Introduction
Gastric cancer remains a significant worldwide
health problem, as the fourth most common type of cancer and the
second leading cause of cancer-related mortality worldwide (one
million diagnoses of stomach cancer were made in 2008, with 740,000
related fatalities) (1–4). Although there has been a reduction in
stomach cancer incidence in multiple countries, early detection
remains the key to a better prognoses. However, in the early stages
of gastric cancer, the majority of patients are asymptomatic and
thus patients are commonly diagnosed at an advanced stage, leading
to a low five-year survival rate (<10%) (4). The etiology of gastric cancer,
similar to the majority of other types of cancer, remains to be
defined, and the susceptibility of the individual to cancer may be
altered by a combination of factors, including lifestyle and age,
in addition to environmental and genetic aspects (5). For example, consumption of nitrate-
or nitrite-rich food (grilled, salted or pickled foods) (6), presence of Helicobacter pylori
infection (7), an age of >60
years and a history of stomach disorders or gastric cancer, have
been reported to be possible variables that can lead to gastric
cancer (8). By contrast, vitamin
C, carotenoids and green tea have been implied to have preventive
effects in gastric cancer (9).
Furthermore, genetic susceptibility has been extensively
investigated as an important contributor to inter-individual
variation of gastric cancer risk (10). Accumulation of genetic and
epigenetic changes (such as mutation and hypermethylation of tumor
suppressor genes) has been confirmed to be involved in the
development and progression of gastric cancer. A number of these
genes, including p53, APC and c-erbB-2, are not gastric
tissue-specific. The gastric tissue-specific genes may serve an
essential role in the development and progression of gastric
cancer. Thus, investigation of these genes may be useful to improve
the understanding of the pathogenesis of gastric cancer, and to
develop novel treatments.
The novel gastrointestinal tract-specific gene GDDR
is abundantly expressed in normal gastric mucosae, but is
downregulated or completely knocked out in gastric cancer (11). GDDR was originally cloned in our
laboratory in 2002, by suppression-subtractive hybridization
between the gastric carcinoma tissues and corresponding normal
gastric mucosae and the ends-Marathon rapid amplification of cDNA
ends (11). GDDR is a
stomach-specific secreted protein and is a member of the gastrokine
gene family. The GDDR protein is well-conserved and contains one
BRICHOS domain with a pair of conserved cysteine residues, and is
proposed to function in folding and intracellular transport or
secretion (12). It possesses
similarities to another gastric foveolar protein termed
gastrokine-1 (13), thus GDDR has
been renamed gastrokine-2 (14).
Functionally, gastrokine-2 protein is involved in the replenishment
of the surface lumen epithelial cell layer and maintenance of the
mucosal integrity. Previous studies have demonstrated that
expression of gastrokine-2 inhibits the proliferation of gastric
cancer cells (15) and the
progression of gastric cancer in vivo, in a trefoil factor
1-dependent manner (16,17). Thus, in the present study, the loss
of expression of gastrokine-2 protein in human gastric cancer
tissue samples was confirmed, and then a functional-grade purified
anti-human CD95 (APO/Fas) antibody was used to activate, and an
anti-Fas (human, neutralizing, clone ZB4) antibody was used to
block the extrinsic pathway following transfection of gastrokine-2.
The effects of gastrokine-2 protein on the regulation of gastric
cancer cell viability and the underlying mechanism were
investigated.
Materials and methods
Tissue samples
A total of 76 cancer and corresponding normal
gastric tissues were collected from the Department of
Gastrointestinal Cancer (The Drum Tower Clinical College of Nanjing
Medical University, Nanjing, China) between November 2011 and June
2012. The clinicopathological characteristics of the patients with
gastric carcinoma are outlined in Table I. All patients were pathologically
confirmed to have gastric adenocarcinoma. The current study was
approved by The Ethics Committee of The Drum Tower Clinical College
of Nanjing Medical University. All patients or their legal
guardians signed an inform consent form prior to participation in
the study. Fresh tissue samples were obtained, snap-frozen using
liquid nitrogen and stored at −80°C until use.
 | Table IGastrokine-2 protein expression in
gastric carcinoma. |
Table I
Gastrokine-2 protein expression in
gastric carcinoma.
| Gastrokine-2 protein
expression | |
|---|
|
| |
|---|
| Characteristic | + | − | P-value |
|---|
| Tumor location | | | 0.699 |
| Total | 1 | 4 | |
| Upper | 7 | 23 | |
| Middle | 4 | 10 | |
| Lower | 6 | 21 | |
| Depth of
invasion | | | 0.689 |
| T0 or T1 | 2 | 4 | |
| T2 | 2 | 3 | |
| T3 | 10 | 43 | |
| T4 | 4 | 8 | |
| TNM stage | | | 0.691 |
| N0 (0) | 3 | 10 | |
| N1 (1–6) | 6 | 11 | |
| N2 (7–15) | 4 | 11 | |
| N3 (>15) | 5 | 26 | |
| Lauren’s
classification | | | 0.187 |
| Intestinal | 7 | 32 | |
| Diffuse | 7 | 19 | |
| Mixed-type | 4 | 7 | |
| Tumor stage | | | 0.667 |
| I | 1 | 4 | |
| II | 5 | 15 | |
| III | 12 | 35 | |
| IV | 0 | 4 | |
| Anti-H.
pylori IgG | | | 0.039 |
| + | 4 | 36 | |
| − | 14 | 22 | |
Cell lines and culture
The SGC-7901 and AGS human gastric cancer cell lines
were purchased from the Shanghai Institute of Cell Biology at the
Chinese Academy of Sciences (Shanghai, China) and cultured in
RPMI-1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10%
fetal bovine serum (HyClone Laboratories, Logan, UT, USA),
1×105 U/l penicillin and 100 mg/l streptomycin (CC033,
Zhongke, Beijing, China)at 37°C in a humidified atmosphere with 5%
CO2.
Construction of expression vector and
gene transfection
Human GDDR cDNA (Invitrogen, Carlsbad, CA, USA) was
cloned into BamHI/EcoRI restriction sites of the
eukaryotic expression vector pcDNA3.1/Myc-His(+) (Invitrogen).
Specifically, a primer (5′-GGAATTCTAATGAAAATACTTGTGGCAT-3′)
containing a BamHI linker in front of the initial GKN1 Met
and 5′-CGGGATCCAACATGAATGTCTGCACAGA-3′ that abolished the GDDR stop
codon for PCR amplification of the GDDR open reading frame were
used. This PCR amplicon was then cloned into a pcDNA3.1/Myc-His(+)
vector. Following sequence confirmation, the vector was termed
pcDNA-GDDR. For gene transfection, the cells were subcultured and
grown to the logarithmic growth phase then transiently transfected
with pcDNA-GDDR or pcDNA3.1 (control) using Lipofectamine 2000
(Invitrogen), according to the manufacturer’s instructions. The
transfection efficiency was evaluated by a parallel transfection
using an EGFP vector (Invitrogen).
