Introduction
Osteosarcoma (OS), the eighth most common form of
childhood cancer, derives from primitive bone forming mesenchymal
cells and is the most prevalent primary bone malignancy. The
complex etiology involves a combination of environmental factors
and genetic impairments. OS has a bimodal age distribution, with
the first peak during adolescence and the second peak in later
adulthood. The first peak occurs in 10–14-year-olds, coinciding
with the pubertal growth spurt which suggests a close correlation
between the adolescent growth spurt and OS (1). Since the development of medical
technology, the five-year survival rate of patients carrying OS has
been significantly improved (2),
while the cure rate of patients carrying OS remains poor (3).
MicroRNA (miRNA) is a short non-coding regulatory
RNA that represses gene expression by imperfectly base pairing to
the 3′ untranslated region (3′UTR) of target mRNAs. Evidence has
shown that alterations in the expression of miRNA are involved in
the initiation, progression, and metastasis of human cancer. It is
believed that miRNAs can function as tumor suppressors as well as
oncogenes during cancer development (4,5).
Studies have indicated that miRNAs have an important role in OS
pathogenesis and progression (6–8).
In the present study, in order to gain insight into
the role of miR polymorphism in OS, the coding regions of three
miRNAs (miR-21, miR-34a and miR-146a) were screened in 103
patients; these miRNAs are associated with numerous types of cancer
pathogenesis (9–11). The effect of variation in
pre-miR-34a coding regions on miR-34a expression and OS cell
proliferation were investigated in vitro and compared with
data from tissue and blood serum samples of patients with OS were
analyzed. Furthermore, the effect of site variation on the
expression of the c-Met oncogene, a target gene of miR-34a, was
investigated using western blot analysis and a luciferase reporter
assay.
Materials and methods
Study population and tissue samples
A total of 65 pairs of surgically resected OS (prior
to neoadjuvant chemotherapy administration) and adjacent normal
bone tissue were acquired from Yantai Yuhuangding Hospital (Qingdao
University, Shandong, China) between January 2010 and June 2012.
Written informed consent was obtained from all patients.
The peripheral blood samples of 103 OS patients were
also obtained from Yantai Yuhuangding Hospital. The control group
consisted of samples from 201 Han-Chinese individuals and were also
collected from Yantai Yuhuangding Hospital. The present study was
approved by the Ethics Committee of Yantai Yuhangding Hospital
(Yantai, China).
DNA collection and genotyping
DNA from the adjacent normal tissues and tumor
tissues of the OS cancer cohort were isolated by using the TIANamp
Genomic DNA kit (Tiangen, Beijing, China). DNA from blood samples
was extracted using the TIANamp Blood DNA kit (Tiangen).
DNA specimens were amplified using standard
polymerase chain reaction (PCR) protocols. The PCR products were
sequenced in the forward direction with the ABI 3730xl sequencing
platform (Applied Biosystems, Foster City, CA, USA). The sequencing
results were analyzed by using DNAMAN version 5.2.9 (Lynnon
Corporation, Quebec, Canada) and Chromas Lite software version 2/22
(Technelysium Pty, Ltd., Shannon Ireland). The PCR primers used for
miR-34a sequencing were 5′-CCCACATTTCCTTCTTATCAACAG-3′ and
5′-GGCATCTCTCGCTTCATCTT-3′.
Quantitative polymerase chain reaction
(qPCR)
qPCR analysis was used to determine the relative
expression levels of miR-34a-5p. Total RNA was extracted from
tissues and cells using TRIzol (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
The expression levels of miR-34a-5p were detected using TaqMan
miRNA RT-Real Time PCR. Single-stranded complementary DNA (cDNA)
was synthesized by using TaqMan MicroRNA Reverse Transcription kit
(Applied Biosystems) and then amplified using TaqMan Universal PCR
Master Mix (Applied Biosystems) together with miRNA-specific TaqMan
dihydrocyclopyrroloindole tripeptide minor groove binder probe:
miR-34a-5p (Applied Biosystems). The U6 small nuclear RNA (snRNA)
was used for normalization. Each sample in each group was measured
in triplicate and the experiment was repeated three or more times
for the detection of miR-34a-5p. Results are expressed as the mean
± standard error of the mean.
