Introduction
Due to their high levels of reperfusion, the kidneys
are prone to ischemia reperfusion injury (IRI), and this condition
may cause or aggravate renal dysfunction. Renal ischemia may be
caused by arterial occlusion, shock and organ transplantation, and
leads to renal cell death, renal failure, delayed graft function
and graft rejection (1). These
events contribute substantially to renal-associated morbidity and
mortality, with a 30–50% death rate, following acute renal failure
(ARF) (2). In addition, ~10% of
renal allografts fail during the first year after transplantation,
and the risk increases by 3–5% each year (3). Since the first report by Zhao et
al (4), several recent studies
have shown that brief ischemia during the onset of reperfusion,
ischemic postconditioning (IPo) is protective in various organs
(5–7). IPo has therefore become a clinical
intervention to significantly reduce IRI (8–10).
In a previous study by our group (11), it was demonstrated that IPo
significantly reduced renal IRI by attenuating renal lipid
peroxidation and cell apoptosis (11). Miklós et al (12) reported that IPo attenuated
inflammatory response by reducing serum and tubular tumor necrosis
factor-α (TNF-α) expression (12).
Renal IPo in the present study was based on the methods described
by Liu et al (11) and
Heusch et al (13),
employing 10 sec of reperfusion immediately following release of
ischemia, followed by 10 sec of ischemia. The process was repeated
three times.
Endogenous heat shock proteins (HSPs), categorized
into various subfamilies based on their molecular weight, are
increasingly expressed upon tissue stress, as a cellular protective
mechanism. HSP70 is a chaperone protein which has a key role in
stress tolerance (14). HSP27 is a
member of the small molecule family of HSPs, and also has an
important role in individual stress tolerance at the cellular level
and maintenance of integrity (15). Heme oxygenase-1 (HO-1) has been the
focus of research in organ transplantation and protection, due to
its anti-oxidant and anti-inflammatory activity, as well as the
ability to improve microcirculation, inhibit immunological
rejection and induce immunological tolerance (16). As an endogenous protective
mechanism, the expression of HSP70, HSP27 and HO-1 protect against
the progression of IR. Zhang et al (17) showed that HSP levels, particularly
those of HSP70, HSP27 and HO-1, are highly sensitive to IRI in rat
kidneys. Several studies have shown that postconditioning induces
expression of HSPs and is protective to the brain and lung
(18,19).
The potential application of HSP induction by IPo in
the kidney has not been demonstrated, to the best of our knowledge.
The present study was designed to determine whether IPo induced
higher expression levels of HSP70, HSP27 and HO-1, thereby
attenuating renal lipid peroxidation, inflammatory responses and
cellular apoptosis, and reducing IRI in the kidneys of rats.
Materials and methods
Animals
Male Sprague Dawley rats (n=140), weighing 250–280
g, aged 6–8 weeks, were obtained from the Hebei Laboratory Animal
Center (Hebei, China). Rats were housed in a standard environment,
under a 12-h light/dark cycle, with access to water and a standard
laboratory diet ad libitum. All procedures and protocols
used in the present study were approved by the Experimental Animal
Ethics Committee of Hebei Medical University (Hebei, China), and
the guidelines of the National Institutes of Health Guide for the
Care and Use of Laboratory Animals were followed.
Experimental protocol
The rats (n=140) were anesthetized by ether
inhalation. Briefly, the peritoneal cavity was opened through a
midline incision. Both kidneys were separated and the bilateral
renal pedicles were occluded for 45 min using an atraumatic
mini-clamp, followed by reperfusion of various durations (1, 3, 6,
12, 24 or 48 h; n=5/time point) (IR group) (11). One group of rats received three
cycles of ischemia (10 sec) followed by 10 sec reperfusion
following the 45-min ischemia, but prior to restoring full
perfusion (IPo group) (11,13).
Another group of rats (n=35) was subjected to the IPo procedure,
using 100 mg/kg quercetin (HSP inhibitor), injected
intraperitoneally at 1 h prior to ischemia (quercetin + IPo group;
n=35). Control rats receiving sham operations were used as the
negative controls. In these animals, the kidneys were exposed
bilaterally for 45 min through a midline incision, but without
clamping their pedicles (sham group). Animals in the four groups
were sacrificed at the end of ischemia (T0) (corresponding to the
end of IPo) and at 1, 3, 6, 12, 24 and 48 h (T1-6) of reperfusion
(n=5 rats at each time-point).