Reverse transcription-polymerase chain
reaction (RT-PCR)
SGC-7901 cells were divided into the control (Con),
control vector-transfected (P) and GDDR cDNA-transfected (G)
groups. At the end of experiments, total RNA (20–50 μg) was
extracted from SGC-7901 human gastric cancer cells using TRIzol
reagent (Invitrogen), and it was reverse transcribed into cDNA
using an RNA PCR kit (DRR036A; Takara Bio, Inc., Otsu, Japan),
according to the manufacturer’s instructions. These cDNA samples
were amplified by PCR using a thermal cycler (Bio-Rad Laboratories,
Hercules, CA, USA) with the following conditions: Initial
denaturation at 94°C for 30 sec; followed by 40 cycles of 95°C for
5 sec, 65°C for 30 sec and 72°C for 30 sec; and a final extension
at 72°C for 10 min. PCR fragments were separated by electrophoresis
on a 1.5% agarose gel and visualized with ethidium bromide. Primer
sequences (Invitrogen) were as follows: Forward:
5′-GACCCCTTCATTGACCTCAACTACA-3′ and reverse:
5′-GTCCACCACCCTGTTGCTGTAGCCA-3′ for GAPDH; forward:
5′-GTGGCATTTTGGTGGTG-3′ and reverse: 5′-CATTGTTGCTTGGGCTGA-3′ for
GDDR; forward: 5′-AGACTGCGTGCCCTGCCAAGA-3′ and reverse: 5′-GGC
CTGCCTGTTCAGTAACT-3′ for Fas; forward: 5′-GAGACA GCCAGGAGAAATCA-3′
and reverse: 5′-CCTGTGGAT GACTGAGTA-3′ for bcl-2; and forward:
5′-GACCCGGTG CCTCAGGATGC-3′ and reverse: 5′-GTCTGTGTCCAC
GGCGGCAA-3′ for Bax.
Protein extraction and western blot
analysis
SGC-7901 cells were prepared as the Con, P and G
groups. At the end of the experiments, total cellular protein was
extracted from tissue specimens and gastric cancer cells using a
lysis buffer containing 1X Protease Inhibitor Cocktail (Roche
Diagnostics GmbH, Mannheim, Germany). Protein concentration was
quantified using the Bicinchoninic Protein Assay kit (KeyGEN,
China). Equal quantities of protein samples were resolved by 15%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and
electroblotted onto polyvinylidene fluoride membranes (Millipore,
Billerica, MA, USA). The membranes were then blocked in 5% non-fat
milk overnight, and the following day, membranes were incubated
with a rabbit polyclonal anti-GDDR (ab70480, Abcam, Cambridge, UK),
rabbit monoclonal anti-Fas (5709-1, Epitomics, Inc., CA, USA),
anti-bcl-2 (BS1511, Bioworld, St. Louis, MN, USA), anti-Bax (BS2538
Bioworld) or anti-GAPDH (BSAP0063 Bioworld) for 4 h. Following
washing with phosphate-buffered saline (PBS) with Tween-20 four
times, and incubation with goat anti-rabbit secondary antibody
(GAR0072, LiankeBio, Hangzhou, China) for 2 h at room temperature,
the protein bands were visualized using an enhanced
chemiluminescence kit (EMD Millipore, Billerica, MA, USA).
Flow cytometry
SGC-7901 cells were prepared as Con, P and G groups,
and then subjected to evaluation of Fas (also known as CD95)
receptor expression. Briefly, cells (1–5×105/100 μl)
were scraped, subsequent to trypsin digestion without EDTA
addition, washed twice with ice-cold PBS and the binding buffer,
resuspended in the presence of an anti-human CD95 (APO-1/Fas)
phycoerythrin (PE) antibody (12-0959, eBioscience, Inc., San Diego,
CA, USA) and incubated in the dark for 30 min. The cell suspension
was then washed with the binding buffer and resuspended in 200 μl
binding buffer. For each sample, 2×104 events were
acquired by a CantoTM Flow Cytometer (BD Biosciences, Franklin
Lakes, NJ, USA). The experiments were conducted in triplicate and
repeated three times.
Tumor cell viability assay
SGC-7901 cells were prepared as Con, P and G groups.
A G+F group was created by coincubation of G group cells with a
functional grade purified anti-human CD95 (APO/Fas; Epitomics,
Burlingame, CA, USA) antibody at 5 mg/ml for 24 h. In the G+F+Z
group, cells underwent the 48-h GDDR vector transfection plus
coincubation with the CD95 (APO/Fas) antibody and an anti-Fas
(human, neutralizing, clone ZB4 at 1 mg/ml; Merck Millipore,
Darmstadt, Germany)] antibody. These cells were seeded into 96-well
plates at 5×103 cells/well and grown for up to 72 h. At
the end of the experiments,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
KeyGEN, Nanjing, China) at 100 μg/well was added to the cell
culture, and the cells were incubated for another 4 h. A volume of
150 μl dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO, USA) was
added to each well subsequent to removal of the supernatant. After
shaking the plate for 20 min on a shaking board, cell viability was
assessed by measuring the absorbance at 490 nm using an
enzyme-labeling instrument (680 model; Bio-Rad Laboratories,
Hercules, CA, USA). The experiments were conducted in quintuplicate
and repeated three times. Growth inhibition (IR%) was calculated
according to the following formula: IR% = [(the absorbance of blank
control group-the absorbance of experimental group)/the absorbance
of blank control group] × 100.
Annexin V-fluorescein isothiocyanate
(FITC) apoptosis assay
An Annexin V-FITC Apoptosis Detection kit with
propidium iodide (eBioscience) was used to detect apoptosis. In
brief, SGC-7901 cells were prepared as the Con, P and G groups. At
the end of experiments, cells were harvested by centrifuging at
2,400 × g for 5 min, washed once in PBS, then once in 1X binding
buffer, pelleted and resuspended at a concentration of
1×106 in 100 μl of 1X binding buffer. A volume of 5 μl
Annexin V-FITC was added to the cell solution, followed by
incubation for 15 min at room temperature. It was then pelleted,
washed with 1X binding buffer, and resuspended in 200 μl of 1X
binding buffer. Next, 5 μl propidium iodide solution was added to
the cells for a 15-min incubation at room temperature in the dark
followed by the addition of 300 μl of 1X binding buffer. A minimum
of 10,000 cells were subjected to flow cytometric analysis of the
viable, apoptotic and necrotic cell populations. The results were
quantified using Cell Quest software with FCS 2.0 files (Flowjo
7.6.5.1, BD Biosciences), according to the manufacturer’s
instructions.
