Introduction
Retinopathy of prematurity (ROP) is a
vasoproliferative retinal disease that affects premature babies and
is a major cause of blindness in children (1). ROP has two discrete phases: The first
is a phase of delayed vascular growth and the second phase is one
of retinal neovascularization (RNV) (1,2). RNV
is one of the main contributors to the pathogenesis of ROP
(3). Additionally, low birth
weight and prematurity are strongly associated with the risk of the
disease (2). Previous studies have
also revealed that use of recombinant human erythropoietin (rhEPO)
is a high risk factor for development of ROP (4–6).
Erythropoietin (EPO) is a glycoprotein that
stimulates production of red blood cells, and rhEPO has been used
for the treatment of neonatal anemia (7). In addition, rhEPO possesses
angiogenic properties, and has been reported to be associated with
a high risk of developing ROP (4).
In an oxygen-induced retinopathy (OIR) model, suppression of EPO by
small interfering RNA inhibited RNV in mice (8), suggesting that EPO promotes
neovascularization in OIR. However, the mechanisms by which EPO
induces neovascularization remain undetermined. It has been
reported that EPO may stimulate postnatal neovascularization in
ischemic brain tissues, thereby enhancing blood supply and reducing
hypoxia in the area of infarction (9). Therefore, EPO may increase the
susceptibility to ROP by increasing RNV.
In the present study, the molecular pathways in ROP
were investigated using a mouse model of OIR. The purpose of this
study was to examine the effect of exogenous administration of
rhEPO on RNV, and to explore possible mechanisms underlying
rhEPO-induced neovascularization.
Materials and methods
Animal model
All experimental protocols were approved by the
Institutional Animal Care and Use Committee of Wuhan University
(Wuhan, China). Neonate mice (C57BL/6) were obtained from the
Animal Center of Wuhan University. All animals (n=132) were housed
at room temperature (23±2°C). The animals were assigned to six
groups. For the control group, animals were housed with room air.
For groups 2–6, the OIR groups (OIR, OIR + vehicle control, and OIR
+ rhEPO 10, 50, and 100 IU), litters of postnatal day 7 (P7) pups
were housed with their nursing dams in a sealed oxygen chamber,
which was controlled to maintain the oxygen concentration at 75±2%,
with a flow rate of 1 l/min. The oxygen chamber was opened every
two days for cleaning and changing of food and water, and every 6 h
for substitution of nursing dams. On postnatal day 12 (P12), the
animals were returned to room air until postnatal day 17 (P17).
Animals in group 2 were not treated with intraperitoneal (IP)
injection of saline or rhEPO. Animals in groups 3–6 received an IP
injection of 0.1 ml saline (group 3) or rhEPO at doses of 10, 50
and 100 IU (group 4, 5 and 6, respectively), which were
administered daily from P7 to P12. On P17, the animals were
sacrificed by intravenous injection of pentobarbital (100 mg/kg)
for the following experiments.
Fluorescein retinal angiography
assessment of new vessel formation
On P17, four mice from each group were sacrificed,
and the eyes were enucleated and fixed in 4% paraformaldehyde for 1
h at room temperature. Retinas were isolated and stained overnight
at 23°C with Isolectin B4-594 Alexa Fluor 594 Conjugate
(I21413; Molecular Probes, Eugene, OR, USA; 1:200 dilution) in 1 mm
CaCl2 in phosphate-buffered saline. Retinas were then
divided into four equal-sized quadrants and whole mounted. Images
of each of the four quadrants of the whole mounted retinas were
captured at ×4 magnification using a fluorescence microscope
(MP5.0-RTV-CLR-10-C; QImaging, Surrey, BC, Canada). These images
were imported and merged together to produce an image of the entire
retina for further analysis, using Photoshop CS5 software (Adobe
Systems, San Jose, CA, USA). Neovascular tuft formation was
measured by comparing the number of pixels in the affected areas
with the total number of pixels in the retina. Retinal
neovascularization was quantified by measuring the ratio of the
neovascular tuft area to the total retinal area. RNV was measured
by a researcher blind to the experimental condition.