Serum and kidney specimens
Serum was extracted from cardiac blood and kidneys
were removed at each time-point. Serum samples were stored at −20°C
for biochemical analysis for creatinine (Cr), blood urea nitrogen
(BUN) and expression level analysis of TNF-α. Tissue samples were
divided into two parts. One part of each specimen was stored at
−80°C for measurements of HSP70, HSP27, HO-1
and caspase-3 mRNA by quantitative polymerase chain reaction
(qPCR), as well as determination of malondialdehyde (MDA) content
and superoxide dismutase (SOD) activity. The other part of each
specimen was fixed in 4% paraformaldehyde and embedded in paraffin
for histopathology, apoptosis and immunohistochemical analyses.
RNA isolation, reverse transcription and
qPCR
Total RNA was extracted from 50 mg of each renal
tissue specimen using TRIzol™ reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer’s
instructions. RNA purity and content were detected by measuring the
optical density (OD) ratio at 260/280 nm using a 756-ultraviolet
spectrophotometer (Aucy Technology Instrument Co., Ltd., Shanghai,
China). Pure RNA from the reverse transcription was determined by a
score of 1.8–2.0 from the 260/280 ratio. Random primers (Promega
Corporation, Madison, WI, USA) were used to synthesize first-strand
cDNA with Moloney murine leukemia virus Reverse Transcriptase
(Promega Corporation). The cDNA was then amplified by qPCR using a
Hot Start Fluorescent PCR Core Reagent kit (SYBR® Green
I) (Boston Biomedical, Cambridge, MA, USA) and the ABI 7300
Real-Time PCR system (Applied Biosystems Life Technologies, Foster
City, CA, USA). Specific primers for HSP70, HO-1, HSP27 and
caspase-3 are listed in Table I.
qPCR was carried out as follows: Initial denaturation at 96°C for 4
min, followed by 40 cycles of amplification at 94°C for 30 sec,
hybridization at 58°C for 30 sec and extension at 72°C for 30 sec.
Relative mRNA in each sample was then quantified automatically by
reference to the standard curve constructed each time according to
SDS v1.3 software (Applied Biosystems Life Technologies). The mRNA
levels were calculated with reference to external standard curves
constructed by plotting the log number of 10-fold serially diluted
cDNA samples against the respective threshold cycle by the second
derivative maximum method. The expression of mRNA levels in each
sample was normalized against the mRNA expression levels of
GAPDH.
 | Table IPrimer sequences used in quantitative
polymerase chain reaction. |
Table I
Primer sequences used in quantitative
polymerase chain reaction.
| Primer | | Sequence 5′ to
3′ |
|---|
| HSP70 | Forward |
GGGTTTGGGTACTTTGGTTA |
| Reverse |
CCCATAAGTTGGGAAACAGT |
| HO-1 | Forward |
GAGGAGATAGAGCGAAACAAGC |
| Reverse | GTGGCTGGT
GTGTAAGGGAT |
| HSP27 | Forward | AGCAGCGGTGTG
TCAGAGAT |
| Reverse |
GCCTTCCTTGGTCTTCACTGT |
| Caspase-3 | Forward |
GACAACAACGAAACCTCCG |
| Reverse |
AGGGTTAGCTGCATCGACA |
| GAPDH | Forward |
TGAACGGGAAGCTCACTGG |
| Reverse |
GCTTCACCACCTTCTTGATGTC |
Immunohistochemistry
Immunohistochemical staining was performed using
rabbit antibodies against rat HSP70 (BS2741; Bioworld Technology,
Inc., St. Louis Park, MN, USA), HO-1 (BS-0827R; Beijing Bioss
Biotechnology Co., Ltd. Beijing, China), HSP27 (BS3435; Bioworld
Technology, Inc.) or nuclear factor kappa-light-chain-enhancer of
activated B cells p65 (NF-κB-p65, BS1253; Bioworld Technology,
Inc.) on paraffin sections according to the manufacturer’s
instructions. The staining was analyzed using the Leica Q-500 Image
Analysis system (Leica Microsystems GmbH, Wetzlar, Germany).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL) assay
Apoptosis in kidney cells was identified by TUNEL
assays, performed according to the manufacturer’s instructions
(Boehringer Ingelheim, Mannheim, Germany). Apoptotic renal tubular
epithelial cells were examined by light microscopy (BX53; Olympus
Corporation, Tokyo, Japan) at ×400 magnification. The apoptotic
index (AI) was defined as the percentage of stained
cells/high-power field.