Secondary structure prediction
The secondary structure of a 110-base pair (bp)
pre-miR-34a sequence including mutation site was predicted using
the RNA fold web server (http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi).
MiR-34a expression vectors
To construct mir-34a expression vectors, fragments
(533 nt) corresponding to pre-mir-34a and its flanking regions
(previously determined to have the two genotypes) were amplified
from cDNA and cloned into the pcDNA3.1 vector (Invitrogen Life
Technologies). The sequences of these two vectors were confirmed by
direct sequencing; the only difference was in the mutation site.
The primers were miR-34a-F/XhoI
5′-CCGCTCGAGGTCACCATGCCTGGCTAATTGAGGAGG-3′ and mir-34a-R/XbaI
5′-GCTCTAGAACTATTCTCCCTACGTGCAAAC-3′.
Dual luciferase assay
The full length of the 2262-bp c-MET 3′UTR were
cloned downstream of the firefly luciferase coding region in the
pmirGLO vector (Promega, Madison, WI, USA) to generate the
luciferase reporter vector. For luciferase reporter assays, SAOS-2
and U2OS cells, obtained from the Cell Resource Center of Peking
Union Medical College (Beijing, China), were seeded in 48-well
plates at a density of 1×104. miR-34a expression and
luciferase reporter vectors were co-transfected by using
Lipofectamine 2000 (Invitrogen Life Technologies). Two days later,
cells were harvested and assayed with the Dual-Luciferase Reporter
Assay system (Promega). Each treatment was performed in triplicate
in three independent experiments. The results were expressed as
relative luciferase activity (Firefly LUC/Renilla LUC).
MTT cell proliferation assay
The proliferation capacity of cells was evaluated
using the MTT assay, performed according to the manufacturer’s
instructions (Sigma, St. Louis, MO, USA), in 96-well plates. In
brief, cells were seeded at a density of 2,000 cells per well
containing 100 μl culture medium and cultured overnight. Every 24 h
interval, 20 μl 5 mg/ml MTT reagent was added to each well and
cells were incubated for a further 4 h at 37°C. The medium was then
removed, and 100 μl dimethyl sulfoxide (DMSO; Sigma) was added to
each well to dissolve the formazan. Optical density (OD) was
evaluated by measuring the absorbance at a test wavelength of 490
nm and a reference wavelength of 630 nm. Wells without cells (DMSO
alone) were used as blanks. Each group contained six wells;
experiments were repeated three times independently and the results
are expressed as the mean ± standard deviation.
Western blot analysis
Protein extracts were boiled in an
SDS/β-mercaptoethanol sample buffer (Sigma), and 30 μg sample was
loaded into each lane of 8% polyacrylamide gels. The proteins were
separated by electrophoresis, and the proteins in the gels were
blotted onto polyvinylidene difluoride membranes (Amersham
Pharmacia Biotech, St. Albans, UK) by electrophoretic transfer. The
membrane was incubated with rabbit anti-c-Met polyclonal antibody
(1:5,000; Abcam, Cambridge, MA, USA) and/or mouse anti-β-actin
monoclonal antibody (1:5,000; Santa Cruz Biotechnology Inc., Santa
Cruz, CA, USA) for 1 h at 37°C. The specific protein antibody
complex was detected using horseradish peroxidase-conjugated goat
anti-rabbit and rabbit anti-mouse immunoglobulin G (1:5,000; Santa
Cruz Biotechnology, Inc.). Detection by the chemiluminescence
reaction was carried using the enhanced chemiluminescence kit
(Pierce, Appleton, WI, USA). The β-actin signal was used as a
loading control.
Detection of serum c-Met concentration
using ELISA
Serum c-Met levels were detected using the sandwich
ELISA method with rabbit and mouse anti-c-Met antibodies (Abcam).
The relative concentrations were compared using OD values directly.
The results were analyzed using the Mann-Whitney U test. P<0.05
was considered to indicate a statistically significant difference
between values.
Statistical analysis
Data were analyzed by using SPSS Statistical Package
version 16 (International Business Machines, Armonk, NY, USA).
