Introduction
Aging is the result of complex changes in the
structure and function of molecules, cells, tissues and whole body
systems. Age-related hearing loss (ARHL), also known as
presbycusis, is believed to result from age-related degeneration of
the peripheral and central components of the auditory system
(1,2). Investigations into ARHL in humans is
limited due to the inaccesibility of auditory system tissue and the
complexity of the genetic and environmental background of
individuals with hearing loss. Numerous animal models have been
established in order to facilitate research into the molecular
mechanisms of ARHL. Among them, an animal model using the chronic
administration of D-galactose (D-gal) is widely used for studying
the mechanisms of ARHL (3–11). These animals exhibit increased
oxidative stress and mitochondrial DNA (mtDNA) common deletion (CD)
in the peripheral and central auditory system (PAS and CAS,
respectively); however, the source of the causative reactive oxygen
species (ROS) in the CAS has not been fully investigated.
NADPH oxidases (NOXs) are one of the main
ROS-generating sites. It is now clear that NOXs are not restricted
to the immune system and that alternative isoforms may be active in
numerous cell types as essential components of cellular signalling,
gene expression regulation and cell differentiation. These enzymes
are able to transport electrons across the plasma membrane,
generating superoxide and other downstream ROS (12). The expression of NOX3 is higher in
the PAS than that in any other tissue (13). In a previous study (5), it was demonstrated that NOX3 may be
an important source of ROS in the PAS of rats with D-gal-induced
aging and that chronic injection of D-gal could increase
NOX3-dependent oxidative stress, mitochondrial damage and apoptosis
in the PAS. NOX2 is not restricted to phagocytic cells, but is
present in numerous non-phagocytic cells and tissues, including
neurons of the CAS (14,15). The effects of NOX2 in the auditory
cortex of the CAS of rats with D-gal-induced aging have yet to be
elucidated.
Mitochondria are another site of predominant ROS
generation in cells (16).
Mitochondrial ROS generation is sensitive to the proton-motive
force across the mitochondrial inner membrane produced by the
electron transport chain, and mild uncoupling caused by the
activation of uncoupling protein 2 (UCP2) may cause a reduction in
the proton-motive force, attenuate mitochondrial ROS generation and
protect cells against ROS-related cellular damage (17). It is therefore hypothesised that an
overexpression of UCP2 may indirectly increase mitochondrial ROS
generation. The expression of UCP2 in the CAS of rats with
D-gal-induced aging, however, is unclear.
In the present study, the expression of NOX2, UCP2
and 8-hydroxy-2-deoxyguanosine (8-OHdG), a biomarker of DNA
oxidative damage (18,19), as well as the mitochondrial total
antioxidant capabilities (T-AOCs) and the levels of the mtDNA CD,
were investigated in the auditory cortex of the CAS in a rat model
with D-gal-induced aging. It was hypothesised that NOX- and
mitochondria-dependent ROS generation and mtDNA oxidative damage
may be primary etiological events in the degeneration of the CAS of
rats with D-gal-induced aging.
Material and methods
Animals and treatments
Eighty-eight one-month-old male Sprague Dawley rats
were obtained from the Experimental Animal Centre of Guangxi
Medical University (Nanning, China). The rats were individually
housed in temperature-controlled (20–22°C) conditions with a 12-h
light/dark cycle, with free access to food and water. The body
weight of each of the rats was monitored throughout the experiment
as an indicator of health. Injections of D-gal to induce aging were
administered according to established methodology (9). Following acclimation for two weeks,
the rats were randomly divided into four groups (n=22 for each
group), in which they were administered daily doses of D-gal
(Sigma, St. Louis, MO, USA) or saline by subcutaneous injection for
eight weeks. The three D-gal groups were referred to as the low-
(150 mg/kg), medium- (300 mg/kg) and high- (500 mg/kg) dose groups
and the fourth group was a control group (administered 0.9% saline
at the same volume). At the end of the eight-week protocol, the
rats were sacrificed by a terminal intraperitoneal injection of
ketamine (30 mg/kg) and an intramuscular injection of
chloropromazine (15 mg/kg). The auditory cortex was dissected and
used for total RNA, genomic DNA and protein extraction and the
examination of mitochondrial T-AOCs. Alternatively, the rats were
perfused with 4% paraformaldehyde for immunohistochemical analysis.