MDA content and SOD activity
The MDA levels and SOD activity in nephridial
tissues were detected using thiobarbituric acid (TBA) and xanthine
oxidase methods, according to the manufacturer’s instructions
(Nanjing Jiancheng Bioengineering Institute, China). The homogenate
(0.1 ml) was used to detect the MDA content. The condensation of
MDA and TBA resulted in a red product, with a maximum absorption
peak at 532 nm (NanoDrop2000 spectrophotometer; Thermo Fisher
Scientific, Wilmington, DE, USA). The MDA content was calculated by
measuring the absorbance at 532 nm and expressed as nmol/mg protein
(nmol/mg prot). SOD activity was determined by detecting the
absorbance at 550 nm. SOD activity was expressed as U/mg prot.
Detection of TNF-α by enzyme-linked
immunosorbent assay (ELISA)
The levels of TNF-α in the serum were measured by
ELISA according to the manufacturer’s instructions (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China).
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical analyses were performed using SPSS 13.0
(SPSS, Inc., Chicago, IL, USA). Analysis of variance was conducted
for comparison of parameters among groups and the
Student-Newman-Keuls test for comparison of parameters between two
groups after normality testing (quantile-quantile plot, Q-Q plot)
and tests for homogeneity of variance (Levene’s test). P<0.05
was considered to indicate a statistically significant
difference.
Results
IPo increases expression of HSPs
Expression levels of HSP70, HSP27 and HO-1 in the
kidney were induced by IR at the mRNA and protein level. The
expression levels were further elevated by IPo at each time-point,
reaching a peak at 6 h after reperfusion. Quercetin inhibited the
IPo-mediated increases of HSP70, HSP27 and HO-1 mRNA and protein
levels by (Fig. 1A–F).
 | Figure 1Relative expression of (A) HSP70 mRNA,
(B) HO-1 mRNA, (C) HSP27 mRNA, (D) HSP70 protein, (E) HO-1 protein
and (F) HSP27 protein, in renal tissue immediately following
reperfusion (0 h) or 1, 3, 6, 12, 24 and 48 h after reperfusion
(mRNA and protein levels were detected by quantitative polymerase
chain reaction and immunohistochemistry). In D-F, the value of the
sham group was defined as 0, and the values of the other groups
were presented relatively to the sham group. #P<0.05,
as compared with the sham group; *P<0.05, as compared
with IR group; ▲P<0.05, as compared with the IPo
group. HSP, heat shock protein; HO-1, heme oxygenase-1; S, control
group; IR, ischemia-reperfusion; IPo, ischemic postconditioning;
IHC, immunohistochemistry. |
Expression of HSPs is involved in the
reduction of renal IRI by IPo
To assess functional renal impairment, changes in
renal pathology were observed by microscopy, and levels of Cr and
BUN were measured in the serum. Renal IR led to severe pathological
and morphological changes, including tubular dilatation and
cellular edema, with partly visible necrosis and tubular cells.