Quantitation of caspase-3, -8 and -9
activity
SGC-7901 cells were prepared as the Con, P and G
groups. At the end of the experiments, caspase activity was then
measured using CaspGLOW Fluorescein Active Caspase-3, CaspGLOW Red
Active Caspase-9 and CaspGLOW Red Active Caspase-8 Staining kits
(#K183, #K199 and #K198, respectively; BioVision, Inc., Milpitas,
CA, USA), according to the manufacturer’s instructions. Briefly,
cells were resuspended in 300 μl complete growth medium at a
concentration of 1×106/ml, and incubated in a 37°C
incubator for 45 min with the anti-caspase-3, -8 and -9 antibodies.
The lysate was centrifuged at 4,800 × g for 5 min at 4°C, washed
twice with the ice-cold wash buffer and the activity of caspase-3,
-8 and -9 measured using the substrate peptides from the staining
kits (FITC-DEVD-FMK, Red-IETD-FMK and Red-LEHD-FMK). The caspase
activity was quantified by determining absorbance with the
Multiskan Spectrum spectrophotometer (Thermo Fisher Scientific,
Waltham, MA, USA) at Ex/Em = 540/570 nm. Analyses were performed in
triplicate with at least three separate experiments.
Statistical analysis
All experimental data were obtained from at least
three independent experiments. The results are expressed as the
mean ± standard deviation and were evaluated using one-way analysis
of variance followed by Student’s t-test. Statistical analysis was
performed using the SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) for
Windows software. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of gastrokine-2 in human
gastric tissues and gastric cancer cell lines
Gastrokine-2 expression was analyzed in 76 primary
gastric cancer and corresponding normal tissues using western blot
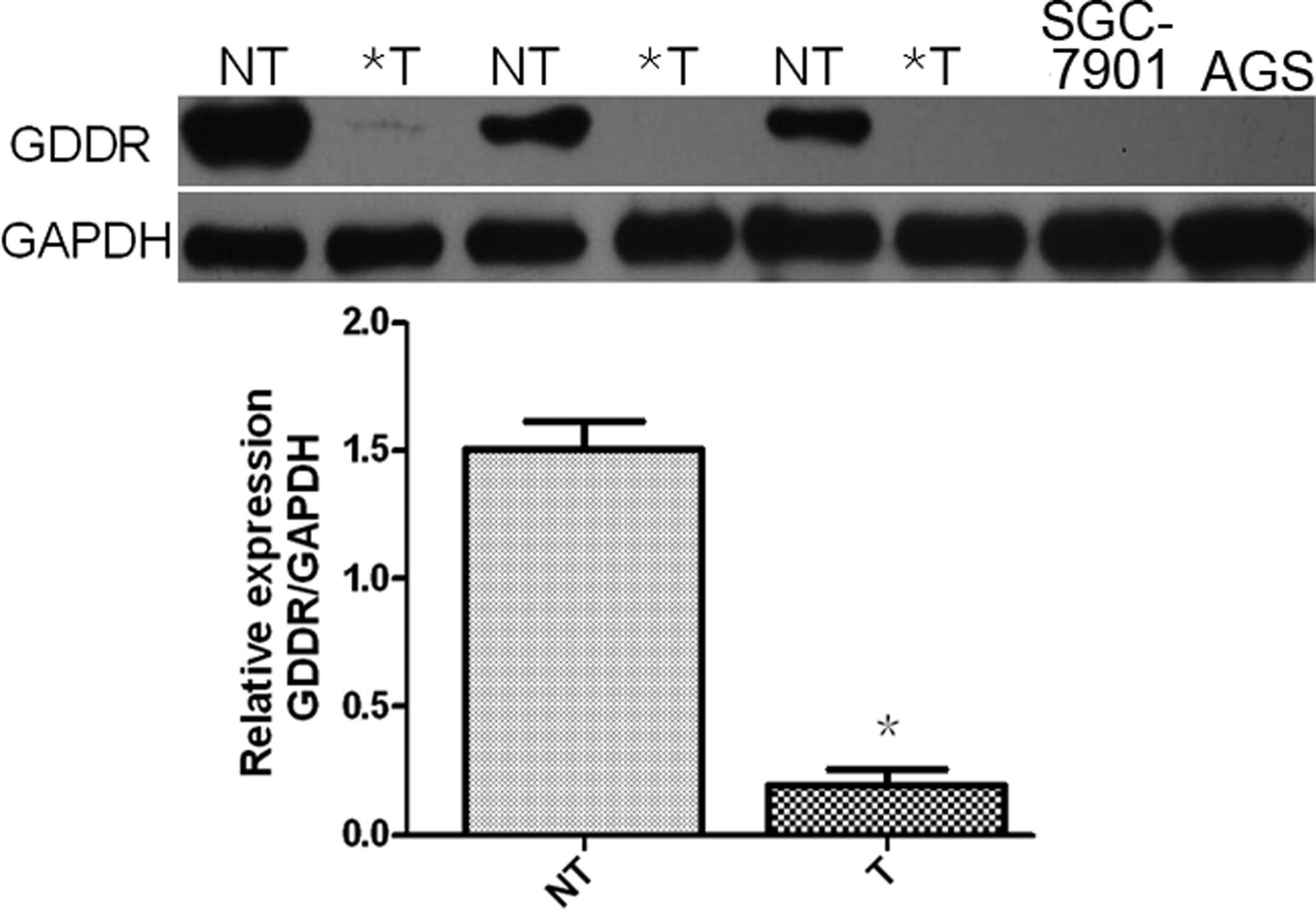
analysis. It was demonstrated that gastrokine-2 protein expression
was reduced in 58 (84.0%) of the 76 cancer tissue samples compared
with the corresponding gastric mucosal tissue samples (Fig. 1). Specifically, gastrokine-2
expression was reduced in 19 (73.07%), 32 (82.05%) and 7 (63.64%)
of the 26 diffuse-, 39 intestinal- and 11 mixed-type gastric cancer
samples, respectively. Expression of gastrokine-2 protein was
indicated to be significantly lower in H. pylori-positive
patients than the level in H. pylori-negative subjects
(P<0.05; Table I), however,
gastrokine-2 protein expression was not associated with tumor
location, depth of invasion, lymph node metastasis, Lauren’s
classification or tumor stage (P>0.05).
Gastrokine-2 expression was then analyzed in the two
gastric cancer cell lines, and it was demonstrated its expression
was absent in the SGC-7901 and AGS cells (Fig. 1).