All experiments were carried out in strict accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The protocol was
approved by the Committee on the Ethics of Animal Experiments of
Guangxi Medical University.
RNA preparation and SYBR®
Green quantitative polymerase chain reaction (qPCR)
The mRNA expression levels of NOX2 and UCP2 were
determined using a SYBR Green qPCR assay (Invitrogen Life
Technologies, Carlsbad, CA, USA). Following the final injection of
D-Gal, 24 rats (n=6 per group) were sacrificed, and both sides of
the auditory cortex from each rat were rapidly removed. One side of
the auditory cortex was used for RNA extraction, and the other side
was used for mtDNA analysis. Total RNA was extracted with
TRIzol® reagent (Takara, Dalian, China) according to the
manufacturer’s instructions. cDNA was reverse transcribed using a
PrimeScript RT Reagent kit (Takara). The RNA and cDNA of each
sample were analysed using a GeneQuant Pro DNA/RNA Calculator
(Biochrom, Cambridge, UK) to assess the concentrations and purity.
The cDNA samples were stored at −20°C until required. qPCR was
performed by applying the SYBR Green qPCR technology with the use
of a StepOnePlus™ Real-Time PCR system (Applied Biosystems, Foster
City, CA, USA). The primer pairs for NOX2, UCP2 and β-actin (as an
internal standard) were as follows: NOX2 forward,
5′-ACATTTTCGTCAAGCGTCCC-3′ and NOX2 reverse,
5′-CCCAGCTCCCACTAACATCA-3′; UCP2 forward,
5′-TGCTGGGCACCATCCTAACC-3′ and UCP2 reverse,
5′-CCTGGAAGCGGACCTTTACC-3′; β-actin forward,
5′-CCTGGAGAAGAGCTATGAGC-3′ and β-actin reverse,
5′-ACAGGATTCCATACCCAGG-3′. The amplification conditions were as
follows: 30 sec at 95°C, then 40 cycles of 5 sec at 95°C, 30 sec at
60°C and 30 sec at 72°C. The amplification of β-actin as an
internal standard was used to normalise the relative gene
expression levels. A melting curve analysis was performed for each
gene, and the specificity and integrity of the PCR products were
confirmed by the presence of a single peak. The relative expression
levels were calculated from the differences in the cycle threshold
(Ct) values between the target mRNA and β-actin. The change in the
relative mRNA levels between the experimental group and the control
group was analysed using the 2−ΔΔCt method, as
previously reported (20).
Immunohistochemical analysis
The protein levels of NOX2 and the expression of
8-OHdG were determined by immunohistochemistry. Sixteen rats (n=4
per group) were sacrificed, and the brains were removed and fixed
with 4% buffered paraformaldehyde overnight, dehydrated and
embedded in paraffin wax. Serial sections of the brainstem were
subsequently cut at a thickness of 5 μm at the level of the
auditory cortex. The sections were then deparaffinised in xylene
and rehydrated through graded concentrations of ethanol. The
samples were incubated with anti-NOX2 (diluted 1:200; Boster
Biological Technology Co., Ltd., Wuhan, China) and anti-8-OHdG
(diluted 1:4,000; Abcam, Cambridge, MA, USA) antibodies overnight
at 4°C. Following washes in phosphate-buffered saline, the slides
were incubated with fluorescein isothiocyanate-conjugated
anti-rabbit and Cy3-conjugated anti-mouse secondary antibodies
(diluted 1:200; Boster Biological Technology Co., Ltd.) for 30 min
at room temperature and the nuclei were counterstained using DAPI
staining solution (Beyotime Institute of Biotechnology, Haimen,
China) for 5 min at room temperature. Images were captured using a
laser scanning confocal microscope (Nikon, Tokyo, Japan) and
analysed using Image-Pro Plus 6.0 software (Media Cybernetics,
Inc., Rockville, MD, USA). As a negative control, sections were
treated in the same manner but without the incubation with primary
antibody.