Hematoxylin-eosin (HE) staining
A total of 6 mice from each group were selected for
HE staining. The eyeball was removed, fixed, dehydrated, and
embedded in paraffin. Serial sections (3 μm) of eyes were cut
sagittally at 30 μm intervals, and five sections were selected from
each eye. The sections were then dried, deparaffinized, rehydrated,
and stained with HE. Subsequently the sections were, in turn,
dehydrated, cleared, mounted, and viewed under a microscope. The
number of endothelial nuclei of newly formed blood vessels that
penetrated the inner limiting membrane (ILM) was counted.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was isolated from retinal tissues (n=6
from each group) using TRIzol (Invitrogen, Carlsbad, CA, USA),
according to the manufacturer’s instructions. RNA was reverse
transcribed into complementary DNA using a First Strand cDNA
Synthesis kit (Tiangen Biotech Co., Ltd., Beijing, China). qPCR was
performed in a reaction mixture containing 4 μl complementary DNA,
0.4 μl of each primer (100 μM), 10 μl SYBR Green/Fluorescein
(Thermo Fisher Scientific, Pittsburgh, PA, USA), and 5.2 μl
double-distilled H2O. Primers used for amplification of
endothelial nitric oxide synthase (eNOS), neuronal nitric oxide
synthase (nNOS), and vascular endothelial growth factor (VEGF) are
presented in Table I. β-actin was
used as a housekeeping gene. The reaction conditions were as
follows: 50°C for 2 min, 95°C for 10 min, and then 40 cycles of
95°C for 30 sec and 60°C for 30 sec. Melting curve analyses were
performed to verify the amplification specificity. The gene
expression ΔCt values of messenger RNA (mRNA) of each sample were
calculated by normalizing with β-actin as an internal control. The
relative expression levels of eNOS, nNOS and VEGF were calculated
using the 2−ΔΔCT method.
 | Table IPrimer sequences and annealing
temperatures of genes. |
Table I
Primer sequences and annealing
temperatures of genes.
| Primer | Sequence (5′-3′) | Annealing temperature
(°C) | Length (bp) |
|---|
| Mouse β-actin | F:
GTCCCTCACCCTCCCAAAAG
R: GCTGCCTCAACACCTCAACCC | 60 | 265 |
| Mouse nNOS | F:
CCTGTGTTCCACCAGGAGAT
R: GTCCCTGGCTAGTGCTTCAG | 60 | 249 |
| Mouse eNOS | F:
AGCATACCCCCACTTCTGTG
R: GAAGATATCTCGGGCAGCAG | 60 | 208 |
| Mouse VEGF | F:
CAGGCTGCTGTAACGATGAA
R: GCCTTGGCTTGTCACATTTT | 60 | 189 |
Western blot analysis
Retinal tissues (n=6 from each group) were
homogenized with cold lysis buffer (on ice). Proteins were isolated
by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and
transferred onto polyvinylidene fluoride membranes by
electroblotting. Membranes were incubated with the following
primary antibodies: Polyclonal rabbit anti-mouse eNOS (dilution
1:300; Beijing Bioss Biotechnology Co., Ltd., Beijing, China),
polyclonal rabbit anti-mouse nNOS (dilution 1:300; Beijing
Biosynthesis Biotechnology Co. Ltd., Beijing, China) and monoclonal
rabbit anti-mouse VEGF (dilution 1:500; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA). The membranes were incubated at 4°C
overnight. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was
used as loading control. Membranes were then incubated with
horseradish peroxidase-linked goat anti-rabbit secondary antibodies
(dilution 1:5,000; Wuhan Boster Biological Technology, Ltd., Wuhan,
China) at room temperature for 2 h. Bands were visualized using a
chemiluminescence detection system (Wuhan Boster Biological
Technology, Ltd.), and analyzed using BandScan 5.0 software (Glyko,
Novato, CA, USA).
Statistical analyses
Data analyses were performed using SPSS statistical
software, version 13.0 J (SPSS, Inc., Chicago, IL, USA). All values
are presented as the mean ± standard deviation. Analysis of
variance was used to compare differences among the six groups.
