Introduction
Glioma, the most common malignant tumor of the
central nervous system, accounts for 30–50% of intracranial tumors.
It is characterized by high cell proliferation rates and
invasiveness. Treatment of glioma includes surgery, radiation and
chemotherapy. However, the median survival rate of glioma patients
is only 9–15 months (1–5). Furthermore, the therapeutic effects
of such treatments are limited, particularly for high grade
gliomas, where the incidence and mortality rate remain high with a
post-operative median survival time of less than one year (6). Difficulty in completely removing
tumors and the recurrence of cancer after treatment remain a
significant barrier to long-term survival. To overcome these
challenges, focus on the development of novel therapies for the
treatment of glioma is required.
Novel treatments include targeting molecular
pathways specifically activated within glioma cells. Studies have
shown that glioma is in part caused by deregulation of the Wnt
signaling pathway and this deregulation is crucial in regulating
processes, such as the initiation, proliferation and development of
glioma cells (7–9). Inhibition of activated abnormal tumor
signaling pathways may be an effective therapy with which to kill
tumor cells, suppress cell proliferation and induce cellular
differentiation.
One such signaling pathway involved in the
development of glioma is the Notch signaling pathway (10). This is activated following binding
of the epidermal growth factor (EGF) repeat region in Notch
receptors (Notch 1–4) to their ligands, δ-like-1, 3 and 4, and
Jagged 1 and 2, which are commonly expressed on adjacent cells
(11). Notch receptors are
important members of a family of proteins involved in the growth
and development of vertebrates and invertebrates, and are involved
in cell proliferation and differentiation (11). Following binding, two enzymatic
cleavages occur to release the Notch intracellular domain (NICD)
from the plasma membrane, thus translocating the NICD into the cell
nucleus, allowing this domain to perform its regulatory role
(12,13).
Studies have reported that the Notch signaling
pathway is involved in the initiation and development of tumors
(14–21). The role of Notch signaling as an
oncogenic or tumor suppressor varies with tumor type (22–27).
Notch signaling can promote cell proliferation in acute T-cell
leukemia, breast cancer, renal epithelial urothelial cancer and
pancreatic cancer (16,18,19,21,28,29).
By contrast, Notch signaling can induce cell cycle arrest in small
cell lung cancer (19,30). Activation of different Notch1
receptors inhibits tumor growth, while activation of Notch2
receptors promotes tumor growth, leading to different effects on
embryonic brain tumor cell proliferation (31). Notch receptors are important in
glioma progression. It has been reported that the Notch1 receptor
enhanced the proliferation of glioma cells (12). However, whether the Notch2 receptor
also promotes cell proliferation remains unknown (31,32).
Studies by Chen et al (10,32),
Reichrath et al (33) and
Sivasankaran et al (30)
have shown that substantial levels of Notch2 mRNA and Notch2
protein are detected in U87 human brain glioma cells. However, it
has been reported that Notch2 expression varies in different glioma
cells, such as astrocytoma and medulloblastoma cells (10,32–34).
Xu et al (35,36) investigated the role of Notch2 in
the U251 glioblastoma astrocytic cell line and found low expression
of Notch2 (35,36). Consequently, it is clear that
Notch2 expression levels vary depending on the type of glioma.
The aim of the current study was to determine the
role of Notch2 in human glioma cell proliferation in an attempt to
provide a direction for the development of a novel molecular
therapy. To investigate the role of Notch2 in glioma cell
proliferation, the U87 cell line was used. This is a primary human
glioblastoma cell line with epithelial morphology, which was
originally obtained from a 44-year-old patient with stage IV
disease. Notch2 expression was downregulated in the U87 human
glioma cells using the RNA interference method. Mini chromosome
maintenance complex (MCM)2, p21 and cyclin-D1 are involved in the
cell cycle, however, the impact of the Notch receptors remains
unclear. Therefore, cell proliferation, cell cycle distribution,
cell cycle-related proteins and cell apoptosis of U87 cells in
vitro and in vivo, prior to and after RNA interference,
were investigated.
Materials and methods
Cell culture
The U87 human glioblastoma cell line was obtained
from the Shanghai Cell Bank of the Chinese Academy of Medical
Science (Shanghai, China). The U87 cells were cultured with
Dulbecco’s modified Eagle’s medium (DMEM; Gibco Inc., Billings, MT,
USA) containing 10% fetal bovine serum (Invitrogen, Carlsbad, CA,
USA), 100 U/ml penicillin and 100 U/ml streptomycin (Beyotime,
Shanghai, China) in an incubator containing 5% CO2 at
37°C.
Animals
Thirty specific pathogen free BALB/c female nude
mice (age, 6 weeks; body weight, 20.0±2.5g) were purchased from
(Beijing HFK Bioscience Co., Ltd., Beijing, China). Mice were
housed at 20–25°C with 50±5% humidity, access to food and water
ad libitum and a 12:12h light/dark cycle. Experiments were
approved by the Medical Ethics Committee of the Second Affiliated
Hospital of Hebei Medical University (Shijiazhuang, China). All
procedures involving mice conformed to the Guide for the Care and
Use of Laboratory Animals published by the National Institutes of
Health (NIH Publication No. 85–23, revised 1996).