Analysis of two independent groups were performed using a Student’s
t-test. Results of tissue miR-34a levels were analyzed using the
Mann-Whitney U test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Genotypes and risk of OS
Coding regions (pre-miR-146a, -21 and -34a) were
scanned in 103 OS patients and 201 healthy controls in order to
investigate the correlation between nucleotide variants of these
three candidate miRNA coding regions and the pathogenesis of OS.
Although no sequence changes had previously been described, the
present study identified that the rare allele A of rs72631823 was
highly correlated to OS (OR=15.65, 95% CI=[5.41, 45.29]) (Table I).
 | Table IGenotype frequencies of rs72631823 in
patients and controls and their association with osteosarcoma. |
Table I
Genotype frequencies of rs72631823 in
patients and controls and their association with osteosarcoma.
| Genotype | Patients (n=103),
freq | Controls (n=201),
freq | OR (95% CI) | P-value |
|---|
| rs72631823
(G>A) |
| A | 28 (0.14) | 4 (0.010) | 15.65 (5.41,
45.29) | <0.001 |
| G | 178 (0.86) | 398 (0.99) | 0.064 (0.022,
0.18) | |
| A A | 4 (0.04) | 0 (0.00) | | |
| A G | 20 (0.19) | 4 (0.020) | 11.87 (3.94,
35.79) | <0.001 |
| G G | 79 (0.77) | 197 (0.98) | 0.067 (0.023,
0.20) | |
| rs2910164
(G>C) |
| C | 27 (0.131) | 72 (0.179) | 0.69 (0.43,
1.12) | 0.13 |