Fisher’s least significant difference t-test was used to compare
differences between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
rhEPO increases retinal
neovascularization
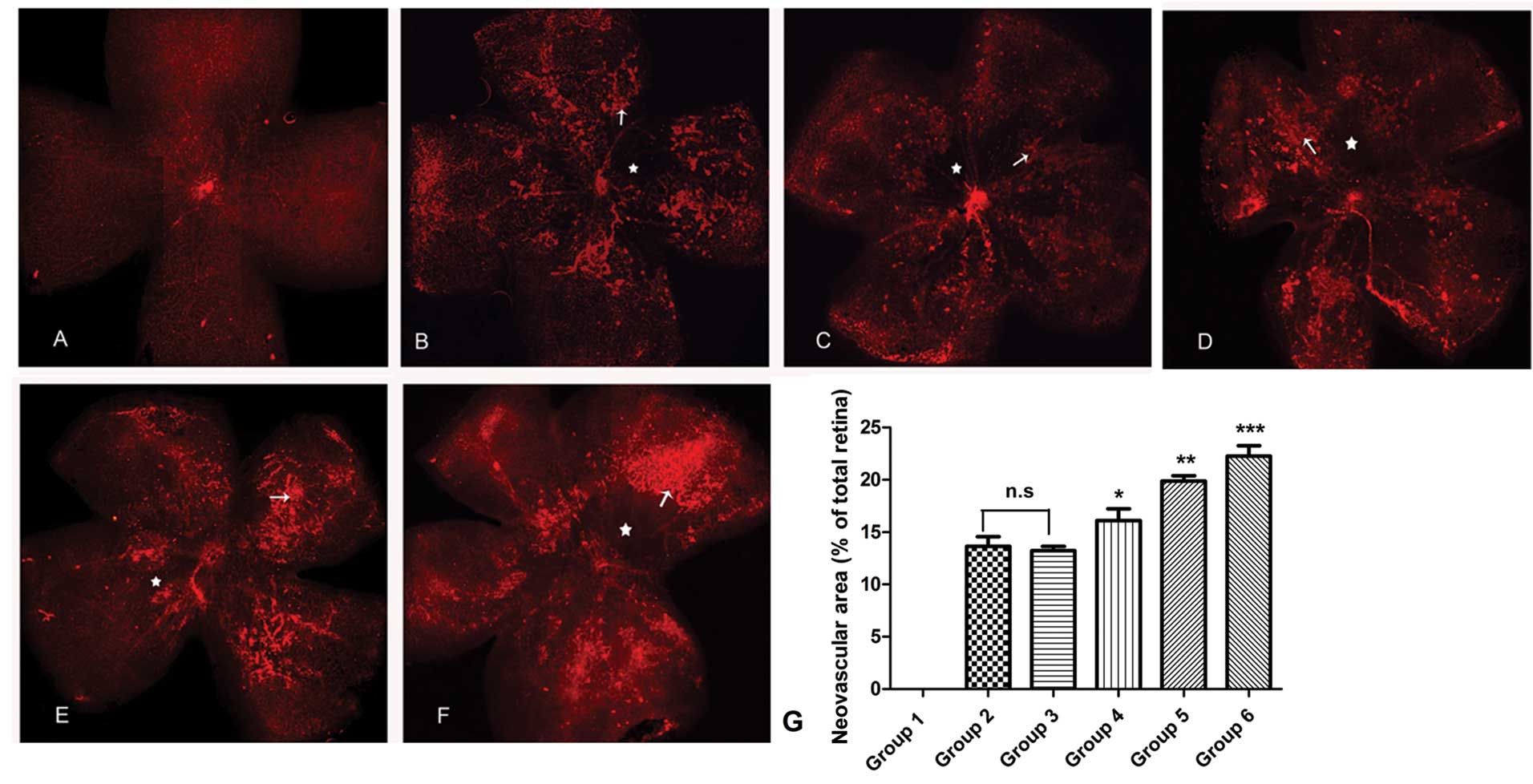
In retinas from control mice, the vessels extended
from the optic disc to the periphery, forming a polygonal reticular
pattern. Neovascularization and avascular zones were not observed
(Fig. 1A). In the retinas from
mice in groups 2–6 (OIR groups), dilated and twisted vessels were
observed and certain vessels in the vascular networks were
occluded. The normal polygonal reticular pattern and the radial
branching pattern were lost in a number of retinal areas and large
avascular zones were identified towards the center.
Neovascularization was observed between the vascular and avascular
zones (Fig. 1B–F). Retinal
neovascular areas were measured and compared among groups 1–6. No
significant difference in the neovascular area between groups 2 and
3 was identified (P>0.05). Compared with that of group 3, rhEPO
treatment significantly increased the neovascular areas in groups
4–6 (P<0.05). rhEPO dose-dependently increased the neovascular
areas in group 5 versus group 4 (P<0.05) and in group 6 versus
group 5 (P<0.05) (Fig. 1G).