Construction and identification of U87
cells stably transfected with plasmids
The p green fluorescent protein (GFP)-V-RS Notch2
short hairpin RNA (shRNA) plasmid was purchased from Beijing
OriGene Technologies Co., Ltd. (Beijing, China). In this study,
three treatments were designed. The U87 cells with no treatment
were considered as a blank control, termed the nontransfection
group. The plasmid pGFP-V-RS Notch2-shRNA containing
Notch2-specific shRNA and the plasmid pGFP-V-RS negative-shRNA
containing unspecific shRNA (Beijing OriGene Technologies Co.,
Ltd.) were considered as the Notch2-shRNA and negative-shRNA
groups, respectively. These plasmids were transfected into U87
cells. Briefly, U87 cells were inocculated into 4-well plates (a
density of 1×105 cells/ml, 150 μl/well) and incubated
overnight. The pGFP-V-RS Notch2-shRNA plasmid or pGFP-V-RS
negative-shRNA plasmid, together with Lipofectamine 2000 and
optimem (both Invitrogen; 1:2.5:250) were incubated for 20 min at
room temperature (RT), forming a DNA-liposome complex. The complex
(100 μl) was added to the 24-well plate after the culture media was
removed and mixed evenly. The U87 cells were incubated in the media
containing the complex for 6 h. After the supernatant was
discarded, DMEM was added to the plate. Cells were incubated in the
media containing the complex and DMEM for 24 h until they were
ready to be passaged at a ratio of 1:10. The transfected cells were
passaged into a vessel containing growth media of 1 μg/ml puromycin
(Corning Inc., New York, NY, USA) and incubated until clonal cells
of U87 were present. Cell clones were selected and inoculated onto
a 96-well plate for incubation. During the incubation, puromycin
was maintained at 1 μg/ml. When cells achieved 70% confluence,
stably transfected cells were transferred to incubation flasks and
analyzed by a CKX31-A11RC fluorescence microscope (OLYMPUS, Tokyo,
Japan) for visualization of the green fluorescent protein included
in the plasmid vector.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA in stably transfected cells was extracted
with the TRIzol (Invitrogen) method. RNA purity was determined
using absorbance at 260 and 280 nm (A260/280) using a Nanodrop
spectrophotometer (ND-2000; Thermo Scientific, Pittsburgh, PA,
USA), and the integrity of the RNA was verified by electrophoresis
on formaldehyde gels. The first cDNA sequence was synthesized
according to the manufacturer’s instructions (Invitrogen). This
cDNA sequence was used as a template for PCR amplification. Primer
sequences were as follows: Forward: 5′-CCC AAT GGG CAA GAA GTC
TA-3′ and reverse: 5′-CAC AAT GTG GTG GTG GGA TA-3′ for Notch2; and
forward: 5′-CCA CCC ATG GCA AAT TCC ATG GCA-3′ and reverse 5′-TCT
AGA CGG CAG GTC AGG TCC AC-3′ for the glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) control. All reactions involved initial
denaturation at 94°C for 15 min followed by 30 cycles of 94°C for
60 sec, 58°C for 60 sec and 72°C for 60 sec. PCR products were
separated on 1.5% agarose gel electrophoresis, examined under UV
light and photographed by a UV transilluminator (Imagemaster,
Pharmacia Biotech, Piscataway, NJ, USA). The mean gray value of
each group was determined by Image J software (National Institutes
of Health, Bethesda, MD, USA). Relative mRNA expression was
normalized to GAPDH expression.
Western blot analysis
Stably transfected cells were lysed in lysis buffer
(Nanjing KeyGen Biotech., Co., Ltd., Nanjing, China) on ice for 10
min and centrifuged at 20,000 × g at 4°C for 10 min. Proteins were
quantified using a Bicinchoninic Acid Protein Assay kit (Beyotime,
Nantong, China). A total of 40 μg protein was loaded for 15% sodium
lauryl sulfate-polyacrylamide gel electrophoresis. Proteins were
transferred onto polyvinylidene fluoride fiber membrane (Millipore,
Billerica, MA, USA), blocked with 5% non-fat milk powder in
Tris-buffered saline with 0.05% Tween-20 (TBST) for 2 h at RT and
incubated with the following primary antibodies at 4°C overnight:
Rabbit polyclonal antibodies against Notch2, cyclin-D1, p21 and
MCM2; and mouse polyclonal antibody against β-actin (Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA). Membranes were rinsed in
TBST three times, and incubated with the respective secondary
antibodies for 1 h at RT (horseradish peroxidase-conjugated
monoclonal goat anti-rabbit IgG and monoclonal goat anti-mouse IgG;
Santa Cruz Biotechnology Inc.). Membranes were rinsed in TBST again
and protein expression was detected with 3,3′-diaminobenzidine
(DAB; Beyotime, Jiangsu, China). The mean gray value of each group
was determined by Image J software. Relative mRNA expression was
normalized to β-actin.