Protein accumulation in the fluid within lumen, perivascular
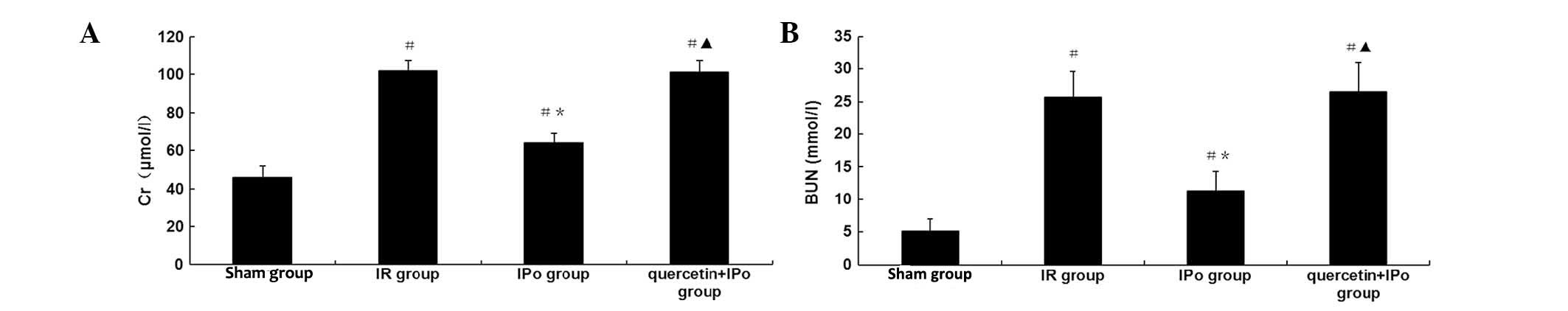
dilatation and congestion (Fig. 2)
as well as increased serum Cr expression levels (102±5 vs. 46±6
μmol/l, P<0.05) and BUN (25.7±3.9 vs. 5.1±1.9 mmol/l, P<0.05)
concentrations at 6 h after reperfusion were also observed. IPo
attenuated the pathological changes and decreased Cr expression
levels (64±5 vs. 102±5 μmol/l, P<0.05) and BUN concentrations
(11.3±3.0 vs. 25.7±3.9 mmol/l, P<0.05) (Fig. 3A and B). The renoprotective effects
of IPo were significantly attenuated by quercetin (Cr, 101±6 vs.
64±5 μmol/l, P<0.05; BUN, 26.5±4.5 vs. 11.3±3.0, P<0.05).
Upregulation of HSPs mediates a decrease
of MDA content and increase of SOD activity by IPo
To assess the levels of lipid peroxidation
associated with renal IR, the MDA content and SOD activity in
kidney tissue were determined. Following 6 h of reperfusion, the
MDA content was significantly increased (2.20±0.23 vs. 1.02±0.19
nmol/mg prot, P<0.05), while the SOD activity was significantly
decreased (104±6 vs. 147±6 U/mg prot, P<0.05). IPo attenuated
the pathology associated with lipid peroxidation of renal IR (MDA
1.35±0.13 vs. 2.20±0.23 nmol/mg prot, P<0.05; SOD 124±4 vs.
104±6 U/mg prot, P<0.05). This effect was significantly
restrained by quercetin (MAD 2.25±0.16 vs. 1.35±0.13 nmol/mg prot,
P<0.05; SOD 106±5 vs. 124±4 U/mg prot, P<0.05) (Fig. 4).
Upregulation of HSPs mediates decrease in
NF-κB and TNF-α levels by IPo
To determine the extent of pathological inflammation
in renal IR, the renal tissue expression levels of NF-κB and the
serum expression levels of TNF-α were evaluated. After 6 h of
reperfusion, the NF-κB expression (6.0±1.4 vs. 1.5±0.5, P<0.05)
in the kidney and the TNF-α levels (2.29±0.18 vs. 1.13±0.14 ng/ml,
P<0.05) in the serum were significantly increased. IPo
attenuated the inflammation due to renal IR (NF-κB expression,
3.4±1.1 vs. 6.0±1.4, P<0.05; TNF-α levels, 1.76±0.13 vs.
2.29±0.18 ng/ml, P<0.05). The effect by IPo was significantly
inhibited by quercetin (NF-κB expression, 5.8±1.8 vs. 3.4±1.1.