Quantification of proliferative
retinopathy
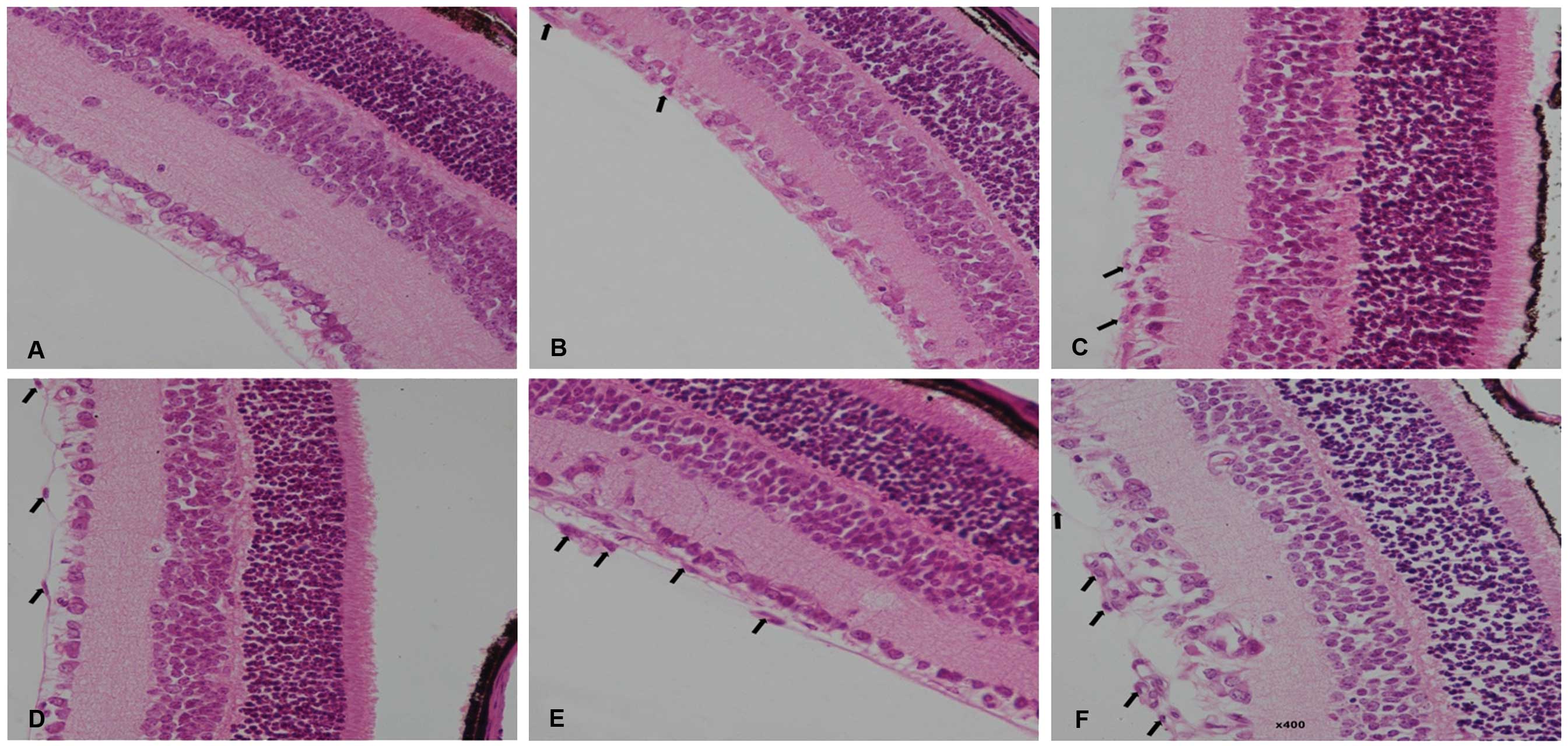
In paraffin sections of retinas from control mice,
no vascular endothelial cells were observed in the vitreous
chamber, which is adjacent to the retinal ILM (Fig. 2A). In retinal slices from mice in
groups 2–6, cellular nuclei of the vascular endothelium were
observed breaking through the retinal ILM (Fig. 2B–F). The degree of
neovascularization was quantified by counting the number of
endothelial cell nuclei (ECN) which had broken through to the
vitreous side of the ILM. In control mice, there was an average of
0.40±0.70 nuclei extending into the vitreous chamber, which was
significantly lower compared with those of groups 2 (35.37±2.78)
(P<0.001) and 3 (38.10±1.90) (P<0.001). No significant
difference regarding the number of ECN was identified between
groups 2 and 3 (P=0.204). Compared with that of group 3, rhEPO
treatment increased the number of ECN extending into the vitreous
chamber to 42.23±2.15 in group 4 (P=0.065), 47.56±3.16 in group 5
(P=0.001), and 55.82±3.27 in group 6 (P<0.001). In summary,
rhEPO dose-dependently increased the number of ECN extending into
the vitreous chamber and there were significant differences in the
number of ECN extending into the vitreous chamber among groups 4–6
(P=0.004).
rhEPO increases the mRNA and protein
expression of eNOS, nNOS and VEGF in OIR mice
The effects of rhEPO on the mRNA and protein
expression levels of eNOS, nNOS and VEGF in OIR mice were examined
using qPCR and western blot analysis. qPCR showed that compared
with those of group 1, the mRNA levels of nNOS and VEGF were
significantly increased in group 2 (P=0.042 and P=0.006,
respectively), but the mRNA levels of eNOS were not significantly
increased (P=0.124). No significant differences in mRNA expression
levels of eNOS, nNOS and VEGF were identified between groups 2 and
3 (P=0.906, P=0.840 and P=0.847, respectively). Compared with those
of group 3, mRNA expression levels of eNOS, nNOS and VEGF were
increased in groups 5 and 6 (P=0.010, and P=0.000 for eNOS, P=0.000
and P=0.000 for nNOS and P=0.000 and P=0.000 for VEGF,
respectively). The upregulation of eNOS, nNOS and VEGF expression
levels by rhEPO was dose-dependent (Fig. 3).
