Introduction
Prostate cancer is the most commonly diagnosed
cancer and the second leading cause of cancer-associated mortality
in males, having remained unchanged for >20 years in the USA
(1). Androgen ablation therapy has
been shown to be effective at the initial stages of prostate
cancer; however, almost all patients progress to an
androgen-independent stage or hormone-refractory prostate cancer
(HRPC), which is unresponsive to hormone deprivation (2). Currently, the standard treatment of
patients with HRPC is with docetaxel, a paclitaxel (Pac; also known
as taxol) derivative-based chemotherapeutic (3). However, the efficiency of this
therapy is frequently impaired by drug resistance, a notable cause
of mortality in this type of cancer (4). Certain genes, including
octamer-binding transcription factor 4 (OCT4), have been
demonstrated to be of vital importance in the formation of
drug-resistant cells in prostate cancer (5,6).
Therefore, since it is difficult to find novel drugs for
chemotherapy, identifying the molecules involved in drug resistance
and applying targeted methods may improve the efficacy of prostate
cancer chemotherapy.
Sex determining region Y-box 2 (Sox2), a member of
the SOX family of transcription factors (7–9), is
critical in the self-renewal of embryonic stem cells (10,11),
maintenance of pluripotency, generation of induced stem cells
(12–14) and apoptosis (15–17).
Aberrant overexpression of Sox2 has been reported in neural
(16), respiratory (18,19),
reproductive (20,21) and digestive system tumors (17,22).
In gastric and colorectal cancer stem-like cells, Sox2 enhanced
tumorigenicity and chemoresistance (23,24).
It has been demonstrated that Sox2 promotes esophageal carcinoma
growth by regulating the phosphoinositide 3-kinase (PI3K)/Akt
signaling pathway, which is key in the cell survival process
(25). In addition, expression of
Sox2 has been shown to be significantly increased in prostate
cancer tissue compared with normal and hyperplastic tissues. As an
androgen receptor-repressed gene, Sox2 promotes the formation of
HRPC (26). Thus, targeted therapy
against Sox2 may improve the efficiency of chemotherapy in patients
with drug-resistant HRPC.
Pac and its derivatives are a wide class of
well-known microtubule stabilizers and have been utilized as
front-line chemotherapeutic agents for several types of cancer,
including prostate cancer (3).
However, these drugs frequently induce drug resistance. The
molecular mechanism of Pac resistance has not been clarified,
although a large amount of evidence has revealed that the PI3K/Akt
signaling pathway is key in the formation of drug resistance in
cancer via promotion of the expression of genes imperative for cell
survival, consequently providing protection against apoptosis
(27–30). In ovarian cancer cells, the
constitutively activated PI3K/Akt signaling pathway conferred
resistance to Pac, which was reversed by the PI3K/Akt inhibitor
LY294002 (31). As a tumor
suppressor gene, phosphatin and tensin homolog (PTEN) acts as a
negative regulator of the PI3K/Akt signaling pathway and its loss
of function is associated with the progression and aggressive
behavior of numerous types of cancer (32,33).
Regulation of the PI3K/Akt signaling pathway through PTEN has been
reported to overcome sunitinib resistance in prostate cancer cells
(34). For this reason, clarifying
the roles of certain genes in the PI3K/Akt signaling pathway may be
a rational way to approach drug-resistant cancer.
In the present study, the impact of Sox2 on the
effects of Pac treatment, which include induction of apoptosis and
inhibition of cell proliferation, were investigated in a prostate
cancer cell line. In addition, the underlying mechanism of the
PI3K/Akt signaling pathway was analyzed. Targeted therapy against
Sox2 administered as a co-treatment with Pac may be a promising
therapy in drug-resistant HRPC.
Materials and methods
Materials
Pac and LY294002 were supplied by Sigma (St. Louis,
MO, USA). Sox2 primary antibody (ab97959, rabbit, polyclonal,
1:500) was obtained from Abcam (Cambridge, MA, USA) and antibodies
against Akt (9272s, rabbit, polyclonal, 1:500) and phosphorylated
(p)-Akt (4058s, rabbit, monoclonal, 1:200) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Antibodies against
cyclin E (sc-198, rabbit, polyclonal, 1:1,000), were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Antibody to
α-tubulin (mouse, monoclonal, 1:1,000), and anti-rabbit (goat,
1:2,000) and anti-mouse (goat, 1:2,000) secondary antibodies were
obtained from Beyotime Institute of Biotechnology (Haimen, China).
The MTT Cell Viability Detection kit, lactate dehydrogenase (LDH)
Assay kit, Annexin V-fluorescein isothiocyanate (FITC) &
propidium iodide (PI) Double Staining Apoptosis Detection kit for
flow cytometry (FCM), Cell Mitochondria Isolation kit, Propidium
Iodide, Caspase-3/9 Activity Assay kit and JC-1 probe were
purchased from Beyotime Institute of Biotechnology.
Cell culture and transfection
PC-3 human prostate cancer cell lines were purchased
from American Type Culture Collection (Manassas, VA, USA). The
cells were maintained in RPMI-1640 medium supplemented with 10%
fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA) and
100 U/ml penicillin/streptomycin (Invitrogen Life Technologies,
Carlsbad, CA, USA), at 37°C in a humidified atmosphere (5%
CO2/95% air). The human Sox2-coding-sequence was cloned
into a pcDNA3.0 vector (Invitrogen Life Technologies) and termed as
pcDNA3.0 Sox2; transfection was performed using
Lipofectamine® 2000 (Invitrogen Life Technologies). A
total of 850 μg/ml G418 (Calbiochem, San Diego, CA, USA) was
applied to select the G418-resistant cells. The cells were treated
with Pac or dimethyl sulfoxide (DMSO) as a vehicle (Veh) for 48 h,
and in several cases, LY294002 was added 2 h prior to Pac
treatment.