Detection of cell proliferation with a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Stably transfected cells were inoculated onto a
96-well plate at a density of 3,000 cells/well. Cell proliferation
was determined by an MTT assay each day for one week. In brief, 5
mg/ml MTT (Nanjing KeyGen Biotech., Co., Ltd.; 20 μl) was added to
each well, incubated for 4 h and then centrifuged at 3,000 × g for
5 min. The supernatant was discarded and 150 μl dimethyl sulfoxide
was added to each well. The plate was incubated for 10 min on a
shaker (Bühler GmbH, Braunschweig, Germany). The absorbance at 490
nm was determined using an automatic Enzyme Labeling instrument
(Beijing Putian Instrument Ltd., Beijing, China). A cell
proliferation curve was generated.
Cell cycle distribution and
apoptosis
Stably transfected cells were collected and fixed in
precooled 70% ethanol overnight at 4°C. After washing with
phosphate-buffered saline (PBS), RNAase A (125 U/ml; Molecular
Probes, Eugene, OR, USA) and propidium iodide (50 μg/ml; Molecular
Probes) were added, and cells were incubated in the dark at 4°C for
30 min. Cells were analyzed using a FACSCalibur flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA), and cell cycle distribution
(in the G0/G1, S and G2/M phases)
was calculated using the ModFit LT 3.2 software (BD Biosciences).
Sub-G1 was taken to represent apoptosis.
U87 human glioblastoma mouse xenograft
tumor model
Stably transfected U87 cells at logarithmic growth
phase were collected and washed with serum free DMEM. Cell
concentration was adjusted to 5×106 cells/ml. Thirty
nude mice were randomly assigned to the three groups with ten mice
in each group. Under a sterile hood, mice were sterilized with 75%
ethanol and injected with 0.1 ml U87 cells (the nontransfection
group), U87 cells stably transfected with Notch2-shRNA (the
Notch2-shRNA group) or scramble-shRNA (the negative-shRNA group)
into the back of the neck. The diameter of the tumor in the
greatest axis and shortest axis was measured with a vernier caliper
every five days. The tumor volume was calculated according to the
formula: Tumor volume (mm3) = DGD × DSD2 ×
0.5. The growth curve of the tumor was drafted as a plot of tumor
volume against inoculation.
Immunohistochemistry (IHC) analysis of
mouse tumor specimens
On day 40 after inoculation, four mice were selected
from each group and sacrificed by cervical dislocation. The
specimens were observed, weighed, fixed in 10% buffered formalin
for 8 h and embedded in wax. Sections (3 μm-thick) from tumor
tissue samples, were mounted on glass slides precoated with
3-aminopropyltriethoxysilane and dried for the IHC analysis (IHC
kit, Mai Bio Co., Ltd., Shanghai, China) of Notch2 protein.
Antibodies against Notch2 for IHC were the same as those used for
western blot analysis. Integrated Optical Density was determined by