Restoration of gastrokine-2 expression in
SGC-7901 gastric carcinoma cells
To determine the role of gastrokine-2 in gastric
cancer cells, pcDNA3.1-GDDR or control pcDNA31 were transiently
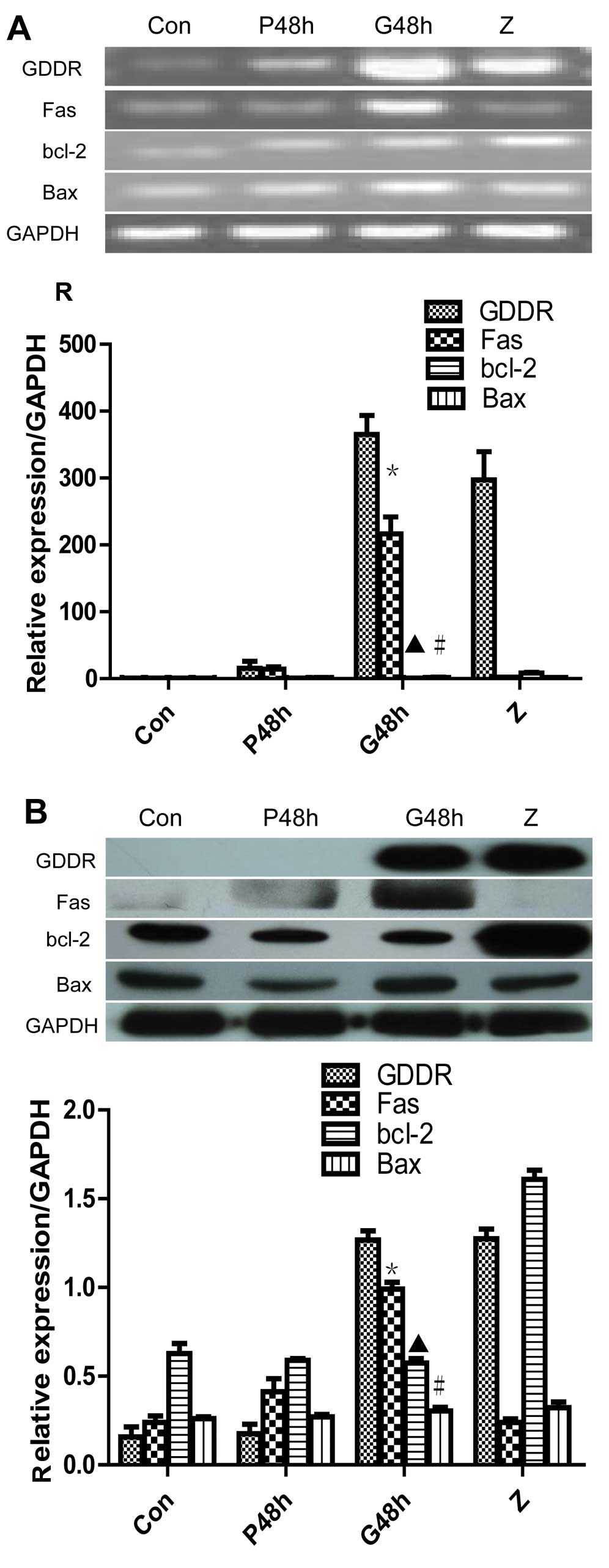
transfected into SGC-7901 cells. The results demonstrated that
pcDNA3.1-GDDR restored gastrokine-2 expression levels in gastric
cancer cells (Fig. 2A). The
altered gene expression was then assessed, and it was indicated
that the level of Fas mRNA was significantly upregulated 48 h after
gene transfection (P<0.05 vs. non-transfected control and vector
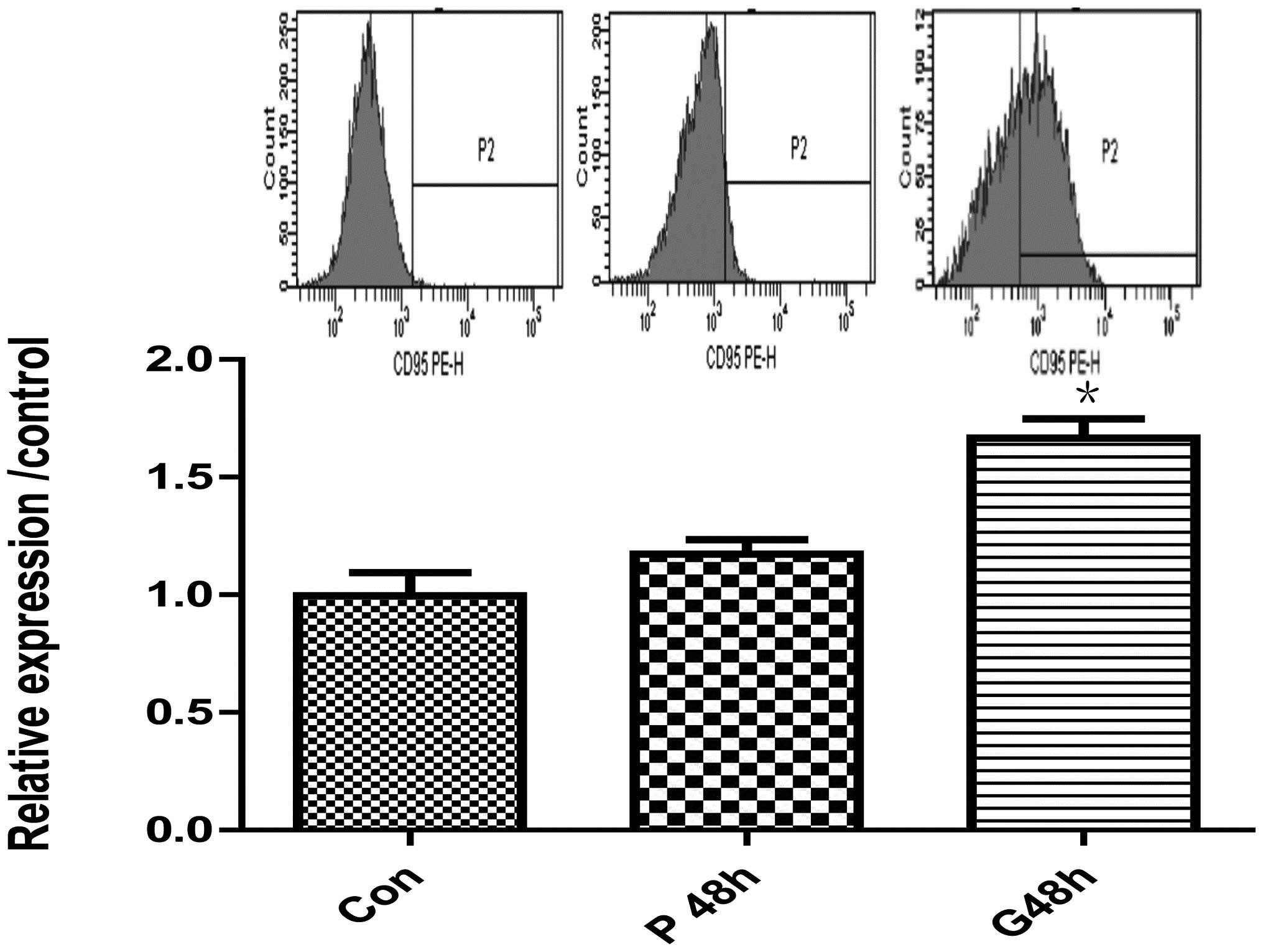
control; Fig. 2A). Fas protein
level was also increased, as detected by western blot analysis
(Fig. 2B) and flow cytometry
(Fig. 3) with a rabbit monoclonal
anti-Fas/CD95 and anti-human CD95 (APO-1/Fas) PE (Fig. 3). However, expression of bcl-2 and
Bax mRNA and protein was not identified to significantly change
from control levels.