Cell viability analysis
The empty vector-transfected (PC-3 Mock) and
pcDNA3.0 Sox2-transfected (PC-3 Sox2) cells (2×105
cells/ml in 96-well plates) were treated with DMSO (1:1,000) or 5
μM Pac for 48 h. Cell viability was measured using the MTT Cell
Viability Detection kit, following the manufacturer’s instructions.
Absorbance was read at 450 nm on a microplate spectrophotometer
(Spectra Max M3; Molecular Devices, Sunnyvale, CA, USA).
Clone formation assay
The PC-3 Mock and PC-3 Sox2 cells were seeded at 500
cells/well in six-well culture plates and treated with DMSO
(1:1,000) or 5 μM Pac for 48 h. Following 10 days of incubation,
the cell colonies were stained with crystal violet, counted and
images were captured by a digital camera (BioSpectrum 810 Imaging
System; UVP, Upland, CA, USA) (35). The ratio of clone formation was
calculated using the following equation: Rate of clone formation
(%) = (clone quantity/500) × 100. The relative clone formation
ratio was normalized to the PC-3 Mock DMSO group.
LDH measurement
Leakage of LDH into the cell culture medium
indicated cell membrane damage. The PC-3 Mock and PC-3 Sox2 cells
were exposed to DMSO (1:1,000) or 5 μM Pac for 48 h, then the
culture medium was centrifuged at 250 g for 10 min, and the
supernatant was transferred to a 96-well culture plate to determine
the quantity of LDH according to the manufacturer’s instructions.
The LDH activity was reported as the percentage relative to the
control level (36). Absorbance
was measured at 450 nm on the SpectraMax M3 microplate
spectrophotometer (Molecular Devices, LLC, Sunnyvale, CA, USA).
Cell cycle and apoptosis analysis
A total of 1×106 cells were seeded into a
60 mm dish 24 h before treatment, then Pac (5 μM)/Veh was added for
48 h (in several cases, LY294002 was added 2 h before Pac
treatment). For the cell cycle analysis, cells were harvested,
fixed with 70% ethanol and stored at 4°C overnight, then incubated
with RNase (25 μg/ml) at 37°C for 30 min, followed by staining with
PI (50 μg/ml) for 30 min in the dark. For the apoptosis analysis,
Annexin V-FITC and PI staining was performed according to the
manufacturer’s instructions (Beyotime Institute of Biotechnology).
The stained cells were counted using a FACSCalibur Flow Cytometer
(BD Biosciences, Franklin Lakes, NJ, USA). All data were analyzed
and visualized by FlowJo® software Ver. 7.6.1 for
Windows (TreeStar, Inc., Ashland, OR, USA).
Mitochondrial membrane potential
assay
The JC-1 probe was used to measure mitochondrial
depolarization in the cells. Briefly, the mitochondria were
separated from the cells following the indicated treatments using
the Cell Mitochondria Isolation kit, were then incubated with 1 ml
JC-1 staining-solution (5 μg/ml) for 20 min at 37°C and rinsed
twice with phosphate-buffered saline. The mitochondrial membrane
potentials were measured using the relative quantities of dual
emissions from mitochondrial JC-1 monomers or aggregates using the
Spectra Max M3 microplate spectrophotometer. The excitation
wavelength was set at 485 nm. Fluorescence intensity was detected
at 525 nm for monomers and 590 nm for aggregates. Mitochondrial
depolarization was indicated by an increase in the 525/590 nm
fluorescence intensity ratio.
Caspase-3 and -9 activity
measurement
Caspase activity was determined using the
Caspase-3/9 Activity Assay kit (Beyotime Institute of
Biotechnology) following the manufacturer’s instructions. The
present study used 96-well microplates for incubating 10 μl cell
lysate in 80 μl reaction buffer containing 10 μl caspase substrate
(2 mM). The lysates were incubated at 37°C for 4 h. Data were
collected using the SpectraMax M3 microplate reader at an
absorbance of 405 nm. Caspase activity was expressed as the ratio
of treated to vehicle control cells.
Transmission electron microscopy
observation
The cells were fixed according to previous methods
(37). The ultrastructure of the
cells was examined by transmission electron microscopy (Hitachi
H-600; Hitachi, Ltd., Tokyo, Japan).
Western blot analysis
Whole-cell lysate preparation and western blot
analysis were conducted as described previously (38).
Statistical analysis
Each experiment was repeated in triplicate.
Statistical analyses were performed using SPSS 20.0 software for
Windows (IBM, Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference and the results are
expressed as the mean ± the standard error of the mean.