Western blot analysis
The protein levels of UCP2 in the auditory cortex
were determined using western blot analysis. Twenty-four rats (n=6
per group) were sacrificed, and both sides of the auditory cortex
from each rat were dissected. The total protein was extracted using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) according to the manufacturer’s instructions.
Protein concentrations were determined using an Enhanced
Bicinchoninic Acid Protein Assay kit (Beyotime Institute of
Biotechnology). Protein lysate (30 μg) was separated by 12%
SDS-PAGE and transferred to polyvinylidene difluoride membranes.
The membranes were incubated for 1 h in a blocking solution
[Tris-buffered saline (TBS) containing 5% skimmed milk], then
washed briefly in TBS and incubated overnight at 4°C with the
appropriate dilution of primary antibodies: Anti-UCP2 (diluted
1:500; Abcam) or anti-β-actin (diluted 1:1,000; Bioworld
Technology, Inc., Minneapolis, MN, USA). The membranes were then
washed to remove excess primary antibody and incubated for 1 h at
room temperature with the appropriate horseradish
peroxidase-conjugated secondary antibody (diluted 1:5,000; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The membranes were
visualised by the enhanced chemiluminescence method using BeyoECL
Plus (Beyotime Institute of Biotechnology) reagent. A
quantification of the detected bands was performed using Image-Pro
Plus 6.0 software. β-actin was used as an internal control.
Mitochondrial T-AOC determination
Twenty-four rats (n=6 per group) were sacrificed,
and both sides of the auditory cortex from each rat were dissected.
Mitochondria in the auditory cortex were quickly extracted using a
Tissue Mitochondria Isolation kit (Beyotime Institute of
Biotechnology) and subsequently used for the analysis of the
T-AOCs. Mitochondrial T-AOCs in the auditory cortex were detected
using a Total Antioxidant Capacity Assay kit in combination with
the fluorescence recovery after photobleaching method, according to
the manufacturer’s instructions (Beyotime Institute of
Biotechnology).
DNA isolation and TaqMan®
qPCR
Total DNA was extracted using the Genomic DNA
Purification kit (Tiangen Biotech Co., Ltd, Beijing, China)
according to the manufacturer’s instructions. The DNA concentration
of each specimen was measured using the GeneQuant Pro DNA/RNA
Calculator and the mtDNA CD levels were determined using a TaqMan
qPCR assay. The displacement (D)-loop in the non-coding region of
mtDNA, representing the conserved segment of mtDNA, was used as a
measure of copy number. Primers and probes for the mtDNA D-loop and
the mtDNA CD were designed as previously described (21). The PCR amplification was performed
on a StepOnePlus Real-Time PCR system in a 20-μl reaction volume
consisting of 10 μl 2× TaqMan PCR mix (Takara), 0.4 μl 50× ROX™
reference dye, 0.4 μl each forward and reverse primer (10 μM), 0.2
μl each probe (10 μM), 4 μl sample DNA (10 ng/μl) and 4.6 μl
distilled water. The cycling conditions consisted of an initial
phase at 95°C for 30 sec, then 40 cycles at 95°C for 5 sec and at
60°C for 30 sec. The cycle number at which a significant increase
in the normalised fluorescence was first detected was designated as
the Ct value. The ratio of the mtDNA CD to the mtDNA was calculated
by the formula ΔCt=(CtmtDNA deletion - CtmtDNA
D-loop). The relative expression (RE) was used to indicate
the factorial difference in the deletions between the experimental
and control groups. The RE was calculated according to the
2−ΔΔCt method, where ΔΔCt = ΔCtmtDNA deletion in
experimental group - ΔCtmtDNA deletion in control
group.
Statistical analysis
The data are presented as the mean ± standard
deviation. The analysis was performed using SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA). Statistical significance was tested
by a one-way analysis of variance. The least significant difference
post hoc test was used to evaluate the differences between groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
D-gal induces an increase in NOX2 and
UCP2 mRNA levels
To investigate the mRNA levels of NOX2 and UCP2 in
the auditory cortex, SYBR Green qPCR was performed. As shown in
Fig. 1, the mRNA levels of NOX2
and UCP2 were significantly higher in the D-gal-treated groups as
compared with those in the control group (P<0.01).