Restoration of gastrokine-2 expression
reduces tumor cell viability in vitro
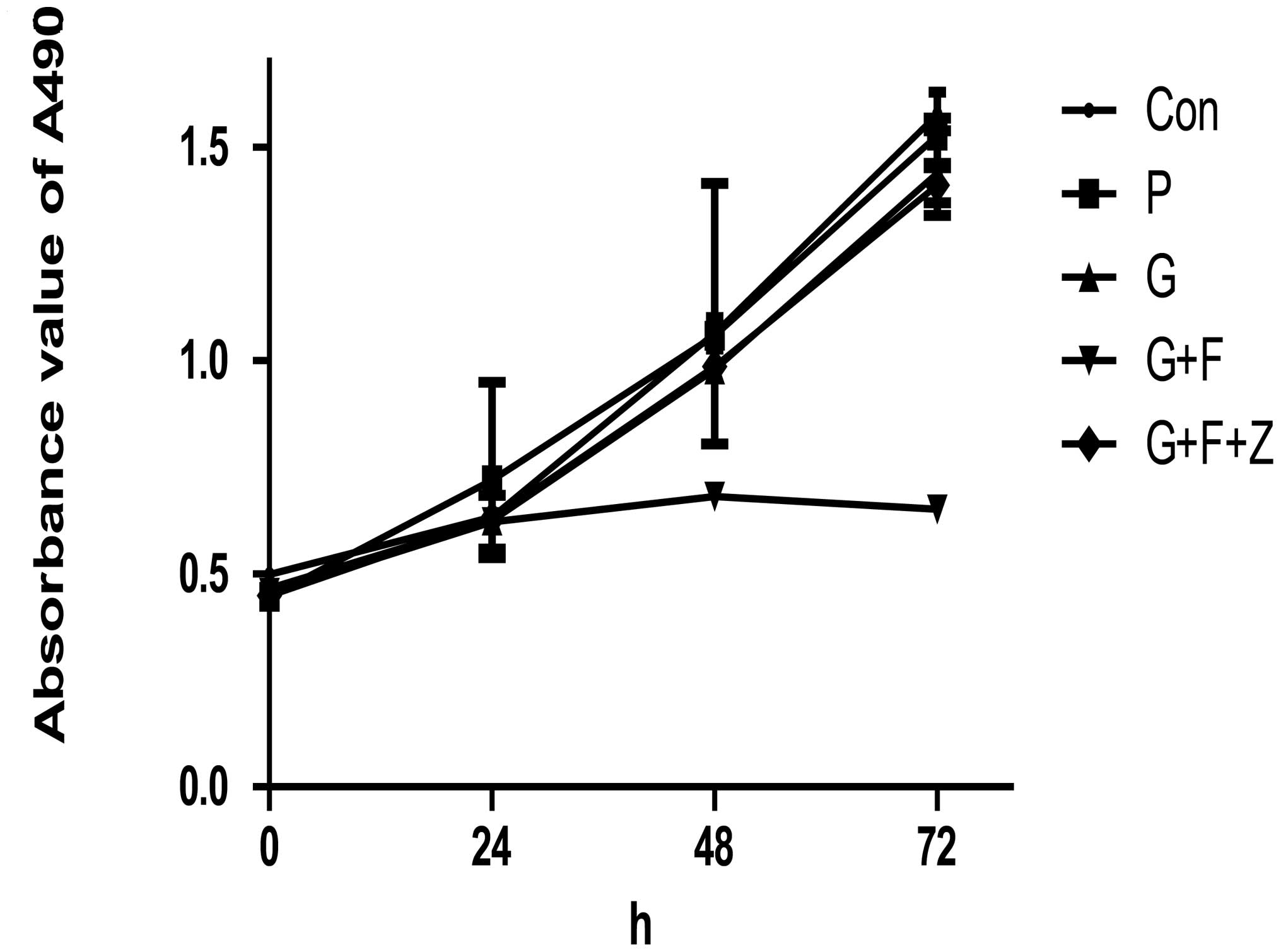
Following transfection, the altered phenotypes of
these gastric cancer cells was evaluated. A cell viability MTT
assay was performed, and the results indicated that restoration of
gastrokine-2 expression significantly reduced tumor cell viability
in the monolayer culture. In brief, the inhibitory rate of G+F was
35.67±5.76 and 58.67±1.78% at 48 and 72 h, respectively. The P and
G groups displayed reduced viability of 0.97±3.71 and 3±3.86%, and
13.69±2.29 and 7.72±5.28%, respectively (P<0.05). Additionally,
the viability of G+F+Z cells was reduced compared with G+F cells
(10.46±0.78 vs. 7.14±3.00% at 48 and 72 h, respectively; P<0.05;
Fig. 4).