ImagePro Plus 6 software (Media Cybernetics, Rockville, MD,
USA).
Cumulative survival rate of nude
mice
Of the ten mice in each group, four mice were
selected from each group for IHC, and the remaining six mice
continued to be housed under standard conditions. Each day, tumor
growth was examined until the mice succumbed to the tumors.
Kaplan-Meier survival plots were used to determine the cumulative
survival rate of nude mice for each group. Tumor weight was
measured 50 days after the inoculation as pre-experiments revealed
that significant differences in tumor weight appeared at this time
period following inoculation.
Statistical analysis
All data are expressed as the mean ± standard
deviation and analyzed with an SPSS 13.0 software package (SPSS
Inc., Chicago, IL, USA). Statistical significance was evaluated by
one-way analysis of variance with the least significance difference
test for post hoc analysis. Kaplan-Meier survival plots were
generated, and comparisons between survival curves were made with
the log-rank statistics. P<0.05 was considered to indicate a
statistically significant difference.
Results
Notch2 mRNA and protein expression was
stably and effectively downregulated by shRNA in U87 glioma
cells
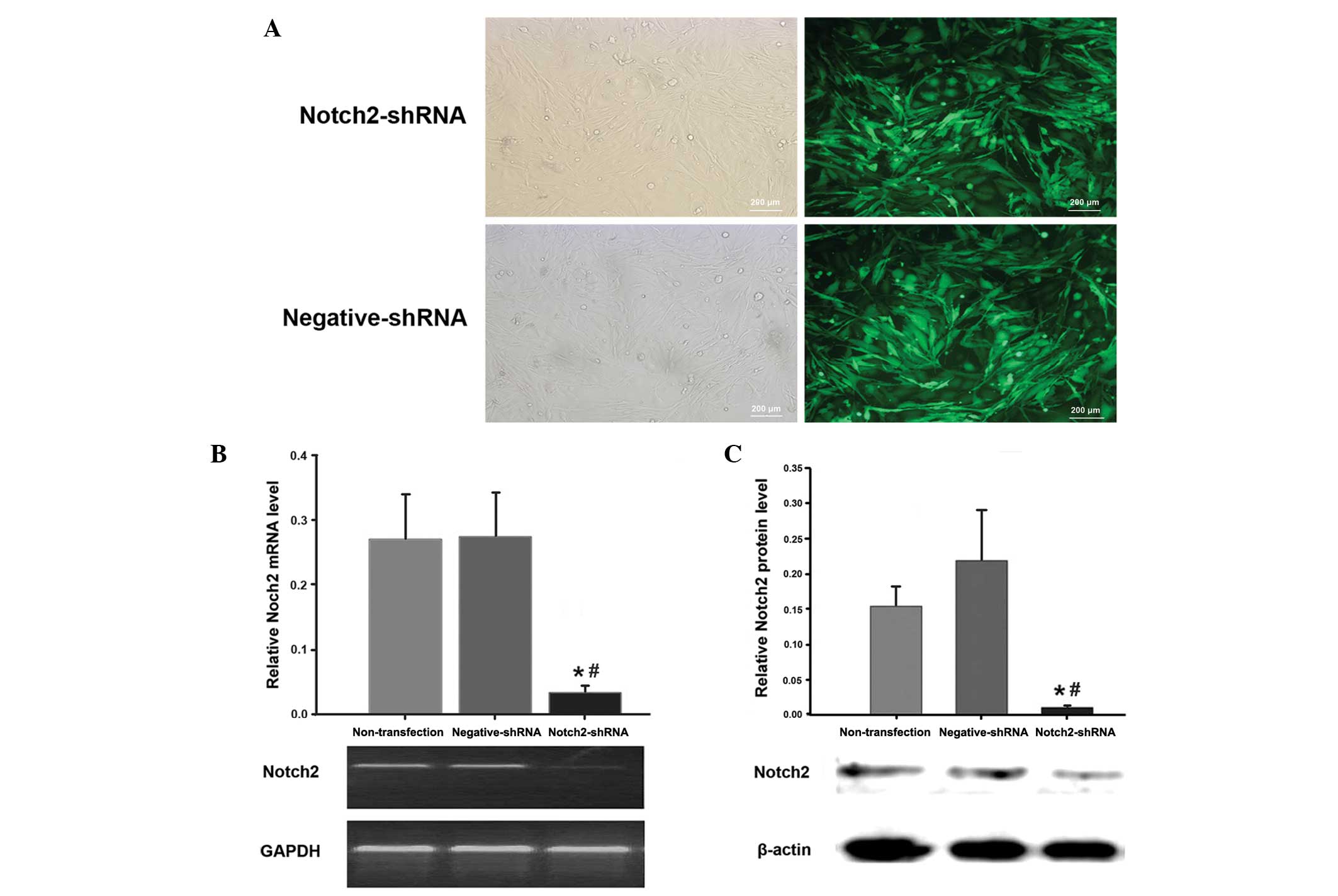
Transfection efficiency as monitored by GFP is shown
in Fig. 1A. Notch2 mRNA and
protein expression in U87 cells was determined by RT-PCR and
western blot analysis, respectively. Compared with the
negative-shRNA group, Notch2 mRNA (Fig. 1B) and protein (Fig. 1C) expression in the Notch2-shRNA
group were reduced by 87.6 and 94.5%, respectively (P<0.05). In
the nontransfection group and the negative-shRNA group, U87 cells
showed no significant changes in the levels of Notch2 mRNA or
protein (P>0.05).
Effects of Notch2 knockdown on cell
proliferation, cell cycle distribution and cell apoptosis in U87
cells (Fig. 2)
Compared with the nontransfection group and the
negative-shRNA group, Notch2 knockdown significantly inhibited U87
cell proliferation after three days of culture (P<0.05). The
highest inhibition rate was ~33.3% on day seven of culture
(Fig. 2A). No significant
difference was identified in the cell proliferation between the
nontransfection group and the negative-shRNA group (all
P>0.05).
Compared with the negative-shRNA group, the
proportion of cells in S phase in the Notch2-shRNA group was
significantly lower (18.1±2.7 vs. 33.7±3.3%; P<0.05), whilst the
proportion in G0/G1 phase was significantly
higher (59.4±4.1 vs 41.9±3.3%; P<0.05). The proportion of cells
in the G2/M phase was not significantly different
between the Notch2-shRNA group and negative-shRNA group (P>0.05;
Fig. 2B and C). There was no
significant difference in the cell cycle distribution between the
nontransfection group and the negative-shRNA group (P>0.05).