P<0.05; TNF-α levels, 2.31±0.17 vs. 1.76±0.13 ng/ml, P<0.05)
(Fig. 5).
 | Figure 5(A) Expression of NF-κB and (B) TNF-α
expression levels, in the four experimental groups at 6 h
post-reperfusion. #P<0.05, as compared with the sham
group; *P<0.05, as compared with the IR group;
▲P<0.05, as compared with the IPo group. S, control
group; IR, ischemia-reperfusion; IPo, ischemic postconditiong; IHC,
immunohistochemistry; TNF-α, tumor necrosis factor-α; NF-κB, renal
nuclear factor kappa-light-chain-enhancer of activated B cells. |
Upregulation of HSPs reduces apoptosis of
renal tubular epithelial cells by IPo
To observe apoptosis and to evaluate the AI, the
expression of caspase-3 mRNA in renal tubular epithelial
cells was assessed by qPCR and TUNEL assays. 6 h after reperfusion,
the expression of caspase-3 mRNA (2.80±0.04 vs. 0.86±0.08,
P<0.05) and AI (30.5±4.1 vs. 5.6±1.5%, P<0.05) were
significantly increased. An increase of TUNEL-positive renal
tubular epithelial cells was observed. IPo decreased the expression
of caspase-3 mRNA (1.60±0.08 vs. 2.80±0.04, P<0.05) and
AI (19.3±4.4 vs. 30.5±4.1%, P<0.05), and fewer TUNEL-positive
renal tubular epithelial cells were observed. The decrease of
apoptosis in the IPo-treated group was significantly attenuated by
quercetin (caspase-3 mRNA, 2.82±0.06 vs. 1.60±0.08,
P<0.05; AI 29.9±4.8 vs. 19.3±4.4%, P<0.05) (Fig. 6A and B; Fig. 7).
Discussion
HSPs have been identified to have various biological
functions with protective effects in cells. Previous studies have
demonstrated the association of HSPs with the effects of organ IR
(17). In the present study, the
expression of three HSPs was observed at various time-points
following IR. The results suggested that IPo induced higher
expression of these proteins.
Quercetin is an inhibitor of HSPs, which acts by
interfering with their transcription (20,21).
In the present study, quercetin was injected intraperitoneally (100
mg/kg) at 1 h prior to the surgically-induced ischemia, based on
methods described previously by Yang et al (22) and Yao et al (23). Significant increases occurred in
the HSP expression in the IPo group as compared with the IR group.
However, no differences were detected between the quercetin + IPo
group and the IR group. The results indicated that quercetin
inhibited IPo-induced HSP expression.
An induction of several HSPs in nephridial tissue
following IR was observed, which was seen as a protective mechanism
against functional injury to the cells. These results were
consistent with those of Zhang et al (17), who used gene microarrary analysis
to report an increased expression of 21 genes, including HSP70
(43-fold), HSP27 (12-fold) and HO-1 (10-fold), in rat kidneys
subjected to early IRI. Furthermore, at each time-point, the
expression levels of HSPs were significantly higher in the IPo
group as compared with the IR group at the corresponding
time-point. The results also indicated that the expression of HSPs
was, in part, time-dependent. HSP expression in the tissues in
response to stress stimuli peaked at 6 h post-reperfusion, but
decreased at 24 h following reperfusion, suggesting activation of
the endogenous protective mechanism early during IRI, in order to
protect cellular functions.
As HSP expression levels peaked at 6 h
post-reperfusion, the serum Cr and BUN levels, renal tubular
epithelial cell apoptosis and histopathological changes were
determined to confirm the protective effects of IPo. The excessive
generation of oxygen radicals causing lipid peroxidation of cell
membranes, protein and enzyme oxidation and irreversible DNA
changes, lead to the inactivation of key cellular functions and
ultimately to cell death (2).
HSP70 regulates the activities of anti-oxidative enzymes by
protective SOD activity (24),
attenuating lipid peroxidation (25) and repairing proximal tubule
structure following renal ischemia (26). The excessive formation of oxygen
radicals is known to destroy the equilibrium of oxidation-reduction
reactions in an organism. A previous study (27) has suggested that HSP70 regulates
the cellular redox status by modulating glutathione-associated
enzyme activities.
HO-1 has been the focus of research in organ
transplantation and protection, as it has anti-oxidant and
anti-inflammatory functions, as well as the ability to improve
microcirculation, inhibit immunological rejection and induce
immunological tolerance. Overexpression of HO-1 has been associated
with a decreased generation of oxygen radicals, increased SOD
levels in serum, attenuated oxidative stress, decreased
infiltration of neutrophilic granulocytes and release of
inflammatory factors, as well as protection against IRI in the
kidney (28,29) and other organs (30–32).