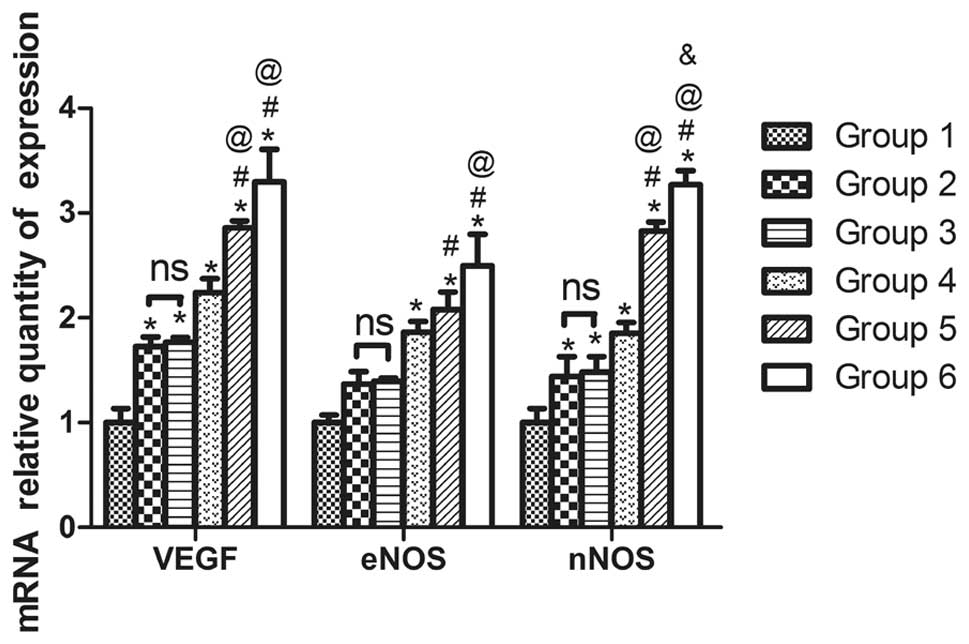 | Figure 3Recombinant human erythropoietin
(rhEPO) promotes mRNA expression of endothelial nitric oxide
synthase (eNOS), neuronal nitric oxide synthase (nNOS), and
vascular endothelial growth factor (VEGF) in an oxygen-induced
retinopathy (OIR) mouse model. Quantitative polymerase chain
reaction results show the relative expression levels of eNOS, nNOS,
and VEGF in retinas from P17 mice in control (group 1), OIR (group
2), OIR + vehicle control (group 3), OIR + rhEPO 10 IU (group 4),
OIR + rhEPO 50 IU (group 5), and OIR + rhEPO 100 IU (group 6)
groups. (n=6; *P<0.05 vs. group 1,
#P<0.05 vs. group 3, @P<0.05 vs. group
4, &P<0.05 vs. group 5). |
Consistent with the mRNA levels, protein expression
levels of eNOS, nNOS, and VEGF were also significantly upregulated
in the OIR + rhEPO groups (groups 4–6) compared with those in the
control group (group 1) (P=0.023, P=0.000 and P=0.000 for eNOS,
P=0.012, P=0.002 and P=0.000 for nNOS and P=0.000, P=0.000 and
P=0.000 for VEGF, respectively). Additionally, protein expression
levels of eNOS and VEGF were significantly upregulated in groups
4–6 compared with those in group 3 (P=0.003, P=0.000 and P=0.000
for eNOS and P=0.012, P=0.000 and P=0.000 for VEGF). Expression
levels of nNOS were increased in group 6 compared with those in
group 3 (P=0.014). rhEPO dose-dependently increased protein
expression of eNOS, nNOS and VEGF (Fig. 4).