D-gal induces an increase in NOX2 and
8-OHdG protein expression
Immunohistochemical analysis was performed in order
to investigate the protein levels of NOX2 and 8-OHdG expression in
the auditory cortex. As shown in Fig.
2A and B, the NOX2 and 8-OHdG expression in the auditory cortex
was significantly increased in the D-gal-treated groups as compared
with that in the control group. Furthermore, the expression of
8-OHdG was predominantly localised to the cytoplasm of the cells in
the auditory cortex (Fig. 2A)
(P<0.01).
D-gal induces an increase in UCP2 protein
expression
Western blot analysis was performed in order to
investigate the protein levels of UCP2 in the auditory cortex. As
shown in Fig. 3A and B, the
protein levels of UCP2 were significantly higher in the
D-gal-treated groups as compared with those in the control group
(P<0.01).
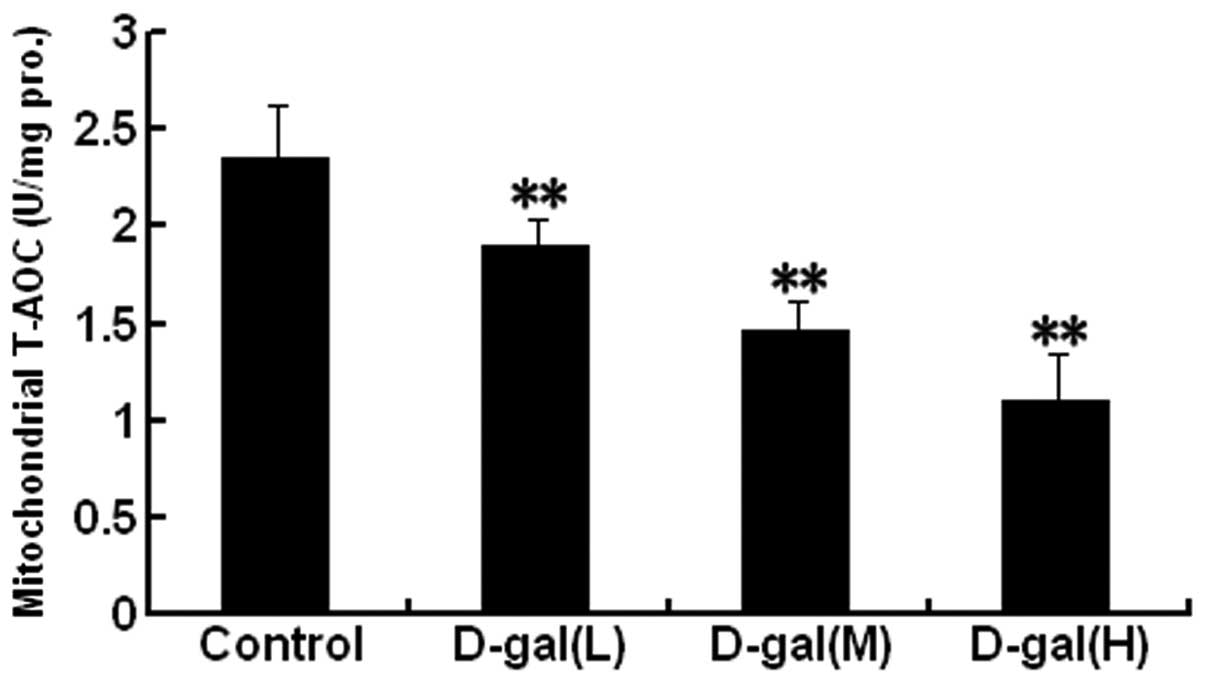
D-gal induces a decrease in mitochondrial
T-AOC
Mitochondrial T-AOC was analysed in the auditory
cortex. As shown in Fig. 4, the
mitochondrial T-AOCs were significantly decreased in the
D-gal-treated groups as compared with those in the control group
(P<0.01).