Results
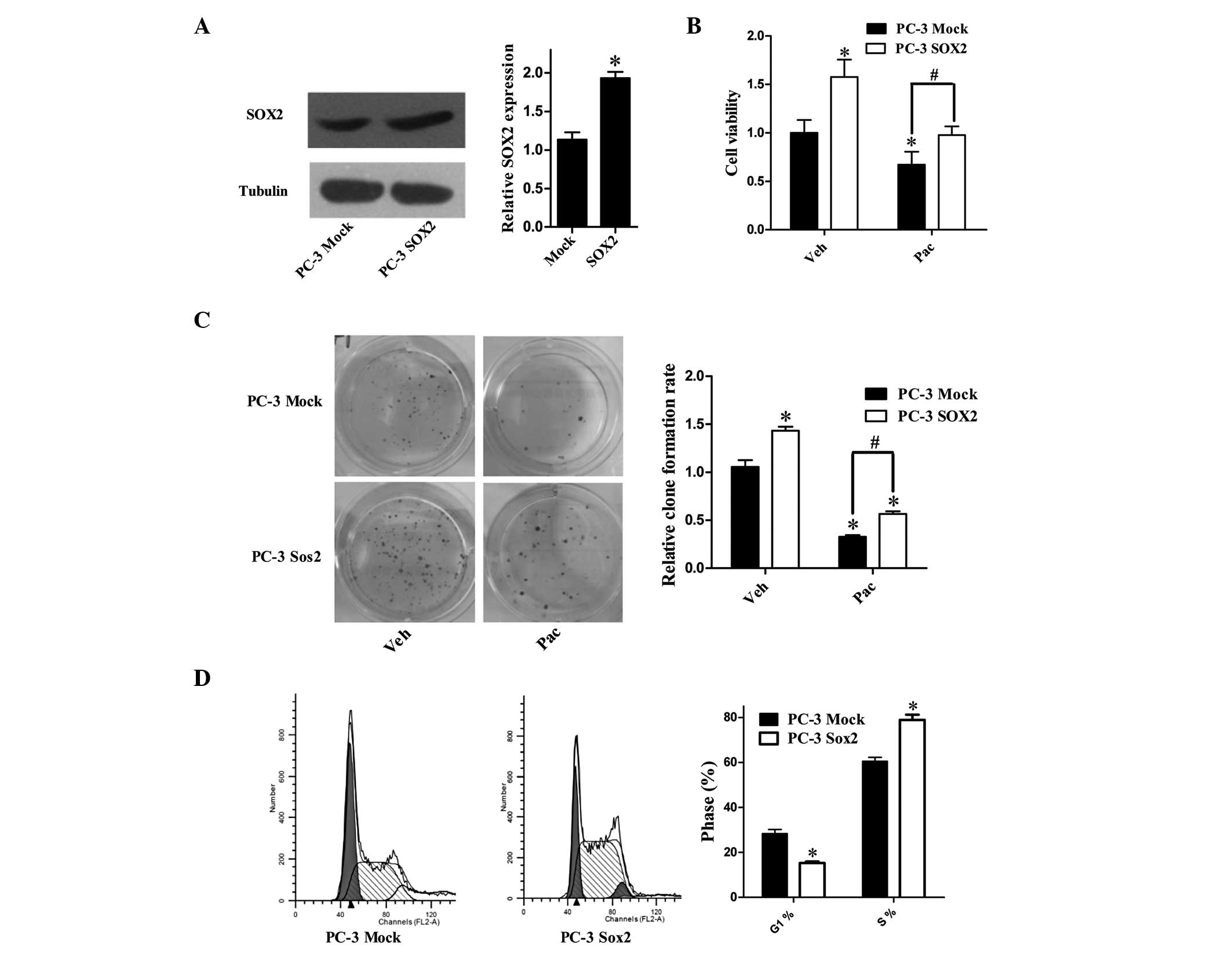
Overexpression of Sox2 promotes cell
proliferation and impairs the cell cycle arrest induced by Pac
To verify the effects of Sox2 on PC-3 cells, cells
were stably transfected with Sox2-expressing vector and termed PC-3
Sox2, in contrast to the empty vector PC-3 Mock cell line (Fig. 1A). To examine whether Sox2 impacted
the effect of Pac on cell proliferation, MTT and clone formation
assays were performed to measure the cell proliferation status
(Fig. 1B and C). The
vehicle-treated PC-3 Sox2 cells exhibited increased proliferation
as compared with the mock-transfected cells, and the decreases in
cell growth following Pac-treatment were significantly attenuated
in PC-3 Sox2 cells as compared with the mock-transfected group. An
FCM-based cell cycle assay was conducted to detect the cell cycle
distribution of PC-3 Mock and PC-3 Sox2 cells (Fig. 1D). The PC-3 Sox2 cells exhibited an
increased percentage of cells in S-phase as compared with the
mock-transfected cells. This implies that the resistance of
Sox2-overexpressing cells may be based on their upregulation of
proliferation and DNA synthesis. In conclusion, the data revealed
that in the Sox2-overexpressing cell line, the cell growth
inhibition caused by Pac was partly attenuated.
Sox2-expression leads to evasion of
apoptosis induced by Pac treatment
To examine the impact of Sox2 on Pac-induced
apoptosis, Annexin V/PI double staining and FCM analysis were used
to measure the apoptotic rate of the cells (Fig. 2A). In the Mock-transfected group,
the apoptotic rate was increased following incubation with Pac,
which was significantly attenuated in the Sox2-overexpressing cell
line. The activities of caspase-3 and caspase-9 increased following
48 h Pac treatment in the PC-3 cells (Fig. 2B), which was significantly
attenuated in the Sox2-overexpressing cells, while caspase-8
activity was not significantly induced (data not shown). JC-1
aggregated in normal mitochondria and exhibited red fluorescence.
Exposure of the cells to Pac for 48 h also resulted in dissipation
of the inner mitochondrial membrane potential, which was shown as
an increased green/red fluorescence ratio. By contrast to
mock-transfected cells, Sox2-overexpression completely inhibited
the depolarization of the mitochondrial membrane following Pac
treatment (Fig. 2C). These results
indicated that Sox2 prevented Pac-induced apoptosis. In addition,
photomicrographs of PC-3 cells exposed to Pac captured by
transmission electron microscopy revealed typical apoptotic
morphology with chromatin condensation and formation of apoptotic
bodies (Fig. 2D and E).