HSP27 overexpression in tissues has been shown to
inhibit the release of proinflammatory factors, such as TNF-α and
macrophage inflammatory protein 2 (MIP2), as well as the
infiltration of neutrophilic granulocytes; these events are known
to protect against IRI-induced damage (33 35). However, a previous
study suggested that a systemic increase of HSP27, instead of a
local increase, in transgenic mice counteracts this protection by
exacerbating renal and systemic inflammation (36). HSP27 has been shown to inhibit the
disassociation of actin and microfibrils, offering protection and
stabilization to the cytoskeleton (34,35).
This function of HSP27 is important in the tolerance of individual
cells and organs to different stresses by maintaining the integrity
of the endothelium and epithelium.
The MDA content reflects the degree of lipid
oxidative reactions, whereas the SOD activity may reflect the
ability of the body to scavenge oxygen free radicals. In the
present study, MDA levels were decreased and the activity of SOD
was increased following renal IPo, which was reversed in the
presence of quercetin. This suggested that IPo elevated the
expression of HSPs and attenuated lipid peroxidation in renal
IRI.
Previous studies have shown that HSP70 and HO-1 can
inhibit the activation of NF-κB, increase the expression of nuclear
factor of kappa light polypeptide gene enhancer in B-cells
inhibitor, downregulate the expression of TNF-α and attenuate IRI
(37,38). In the present study, IPo induced
the expression of HSPs and reduced the levels of NF-κB and TNF-α.
In the presence of quercetin these effects of IPo were inhibited.
These findings suggested that IPo attenuated inflammatory
reactions, following renal IR, and that the renal protection was
associated with the expression of HSPs.
In previous studies, HSP70, HSP 27 and HO-1
(34,39,40)
were identified to reduce organ IRI through the inhibition of
mitochondrial cytochrome C release, caspase-3 activation,
inhibition of B-cell lymphoma 2 (Bcl-2)-associated X, elevation of
Bcl-2 and Bcl-2 extra large gene expression and reduction of
apoptosis. In the present study, analysis of renal tubular
epithelial cell apoptosis indicated that in the IPo group, the
caspase-3 mRNA levels and the AI decreased. The addition of
quercetin attenuated these effects, followed by a decrease in the
expression of HSPs. This suggested that IPo increased the
expression of HSPs, reduced apoptosis, thereby reducing renal
IRI.
In conclusion, the present study indicated that IPo
induced HSP70, HSP27 and HO-1 expression. The subsequent reduction
of the generation of superoxide anions and peroxides upon sudden
reperfusion following ischemia, attenuating lipid oxidation,
reducing the levels of NF-κB and TNF-α, inflammatory response, and
cellular apoptosis, as well as renal IRI.
Acknowledgements
This study was supported by a grant from the Natural
Science Foundation of Hebei Province (no. C2011307006).
References
|
1
|
Serviddio G, Romano AD, Gesualdo L, et al:
Postconditioning is an effective strategy to reduce renal
ischaemia/reperfusion injury. Nephrol Dial Transplant.
23:1504–1512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kunduzova OR, Bianchi P, Pizzinat N, et
al: Regulation of JNK/ERK activation, cell apoptosis, and tissue
regeneration by monoamine oxidases after renal
ischemia-reperfusion. FASEB J. 16:1129–1131. 2002.PubMed/NCBI
|
|
3
|
Akoh JA: Transplant nephrectomy. World J
Transplant. 1:4–12. 2011. View Article : Google Scholar
|
|
4
|
Zhao ZQ, Corvera JS, Halkos ME, et al:
Inhibition of myocardial injury by ischemic postconditioning during
reperfusion: comparison with ischemic preconditioning. Am J Physiol
Heart Circ Physiol. 285:H579–H588. 2003.PubMed/NCBI
|
|
5
|
Penna C, Tullio F, Moro F, et al: Effects
of a protocol of ischemic postconditioning and/or captopril in
hearts of normotensive and hypertensive rats. Basic Res Cardiol.
105:181–192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wever KE, Menting T, Masereeuw R, et al:
Local and remote ischemic postconditionings have synergistic
protective effects on renal ischemia-reperfusion injury.
