annexin V (20 μg/ml) were added to each
sample, and the samples were incubated in the dark for 30 min.
After 5 μl propidium iodide was added, the cell apoptosis rate was
analyzed using a FACScan flow cytometer (Becton-Dickinson, Franklin
Lanes, NJ, USA).
Measurement of caspase-3 activity
The caspase-3 activity was evaluated using a
commercialized caspase-3 assay kit (Biovision, Mountain View, CA,
USA). In total, ~1×106 cells were harvested, and the
pellet was resuspended in lysis buffer. The protein levels were
determined through the bicinchoninic acid assay according to the
manufacturer’s instructions. The caspase-3 activity was measured as
the optical density. The absorbance at 405 nm of the released
peptide nucleic acid (pNA) was monitored using a
spectrophotometer.
Quantitative polymerase chain reaction
(qPCR)
The total RNA from H9c2 cells was isolated using a
Small-Scale Phenol-Free Total RNA Isolation kit (Ambion, Austin,
TX, USA). Reverse transcription was carried out in a volume of 20
μl containing 1 μg total RNA. The expression of Frizzled-2, Wnt5a
and GAPDH was quantitatively checked in H9C2 cells using an ABI
7500 RT-PCR System (Applied Biosystems, Foster, CA, USA) as
described previously (27). The
Power SYBR Green PCR Master Mix (Lingke, Shanghai, China) was used
as a double-stranded DNA-specific dye according to the
manufacturer’s instructions. The PCR conditions were 95°C for 1
min, 40 cycles of 95°C for 15 sec and 55°C for 1 min for
Frizzled-2, and 95°C for 1 min, 40 cycles of 95°C for 15 sec and
60°C for 1 min for Wnt5a and GAPDH. The levels of Frizzled-2 and
Wnt5a mRNA were calculated based on the 2−ΔΔCT of the
intervening and the control groups. The following primer sequences
were used: GAPDH, forward 5′-AGG GCT GCC TTC TCT TGT GA-3′ and
reverse 5′-AAC TTG CCG TGG GTA GAG TCA-3′; Frizzled-2, forward
5′-TCG TTT TGC CCG TCT CT-3′ and reverse 5′-TAG CGG AAT CGC TGC
AT-3′; Wnt5a, forward 5′-CGG AGA TTG TGG ATC AGT TC-3′ and reverse
5′-GGT TCC AGC TGC AAT TCT TG-3′. The primers were synthesized by
the Guangzhou Land Biology Technology Company (Guangzhou,
Guangdong, China).
Western blot analysis
The protein samples were prepared using the
ProteoExtract Transmembrane Protein Extraction kit (Novagen, Merck
KgaA, Darmstadt, Germany), and the protein concentration was
measured using a bicinchoninic acid protein assay kit (Bocai,
Shanghai, China). Following blocking for 1 h in Tris-buffered
saline and Tween-20 (TBST) containing 5% bovine serum albumin, the
membranes were washed in TBST and probed with the following primary
antibodies overnight at 4°C: Rabbit polyclonal antibody to
Frizzled-2, rabbit polyclonal antibody to Wnt5a and goat polyclonal
antibody to human GAPDH (Invitrogen, Carlsbad, CA, USA). The
membranes were then incubated with goat anti-rabbit immunoglobulin
G peroxidase-conjugated secondary antibody (Sigma Aldrich) and
visualized by enhanced chemiluminescence according to the
manufacturer’s instructions. GAPDH was used as the internal loading
control. For quantification, the Image-Pro Plus 6.0 software (Media
Cybernetics, Silver Spring, MD, USA) was used to measure the
integrated optical density (IOD) of the bands. The relative protein
levels are expressed as the ratio to the levels of GAPDH.
Laser scanning confocal microscopy
The intracellular Ca2+ was detected by
laser scanning confocal microscopy (Carl-Zeiss, Jena, Germany). The
cells were incubated with Fluo-3AM at 37°C for 60 min, and the
fluorescence was monitored at 528 nm. Images (512×512 pixels) were
acquired, and the quantitative analysis of the laser scanning
confocal microscopy data was conducted using the Image-Pro Plus 6.0
software. The values are expressed as the IOD and represented as
the mean ± standard error of the mean (SEM).
Statistical analysis
The data were analyzed using the SPSS 16.0
statistical software (SPSS, Inc., Chicago, IL, USA) and are
expressed as the mean ± SEM. The statistical analysis was completed
using one-way analysis of variance with Fisher’s least-significant
difference post-hoc tests for multiple comparisons of the means.
Differences were considered to be statistically significant if
P<0.05.