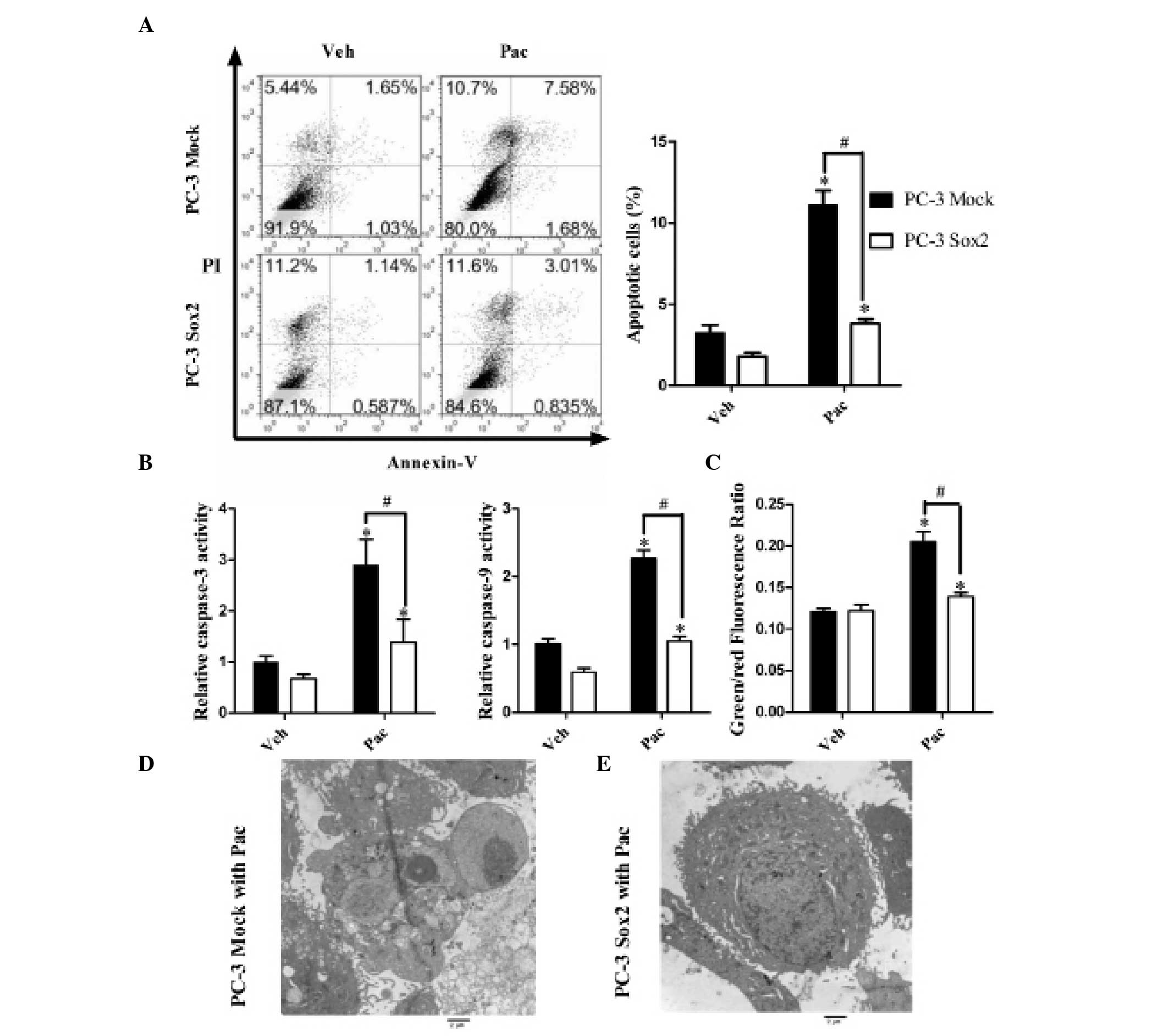 | Figure 2Sox2 inhibits the apoptotic effect
induced by Pac treatment. (A) Apoptotic cell ratio of empty vector
(PC-3 Mock)/Sox2 overexpression cells (PC-3 Sox2) under Pac or Veh
treatment, measured by flow cytometry-based Annexin V-fluorescein
isothiocyanate and PI double staining analysis. (B) Relative
activity of caspase-3, 9 in PC-3 Mock/PC-3 Sox2 cells treated with
Pac or Veh. (C) Mitochondrial membrane potential of PC-3 Mock and
PC-3 Sox2 cells under Pac or Veh treatment, measured by JC-1 probe.
An increase in the green/red ratio indicated depolarization of the
mitochondrial membrane. (D and E) Transmission electron microscopy
of the morphological changes of apoptosis induced by Pac. (D) PC-3
Mock cells treated with Pac (magnification, ×8,000). (E) PC-3 Sox2
cells treated with Pac (magnification, ×8,000). Results were
representative of five independent experiments. All data were
analyzed by one-way analysis of variance and are presented as the
mean ± standard error of the mean of three independent experiments.
*P<0.05 vs. PC-3 Mock with Veh; #P<0.05
vs. PC-3 Sox2 with Pac. PI, propidium iodide; Sox2, sex determining
region Y-box 2; Pac, paclitaxel; Veh, vehicle
(dimethylsulfoxide). |
An LDH assay was also performed to measure the
cytotoxic effect of Pac on cells; however, no significant
difference was observed between the PC-3 Mock (121.4±9.8%) and PC-3
Sox2 groups (119.7±8.6%).
The PI3K/Akt signaling pathway is
involved in the Sox2-mediated anti-apoptotic and cell
proliferation-promoting effect during Pac treatment
To investigate the underlying mechanism of Sox2 on
Pac-treated cells, several signaling pathways and apoptosis- and
proliferation-associated proteins were examined. p-Akt, cyclin E
and survivin expression levels were found to be upregulated in PC-3
Sox2 cells as compared with levels in mock-transfected cells
(Fig. 3), while B-cell lymphoma-2
and p21 expression levels remained unchanged (data not shown). As
cyclin E and survivin have been reported as Akt-regulated proteins,
a PI3K inhibitor, LY294002, was used to suppress the PI3K/Akt
signaling pathway activity of each group, respectively. Inhibition
of the PI3K/Akt signaling pathway reduced the expression levels of
p-Akt, cyclin E and survivin (Fig.
3), and partially attenuated the effects of Sox2 on preventing
apoptosis and promoting cell proliferation under Pac treatment
(Fig. 4).