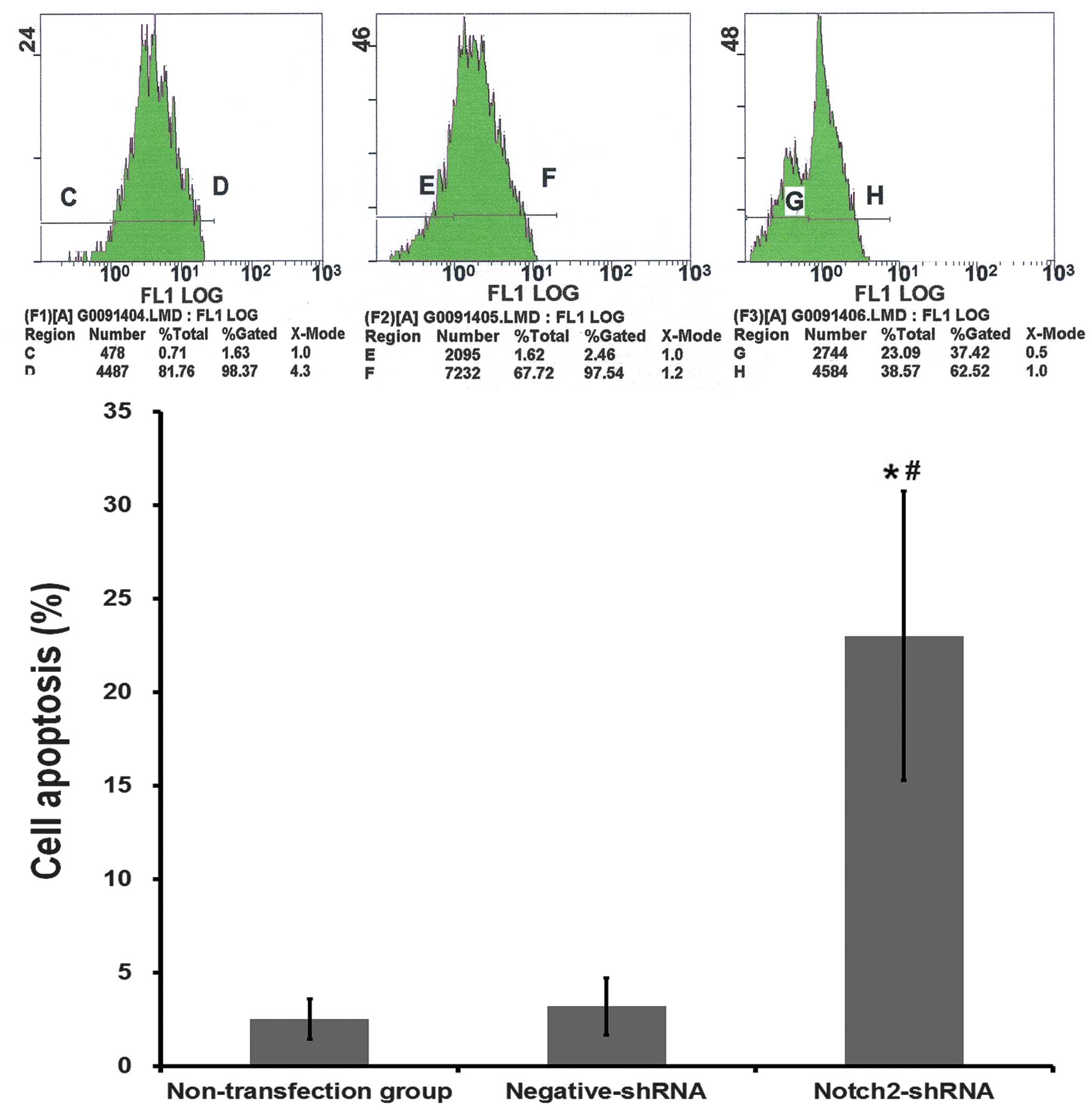
Compared with the negative-shRNA group, the
percentage of cells that had undergone apoptosis in the
Notch2-shRNA group was significantly higher (23.00±7.74 vs.
3.21±1.53%; P<0.001; Fig. 3).
There was no significantly difference in the percentage of cells
that had undergone apoptosis between the nontransfection group and
the negative-shRNA group (P>0.05).
Effects of Notch2 knockdown on cell
cycle-related protein expression
Western blot analysis showed that Notch2 silencing
significantly inhibited protein expression of MCM2 and cyclin-D1,
but significantly increased expression of p21, compared with the
negative-shRNA group (all P<0.01; Fig. 2D and E). No significant difference
was identified in the cell cycle-related protein expression between
the nontransfection group and the negative-shRNA group (all
P>0.05).
Effects of Notch2 knockdown on tumor
growth and cumulative survival rate in nude mouse xenograft tumor
models
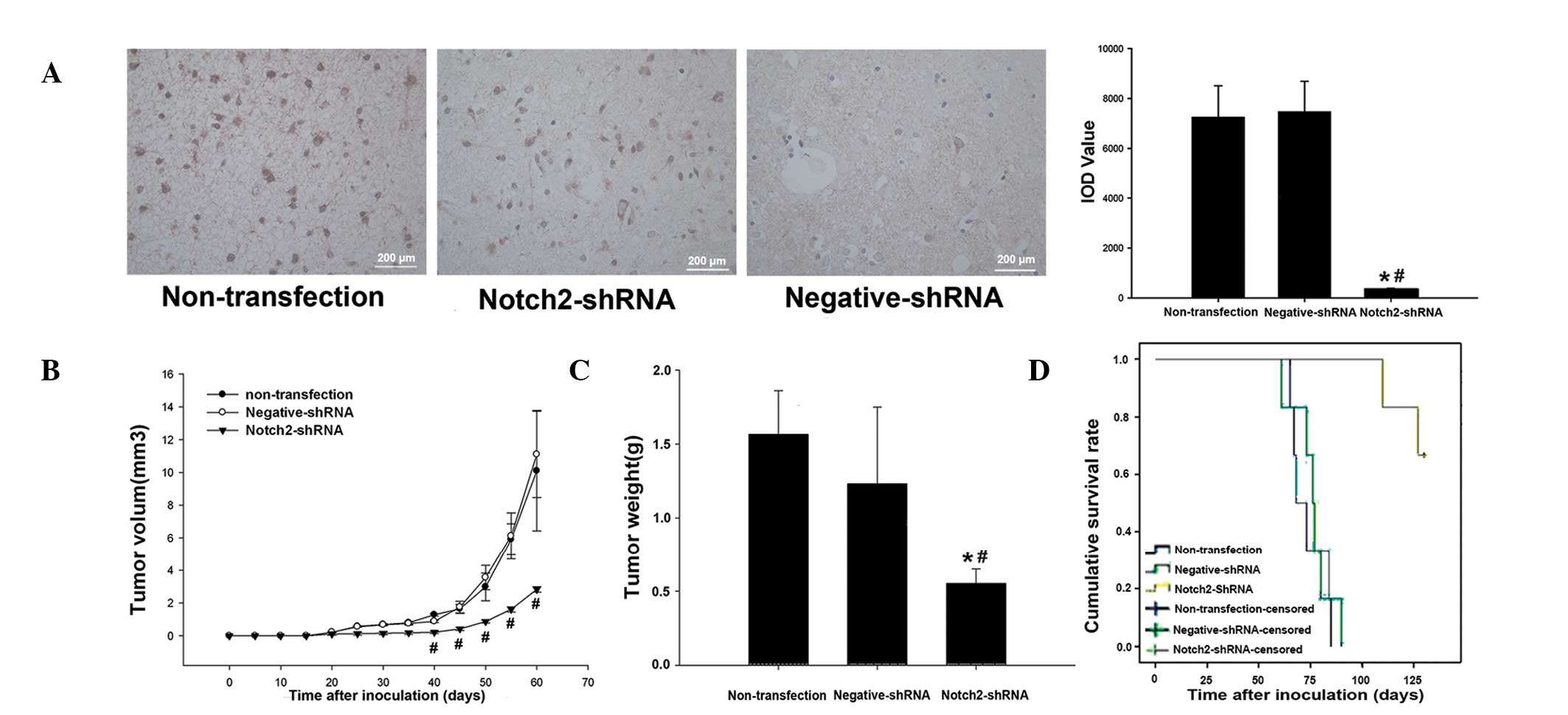
IHC analysis showed relatively high protein
expression of Notch2 in the tumor tissues inoculated with naive U87
cells or U87 cells stably transfected with negative-shRNA
(yellow-brown to brown color), but almost no Notch2 protein
expression was observed in the tumor tissues inoculated with U87
cells stably transfected with Notch2-shRNA on day 40 after
inoculation (Fig. 4A). This was a
statistically significant reduction (both P<0.05; Fig. 4A).
The tumor growth curve showed that tumor volume was
significantly lower in the Notch2-shRNA group than that in the
nontransfection and negative-shRNA groups 40 days after inoculation
(P<0.05; Fig. 4B). On day 50
after inoculation, tumor weight in the Notch2-shRNA group was
significantly lower than that in the nontransfection and
negative-shRNA groups (0.55±0.1 vs. 1.57±0.29 and 1.23±0.52g,
respectively; P<0.01; Fig.
4C).
The effect of Notch2 silencing on the cumulative