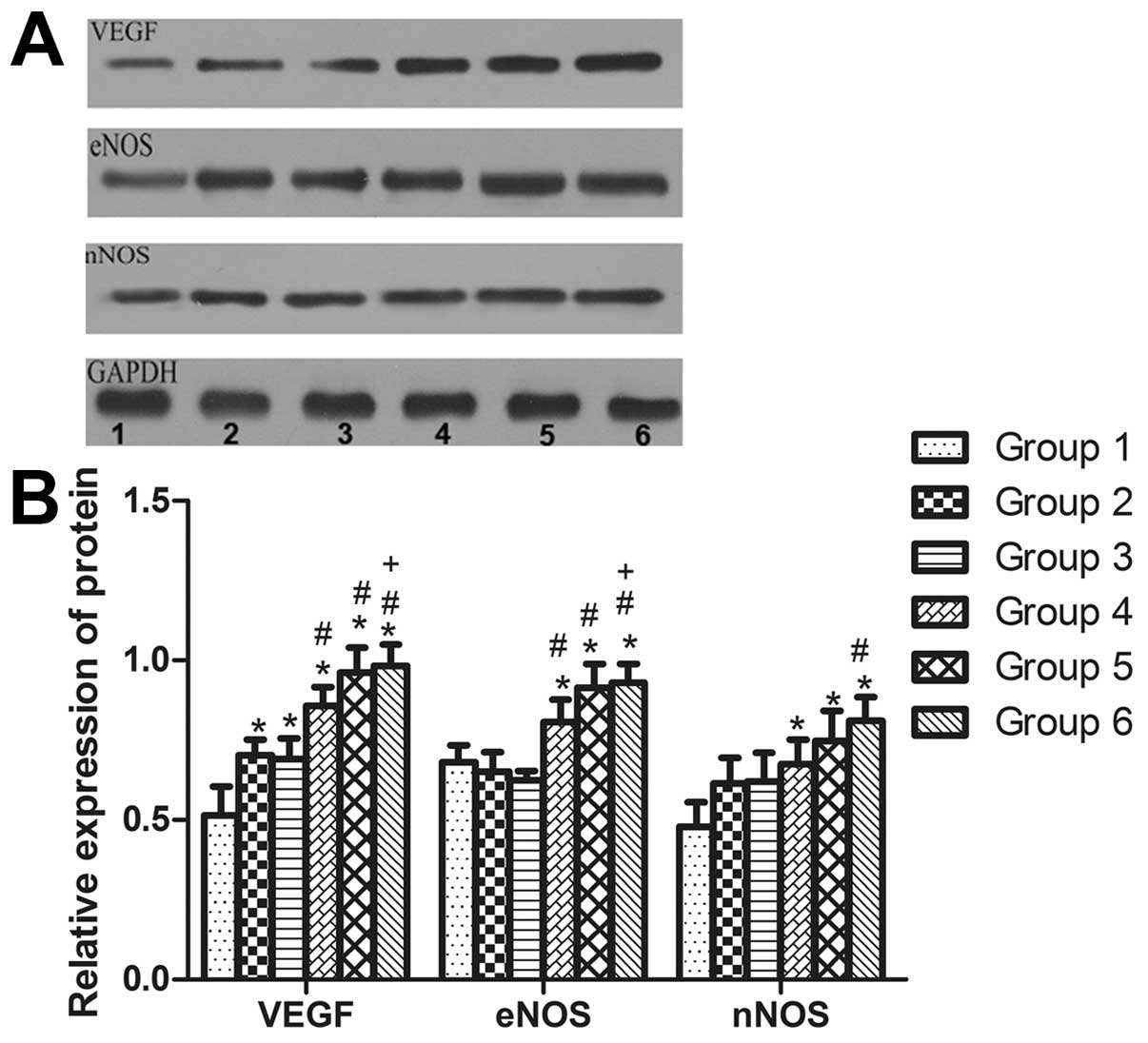 | Figure 4Recombinant human erythropoietin
(rhEPO) promotes protein expression of endothelial nitric oxide
synthase (eNOS), neuronal nitric oxide synthase (nNOS), and
vascular endothelial growth factor (VEGF) in an oxygen-induced
retinopathy (OIR) mouse model. (A) Representative western blots
showing the expression of eNOS, nNOS, and VEGF in retinas from P17
mice. Lane 1: Control (group 1), lane 2: OIR (group 2), lane 3: OIR
+ vehicle control (group 3), lane 4: OIR + rhEPO 10 IU (group 4),
lane 5: OIR + rhEPO 50 IU (group 5), and lane 6: OIR + rhEPO 100 IU
(group 6). (B) Quantitative western blot analysis of the relative
expression of eNOS, nNOS, and VEGF of retinas from P17 mice.
Glyceraldehyde 3-phosphate dehydrogenase was used as the loading
control. (n=6; *P<0.05 vs. group 1,
#P<0.05 vs. Group 3, +P<0.05 vs. group
4). |
Discussion
rhEPO has previously been used in the treatment of
anemia, and has subsequently been identified as an effective
treatment for neonatal anemia and anemia caused by cancer treatment
(7,10–12).
Numerous clinical and preclinical studies have shown that use of
rhEPO is associated with a high risk of developing ROP (4–6,8),
although one small clinical study has reported that such use is not
associated with the incidence and severity of ROP (13).
Certain large scale clinical trials have
demonstrated the association of rhEPO with a high risk of
developing ROP (6,14). Figueras-Aloy et al (6) reported that EPO plus iron
administration was associated with a high risk of grade 1 ROP.