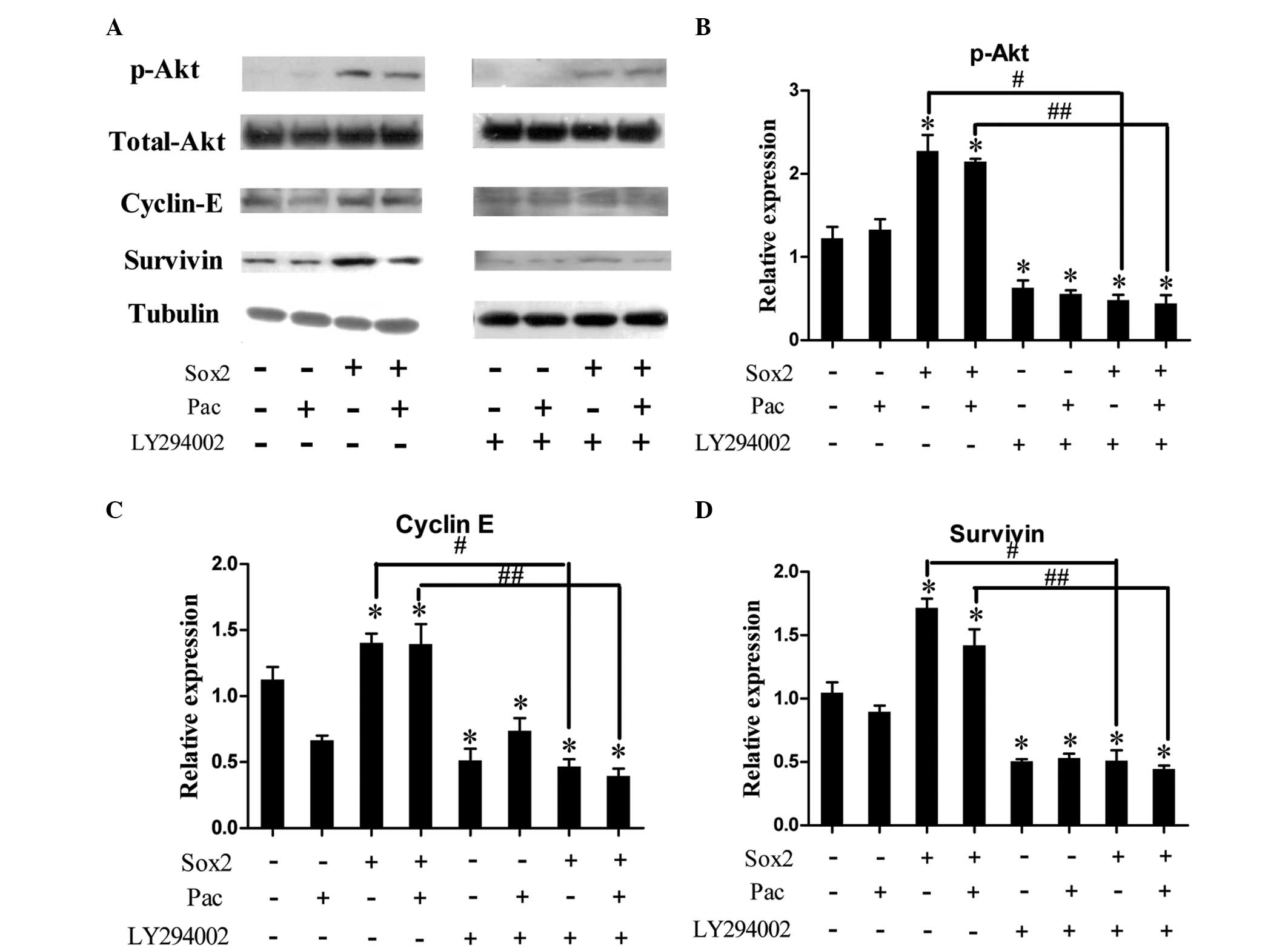 | Figure 3Expression levels of cell cycle- and
apoptosis-associated proteins in PC-3 prostate cancer cell lines
treated with Pac. (A) Expression levels of p-Akt, Akt, cyclin E and
survivin in empty vector (PC-3 Mock; Sox2, −) and Sox2
overexpression (PC-3 Sox2; Sox2, +) cells under Pac treatment (Pac,
+) or dimethyl sulfoxide (Pac, −), with or without pretreatment
with the phosphoinositide 3-kinase/Akt pathway inhibitor LY294002.
(B–D) Relative p-Akt, cyclin E and survivin expression levels in
the different treatment groups. Data were analyzed by one-way
analysis of variance and are presented as the mean ± standard error
of the mean of three independent experiments.
*P<0.05, vs. PC-3 Mock without Pac;
#P<0.05, vs. PC-3 Sox2 without Pac;
##P<0.05, vs. PC-3 Sox2 with Pac. Sox2, sex
determining region Y-box 2; Pac, paclitaxel; p, phosphorylated. |
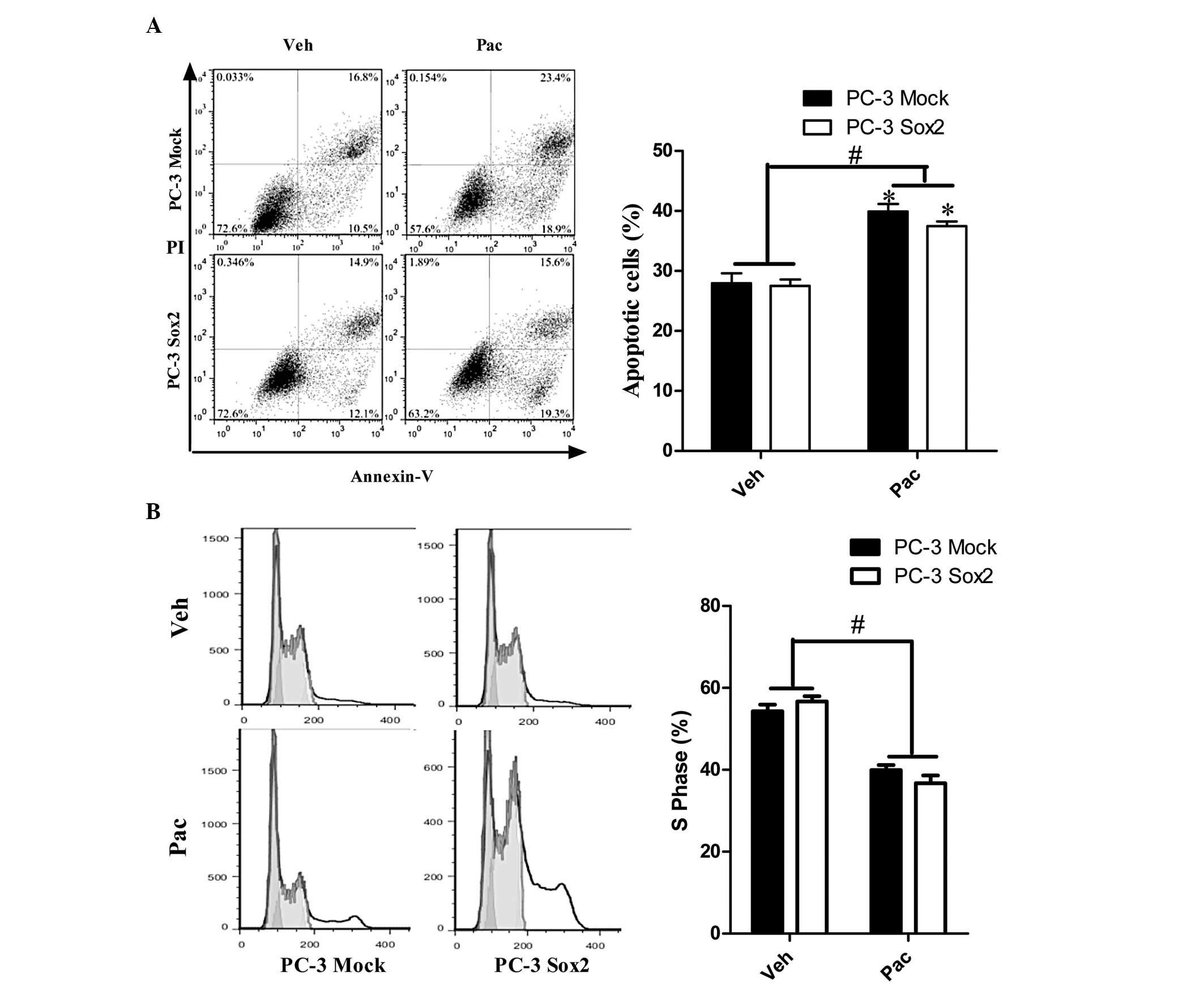 | Figure 4The PI3K/Akt pathway is involved in
the Sox2-mediated anti-apoptotic and cell proliferation-promoting
effects during paclitaxel treatment. (A) Apoptotic cell ratio of
empty vector (PC-3 Mock)/Sox2 over-expression (PC-3 Sox2) cells
under Pac and Veh treatment, with 2 h pre-treatment with 30 μM
LY294002, measured by FCM-based Annexin V-fluorescein
isothiocyanate and PI double staining analysis. (B) Cell cycle
analysis of PC-3 Mock and PC-3 Sox2 cells under Pac or Veh, with 2
h pretreatment with 30 μM LY294002, measured by FCM. Data were
analyzed by one-way analysis of variance and are presented as the
mean ± standard error of the mean of three independent experiments.
*P<0.05, vs. PC-3 Mock with Veh;
#P<0.05, PC-3 Mock and PC-3 Sox2 with Pac vs. PC-3
Mock and PC-3 Sox2 with Veh. FCM, flow cytometry; PI, propidium
iodide; Sox2, sex determining region Y-box 2; Pac, paclitaxel; Veh,
vehicle (dimethylsulfoxide). |
Discussion
Prostate cancer is the most commonly diagnosed
cancer and the second leading cause of cancer-associated mortality
in males (1). In the clinic, the
standard therapy for patients with HRPC is docetaxel, a Pac
derivative-based chemotherapeutic. However, the efficiency of this
drug is frequently impaired due to drug resistance (4). Several genes, including OCT4,
have been reported to be involved in the development of drug
resistance in prostate cancer cells (5,6).
Identifying novel molecular mechanisms underlying this drug
resistance may achieve more effective chemotherapy for prostate
cancer patients.
Pac (taxol) has been used as a chemotherapeutic
agent since the 1990s. The induction of apoptosis and cell growth
inhibition by Pac have been demonstrated, independent to the