survival rate in nude mouse xenograft tumor models is shown in
Fig. 4D. In the nontransfection
and negative-shRNA groups, tumor growth was rapid resulting in
death on day 61–90 after inoculation. The survival time of mice in
these groups was 73.7±3.6 days in the nontransfection group and
76.2±3.9 days in the negative-shRNA group. By contrast, in the
Notch2-shRNA group, on day 130 after inoculation, four mice were
alive and the survival time of the mice was 126.2±3.0 days. The
cumulative survival rate was significantly longer in the
Notch2-shRNA group compared with the negative-shRNA group (log-rank
test, P=0.01). The cumulative survival rate was not identified to
be significantly different between the nontransfection group and
the negative-shRNA group (log-rank test, P=0.59).
Discussion
Currently, the treatment of glioma, one of the most
common malignant tumors of the central nervous system, consists of
surgery, radiation and chemotherapy (1–5).
Difficulty in completely removing tumors and the recurrence of
cancer after treatment remains a significant obstacle to long-term
survival. Thus it is crucial to develop novel therapies for the
treatment of glioma, such as molecular cancer therapy. As it has
been previously reported that the Notch signaling pathway is
important for the initiation and development of tumors (14–21),
it was investigated in U87 primary human glioma cells in the
current study.
In the present study, substantial levels of Notch2
mRNA and Notch2 protein expression were detected in U87 human brain
glioma cells. These results are in agreement with those reported by
Chen et al (10,32), Reichrath et al (33) and Sivasankaran et al
(30). Notably, Chen et al
(10,32) and Reichrath et al (33) reported that Notch2 expression
varied in different glioma cells, for example astrocytoma and
medulloblastoma cells. This indicates that Notch2 may act as an
oncogene or tumor suppressor protein, depending on the type of
glioma (34–36).