D-gal induces increased levels of the
mtDNA CD
TaqMan qPCR assay was used to investigate the levels
of the mtDNA CD in the auditory cortex. The dual-labelled
fluorescent DNA probe was designed to specifically recognise the
fusion sequence, which was present only in mutant mtDNA that
harboured the CD. As shown in Fig.
5, the accumulation of the mtDNA CD was significantly higher in
the D-gal-treated groups as compared with that in the control group
(P<0.01).
Discussion
Oxidative damage to mtDNA has a strong association
with the molecular process of aging (22). To the best of our knowledge, this
study showed for the first time that levels of NOX2 and 8-OHdG, a
biomarker of DNA oxidative damage, were significantly increased in
the auditory cortex of the CAS in rats with D-gal-induced aging.
The expression of 8-OHdG was observed predominantly in the
cytoplasm of cells, suggesting that D-gal induced mtDNA oxidative
damage in the auditory cortex. These findings indicate that D-gal
may induce mtDNA oxidative damage in the CAS in part through the
NOX2 pathway, and NOX2-associated ROS generation may play an
essential role in the aging process of the CAS. NOXs function to
produce ROS, as opposed to mitochondria, which generate ROS as a
byproduct of their metabolism (23). Previous studies have demonstrated
that NOX3 represents the primary source of ROS generation in the
inner ear, which contributes to cisplatin-induced ROS generation in
the PAS (13,24,25).
It has additionally been shown that NOX3-associated ROS generation
may function in the degeneration in the PAS (5), and NOX2-dependent oxidative stress
may contribute to the mtDNA CD and mitochondrial ultrastructural
damage in the hippocampus (26) of
rats with D-gal-induced aging. Therefore, NOX2-associated ROS
generation may be an important source of ROS in the aging process
of the CAS.
Mitochondria are another important source of ROS in
cells (16). UCP2, which is
located in the mitochondrial inner membrane, can act to slightly
lower the proton-motive force across the membrane through a mild
uncoupling and, in this way, attenuate mitochondrial ROS generation
(17). In a previous study, it was
demonstrated that UCP2 mRNA expression was upregulated in the inner
ear ganglia cells of the mouse following aminoglycoside
intoxication, and these responses were blocked by a
co-administration with antioxidant (27). Previous studies have also reported
that UCP2 mRNA expression in the vestibular ganglion of the inner
ear was significantly upregulated following a labyrinthectomy
(28), and UCP2 mRNA and protein
expression was significantly increased in the inner ear of D-gal-
and/or high-fat diet-treated rats (5). These previous studies have indicated
that UCP2 may indirectly reflect the ROS levels of cells and play a
protective role against oxidative damage in the inner ear. In the
present study, UCP2 was found to be overexpressed in the auditory
cortex of rats with D-gal-induced aging, which may also suggest
that increased UCP2 levels reflect increases in ROS generation and
that UCP2 plays protective roles in the aging process of the CAS in
rats with D-gal-induced aging.
mtDNA is highly susceptible to ROS-induced damage
due to its close proximity to the sites of ROS generation and lack
of protective histones (29).
Increases in ROS generation together with decreases in
mitochondrial T-AOCs may induce mtDNA oxidative damage. The most
common type of mtDNA damage associated with aging is the mtDNA
4,977-bp deletion (also known as the CD) in humans and the
corresponding mtDNA 4,834-bp deletion in rats. Therefore, the mtDNA
CD has been widely used as a biomarker for aging (21,30–32).
An association between elevated mtDNA CD levels and ARHL has been
observed in a number of studies (32–34).
Previous studies have demonstrated that the frequency of the mtDNA
CD was increased in the PAS (5,6,8,10,11)
and CAS (4,7,9) of
D-gal-induced aging rats. The present study also found that the
mtDNA CD levels were increased in the auditory cortex of
D-gal-treated rats, and these increased mtDNA CD levels correlated
with mtDNA oxidative damage. Therefore, these findings suggest that
mtDNA damage in the auditory cortex of rats with D-gal-induced
aging may be caused by NOX2- and mitochondria-associated ROS
generation.