Brown et al (14) reported
that rhEPO dose-dependently increased the risk of ROP, and that all
patients who underwent laser coagulation were treated with rhEPO.
However, the mechanisms by which EPO increases the risk of ROP are
not well established. In the present study, it was determined that
rhEPO treatment promoted RNV in a mouse model of OIR, which was
accompanied by an increase in the expression levels of eNOS, nNOS
and VEGF. In this respect, the present study suggests that eNOS,
nNOS and VEGF may mediate neovascularization induced by rhEPO in
ROP.
The development of ROP includes two phases: A first
phase of oxygen-induced vessel attenuation and a second phase of
hypoxia-induced vasoproliferation (1,2). The
initial period of retinal vascular attenuation occurs when an
infant undergoes oxygen therapy, and subsequent RNV is promoted by
exposure of the infant to room air (15). The newly formed blood vessels are
fragile and rupture easily, leading to severe complications such as
intravitreous hemorrhage and retinal detachment. In the present
study, an ROP mouse model was used, in which P7 mice were first
raised in a medium with a high oxygen concentration to mimic the
first stage of ROP, and then subsequently returned to room air on
P12 to mimic the second stage of ROP. This model has been
demonstrated to be a good animal model of human ROP (16).
Once the mouse model of ROP was established,
immunofluorescent microscopy was used to investigate the morphology
of retinal vascular networks in whole-mounted retinas. It was
determined that abnormal neovascularization occurred in the OIR
group, which was accompanied by dilated, twisted and occluded blood
vessels. rhEPO treatment resulted in an increase in the number of
newly formed and severely dilated and occluded vessels. In
addition, the degree of neovascularization was quantified by
measuring the ratio of the neovascular tuft area to the total
retinal area and counting the number of ECN on the vitreous side of
the ILM. In normal conditions, there are no neovascularized or
avascular zones in flat-mounted retinas, and blood vessels in the
vitreous chamber and retinal vessels do not extend out into the
ILM. Therefore, neovascular areas and blood vessels that extend
into the vitreous chamber across the ILM are regarded as abnormal
neovascularization. Counting the number of ECN on the vitreous side
of the ILM has been used as the gold standard for studying RNV. In
the present study, it was revealed that the number of ECN extending
into vitreous chamber was significantly greater in the OIR group
compared with that in the control group, suggesting that the OIR
model successfully induced RNV. Treatment with rhEPO increased the
number of ECN extending into the vitreous chamber in a
dose-dependent manner, further suggesting that EPO promotes
RNV.
VEGF is one of the most potent proangiogenic factors
and it induces endothelial cell proliferation and promotes
angiogenesis (17). VEGF induces
angiogenesis through direct stimulation of VEGF receptors on
vascular cells, leading to increased permeability and
neovascularization in vivo. Sato et al (18) reported that the elevated level of
EPO in eyes with stage 4 ROP was correlated with the level of VEGF,
suggesting that EPO and VEGF contribute to the development of ROP.
Furthermore, VEGF has been shown to mediate angiogenesis induced by
erythropoiesis-stimulating agents (19). The present study revealed that
rhEPO treatment dose-dependently increased RNV in an OIR mouse
model, which was accompanied by an increase in levels of VEGF
expression. The rhEPO-induced upregulation of VEGF was
dose-dependent, suggesting that rhEPO may promote VEGF expression,
thus contributing to the pathogenesis of RNV.
NOSs, including constructive and inducible NOS, are
a family of enzymes that catalyze the production of nitric oxide
from L-arginine. Nitric oxide is an important cellular signaling
molecule that is involved in angiogenesis (20). VEGF stimulates the release of
nitric oxide from cultured human umbilical venous endothelial cells
and upregulates the expression of NOS.
Rezaeian et al (21) reported that EPO promoted
angiogenesis and prevented musculocutaneous tissues from ischemic
damage by upregulating the expression of eNOS and nNOS.
Concurrently, the present study revealed that rhEPO treatment
promoted RNV and induced significantly higher levels of expression
of eNOS and nNOS in an OIR mouse model. Upregulation of eNOS and
nNOS induced by rhEPO was dose-dependent, suggesting that rhEPO may
contribute to the development of ROP by inducing the expression of
eNOS and nNOS.
In conclusion, this study demonstrated that rhEPO
treatment promoted RNV in an OIR mouse model, suggesting that EPO
contributes to the pathogenesis of RNV. It was also revealed that
rhEPO treatment results in an increase in the mRNA and protein
expression levels of VEGF, eNOS and nNOS, suggesting that rhEPO
regulates RNV via upregulation of VEGF, eNOS and nNOS. Thus, this
study provides a theoretic basis for prevention of rhEPO-induced
RNV in a clinical setting.