Results
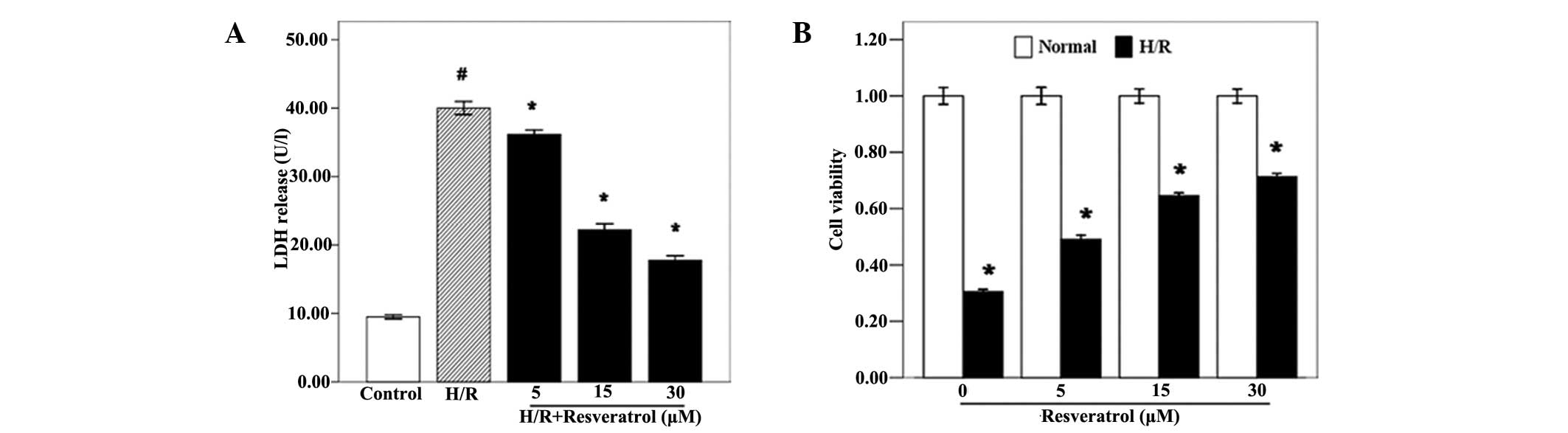
Resveratrol improves cell activity and
reduces LDH release
Initially, the cytotoxic effects of resveratrol on
H9c2 cells were observed through the CCK-8 assay, and the results
indicated that there were no significant differences in cell
viability between the groups treated with different concentrations
of resveratrol (Fig. 1B;
P<0.05). Additionally, resveratrol increased the H/R
cardiomyocyte viability in a dose-dependent manner (Fig. 1B; P<0.05). Furthermore, LDH was
measured in order to determine the extent of cell injury, and the
results revealed that resveratrol significantly decreased the LDH
levels of H/R cardiomyocytes (Fig.
1A; P<0.05).
Effect of resveratrol on apoptosis of
caspase-3 activity in H/R H9c2 cells
To investigate the cardioprotective effect of
resveratrol on H/R cardiomyocytes, the apoptotic rate of
cardiomyocytes was then examined. The cardiomyocytes were treated
with resveratrol (5, 15 or 30 μM) 10 min after the beginning of
reoxygenation, and the levels of apoptosis and caspase-3 were
detected by flow cytometry 24 h after reoxygenation. The flow
cytometry results revealed that the apoptotic rate in the H/R group
was significantly elevated compared with that in the control group,
and resveratrol treatment inhibited H/R-induced cardiomyocyte
apoptosis (Fig. 2A and B). In
order to further demonstrate the anti-apoptotic action of
resveratrol, the caspase-3 activity was determined. Caspase-3 is a
crucial mediator of programmed cell death (19). The caspase-3 activity was
significantly increased in the H/R group compared with that in the
control group. However, resveratrol treatment significantly reduced
the caspase-3 activity in a concentration-dependent manner
(Fig. 2C; P<0.05). Therefore,
resveratrol may protect cardiomyocytes by reducing their apoptotic
rate and inhibiting caspase-3 activity.