| G | 179 (0.869) | 330 (0.821) | 1.45 (0.90,
2.33) | |
| C C | 3 (0.078) | 4 (0.020) | 1.48 (0.32,
6.73) | 0.10 |
| C G | 21 (0.48) | 64 (0.318) | 1.68 (0.98,
2.89) | |
| G G | 79 (0.45) | 133 (0.662) | 0.55 (0.31,
0.96) | |
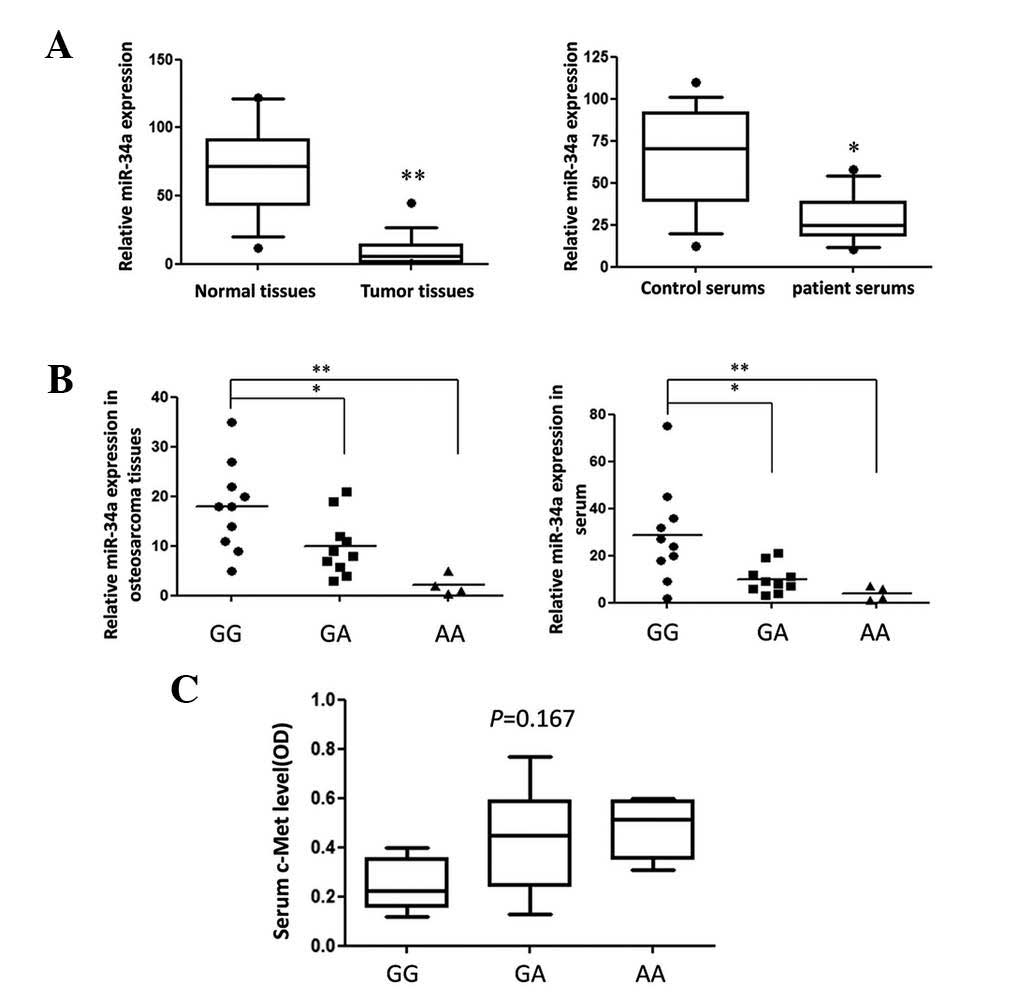
The G to A variation can predict
pri-miR-34a stability and reduce mature miR-34a expression
To further explore the function of the mutation
site, the predicted secondary structures of two pri-miR-34a
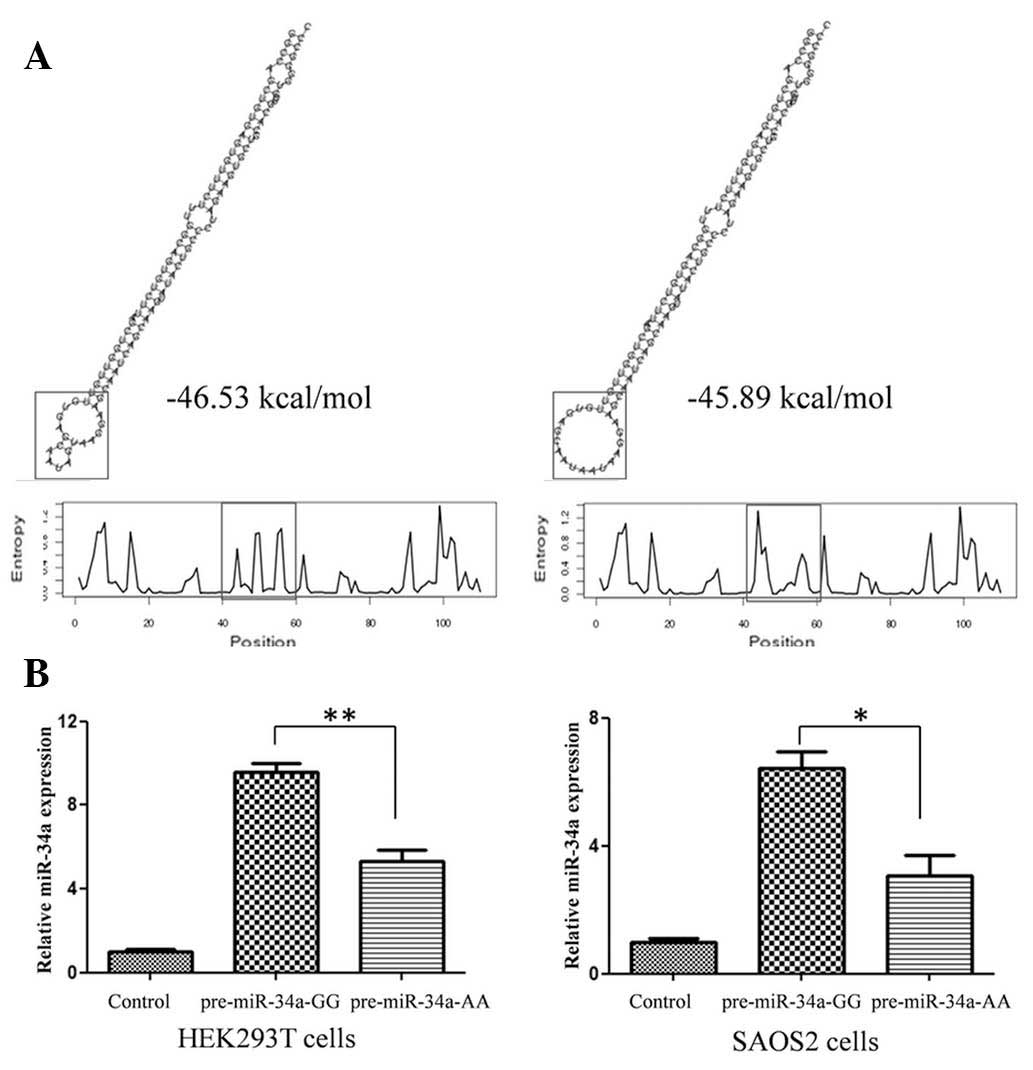
genotype molecules were compared. As shown in Fig. 1A, rare allele A caused an apparent
change in loop size and a higher than predicted ΔG from −46.53
kcal/mol to −45.89 kcal/mol. The expression of mature miR-34a-5p
generated by different miR-34a-5p genotype expression vectors in
two different cell lines was detected using qPCR. The G to A
variation caused an ~50% reduction in mature miR-34a-5p expression
(Fig. 1B), the result of which
correlated with data from OS tissues (Fig. 2B).
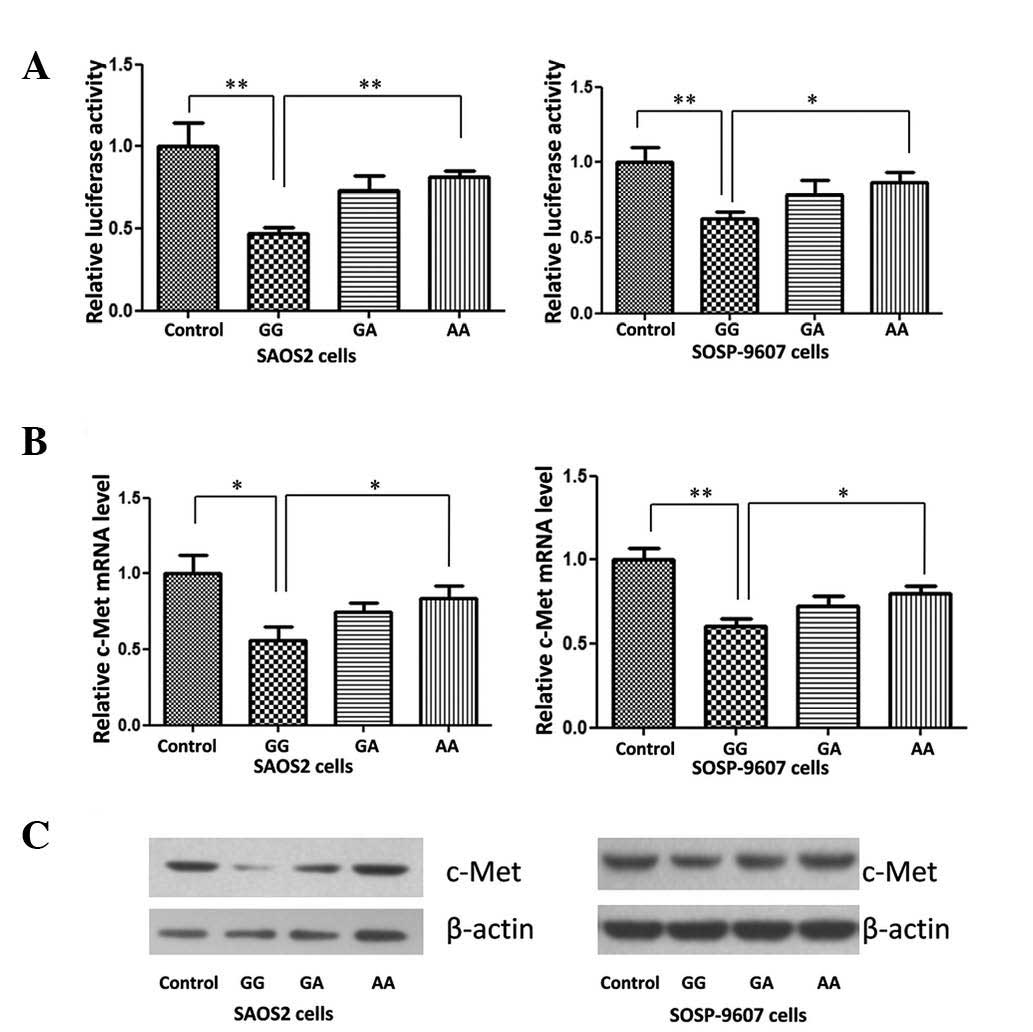
Disturbed miR-34a expression weakens the
suppression effect on c-Met expression
Studies have reported that miR-34a directly
repressed the expression of c-Met in HeLa cells (12), suppressed brain tumor growth
through targeting c-Met (13) and
acted as a tumor suppressor in uveal melanoma cell proliferation