References
|
1
|
Smith LE: Pathogenesis of retinopathy of
prematurity. Growth Horm IGF Res. 14(Suppl A): S140–S144. 2004.
View Article : Google Scholar
|
|
2
|
Hartnett ME and Penn JS: Mechanisms and
management of retinopathy of prematurity. N Engl J Med.
368:1162–1163. 2013.PubMed/NCBI
|
|
3
|
Shrestha JB, Bajimaya S, Sharma A,
Shresthal J and Karmacharya P: Incidence of retinopathy of
prematurity in a neonatal intensive care unit in Nepal. J Pediatr
Ophthalmol Strabismus. 47:297–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Romagnoli C, Tesfagabir MG, Giannantonio C
and Papacci P: Erythropoietin and retinopathy of prematurity. Early
Hum Dev. 87(Suppl 1): S39–S42. 2011. View Article : Google Scholar
|
|
5
|
Suk KK, Dunbar JA, Liu A, et al: Human
recombinant erythropoietin and the incidence of retinopathy of
prematurity: a multiple regression model. J AAPOS. 12:233–238.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Figueras-Aloy J, Alvarez-Domínguez E,
Morales-Ballus M, Salvia-Roiges MD and Moretones-Suñol G: Early
administration of erythropoietin in the extreme premature, a risk
factor for retinopathy of prematurity? An Pediatr (Barc).
73:327–333. 2010.(In Spanish).
|
|
7
|
Shannon K: Recombinant human
erythropoietin in neonatal anemia. Clin Perinatol. 22:627–640.
1995.PubMed/NCBI
|
|
8
|
Xiong SQ, Xia XB, Xu HZ and Jiang J:
Suppression of retinal neovascularization by small-interference RNA
targeting erythropoietin. Ophthalmologica. 223:306–312. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heeschen C, Aicher A, Lehmann R, et al:
Erythropoietin is a potent physiologic stimulus for endothelial
progenitor cell mobilization. Blood. 102:1340–1346. 2003.
View Article : Google Scholar
|
|
10
|
Zuppa AA, Alighieri G, Fracchiolla A, et
al: Comparison between two treatment protocols with recombinant
human erythropoietin (rHuEpo) in the treatment of late anemia in
neonates with Rh-isoimmunization. Pediatr Med Chir. 34:186–191.
2012. View Article : Google Scholar
|
|
11
|
Thomaidis T, Weinmann A, Sprinzl M, et al:
Upper-GI-Group of AIO (Arbeitsgemeinschaft Internistische
Onkologie), Germany: Erythropoietin treatment in
chemotherapy-induced anemia in previously untreated advanced
esophagogastric cancer patients. Int J Clin Oncol. 19:288–296.
2013. View Article : Google Scholar
|
|
12
|
Fenner MH and Ganser A: Erythropoietin in
cancer-related anemia. Curr Opin Oncol. 20:685–689. 2008.
View Article : Google Scholar
|
|
13
|
Shah N, Jadav P, Jean-Baptiste D, et al:
The effect of recombinant human erythropoietin on the development
of retinopathy of prematurity. Am J Perinatol. 27:67–71. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brown MS, Barón AE, France EK and Hamman
RF: Association between higher cumulative doses of recombinant
erythropoietin and risk for retinopathy of prematurity. J AAPOS.
10:143–149. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Patz A: Current concepts of the effect of
oxygen on the developing retina. Curr Eye Res. 3:159–163. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith LE, Wesolowski E, McLellan A, et al:
Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci.
35:101–111. 1994.PubMed/NCBI
|
|
17
|
Neufeld G, Cohen T, Gengrinovitch S and
Poltorak Z: Vascular endothelial growth factor (VEGF) and its
receptors. FASEB J. 13:9–22. 1999.PubMed/NCBI
|
|
18
|
Sato T, Kusaka S, Shimojo H and Fujikado
T: Vitreous levels of erythropoietin and vascular endothelial
growth factor in eyes with retinopathy of prematurity.
Ophthalmology. 116:1599–1603. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McVicar CM, Colhoun LM, Abrahams JL, et
al: Differential modulation of angiogenesis by
erythropoiesis-stimulating agents in a mouse model of ischaemic
retinopathy. PLoS One. 5:e118702010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cooke JP and Losordo DW: Nitric oxide and
angiogenesis. Circulation. 105:2133–2135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rezaeian F, Wettstein R, Egger JF, et al:
Erythropoietin-induced upregulation of endothelial nitric oxide
synthase but not vascular endothelial growth factor prevents
musculocutaneous tissue from ischemic damage. Lab Invest. 90:40–51.
2010. View Article : Google Scholar
|