Restoration of gastrokine-2 expression
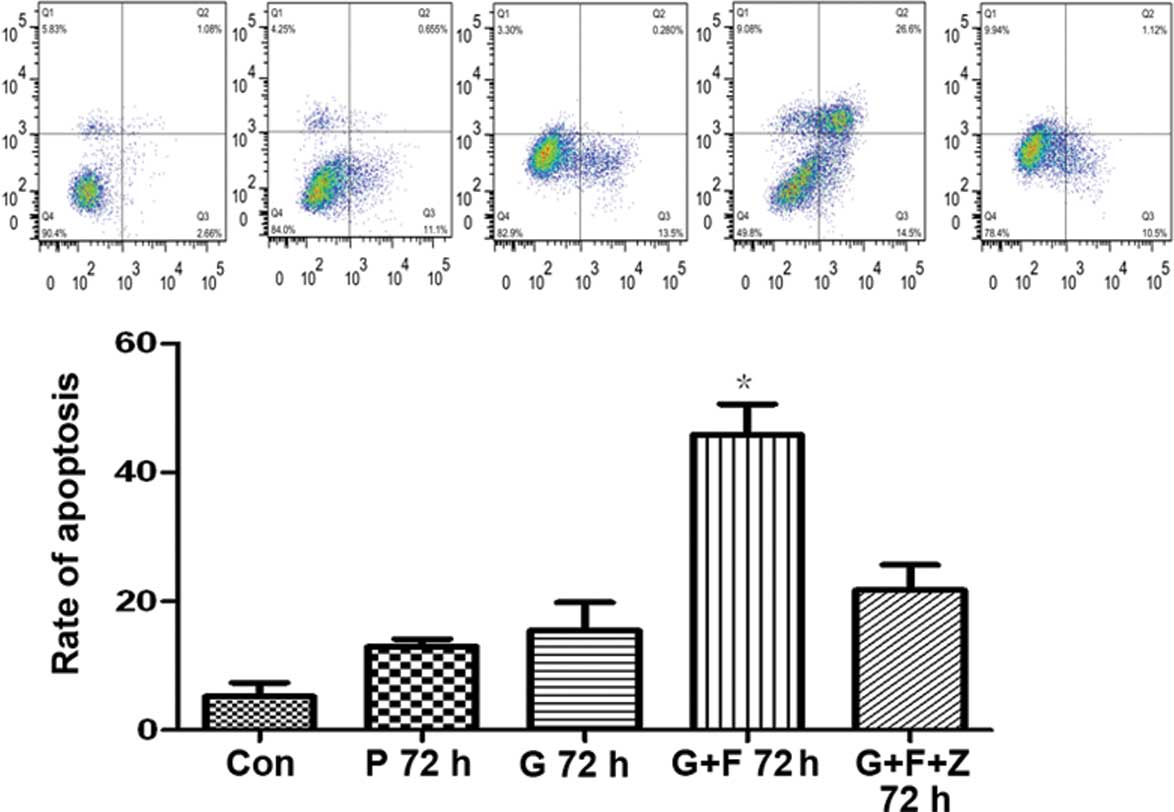
induces apoptosis in gastric cancer cells
To assess the cause of the reduced cell viability,
the rate of apoptosis was evaluated. Following 48-h gastrokine-2
transfection, SGC-7901 gastric cancer cells were incubated with
functional grade purified anti-human CD95 (APO/Fas) for 24 h. The
rate of apoptosis following antibody incubation was 45.89±8.20%,
which was significantly higher than the level in cells transfected
with gastrokine-2 vector (15.48±7.53%), control vector
(12.97±1.99%), and non-transfected controls (5.24±3.71)
(P<0.05). However, when the cells were coincubated with the two
antibodies (CD95 and Fas; G+F+Z cells), the apoptosis rate was
21.71±6.90%, which was significantly reduced compared with the G+F
cells (P<0.05; Fig. 5).
Restoration of gastrokine-2 expression
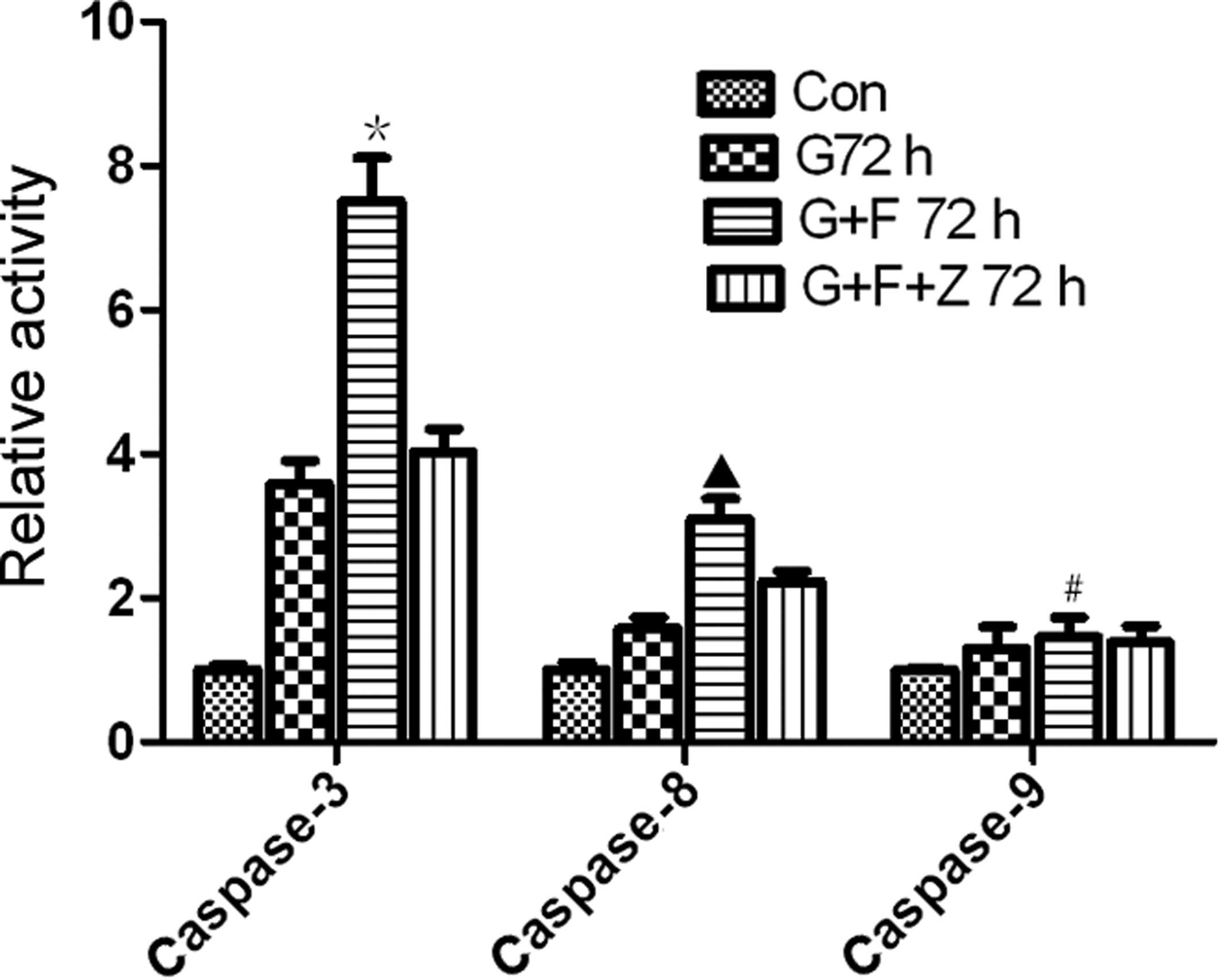
induces activation of caspase-3, -8, and -9
To further assess the effect of gastrokine-2
restoration on the induction of apoptosis, the activity of
caspase-3, -8 and -9 was determined. The data demonstrated that the
relative activity of caspase-3 (7.5±1.04) and caspase-8 (3.09±0.49)
was significantly higher in the G+F group compared with cells of
the G group (3.58±0.57 and 1.58±0.26, caspase-3 and -8,
respectively; P<0.05) and parental cell control (1.00±0.12 and
1.00±0.18 for caspase-3 and -8, respectively; P<0.01). The
relative activity of caspase-3 (4.03±0.55) and caspase-8
(2.23±0.24) was lower in the G+F+Z group compared with the G+F
group (Fig. 6). Furthermore, the
activity levels of caspase-9 were 1.00±0.05, 1.03±0.11, 1.12±0.11,
and 1.04±0.17 in the control, G, G+F and G+F+Z groups,
respectively, indicating no significant differences (P>0.05;
Fig. 6).