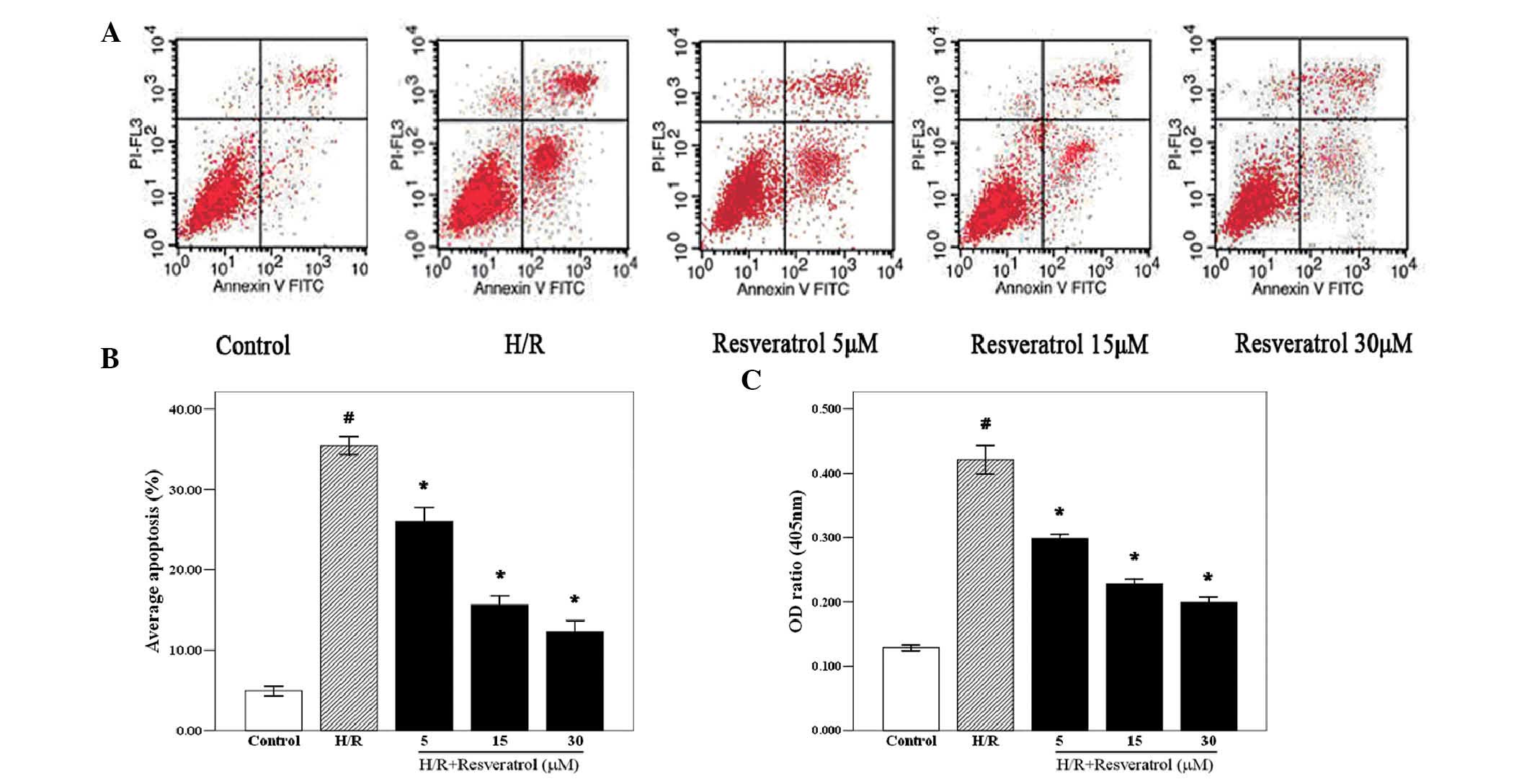 | Figure 2Effects of resveratrol on caspase-3
activity and apoptosis of H/R cardiomyocytes. (A) Cardiomyocytes
were treated with resveratrol (5, 15, or 30 μM) 10 min after the
start of reoxygenation, and the rate of apoptosis was detected by
flow cytometry 24 h after reoxygenation. Resveratrol significantly
decreased the apoptosis of H/R cardiomyocytes (P<0.05). (B)
Quantified apoptotic rates of cardiomyocytes shown in A. (C)
Caspase-3 activity was determined 24 h after reoxygenation, and
resveratrol (5, 15 or 30 μM) inhibited caspase-3 activity. Values
are expressed as the mean ± standard deviation (n=6);
#P<0.05 versus the control group,
*P<0.05 versus the H/R group. H/R,
hypoxia/reoxygenation; FITC, fluorescein isothiocyanate; PI,
propidium iodide; OD, optical density. |
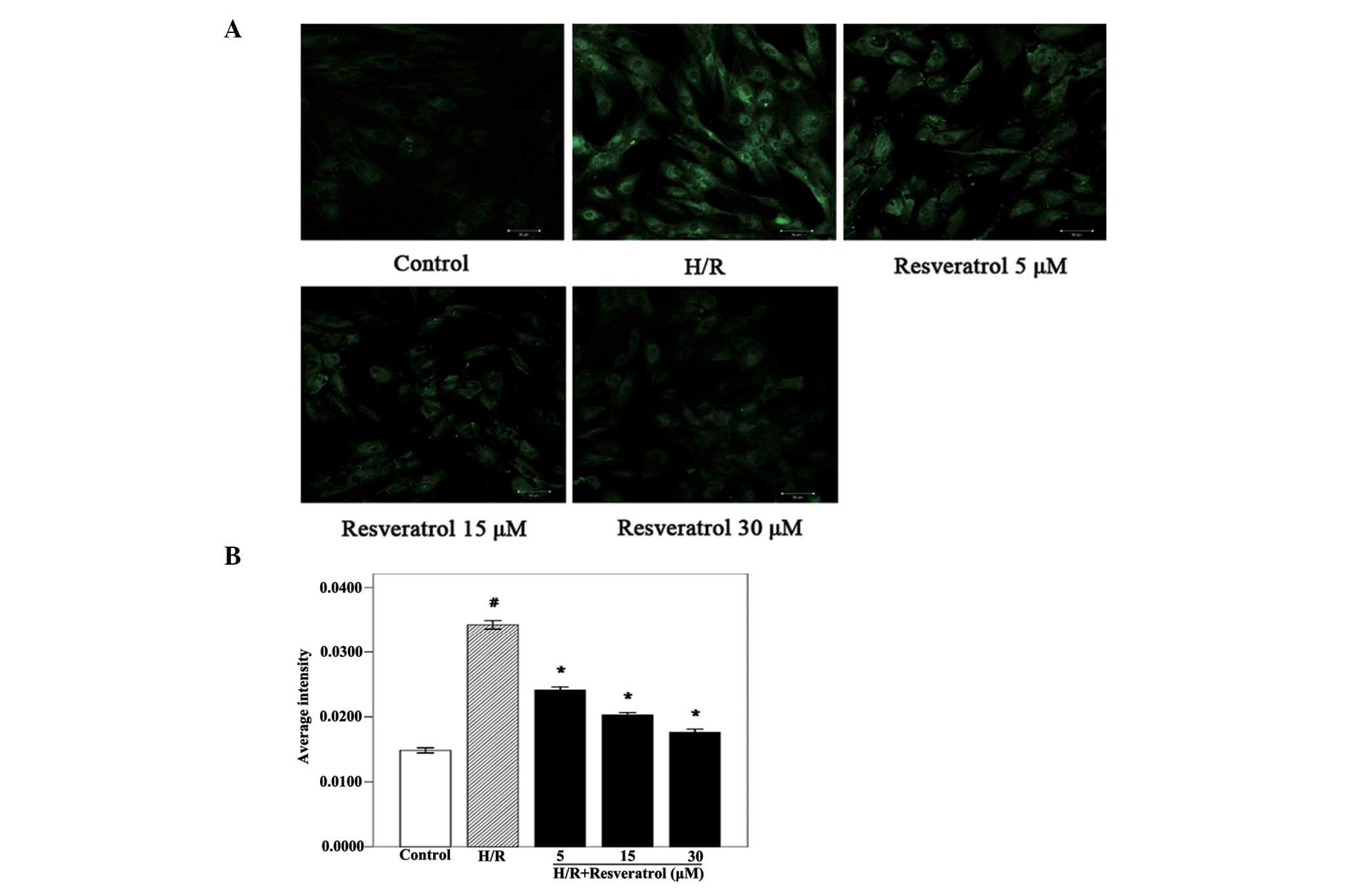
Resveratrol decreases intracellular
Ca2+ accumulation in H9c2 cells
Intracellular Ca2+ accumulation is a
primary cause of cardiomyocyte apoptosis in MIRI (20). To investigate the concrete
mechanisms of cardiomyocyte apoptosis and to determine whether the
anti-apoptotic action of resveratrol is associated with
Ca2+ overload, the effect of resveratrol on
intracellular Ca2+ accumulation was examined. The
intracellular Ca2+ levels of the H/R group were
significantly increased compared with those observed in the control
group (Fig. 3). Of note,
resveratrol treatment (5, 15 or 30 μM) significantly decreased the
intracellular Ca2+ levels in a dose-dependent manner,
similarly to the results observed in the analysis of the apoptotic
rate and caspase-3 activity. Together, these results indicated that
resveratrol protected cardiomyocytes from H/R injury and reduced
apoptosis by inhibiting the intracellular Ca2+
levels.