microtubule stabilizing effect. However, for the majority of
chemotherapeutic agents, including Pac, drug resistance frequently
emerges following usage. To overcome this, a higher dose may be
administered; however, this inevitably induces severe cytotoxicity
in normal tissues. In this regard, a therapeutic strategy involving
dual agents, particularly targeted drugs, has been evaluated to
reach higher therapeutic efficacy (39). The mechanism of Pac resistance is
not well-characterized; however, a number of mechanisms independent
to microtubule stabilization function have been suggested (40). A large body of evidence has
demonstrated that the PI3K/Akt signaling pathway, which regulates a
series of cell survival- and proliferation-associated genes, is key
in the development of chemoresistance (27–30).
Sox2, an important component of the ‘induced
pluripotent stem cell cocktail’, is a member of the SOX family of
transcription factors, and is critical in self-renewal of embryonic
stem cells, maintenance of pluripotency, generation of induced stem
cells and apoptosis. Aberrant over-expression of Sox2 has been
reported in several types of tumor (16–22).
Expression of Sox2 was found to be significantly increased in the
prostate cancer tissues compared with normal and hyperplastic
tissues (41). Sox2 is an androgen
receptor-repressed gene and promotes the formation of HRPC. In a
previous study, Sox2 was shown to be involved in transforming
growth factor-α-induced cell proliferation and exhibit an
anti-apoptotic effect in prostate cancer cells, mediated by cyclin
E, p27 and survivin (38). In the
present study, the results revealed that Sox2 serves as a ‘safe
guard’ for maintaining cell proliferation, which characterizes
tumor cells. In both the PC-3 Mock and PC-3 Sox2 cell groups, Pac
inhibited cell growth, but the MTT and clone formation assay
results revealed that, following Pac treatment, overexpression of
Sox2 in the PC-3 Sox2 cells significantly promoted cell growth in
comparison with the PC-3 Mock group. Furthermore, the FCM cell
cycle assay indicated that overexpression of Sox2 increased the
percentage of cells in S phase, which suggested that Sox2 promoted
G1 to S phase progression. The G1/S checkpoint protein regulated by
Sox2 was thus analyzed. In accordance with previous studies
(38), the expression levels of
cyclin E, which combines with cyclin-dependent kinase 2 and is
essential for DNA replication, and G1/S transition were examined
(42). The results revealed that
cyclin E expression was upregulated in PC-3 Sox2 cells, which
explains the cell cycle-promoting effects of Sox2.