and migration through the downregulation of c-Met (14). Yan et al (15) confirmed that miR-34a repressed OS
cell proliferation and migration in vitro and in
vivo. In order to detect the impact of G>A variation on
miR-34a target genes in the present study, a c-Met reporter system
was constructed. The results of the dual luciferase assay indicated
that in the two OS cell lines, expression of c-Met was
significantly downregulated following transfection with different
miR-34a genotype expression vectors, compared with that of normal
control cells (Fig. 3A).
Detection of c-Met expression using qPCR
and western blot analysis
As shown in Fig. 3B and
C, the expression of c-Met in two OS cell lines was
significantly repressed by the miR-34 GG genotype expression
plasmid. A>G variation reduced the repressive effect of miR-34a,
48 h post-plasmid transfection.
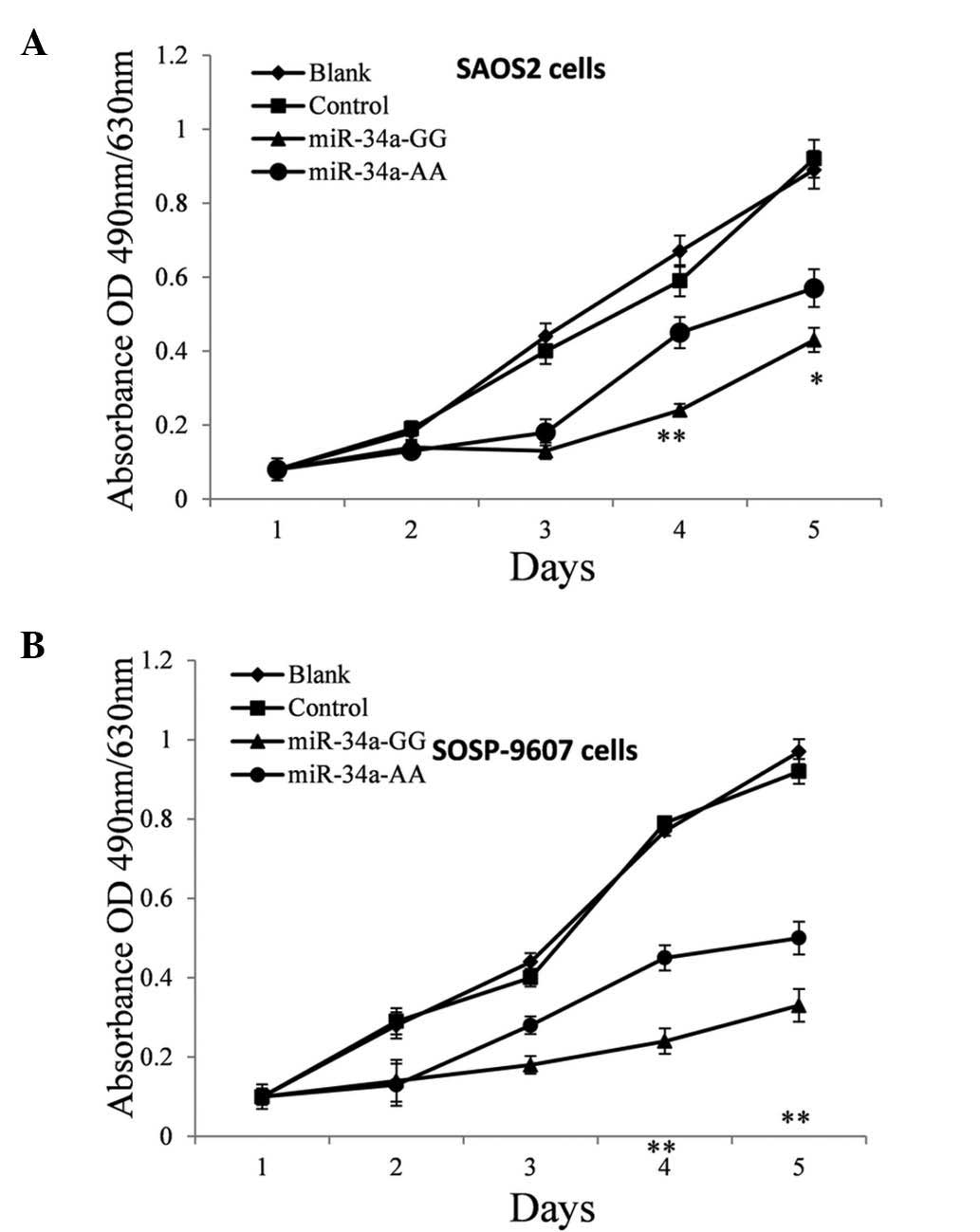
Disturbed miR-34a expression weakens the
suppression of OS cell proliferation in vitro
Based on current knowledge of the function of c-Met,
it was predicted that the reduction of miR-34a expression should
promote cell proliferation (16–17).
Therefore, a proliferation assay was performed on SAOS2 and
SOSP-9607 cells treated with pre-miR-34a of different genotypes in
order to investigate the effect of polymorphism on the anti-tumor
efficacy of miR-34a in OS cells. The MTT assay was performed every