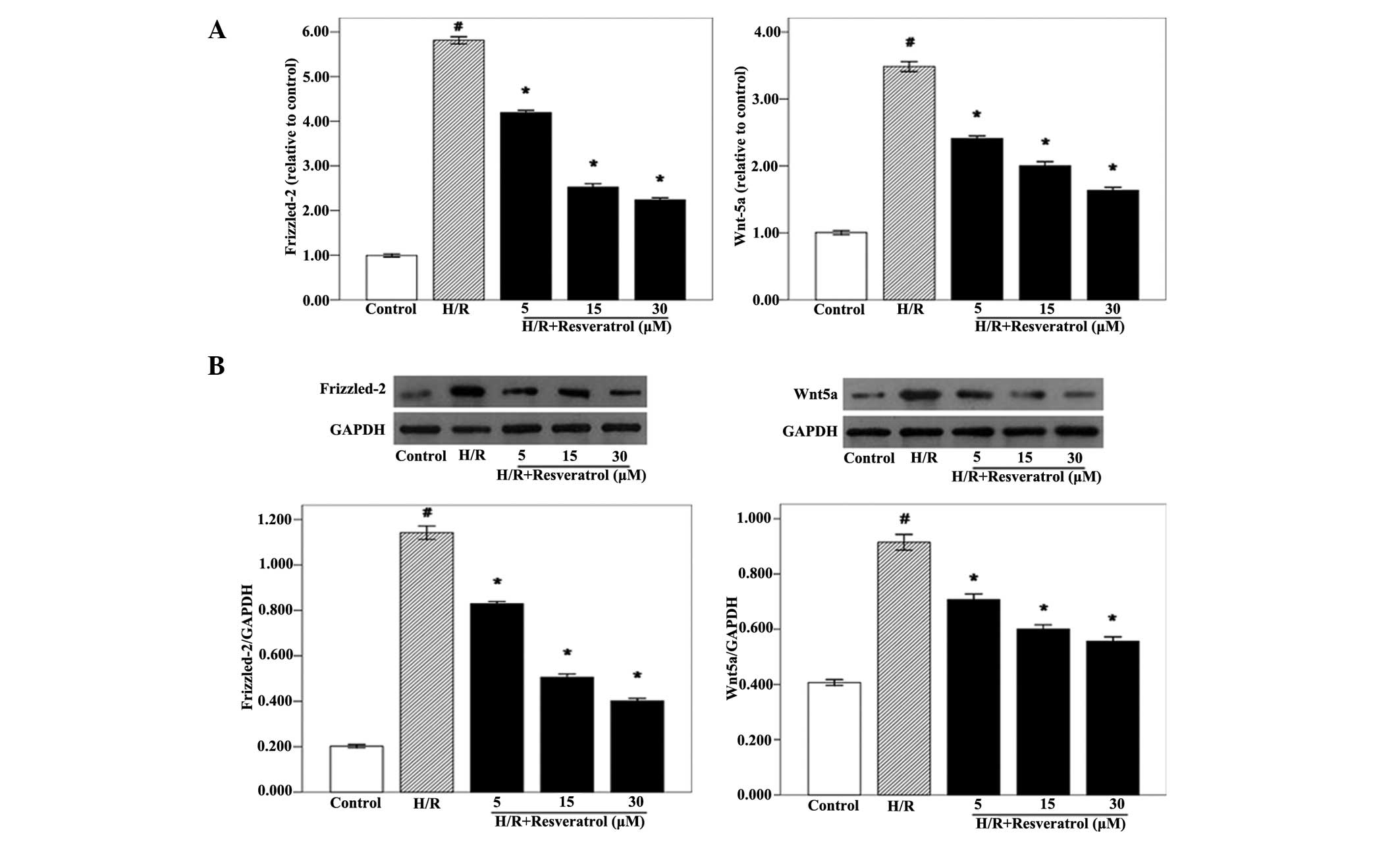
Effects of resveratrol on the gene and
protein expression of Wnt5a and Frizzled-2
It has been demonstrated that the Wnt5a/Frizzled-2
pathway regulates intracellular Ca2+ accumulation
(12). Therefore, the possibility
of resveratrol stimulation affecting intracellular Ca2+
accumulation via the Wnt5a/Frizzled-2 signaling pathway was
examined. qPCR analysis revealed that the expression levels of
Wnt5a and Frizzled-2 mRNA were significantly increased in H/R
cardiomyocytes compared with the control group. However,
resveratrol treatment (5, 15 or 30 μM) significantly reduced the
levels of Wnt5a and Frizzled-2 mRNA in H9c2 cells (Fig. 4A). These results indicated that
resveratrol inhibited Wnt5a and Frizzled-2 mRNA expression in a
concentration-dependent manner. Furthermore, western blot analysis
was performed in order to examine the Wnt5a and Frizzled-2 protein
expression levels. Similar to the qPCR results, the western blot
analysis revealed that the Wnt5a and Frizzled-2 protein levels were
significantly lower in the resveratrol group compared with those in
the H/R group (Fig. 4B). These
results supported the hypothesis that resveratrol has a critical
role in the inhibition of the Wnt5a/Frizzled-2 pathway, which has
been proven to be responsible for intracellular Ca2+
accumulation. Therefore, resveratrol may reduce intracellular
Ca2+ accumulation by inhibiting the Wnt5a/Frizzled-2
pathway.