In addition, the apoptosis-inducing effect of Pac
was inhibited by Sox2 overexpression, as measured by an FCM
apoptosis assay. To verify that this decrease in the apoptotic rate
was not due to decreases in necrosis, the LDH assay was also
performed, and the data did not reveal significant differences
between the PC-3 Mock and Sox2 groups. This finding indicated that
Sox2 affected the apoptosis-inducing effect of Pac, but not cell
necrosis; and that at this concentration of Pac (5 μM), necrosis
induced by Pac was not evident.
By contrast to the PC-3 Mock cells, the
phosphorylation levels of Akt were found to be significantly
upregulated in the PC-3 Sox2 cells, which suggested that the cell
proliferation and anti-apoptotic effects of Sox2 following Pac
treatment may be mediated by activation of the PI3K/Akt signaling
pathway. Survivin, a member of the inhibitor of the
apoptosis-mediating protein family, is capable of regulating cell
proliferation and apoptosis (43),
and has been proven to be downstream of the PI3K/Akt signaling
pathway (44). In the present
study, survivin expression levels were detected at the protein
level, and results indicated that the expression levels of survivin
were significantly increased in PC-3 Sox2 cells, which may account
for the anti-apoptotic effect of Sox2.
To confirm the impact of Akt activation on cell
behavior, the PC-3 cells were pre-treated with LY294002, a PI3K/Akt
inhibitor. The results revealed that LY294002 inhibited the
upregulation of cyclin E and survivin as well as the
phosphorylation of Akt induced by Sox2 over-expression. In
addition, LY294002 inhibited the anti-apoptotic and G1/S transition
promoting effects of Sox2 following Pac treatment. All results
support the hypothesis that the effects of Sox2 are mediated by the
PI3K/Akt signaling pathway. Notably, as determined by FCM, no
significant differences in the percentage of apoptotic cells or the
percentage of cells in S phase were identified between PC-3 Mock
and PC-3 Sox2 cells receiving the same treatment (Veh or Pac),
which indicated that LY294002 completely inhibited the effects of
Sox2. However, markedly different S-phase distribution and
apoptotic percentages were observed between Veh and Pac treatment
in the same cell types (PC-3 Mock or PC-3 Sox2). This suggested
that Pac impacted the cell cycle distribution and cell apoptosis in
multiple ways.
In conclusion, the present study indicated that: (i)
Overexpression of Sox2 exerted a drug-resistance function in PC-3
cells and may antagonize the effects of Pac; (ii) the
Pac-resistance effect of Sox2 is mediated by continuous activation
of the PI3K/Akt signaling pathway; and (iii) under Pac treatment,
the PI3K/Akt signaling pathway promotes cell proliferation and
antagonizes apoptosis via targeting cyclin E and survivin. These
results may indicate novel therapeutic methods for chemoresistant
prostate cancer.
Acknowledgements
This study was supported by the National Youth
Natural Science Foundation of China (grant no. 81101942) and the
Natural Science Foundation of Education Department, Heilongjiang,
China (grant no. 12531300).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Moon C, Park JC, Chae YK, Yun JH and Kim
S: Current status of experimental therapeutics for prostate cancer.
Cancer Lett. 266:116–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petrylak DP: Docetaxel (Taxotere) in
hormone-refractory prostate cancer. Semin Oncol. 27:24–29.
2000.PubMed/NCBI
|
|
4
|
Seruga B, Ocana A and Tannock IF: Drug
resistance in metastatic castration-resistant prostate cancer. Nat
Rev Clin Oncol. 8:12–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Linn DE, Yang X, Sun F, et al: A Role for
OCT4 in Tumor Initiation of Drug-Resistant Prostate Cancer Cells.