24 h post plasmid transfection. As hypothesized, these pre-miR-34a
GG genotypes suppressed the proliferation of SAOS2 (Fig. 4A) and SOSP-9607 (Fig. 4B) cells significantly, most notably
on the fourth day post-transfection (P=0.0015 and 0.0047) and the
A>G mutation reduced the suppressive effect by nearly 20%
(P=0.033) and 35% (P=0.0031), respectively.
Discussion
Recent evidence has demonstrated that altered miRNA
expression correlates with various human diseases, particularly
numerous types of cancer. The behavior of miRNAs is complex as they
regulate hundreds of targets, resulting in the downregulation of
numerous target genes, including oncogenes and tumor suppressors.
Therefore, exploring their clinical potential is particularly
promising for the identification of novel diagnosis and treatment
methods for patients with cancer.
In mammalian cells, following transcription by RNA
polymerase II, primary miRNA (pri-miRNA) is processed by Drosha and
converted into an ~70 nt hairpin precursor miRNA (pre-miRNA).
Through interactions with exportin-5 and Ras-related nuclear
protein-guanosine triphosphate, pre-miRNA is transported into the
cytoplasm, where it is further processed by RNA polymerase III and
Dicer prior to finally being turned into mature miRNA of ~22 nt
(18). Increasing evidence
indicated that nucleotide variation in miRNA sequence can alter
miRNA expression and/or maturation and therefore be involved in the
occurrence of diseases (19,20).
In the present study, three tumor-associated miRNA coding regions
were scanned in Chinese-Han OS patients in order to investigate the
genetic predisposition of OS. It was found that a G>A variation
in the pre-miR-34a coding region was associated with higher
morbidity in patients with OS. The expression of mature miR-34a in
cells transfected with pre-miR-34a expression vectors of different
genotypes was detected using qPCR. It was found that the G>A
variation reduced miR-34a expression in vitro, which
correlated with the data from tumor tissue and patient serum
samples. The function of an miRNA is mainly dependent on which
genes are suppressed by this miRNA. Therefore, in order to
investigate the biological function of G>A variation, a
dual-luciferase assay and western blot were used to detect the site
variation effect on c-Met expression, a target gene of miR-34a. As
hypothesized, G>A variation weakened the suppression of c-Met in
the two OS cell lines. However, the serum c-Met concentration in
patients of different genotypes was detected and no significant
differences were found. This may be due to the fact that miRNA is
only one of the numerous factors that contribute to the regulation
of gene expression.