In conclusion, the present findings indicate that
both NOX- and mitochondria-associated ROS generation may contribute
to mtDNA oxidative damage in the auditory cortex of the CAS of rats
with D-gal-induced aging. NOX2 and UCP2 may therefore be useful
therapeutic targets to prevent or slow the development of ARHL.
Acknowledgements
This study was supported by grants from the Shenzhen
Nanshan Science and Technology Development Foundation (no. 2012014)
and the National Natural Science Foundation of China (no.
61302037).
Abbreviations:
|
ARHL
|
age-related hearing loss
|
|
CAS
|
central auditory system
|
|
CD
|
common deletion
|
|
D-gal
|
D-galactose
|
|
mtDNA
|
mitochondrial DNA
|
|
NOX
|
NADPH oxidase
|
|
PAS
|
peripheral auditory system
|
|
ROS
|
reactive oxygen species
|
|
T-AOC
|
total antioxidant capability
|
|
UCP
|
uncoupling protein
|
|
8-OHdG
|
8-hydroxy-2-deoxyguanosine
|
References
|
1
|
Frisina RD and Walton JP: Age-related
structural and functional changes in the cochlear nucleus. Hear
Res. 216–217:216–223. 2006.PubMed/NCBI
|
|
2
|
Howarth A and Shone GR: Ageing and the
auditory system. Postgrad Med J. 82:166–171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu L, Sun Y, Hu YJ, et al: Increased
p66Shc in the inner ear of D-galactose-induced aging mice with
accumulation of mitochondrial DNA 3873-bp deletion: p66Shc and
mtDNA damage in the inner ear during aging. PLoS One. 7:e504832012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhong Y, Hu Y, Peng W, et al: Age-related
decline of the cytochrome c oxidase subunit expression in
the auditory cortex of the mimetic aging rat model associated with
the common deletion. Hear Res. 294:40–48. 2012.PubMed/NCBI
|
|
5
|
Du Z, Yang Y, Hu Y, et al: A long-term
high-fat diet increases oxidative stress, mitochondrial damage and
apoptosis in the inner ear of D-galactose-induced aging rats. Hear
Res. 287:15–24. 2012. View Article : Google Scholar
|
|
6
|
Zhong Y, Hu YJ, Chen B, et al:
Mitochondrial transcription factor A overexpression and base
excision repair deficiency in the inner ear of rats with
D-galactose-induced aging. FEBS J. 278:2500–2510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen B, Zhong Y, Peng W, et al: Increased
mitochondrial DNA damage and decreased base excision repair in the
auditory cortex of D-galactose-induced aging rats. Mol Biol Rep.
38:3635–3642. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhong Y, Hu YJ, Yang Y, et al:
Contribution of common deletion to total deletion burden in
mitochondrial DNA from inner ear of d-galactose-induced aging rats.
Mutat Res. 712:11–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen B, Zhong Y, Peng W, Sun Y and Kong
WJ: Age-related changes in the central auditory system: comparison
of D-galactose-induced aging rats and naturally aging rats. Brain
Res. 1344:43–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kong WJ, Wang Y, Wang Q, Hu YJ, Han YC and
Liu J: The relation between D-galactose injection and mitochondrial
DNA 4834 bp deletion mutation. Exp Gerontol. 41:628–634. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kong WJ, Hu YJ, Wang Q, et al: The effect
of the mtDNA4834 deletion on hearing. Biochem Biophys Res Commun.
344:425–430. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bánfi B, Malgrange B, Knisz J, Steger K,
Dubois-Dauphin M and Krause KH: NOX3, a superoxide-generating NADPH
oxidase of the inner ear. J Biol Chem. 279:46065–46072.
2004.PubMed/NCBI
|
|
14
|
Quinn MT, Ammons MC and Deleo FR: The
expanding role of NADPH oxidases in health and disease: no longer
just agents of death and destruction. Clin Sci (Lond). 111:1–20.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Serrano F, Kolluri NS, Wientjes FB, Card
JP and Klann E: NADPH oxidase immunoreactivity in the mouse brain.