Genes Cancer. 1:908–916. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu K, Xie D, Zou Y, et al: The mechanism
of DAB2IP in chemo-resistance of prostate cancer cells. Clin Cancer
Res. 19:4740–4749. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pevny LH and Lovell-Badge R: Sox genes
find their feet. Curr Opin Genet Dev. 7:338–344. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wegner M: From head to toes: the multiple
facets of Sox proteins. Nucleic Acids Res. 27:1409–1420. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kamachi Y, Uchikawa M and Kondoh H:
Pairing SOX off: with partners in the regulation of embryonic
development. Trends Genet. 16:182–187. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Masui S, Nakatake Y, Toyooka Y, et al:
Pluripotency governed by Sox2 via regulation of Oct3/4 expression
in mouse embryonic stem cells. Nat Cell Biol. 9:625–635. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fong H, Hohenstein KA and Donovan PJ:
Regulation of self-renewal and pluripotency by Sox2 in human
embryonic stem cells. Stem Cells. 26:1931–1938. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takahashi K, Tanabe K, Ohnuki M, et al:
Induction of pluripotent stem cells from adult human fibroblasts by
defined factors. Cell. 131:861–872. 2007. View Article : Google Scholar
|
|
13
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamanaka S: Strategies and new
developments in the generation of patient-specific pluripotent stem
cells. Cell Stem Cell. 1:39–49. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ben-Porath I, Thomson MW, Carey VJ, et al:
An embryonic stem cell-like gene expression signature in poorly
differentiated aggressive human tumors. Nat Genet. 40:499–507.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gangemi RM, Griffero F, Marubbi D, et al:
Sox2 silencing in glioblastoma tumor-initiating cells causes stop
of proliferation and loss of tumorigenicity. Stem Cells. 27:40–48.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bass AJ, Watanabe H, Mermel CH, et al:
Sox2 is an amplified lineage-survival oncogene in lung and
esophageal squamous cell carcinomas. Nat Genet. 41:1238–1242. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Güre AO, Stockert E, Scanlan MJ, et al:
Serological identification of embryonic neural proteins as highly
immunogenic tumor antigens in small cell lung cancer. Proc Natl
Acad Sci USA. 97:4198–4203. 2000.PubMed/NCBI
|
|
19
|
Hussenet T, Dali S, Exinger J, et al: SOX2
is an oncogene activated by recurrent 3q26.3 amplifications in
human lung squamous cell carcinomas. PLoS One. 5:e89602010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ye F, Li Y, Hu Y, Zhou C, Hu Y and Chen H:
Expression of Sox2 in human ovarian epithelial carcinoma. J Cancer
Res Clin Oncol. 137:131–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jia X, Li X, Xu Y, et al: SOX2 promotes
tumorigenesis and increases the anti-apoptotic property of human
prostate cancer cell. J Mol Cell Biol. 3:230–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saigusa S, Tanaka K, Toiyama Y, et al:
Correlation of CD133, OCT4, and SOX2 in rectal cancer and their
association with distant recurrence after chemoradiotherapy. Ann
Surg Oncol. 16:3488–3498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rao GH, Liu HM, Li BW, et al:
Establishment of a human colorectal cancer cell line P6C with stem
cell properties and resistance to chemotherapeutic drugs. Acta
Pharmacol Sin. 34:793–804. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tian T, Zhang Y, Wang S, Zhou J and Xu S:
Sox2 enhances the tumorigenicity and chemoresistance of cancer
stem-like cells derived from gastric cancer. J Biomed Res.
26:336–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gen Y, Yasui K, Nishikawa T and Yoshikawa
T: SOX2 promotes tumor growth of esophageal squamous cell carcinoma
through the AKT/mammalian target of rapamycin complex 1 signaling
pathway. Cancer Sci. 104:810–816. 2013. View Article : Google Scholar
|
|
26
|
Kregel S, Kiriluk KJ, Rosen AM, et al:
Sox2 is an androgen receptor-repressed gene that promotes
castration-resistant prostate cancer. PLoS One. 8:e537012013.
View Article : Google Scholar
|
|
27
|
Kim SH, Juhnn YS and Song YS: Akt
involvement in paclitaxel chemoresistance of human ovarian cancer
cells. Ann N Y Acad Sci. 1095:82–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fraser M, Leung B, Jahani-Asl A, Yan X,
Thompson WE and Tsang BK: Chemoresistance in human ovarian cancer:
the role of apoptotic regulators. Reprod Biol Endocrinol. 1:662003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smith DA, Kiba A, Zong Y and Witte ON:
Interleukin-6 and oncostatin-M synergize with the PI3K/AKT pathway
to promote aggressive prostate malignancy in mouse and human
tissues. Mol Cancer Res. 11:1159–1165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barile E, De SK, Feng Y, et al: Synthesis
and SAR studies of dual AKT/NF-kappaB inhibitors against melanoma.
Chem Biol Drug Des. 82:520–533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu L, Hofmann J, Lu Y, Mills GB and Jaffe
RB: Inhibition of phosphatidylinositol 3′-kinase increases efficacy
of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer
Res. 62:1087–1092. 2002.
|
|
32
|
Alimonti A, Carracedo A, Clohessy JG, et
al: Subtle variations in Pten dose determine cancer susceptibility.
Nat Genet. 42:454–458. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Trotman LC, Niki M, Dotan ZA, et al: Pten
dose dictates cancer progression in the prostate. PLoS Biol.
1:E592003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Makhov PB, Golovine K, Kutikov A, et al:
Modulation of Akt/mTOR signaling overcomes sunitinib resistance in
renal and prostate cancer cells. Mol Cancer Ther. 11:1510–1517.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Franken NA, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang CC, Aronstam RS, Chen DR and Huang
YW: Oxidative stress, calcium homeostasis, and altered gene
expression in human lung epithelial cells exposed to ZnO
nanoparticles. Toxicol In Vitro. 24:45–55. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang S, Zu Y, Fu Y, Zhang Y and Efferth
T: Activation of the mitochondria-driven pathway of apoptosis in
human PC-3 prostate cancer cells by a novel hydrophilic paclitaxel
derivative, 7-xylosyl-10-deacetylpaclitaxel. Int J Oncol.
33:103–111. 2008.
|
|
38
|
Lin F, Lin P, Zhao D, et al: Sox2 targets
cyclinE, p27 and survivin to regulate androgen-independent human
prostate cancer cell proliferation and apoptosis. Cell Prolif.
45:207–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sandler A, Gray R, Perry MC, et al:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang YI, Lee KT, Park HJ, et al:
Tectorigenin sensitizes paclitaxel-resistant human ovarian cancer
cells through downregulation of the Akt and NFκB pathway.
Carcinogenesis. 33:2488–2498. 2012.PubMed/NCBI
|
|
41
|
Bae KM, Su Z, Frye C, et al: Expression of
pluripotent stem cell reprogramming factors by prostate tumor
initiating cells. J Urol. 183:2045–2053. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sherr CJ: G1 phase progression: cycling on
cue. Cell. 79:551–555. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Altieri DC and Marchisio PC: Survivin
apoptosis: an interloper between cell death and cell proliferation
in cancer. Lab Invest. 79:1327–1333. 1999.PubMed/NCBI
|
|
44
|
Peng XH, Karna P, Cao Z, Jiang BH, Zhou M
and Yang L: Cross-talk between epidermal growth factor receptor and
hypoxia-inducible factor-1alpha signal pathways increases
resistance to apoptosis by up-regulating survivin gene expression.
J Biol Chem. 281:25903–25914. 2006. View Article : Google Scholar
|