In conclusion, the present study established a
correlation between miR-34a and the risk of OS in a Chinese Han
population by identifying one functional single nucleotide
polymorphism site in pre-miR-34a. These findings may give insight
into the mechanisms of OS development and create an opportunity to
approach the diagnosis and treatment of OS.
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
2
|
Klein MJ and Siegal GP: Osteosarcoma:
anatomic and histologic variants. Am J Clin Pathol. 125:555–581.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guise TA, O’Keefe R, Randall RL and Terek
RM: Molecular biology and therapeutics in musculoskeletal oncology.
J Bone Joint Surg Am. 91:724–732. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bang YJ: Advances in the management of
HER2-positive advanced gastric and gastroesophageal junction
cancer. J Clin Gastroenterol. 46:637–648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nicoloso MS, Spizzo R, Shimizu M, Rossi S
and Calin GA: MicroRNAs - the micro steering wheel of tumour
metastases. Nat Rev Cancer. 9:293–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu JQ, Liu P, Si MJ and Ding XY:
MicroRNA-126 inhibits osteosarcoma cells proliferation by targeting
Sirt1. Tumour Biol. 34:3871–3877. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tao T, Wang Y, Luo H, et al: Involvement
of FOS-mediated miR-181b/miR-21 signalling in the progression of
malignant gliomas. Eur J Cancer. 49:3055–3063. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu H, Zhang Y, Cai XH, Huang JF and Cai L:
Changes in microRNA expression in the MG-63 osteosarcoma cell line
compared with osteoblasts. Oncol Lett. 4:1037–1042. 2012.PubMed/NCBI
|
|
9
|
Nugent M: MicroRNA function and
dysregulation in bone tumors: the evidence to date. Cancer
management and research. 6:15–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ziyan W, Shuhua Y, Xiufang W and Xiaoyun
L: MicroRNA-21 is involved in osteosarcoma cell invasion and
migration. Medical oncology. 28:1469–1474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Labbaye C and Testa U: The emerging role
of MIR-146A in the control of hematopoiesis, immune function and
cancer. Journal of hematology & oncology. 5:132012.PubMed/NCBI
|
|
12
|
Bommer GT, Gerin I, Feng Y, et al:
p53-mediated activation of miRNA34 candidate tumor-suppressor
genes. Curr Biol. 17:1298–1307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Guessous F, Zhang Y, et al:
MicroRNA-34a inhibits glioblastoma growth by targeting multiple
oncogenes. Cancer Res. 69:7569–7576. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan D, Zhou X, Chen X, et al: MicroRNA-34a
inhibits uveal melanoma cell proliferation and migration through
downregulation of c-Met. Invest Ophthalmol Vis Sci. 50:1559–1565.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan K, Gao J, Yang T, et al: MicroRNA-34a
inhibits the proliferation and metastasis of osteosarcoma cells
both in vitro and in vivo. PloS one. 7:e337782012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dang Y, Luo D, Rong M and Chen G:
Underexpression of miR-34a in hepatocellular carcinoma and its
contribution towards enhancement of proliferating inhibitory
effects of agents targeting c-MET. PloS one. 8:e610542013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanaka N, Toyooka S, Soh J, et al:
Downregulation of microRNA-34 induces cell proliferation and
invasion of human mesothelial cells. Oncology reports.
29:2169–2174. 2013.PubMed/NCBI
|
|
18
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jazdzewski K, Murray EL, Franssila K,
Jarzab B, Schoenberg DR and de la Chapelle A: Common SNP in
pre-miR-146a decreases mature miR expression and predisposes to
papillary thyroid carcinoma. Proc Natl Acad Sci USA. 105:7269–7274.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saunders MA, Liang H and Li WH: Human
polymorphism at microRNAs and microRNA target sites. Proc Natl Acad
Sci USA. 104:3300–3305. 2007. View Article : Google Scholar : PubMed/NCBI
|