Transplantation. 94:e1–e2. 2012. View Article : Google Scholar
|
|
7
|
Wang JY, Shen J, Gao Q, et al: Ischemic
postconditioning protects against global cerebral
ischemia/reperfusion-induced injury in rats. Stroke. 39:983–990.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu KX, Li YS, Huang WQ, et al: Immediate
postconditioning during reperfusion attenuates intestinal injury.
Intensive Care Med. 35:933–942. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lønborg J, Kelbaek H, Vejlstrup N, et al:
Cardioprotective effects of ischemic postconditioning in patients
treated with primary percutaneous coronary intervention, evaluated
by magnetic resonance. Circ Cardiovasc Interv. 3:34–41. 2010.
|
|
10
|
Deftereos S, Giannopoulos G, Tzalamouras
V, et al: Renoprotective effect of remote ischemic
post-conditioning by intermittent balloon inflations in patients
undergoing percutaneous coronary intervention. J Am Coll Cardio.
61:1949–1955. 2013. View Article : Google Scholar
|
|
11
|
Liu JJ, Zhao YL and Zhang YD: Effects of
ischemic postconditioning on the renal ischemia-reperfusion injury
in rats. Chin J Anesthesiol. 27:651–654. 2007.
|
|
12
|
Miklós Z, Kürthy M, Degrell P, et al:
Ischaemic postconditioning reduces serum and tubular TNF-α
expression in ischaemic-reperfused kidney in healthy rats. Clin
Hemorheol Microcirc. 50:167–178. 2012.PubMed/NCBI
|
|
13
|
Heusch G, Büchert A, Feldhaus S and Schulz
R: No loss of cardioprotection by postconditioning in connexin
43-deficient mice. Basic Res Cardiol. 101:354–356. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stricher F, Macri C, Ruff M and Muller S:
HSPA8/HSC70 chaperone protein: Structure, function, and chemical
targeting. Autophagy. 9:2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ziemann E, Zembroñ-Lacny A, Kasperska A,
et al: Exercise training-induced changes in inflammatory mediators
and heat shock proteins in young tennis players. J Sports Sci Med.
12:282–289. 2013.
|
|
16
|
Morse D and Choi AM: Heme oxygenase-1: the
‘emerging molecule’ has arrived. Am J Respir Cell Mol Biol.
27:8–16. 2002.
|
|
17
|
Zhang PL, Lun M, Schworer CM, et al: Heat
shock protein expression is highly sensitive to
ischemia-reperfusion injury in rat kidneys. Ann Clin Lab Sci.
38:57–64. 2008.PubMed/NCBI
|
|
18
|
Xing B, Chen H, Zhang M, et al: Ischemic
postconditioning inhibits apoptosis after focal cerebral
ischemia/reperfusion injury in the rat. Stroke. 39:2362–2369. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu B, Gao X, Xu J, et al: Ischemic
postconditioning attenuates lung reperfusion injury and reduces
systemic proinflammatory cytokine release via heme oxygenase 1. J
Surg Res. 166:e157–164. 2011. View Article : Google Scholar
|
|
20
|
Manwell LA and Heikkila JJ: Examination of
KNK437- and quercetin-mediated inhibition of heat shock-induced
heat shock protein gene expression in Xenopus laevis cultured
cells. Comp Biochem Physiol A Mol Integr Physiol. 148:521–530.
2007. View Article : Google Scholar
|
|
21
|
Khomenko IP, Bakhtina LY, Zelenina OM, et
al: Role of heat shock proteins HSP70 and HSP32 in the protective
effect of adaptation of cultured HT22 hippocampal cells to
oxidative stress. Bull Exp Biol Med. 144:174–177. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang CW, Li C, Jung JY, et al:
Preconditioning with erythropoietin protects against subsequent
ischemia-reperfusion injury in rat kidney. FASEB J. 17:1754–1755.
2003.PubMed/NCBI
|
|
23
|
Yao K, Rao H, Wu R, Tang X and Xu W:
Expression of Hsp70 and Hsp27 in lens epithelial cells in contused
eye of rat modulated by thermotolerance or quercetin. Mol Vis.