In the current study, the effect of silencing Notch2
in cell proliferation was investigated using the MTT method. It was
found that the Notch2 receptor was closely correlated with the
level of proliferation of U87 glioma cells. Cell proliferation was
significantly lower in the Notch2-shRNA group compared with the
nontransfection and negative-shRNA groups. Cell cycle, determined
by flow cytometry, showed that the proportion of cells in S phase
was lower, whilst the proportion of cells in G1 phase
was higher, in the Notch2-shRNA group compared with the
nontransfection and negative-shRNA groups. After Notch2-shRNA cells
were transplanted into nude mice, tumor growth was significantly
suppressed, the number of tumors decreased and survival time
increased. Similarly, Chen et al (32) reported that downregulation of
Notch2 inhibited proliferation of U87 glioma cells in vitro.
Jin et al (37) also showed
that inhibiting the Notch signaling pathway with MRK003 can inhibit
proliferation of U251 and U87 cells in vitro. In addition,
it has been reported that suppressing the expression of Notch1 and
Notch2 slows glioma cell proliferation in vitro (10). Here, Chen et al, showed that
suppressing Notch2 expression was more effective in decreasing the
rate of cell proliferation than the suppression of Notch1. By
contrast, several studies have shown that Notch2 inhibits tumor
cell growth by antagonizing Notch1 and that upregulation of Notch2
in U251 cells can suppress glioma cell proliferation (31,32,35,36).
Consequently, it was hypothesized that Notch2 may have a dual
effect on the proliferation of glioma cells. Several studies have
investigated the effect of Notch2 on cell proliferation in
vitro, but not in vivo (10,32,37).
The current study confirmed the inhibition of glioma cell
proliferation by downregulation of Notch2 in vitro and in
vivo, thus providing valuable information for future studies on
the role of Notch signaling in glioma.
The downstream Notch signaling pathways remain to be
fully understood. Studies have shown that Notch signaling can
regulate cell cycle progression and subsequent cell proliferation
by multiple pathways (15,21–27,30,37).
In the present study, it was found that Notch2 signaling in U87
human glioma cells increased the proportion of cells in the S phase
and upregulated MCM2 and cyclin D1 protein expression levels;
however, p21 protein expression was downregulated. This is
consistent with previous studies that have shown that expression of
downstream proteins of the Notch signaling pathway varies in
different carcinoma cells. In small cell lung cancer cells, Notch
signaling can increase p21 and p27 expression and hence induce cell
cycle arrest (14). In mouse
keratinocytes, Notch signaling inhibited cell cycle progression by
increasing CSL-dependent p21 expression (38). A recent study has reported that
Notch signaling induces cell cycle arrest by downregulating MCM2
and MCM6 protein expression (39).
In the current study downregulation of Notch2 inhibited U87 cell
proliferation by altering cell cycle-related protein expression and
thereby regulating cell cycle progression.
The Notch signaling pathway is important for the
initiation and development of tumors. In particular, the Notch2
receptor appears to be vital in the regulation of gliomblastoma
cell proliferation (35,36). The present study indicates that
downregulation of Notch2 mRNA and protein expression suppresses U87
human glioma cell proliferation in vitro and in vivo,
and induces cell cycle arrest at the G0/G1
phase by upregulation of p21 expression, and downregulation of MCM2
and cyclin-D1 expression and cell apoptosis. The results of the
present study indicate that the Notch2 signaling pathway is
important in U87 human glioma cell proliferation. However, the
molecular mechanisms underlying Notch2 regulation of glioma cell
proliferation require further investigation.
Acknowledgements
This study was supported by grants from the Doctoral
Fund of Zhengzhou.
References
|
1
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN: Molecular pathology of malignant
gliomas. Annu Rev Pathol. 1:97–117. 2006. View Article : Google Scholar
|
|
3
|
Hulleman E and Helin K: Molecular
mechanisms in gliomagenesis. Adv Cancer Res. 94:1–27. 2005.
View Article : Google Scholar
|
|
4
|
Konopka G and Bonni A: Signaling pathways
regulating gliomagenesis. Curr Mol Med. 3:73–84. 2003. View Article : Google Scholar
|
|
5
|
Ohgaki H and Kleihues P: Population-based
studies on incidence, survival rates, and genetic alterations in
astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol.
64:479–489. 2005.
|
|
6
|
Claus EB and Black PM: Survival rates and
patterns of care for patients diagnosed with supratentorial
low-grade gliomas: data from the SEER program, 1973–2001. Cancer.
106:1358–1363. 2006.PubMed/NCBI
|
|
7
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cerchia L, Martinez Montero JC and
Monfared P: Signal transduction alterations in glioma: implications
for diagnosis and therapy. J Signal Transduct. 2012:7042472012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mellinghoff IK, Lassman AB and Wen PY:
Signal transduction inhibitors and antiangiogenic therapies for
malignant glioma. Glia. 59:1205–1212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen J, Kesari S, Rooney C, et al:
Inhibition of notch signaling blocks growth of glioblastoma cell
lines and tumor neurospheres. Genes Cancer. 1:822–835. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Strizzi L, Hardy KM, Seftor EA, et al:
Development and cancer: at the crossroads of Nodal and Notch
signaling. Cancer Res. 69:7131–7134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Purow BW, Haque RM, Noel MW, et al:
Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1,
is critical for glioma cell survival and proliferation. Cancer Res.
65:2353–2363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pannuti A, Foreman K, Rizzo P, et al:
Targeting Notch to target cancer stem cells. Clin Cancer Res.