Discussion
The present study revealed that resveratrol
ameliorates the effects of Frizzled-2 mediated by the
Wnt/Ca2+ pathway in H/R-induced cardiomyocytic
Ca2+ overload and protects cardiomyocytes from injury
and apoptosis. The Wnt5a/Frizzled-2 pathway has a critical role in
Ca2+ overload, which is a major factor that induced
cardiomyocyte apoptosis in AMI. Evidence was provided supporting
the conclusion that resveratrol suppresses Ca2+ overload
by downregulating the expression of the Wnt5a/Frizzled-2
pathway.
It has been shown that resveratrol has numerous
functions, including cardioprotective effects during MIRI (21,22).
In the present study, H9c2 cells were selected for the in
vitro H/R cell model due to their wide applications in MIRI
research (15,23). The levels of LDH and cell viability
are used as indicators of cardiomyocyte injury. The cell viability
was found to be decreased in cardiomyocytes undergoing H/R
treatment and resveratrol treatment was identified to be capable of
protecting against H/R-induced cell injury. Similarly, resveratrol
treatment also decreased the LDH levels in H/R cardiomyocytes.
Therefore, as previous studies have demonstrated, it was proven
that resveratrol exerted a protective effect on H/R cardiomyocytes
(18). It is believed that the
cardioprotection induced by resveratrol is achieved through a
preconditioning effect; however, it is difficult to restrict its
administration as a pretreatment (24,25).
Resveratrol was found to still function when applied after the
initiation of reoxygenation, which means that resveratrol exerted
direct protective effects on H/R cardiomyocytes and may be used for
the clinical treatment of MIRI.
It is widely recognized that reperfusion is a
‘double-edged sword’, as reperfusion itself may lead to massive
cardiomyocyte apoptosis due to the I/R procedure (26). Consistent with previous studies, it
was demonstrated that resveratrol significantly decreased the
apoptotic rate of H/R-treated cardiomyocytes. The activity of
caspase-3, which is a key factor of the caspase cascade, was also
inhibited (27,28). However, there is controversy
regarding the resveratrol dosage and its cardioprotective action;
studies have reported that a low dosage of resveratrol resulted in
anti-apoptotic effects, whereas a high dosage of resveratrol may
transfer a death signal, enlarge the myocardial infarction size and
promote cardiomyocyte apoptosis (29,30).
The present study indicated that resveratrol is able to exert
anti-apoptotic effects at a relatively low concentration (5–30 μM);
however, the optimal dosage remains to be determined.
Ca2+ overload is the main pathological
change of MIRI (31). The
elevation in the intracellular Ca2+ levels directly
triggers cell death, and an increase in the mitochondrial
Ca2+ levels may induce depolarization of the inner
mitochondrial membrane and the opening of mitochondrial
permeability transition pores, which would give rise to adenosine
triphosphate depletion and apoptosis (4,32–34).
Thus, the control of the intracellular Ca2+ levels is a
key factor in the treatment of reperfusion injury. A recent study
demonstrated that resveratrol suppressed
H2O2-induced Ca2+ inflow and
indicated that resveratrol attenuated H/R-induced cell injury and
apoptosis by inhibiting the Ca2+ overload (11). Consistent with the abovementioned
study, Ca2+ release was demonstrated to be significantly
decreased when H/R cardiomyocytes were treated with resveratrol.
Additionally, resveratrol treatment also reduced the apoptosis of
cardiomyocytes. Therefore, one plausible explanation is that
resveratrol decreases H/R-induced cell injury by suppressing
Ca2+ overload.
The mechanisms involved in Ca2+ overload
require to be fully elucidated, and mitochondrial dysfunction, an
increase in cell membrane permeability, and catecholamine are
currently the primary known causes of Ca2+ overload
(4,35,36).