12:445–450. 2006.
|
|
24
|
Lepore DA, Knight KR, Anderson RL and
Morrison WA: Role of priming stresses and Hsp70 in protection from
ischemia-reperfusion injury in cardiac and skeletal muscle. Cell
Stress Chaperones. 6:93–96. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Siu PM, Wang Y and Alway SE: Apoptotic
signaling induced by H2O2-mediated oxidative stress in
differentiated C2C12 myotubes. Life Sci. 84:468–481. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bidmon B, Endemann M, Müller T, et al:
Heat shock protein-70 repairs proximal tubule structure after renal
ischemia. Kidney Int. 58:2400–2407. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo S, Wharton W, Moseley P and Shi H:
Heat shock protein 70 regulates cellular redox status by modulating
glutathione-related enzyme activities. Cell Stress Chaperones.
12:245–254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Katavetin P, Inagi R, Miyata T, et al:
Erythropoietin induces heme oxygenase-1 expression and attenuates
oxidative stress. Biochem Biophys Res Commun. 359:928–934. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ferenbach DA, Ramdas V, Spencer N, et al:
Macrophages expressing heme oxygenase-1 improve renal function in
ischemia/reperfusion injury. Mol Ther. 18:1706–1713. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park J, Kang JW and Lee SM: Activation of
the cholinergic anti-inflammatory pathway by nicotine attenuates
hepatic ischemia/reperfusion injury via heme oxygenase-1 induction.
Eur J Pharmacol. 707:61–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saeki I, Matsuura T, Hayashida M and
Taguchi T: Ischemic preconditioning and remote ischemic
preconditioning have protective effect against cold
ischemia-reperfusion injury of rat small intestine. Pediatr Surg
Int. 27:857–862. 2011. View Article : Google Scholar
|
|
32
|
Jia XM, Zhou ZX, Huang JJ, Chu W and Guan
QH: Protective effects of the induction of heme oxygenase-1 on
ischemia reperfusion lung injury: in vivo experiment with
rats. Zhonghua Yi Xue Za Zhi. 87:1211–1213. 2007.(In Chinese).
|
|
33
|
Park SW, Chen SW, Kim M, D’Agati VD and
Lee HT: Human heat shock protein 27-overexpressing mice are
protected against acute kidney injury after hepatic ischemia and
reperfusion. Am J Physiol Renal Physiol. 297:F885–F894. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen SW, Park SW, Kim M, et al: Human heat
shock protein 27 overexpressing mice are protected against hepatic
ischemia and reperfusion injury. Transplantation. 87:1478–1487.
2009. View Article : Google Scholar
|
|
35
|
Park SW, Chen SW, Kim M, D’Agati VD and
Lee HT: Selective intrarenal human A1 adenosine receptor
overexpression reduces acute liver and kidney injury after hepatic
ischemia reperfusion in mice. Lab Invest. 90:476–495. 2010.
View Article : Google Scholar
|
|
36
|
Chen SW, Kim M, Kim M, et al: Mice that
overexpress human heat shock protein 27 have increased renal injury
following ischemia reperfusion. Kidney Int. 75:499–510. 2009.
View Article : Google Scholar
|
|
37
|
Kuboki S, Schuster R, Blanchard J, et al:
Role of heat shock protein 70 in hepatic ischemia-reperfusion
injury in mice. Am J Physiol Gastrointest Liver Physiol.
292:G1141–G1149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Devey L, Mohr E, Bellamy C, et al: c-Jun
terminal kinase-2 gene deleted mice overexpress hemeoxygenase-1 and
are protected from hepatic ischemia reperfusion injury.
Transplantation. 88:308–316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ke B, Shen XD, Gao F, et al: Small
interfering RNA targeting heme oxygenase-1 (HO-1) reinforces liver
apoptosis induced by ischemia-reperfusion injury in mice: HO-1 is
necessary for cytoprotection. Hum Gene Ther. 20:1133–1142. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Manucha W and Vallés PG: Cytoprotective
role of nitric oxide associated with Hsp70 expression in neonatal
obstructive nephropathy. Nitric Oxide. 18:204–215. 2008. View Article : Google Scholar : PubMed/NCBI
|