16:3141–3152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sriuranpong V, Borges MW, Ravi RK, et al:
Notch signaling induces cell cycle arrest in small cell lung cancer
cells. Cancer Res. 61:3200–3205. 2001.PubMed/NCBI
|
|
15
|
Ramdass B, Maliekal TT, Lakshmi S, et al:
Coexpression of Notch1 and NF-kappaB signaling pathway components
in human cervical cancer progression. Gynecol Oncol. 104:352–361.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sjölund J, Johansson M, Manna S, et al:
Suppression of renal cell carcinoma growth by inhibition of Notch
signaling in vitro and in vivo. J Clin Invest. 118:217–228.
2008.PubMed/NCBI
|
|
17
|
Fortini ME: Notch signaling: the core
pathway and its posttranslational regulation. Dev Cell. 16:633–647.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zardawi SJ, O’Toole SA, Sutherland RL and
Musgrove EA: Dysregulation of Hedgehog, Wnt and Notch signalling
pathways in breast cancer. Histol Histopathol. 24:385–398.
2009.PubMed/NCBI
|
|
19
|
Ristorcelli E and Lombardo D: Targeting
Notch signaling in pancreatic cancer. Expert Opin Ther Targets.
14:541–552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Wang C, Meng Q, et al: siRNA
targeting Notch-1 decreases glioma stem cell proliferation and
tumor growth. Mol Biol Rep. 39:2497–2503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dai J, Ma D, Zang S, et al: Cross-talk
between Notch and EGFR signaling in human breast cancer cells.
Cancer Invest. 27:533–540. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Noseda M, Chang L, McLean G, et al: Notch
activation induces endothelial cell cycle arrest and participates
in contact inhibition: role of p21Cip1 repression. Mol Cell Biol.
24:8813–8822. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sarmento LM, Huang H, Limon A, et al:
Notch1 modulates timing of G1-S progression by inducing SKP2
transcription and p27 Kip1 degradation. J Exp Med. 202:157–168.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dakubo GD, Mazerolle CJ and Wallace VA:
Expression of Notch and Wnt pathway components and activation of
Notch signaling in medulloblastomas from heterozygous patched mice.
J Neurooncol. 79:221–227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao N, Guo Y, Zhang M, et al: Akt-mTOR
signaling is involved in Notch-1-mediated glioma cell survival and
proliferation. Oncol Rep. 23:1443–1447. 2010.PubMed/NCBI
|
|
26
|
Berry N, Gursel DB and Boockvar JA: Notch
inhibition via micro-RNA blocks glioma development. Neurosurgery.
70:N20–N22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Muto J, Imai T, Ogawa D, et al:
RNA-binding protein Musashi1 modulates glioma cell growth through
the post-transcriptional regulation of Notch and PI3 kinase/Akt
signaling pathways. PLoS One. 7:e334312012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Palomero T and Ferrando A: Therapeutic
targeting of NOTCH1 signaling in T-cell acute lymphoblastic
leukemia. Clin Lymphoma Myeloma. 9(Suppl 3): S205–S210. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mullendore ME, Koorstra JB, Li YM, et al:
Ligand-dependent Notch signaling is involved in tumor initiation
and tumor maintenance in pancreatic cancer. Clin Cancer Res.
15:2291–2301. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sivasankaran B, Degen M, Ghaffari A, et
al: Tenascin-C is a novel RBPJkappa-induced target gene for Notch
signaling in gliomas. Cancer Res. 69:458–465. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan X, Mikolaenko I, Elhassan I, et al:
Notch1 and notch2 have opposite effects on embryonal brain tumor
growth. Cancer Res. 64:7787–7793. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen L, Zhang R, Li P, et al: P53-induced
microRNA-107 inhibits proliferation of glioma cells and
down-regulates the expression of CDK6 and Notch-2. Neurosci Lett.
534:327–332. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reichrath S, Müller CS, Gleissner B, et
al: Notch- and vitamin D signaling in 1,25(OH)2D3-resistant
glioblastoma multiforme (GBM) cell lines. J Steroid Biochem Mol
Biol. 121:420–424. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu P, Yu S, Jiang R, et al: Differential
expression of Notch family members in astrocytomas and
medulloblastomas. Pathol Oncol Res. 15:703–710. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu P, Zhang A, Jiang R, et al: The
different role of Notch1 and Notch2 in astrocytic gliomas. PLoS
One. 8:e536542013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu P, Qiu M, Zhang Z, et al: The oncogenic
roles of Notch1 in astrocytic gliomas in vitro and in vivo. J
Neurooncol. 97:41–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jin R, Nakada M, Teng L, et al:
Combination therapy using Notch and Akt inhibitors is effective for
suppressing invasion but not proliferation in glioma cells.
Neurosci Lett. 534:316–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rangarajan A, Hong SJ, Gifford A and
Weinberg RA: Species- and cell type-specific requirements for
cellular transformation. Cancer Cell. 6:171–183. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Noseda M and Karsan A: Notch and
minichromosome maintenance (MCM) proteins: integration of two
ancestral pathways in cell cycle control. Cell Cycle. 5:2704–2709.
2006. View Article : Google Scholar : PubMed/NCBI
|