The Wnt5a/Frizzled-2 pathway, which is a well-known
Ca2+-modulating pathway, is activated upon inflammation
or ischemia/anoxia stimulation (37,38).
A previous study by our group proved that the Wnt5a/Frizzled-2
pathway has a vital role in Ca2+ release in H/R
cardiomyocytes (15). To confirm
whether resveratrol suppresses Ca2+ overload by
inhibiting the Wnt5a/Frizzled-2 pathway, the expression levels of
Wnt5a and Frizzled-2 were determined. The results revealed that the
gene and protein levels of both Wnt5a and Frizzled-2 were elevated
by the stimulation of H/R and that resveratrol downregulated their
expression in a dose-dependent manner. Therefore, resveratrol may
decrease Ca2+ overload due to H/R by inhibition of the
Wnt/Frizzled-2 pathway.
In conclusion, the present study determined that
resveratrol directly protected cardiomyocytes from H/R-induced
reperfusion injury and apoptosis through inhibition of the
Ca2+ via the Wnt5a/Frizzled-2 pathway in MIRI. However,
the results only demonstrated the cardioprotective effects of
resveratrol in vitro. Due to the complicated nature of the
in vivo environment and the low bioavailability of
resveratrol, further in vivo studies will be conducted in
order to confirm the effects of resveratrol on Ca2+
overload and reperfusion injury.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81270218).
References
|
1
|
Boateng S and Sanborn T: Acute myocardial
infarction. Dis Mon. 59:83–96. 2013. View Article : Google Scholar
|
|
2
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Turer AT and Hill JA: Pathogenesis of
myocardial ischemia-reperfusion injury and rationale for therapy.
Am J Cardiol. 106:360–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weiss JN, Korge P, Honda HM and Ping P:
Role of the mitochondrial permeability transition in myocardial
disease. Circ Res. 93:292–301. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fulda S and Debatin KM: Sensitization for
tumor necrosis factor-related apoptosis-inducing ligand-induced
apoptosis by the chemopreventive agent resveratrol. Cancer Res.
64:337–346. 2004. View Article : Google Scholar
|
|
6
|
Genade T and Lang DM: Resveratrol extends
lifespan and preserves glia but not neurons of the Nothobranchius
guentheri optic tectum. Exp Gerontol. 48:202–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang SJ, Bo QY, Zhao XH, Yang X, Chi ZF
and Liu XW: Resveratrol pre-treatment reduces early inflammatory
responses induced by status epilepticus via mTOR signaling. Brain
Res. 1492:122–129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hao HD and He LR: Mechanisms of
cardiovascular protection by resveratrol. J Med Food. 7:290–298.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu JM and Hsieh TC: Resveratrol: a
cardioprotective substance. Ann N Y Acad Sci. 1215:16–21. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ungvari Z, Labinskyy N, Mukhopadhyay P, et
al: Resveratrol attenuates mitochondrial oxidative stress in
coronary arterial endothelial cells. Am J Physiol Heart Circ
Physiol. 297:H1876–H1881. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shen M, Wu RX, Zhao L, et al: Resveratrol
attenuates ischemia/reperfusion injury in neonatal cardiomyocytes
and its underlying mechanism. PLoS One. 7:e512232012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuhl M, Sheldahl LC, Park M, Miller JR and
Moon RT: The Wnt/Ca2+ pathway: a new vertebrate Wnt
signaling pathway takes shape. Trends Genet. 16:279–283. 2000.
View Article : Google Scholar
|
|
13
|
Slusarski DC, Corces VG and Moon RT:
Interaction of Wnt and a Frizzled homologue triggers
G-protein-linked phosphatidylinositol signalling. Nature.
390:410–413. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sheldahl LC, Park M, Malbon CC and Moon
RT: Protein kinase C is differentially stimulated by Wnt and
Frizzled homologs in a G-protein-dependent manner. Curr Biol.
9:695–698. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou SS, He F, Chen AH, Hao PY and Song
XD: Suppression of rat Frizzled-2 attenuates
hypoxia/reoxygenation-induced Ca2+ accumulation in rat
H9c2 cells. Exp Cell Res. 318:1480–1491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen M, Sun HY, Li SJ, Das M, Kong JM and
Gao TM: Nitric oxide as an upstream signal of p38 mediates
hypoxia/reoxygenation-induced neuronal death. Neurosignals.
17:162–168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shin EJ, Schram K, Zheng XL and Sweeney G:
Leptin attenuates hypoxia/reoxygenation-induced activation of the
intrinsic pathway of apoptosis in rat H9c2 cells. J Cell Physiol.