Discussion
Apoptosis, also known as programmed cell death, is a
basic biological process that functions to maintain homeostasis of
the human body by removing undesirable cells (18). Apoptosis is controlled by a diverse
range of cell signals, which can be classified into two major
molecular signaling pathways; the extrinsic and intrinsic pathways
(19–22). The extrinsic apoptotic pathway
involves binding of the Fas ligand (FasL) to the Fas receptor
(FasR; also termed CD95) (23,24),
a transmembrane protein of the tumor necrosis factor family. This
results in formation of the death-inducing signaling complex, which
contains the Fas-associated death domain (FADD), caspase-8 and
caspase-10. FADD is an adapter complex that recruits and activates
caspase-8. Cleaved caspase-8 then induces cleavage and activation
of executive caspase-3, and in turn, the activated capase-3 cleaves
DNA molecules, leading to apoptosis (25–27).
Alternatively, the intrinsic (or mitochondrial) pathway is largely
dependent on the bcl-2 family of proteins (such as Bax) to induce
cytochrome c release from the mitochondria. Cytochrome
c binds to apoptotic protease activating factor-1, ATP and
pro-caspase-9 to form a protein complex known as an apoptosome, in
order to activate caspase-3 for induction of apoptosis. Different
stimuli activate one of these apoptotic pathways, or both (23–27).
Previously, it has been demonstrated that the
Fas/FasL pathway exerts a central role in induction of apoptosis,
and alteration of this pathway has been observed in gastric
adenocarcinoma cells (28).
Gastric cancer tissues also indicated a downregulation of Fas, but
increased FasL expression. Indeed, downregulation of Fas receptor
expression in cancer cells can lead to apoptosis resistance and
FasL stimulation (29,30). However, increased expression of
FasL and reduced expression of caspase-3 in gastric cancer cells of
the primary foci serve an important role in gastric carcinogenesis
(27). FasL has also been
implicated in de-differentiation, growth, invasion and metastasis
of gastric cancer cells, through the induction of apoptosis in the
infiltrating lymphocytes. By contrast, chemical substances derived
from the primary foci of gastric cancer tissues and the metastatic
microenvironment may inhibit the growth of metastatic cells by
enhancing caspase-3 expression levels and decreasing those of FasL
(27).
In the present study, the level of gastrokine-2
protein was reduced, or absent, in the majority of gastric cancer
tissues and absent in two gastric cancer cell lines, which is
consistent with the results reported by Du et al (11). Previous studies have not implicated
gastrokine-2 as a putative gastric cancer-specific tumor suppressor
gene (11). However, other studies
have demonstrated that gastrokine-2 is a secretory peptide of human
gastric surface mucous cells (31)
and modulates gut epithelial cell proliferation (32). Gastrokine-2 expression has been
reported to be attenuated in gastric adenocarcinomas (85% of
diffuse and 54% intestinal type tumors) (33), whilst in gastric epithelial cells
it has been indicated to be significantly upregulated following
eradication of Helicobacter pylori, a risk factor for
gastric cancer (34). These data
indicated that gastrokine-2 may serve an important function in
gastric epithelial cell homeostasis and that altered expression of
gastrokine-2 protein may contribute to gastric carcinogenesis.
Gastrokine-1, another member of the gastrokine family, has been
demonstrated to introduce apoptosis in gastric cancer cells mainly
through the Fas/FasL pathway (35).
The current study also demonstrated that restoration
of gastrokine-2 protein expression upregulated Fas expression, but
there was no significant difference in the expression level of
bcl-2 and Bax, indicating that the extrinsic apoptosis pathway
serves a role in gastrokine-2-induced gastric cancer cell
apoptosis. To verify this, a functional grade purified anti-human
CD95 (APO/Fas) antibody was used to promote apoptosis, and an
anti-Fas (human, neutralizing, clone ZB4) antibody was used to
block this extrinsic pathway. The data indicated that apoptosis was
markedly increased in gastric cancer cells transfected with
gastrokine-2 and incubated with functional grade purified CD95
(APO/Fas) antibody (48 h, 72 h), but the increase can be reversed
by treatment with anti-Fas (human, neutralizing, clone ZB4)
antibody. In order to further confirm this hypothesis, the activity
of caspase 3, 8, and 9 was analyzed in these groups of gastric cell
lines, and it was identified that caspase 3 and 8 in extrinsic
apoptosis was activated or inhibited by functional grade purified
CD95 (APO/Fas) and anti-Fas (human, neutralizing, clone ZB4)
antibodies, respectively. However, caspase 9-related intrinsic
apoptotic gene expression was not significantly altered.
In conclusion, to the best of our knowledge, the
data from the current study demonstrated for first time that
restoration of gastrokine-2 expression in SGC-7901 gastric cancer
cells inhibits cell viability and induces apoptosis. Furthermore,
it was demonstrated that apoptosis was induced through activation
of the extrinsic apoptosis pathway. Following further
investigation, gastrokine-2 may prove to be a potential target for
novel molecular therapies for gastric cancer.
Acknowledgements
The authors would like to thank Medjaden Bioscience
Limited, Hong Kong, China, for their assistance in editing this
manuscript.
References
|
1
|
Forman D and Burley VJ: Gastric cancer:
global pattern of the disease and an overview of environmental risk
factors. Best Pract Res Clin Gastroenterol. 20:633–649. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hohenberger P and Gretschel S: Gastric
cancer. Lancet. 362:305–315. 2003. View Article : Google Scholar
|
|
3
|
Leung WK, Wu MS, Kakugawa Y, et al: Asia
Pacific Working Group on Gastric Cancer: Screening for gastric
cancer in Asia: current evidence and practice. Lancet Oncol.