Brain Res. 988:193–198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raha S and Robinson BH: Mitochondria,
oxygen free radicals, disease and ageing. Trends Biochem Sci.
25:502–508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brand MD and Esteves TC: Physiological
functions of the mitochondrial uncoupling proteins UCP2 and UCP3.
Cell Metab. 2:85–93. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kujoth GC, Hiona A, Pugh TD, et al:
Mitochondrial DNA mutations, oxidative stress, and apoptosis in
mammalian aging. Science. 309:481–484. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma Y, Mehta SL, Lu B and Li PA: Deficiency
in the inner mitochondrial membrane peptidase 2-like (Immp21) gene
increases ischemic brain damage and impairs mitochondrial function.
Neurobiol Dis. 44:270–276. 2011. View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
|
|
21
|
Nicklas JA, Brooks EM, Hunter TC, Single R
and Branda RF: Development of a quantitative PCR (TaqMan) assay for
relative mitochondrial DNA copy number and the common mitochondrial
DNA deletion in the rat. Environ Mol Mutagen. 44:313–320. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang CH, Wu SB, Wu YT and Wei YH:
Oxidative stress response elicited by mitochondrial dysfunction:
implication in the pathophysiology of aging. Exp Biol Med
(Maywood). 238:450–460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krause KH: Aging: a revisited theory based
on free radicals generated by NOX family NADPH oxidases. Exp
Gerontol. 42:256–262. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mukherjea D, Jajoo S, Kaur T, Sheehan KE,
Ramkumar V and Rybak LP: Transtympanic administration of short
interfering (si)RNA for the NOX3 isoform of NADPH oxidase protects
against cisplatin-induced hearing loss in the rat. Antioxid Redox
Signal. 13:589–598. 2010. View Article : Google Scholar
|
|
25
|
Mukherjea D, Jajoo S, Sheehan K, et al:
NOX3 NADPH oxidase couples transient receptor potential vanilloid 1
to signal transducer and activator of transcription 1-mediated
inflammation and hearing loss. Antioxid Redox Signal. 14:999–1010.
2011. View Article : Google Scholar
|
|
26
|
Du Z, Hu Y, Yang Y, et al: NADPH
oxidase-dependent oxidative stress and mitochondrial damage in
hippocampus of D-galactose-induced aging rats. J Huazhong Univ Sci
Technolog Med Sci. 32:466–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kitahara T, Li-Korotky HS and Balaban CD:
Regulation of mitochondrial uncoupling proteins in mouse inner ear
ganglion cells in response to systemic kanamycin challenge.
Neuroscience. 135:639–653. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kitahara T, Horii A, Kizawa K, Maekawa C
and Kubo T: Changes in mitochondrial uncoupling protein expression
in the rat vestibular nerve after labyrinthectomy. Neurosci Res.
59:237–242. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Druzhyna NM, Wilson GL and LeDoux SP:
Mitochondrial DNA repair in aging and disease. Mech Ageing Dev.
129:383–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meissner C, Bruse P, Mohamed SA, et al:
The 4977 bp deletion of mitochondrial DNA in human skeletal muscle,
heart and different areas of the brain: a useful biomarker or more?
Exp Gerontol. 43:645–652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yowe DL and Ames BN: Quantitation of
age-related mitochondrial DNA deletions in rat tissues shows that
their pattern of accumulation differs from that of humans. Gene.
209:23–30. 1998. View Article : Google Scholar
|
|
32
|
Markaryan A, Nelson EG and Hinojosa R:
Quantification of the mitochondrial DNA common deletion in
presbycusis. Laryngoscope. 119:1184–1189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bai U, Seidman MD, Hinojosa R and Quirk
WS: Mitochondrial DNA deletions associated with aging and possibly
presbycusis: a human archival temporal bone study. Am J Otol.
18:449–453. 1997.PubMed/NCBI
|
|
34
|
Ueda N, Oshima T, Ikeda K, Abe K, Aoki M
and Takasaka T: Mitochondrial DNA deletion is a predisposing cause
for sensorineural hearing loss. Laryngoscope. 108:580–584. 1998.
View Article : Google Scholar : PubMed/NCBI
|