221:490–497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang C, Lin G, Wan W, et al: Resveratrol,
a polyphenol phytoalexin, protects cardiomyocytes against
anoxia/reoxygenation injury via the TLR4/NF-κB signaling pathway.
Int J Mol Med. 29:557–563. 2012.PubMed/NCBI
|
|
19
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kajstura J, Cheng W, Reiss K, et al:
Apoptotic and necrotic myocyte cell deaths are independent
contributing variables of infarct size in rats. Lab Invest.
74:86–107. 1996.PubMed/NCBI
|
|
21
|
Robich MP, Osipov RM, Nezafat R, et al:
Resveratrol improves myocardial perfusion in a swine model of
hypercholesterolemia and chronic myocardial ischemia. Circulation.
122(Suppl): S142–S149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burstein B, Maguy A, Clément R, et al:
Effects of resveratrol (trans-3,5,4′-trihydroxystilbene) treatment
on cardiac remodeling following myocardial infarction. J Pharmacol
Exp Ther. 323:916–923. 2007.
|
|
23
|
Eguchi M, Liu Y, Shin EJ and Sweeney G:
Leptin protects H9c2 rat cardiomyocytes from H2O2-induced
apoptosis. FEBS J. 275:3136–3144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Das S, Alagappan VK, Bagchi D, et al:
Coordinated induction of iNOS-VEGF-KDR-eNOS after resveratrol
consumption: a potential mechanism for resveratrol preconditioning
of the heart. Vascul Pharmacol. 42:281–289. 2005. View Article : Google Scholar
|
|
25
|
Das S, Tosaki A, Bagchi D, et al:
Resveratrol-mediated activation of cAMP response element-binding
protein through adenosine A3 receptor by Akt-dependent and
-independent pathways. J Pharmacol Exp Ther. 314:762–769. 2005.
View Article : Google Scholar
|
|
26
|
Moens AL, Claeys MJ, Timmermans JP and
Vrints CJ: Myocardial ischemia/reperfusion-injury, a clinical view
on a complex pathophysiological process. Int J Cardiol.
100:179–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bradamante S, Barenghi L, Piccinini F, et
al: Resveratrol provides late-phase cardioprotection by means of a
nitric oxide-and adenosine-mediated mechanism. Eur J Pharmacol.
465:115–123. 2003. View Article : Google Scholar
|
|
28
|
Das S, Cordis GA, Maulik N and Das DK:
Pharmacological preconditioning with resveratrol: role of
CREB-dependent Bcl-2 signaling via adenosine A3 receptor
activation. Am J Physiol Heart Circ Physiol. 288:H328–H335. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shimamoto A, Chong AJ, Yada M, et al:
Inhibition of Toll-like receptor 4 with eritoran attenuates
myocardial ischemia-reperfusion injury. Circulation. 114(Suppl):
I270–I274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dudley J, Das S, Mukherjee S and Das DK:
Resveratrol, a unique phytoalexin present in red wine, delivers
either survival signal or death signal to the ischemic myocardium
depending on dose. J Nutr Biochem. 20:443–452. 2009. View Article : Google Scholar
|
|
31
|
Gasser RN: The interdependence of
hypertension, calcium overload, and coronary spasm in the
development of myocardial infarction. Angiology. 39:761–772. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Borutaite V and Brown GC: Mitochondria in
apoptosis of ischemic heart. FEBS Lett. 541:1–5. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hausenloy DJ and Yellon DM: The
mitochondrial permeability transition pore: its fundamental role in
mediating cell death during ischaemia and reperfusion. J Mol Cell
Cardiol. 35:339–341. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Borutaite V, Jekabsone A, Morkuniene R and
Brown GC: Inhibition of mitochondrial permeability transition
prevents mitochondrial dysfunction, cytochrome c release and
apoptosis induced by heart ischemia. J Mol Cell Cardiol.
35:357–366. 2003. View Article : Google Scholar
|
|
35
|
Pei JM, Kravtsov GM, Wu S, et al: Calcium
homeostasis in rat cardiomyocytes during chronic hypoxia: a time
course study. Am J Physiol Cell Physiol. 285:C1420–C1428. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Penna C, Perrelli MG and Pagliaro P:
Mitochondrial pathways, permeability transition pore, and redox
signaling in cardioprotection: therapeutic implications. Antioxid
Redox Signal. 18:556–599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koval A, Purvanov V, Egger-Adam D and
Katanaev VL: Yellow submarine of the Wnt/Frizzled signaling:
submerging from the G protein harbor to the targets. Biochem
Pharmacol. 82:1311–1319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012.
|