9:279–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Soerjomataram I, Lortet-Tieulent J, Parkin
DM, et al: Global burden of cancer in 2008: a systematic analysis
of disability-adjusted life-years in 12 world regions. Lancet.
380:1840–1850. 2012.PubMed/NCBI
|
|
5
|
Yoshida T, Ono H, Kuchiba A, et al:
Genome-wide germline analyses on cancer susceptibility and GeMDBJ
database: Gastric cancer as an example. Cancer Sci. 7:1582–1589.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mirvish SS: Gastric cancer and salivary
nitrate and nitrite. Nature. 315:461–462. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Parsonnet J, Friedman GD, Vandersteen DP,
et al: Helicobacter pylori infection and the risk of gastric
carcinoma. N Engl J Med. 325:1127–1131. 1991. View Article : Google Scholar
|
|
8
|
Lo SS, Wu CW, Hsieh MC, et al:
Relationship between age and clinical characteristics of patients
with gastric cancer. J Gastroenterol Hepatol. 11:511–514. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hwang H, Dwyer J and Russell RM: Diet,
Helicobacter pylori infection, food preservation and gastric
cancer risk: are there new roles for preventative factors? Nutr
Rev. 52:75–83. 1994.
|
|
10
|
Roberts-Thomson IC and Butler WJ:
Polymorphism and gastric cancer. J Gastroenterol Hepatol.
20:793–794. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Du JJ, Dou KF, Peng SY, et al:
Down-regulated full-length novel gene GDDR and its effect on
gastric cancer. Zhonghua Yi Xue Za Zhi. 83:1166–1168. 2003.(In
Chinese).
|
|
12
|
Sánchez-Pulido L, Devos D and Valencia A:
BRICHOS: a conserved domain in proteins associated with dementia,
respiratory distress and cancer. Trends Biochem Sci. 27:329–332.
2002.PubMed/NCBI
|
|
13
|
Oien KA, McGregor F, Butler S, et al:
Gastrokine 1 is abundantly and specifically expressed in
superficial gastric epithelium, down-regulated in gastric
carcinoma, and shows high evolutionary conservation. J Pathol.
203:789–797. 2004. View Article : Google Scholar
|
|
14
|
Westley BR, Griffin SM and May FE:
Interaction between TFF1, a gastric tumor suppressor trefoil
protein, and TFIZ1, a brichos domain-containing protein with
homology to SP-C. Biochemistry. 44:7967–7975. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Du JJ, Dou KF, Peng SY, et al: Study on
novel gene GDDR related to gastric cancer. Zhonghua Wai Ke Za Zhi.
43:10–13. 2005.(In Chinese).
|
|
16
|
Chu G, Qi S, Yang G, et al:
Gastrointestinal tract specific gene GDDR inhibits the progression
of gastric cancer in a TFF1 dependent manner. Mol Cell Biochem.
359:369–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baus-Loncar M, Lubka M, Pusch CM, et al:
Cytokine regulation of the trefoil factor family binding protein
GKN2 (GDDR/TFIZ1/blottin) in human gastrointestinal epithelial
cells. Cell Physiol Biochem. 20:193–204. 2007.
|
|
18
|
Reed JC: Mechanisms of apoptosis. Am J
Pathol. 157:1415–1430. 2000. View Article : Google Scholar
|
|
19
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163.
2005.PubMed/NCBI
|
|
20
|
Carrington PE, Sandu C, Wei Y, et al: The
structure of FADD and its mode of interaction with procaspase-8.
Mol Cell. 22:599–610. 2006. View Article : Google Scholar
|
|
21
|
Roy MK, Thalang VN, Trakoontivakorn G and
Nakahara K: Mahanine, a carbazole alkaloid from Micromelum
minutum, inhibits cell growth and induces apoptosis in U937
cells through a mitochondrial dependent pathway. Br J Pharmacol.
145:145–155. 2005.
|
|
22
|
Henkler F, Behrle E, Dennehy KM, et al:
The extracellular domains of FasL and Fas are sufficient for the
formation of supramolecular FasL-Fas clusters of high stability. J
Cell Biol. 168:1087–1098. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ashkenazi A and Dixit VM: Death receptors:
signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thorburn A: Death receptor-induced cell
killing. Cell Signal. 16:139–144. 2004. View Article : Google Scholar
|
|
25
|
Houston A and O’Connell J: The Fas
signalling pathway and its role in the pathogenesis of cancer. Curr
Opin Pharmacol. 4:321–326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mollinedo F and Gajate C: Fas/CD95 death
receptor and lipid rafts: new targets for apoptosis-directed cancer
therapy. Drug Resist Updat. 9:51–73. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng HC, Sun JM, Wei ZL, et al:
Expression of Fas ligand and caspase-3 contributes to formation of
immune escape in gastric cancer. World J Gastroenterol.
9:1415–1420. 2003.PubMed/NCBI
|
|
28
|
Boroumand-Noughabi S, Sima HR,
Ghaffarzadehgan K, et al: Soluble Fas might serve as a diagnostic
tool for gastric adenocarcinoma. BMC Cancer. 10:2752010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Z, Wang Z, Zhao Z, et al: Expression of
Fas, FasL and IFN-gamma in gastric cancer. Beijing Da Xue Xue Bao.
35:386–389. 2003.PubMed/NCBI
|
|
30
|
Gryko M, Guzińska-Ustymowicz K, Pryczynicz
A, et al: Correlation between Fas and FasL proteins expression in
normal gastric mucosa and gastric cancer. Folia Histochem Cytobiol.
49:142–147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kouznetsova I, Laubinger W, Kalbacher H,
et al: Biosynthesis of gastrokine-2 in the human gastric mucosa:
restricted spatial expression along the antral gland axis and
differential interaction with TFF1, TFF2 and mucins. Cell Physiol
Biochem. 20:899–908. 2007. View Article : Google Scholar
|
|
32
|
Otto WR, Patel K, McKinnell I, et al:
Identification of blottin: a novel gastric trefoil factor family-2
binding protein. Proteomics. 6:4235–4245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moss SF, Lee JW, Sabo E, et al: Decreased
expression of gastrokine 1 and the trefoil factor interacting
protein TFIZ1/GKN2 in gastric cancer: influence of tumor histology
and relationship to prognosis. Clin Cancer Res. 14:4161–4167. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Resnick MB, Sabo E, Meitner PA, et al:
Global analysis of the human gastric epithelial transcriptome
altered by Helicobacter pylori eradication in vivo. Gut.
55:1717–1724. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rippa E, La Monica G, Allocca R, et al:
Overexpression of gastrokine-1 in gastric cancer cells induces
Fas-mediated apoptosis. J Cell Physiol. 226:2571–2578. 2011.
View Article : Google Scholar : PubMed/NCBI
|