Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignant tumors in the world (1). It is the third common cause of cancer
mortality worldwide, especially in the developing countries. HCC
patients show a poor 5-year survival rate, since most of them are
diagnosed at a late stage (2). HCC
is associated with high potential for vascular invasion,
metastasis, and recurrence even after surgical resection, leading
to poor prognosis (3). HCC is a
multi-step processes including numerous gene changes that associate
with cell proliferation, apoptosis, invasion and metastasis. Thus,
there is an urgent demand for early diagnosis and the development
of novel molecular targets for HCC. In recent years, as the
development of molecular-based therapies has emerged, increasing
research focus has been placed on the identification of biomarkers,
the expression of which is altered during the development of HCC
(4–6). Numerous studies have shown that
biomarkers may be promising molecular targets for the treatment of
HCC (7–9).
The protein glypican-3 (GPC3) is a valuable
diagnostic marker and a potential therapeutic target for HCC
(10). It has been reported that
this protein is almost not expressed in healthy liver or non-tumor
tissues, while strong positive staining was observed for GPC3 at
carcinoma sites (11–14). Furthermore, GPC3 is highly
expressed in melanoma, ovarian clear-cell carcinomas, yolk sac
tumors, neuroblastoma, hepatoblastoma, Wilms’ tumor cells, and
other tumors (15–19). By contrast, the gene appears
silenced in breast cancer, mesothelioma, epithelial ovarian cancer
and lung adenocarcinoma (20–22).
Based on these reports, it was proposed that GPC3 is highly and
specifically expressed in HCC, and its overexpression appears not
to inhibit HCC, but rather, promote it (23). GPC3 exerts positive or negative
effects on cell growth, depending on the cell type (24,25).
The gene was first identified by Filmus et al (26) in 1988, and was named by Pilia et
al (27) in 1996. GPC3
is located on the human X chromosome (Xq26) and encodes a 70-kDa
core protein with 580 amino acids. It is a member of the glypican
family, which has a basic structure consisting of a core protein, a
heparan sulfate chain, and a glycosylphosphatidylinositol (GPI)
anchor via which it attaches to the cell membrane (28,29).
As suggested by a previous study, GPC3 regulates cell morphology,
adhesion, apoptosis, proliferation, migration, survival and
differentiation by receiving signals from receptors on the cell
surface (18). The protein can
crosstalk with a number of signaling pathways during the
oncogenesis of HCC. Although numerous biochemical and genetic
studies have been performed to elucidate the role of GPC3 in
modulating cell biological behavior (25,30),
the molecular mechanisms underlying GPC3-mediated invasion and
migration remain elusive. The role of GPC3 in tumorigensesis
deserves further investigation.
The epithelial-mesenchymal transition (EMT) is a key
event in the tumor invasion process, whereby epithelial cell layers
loose polarity and cell-cell contacts and undergo dramatic
remodeling of the cytoskeleton (31). It is a process involving
dissociation of adherens junctions and changes in cell
morphology.
The present study focused on the role of GPC3 in the
oncogenesis of HCC and the potential molecular events promoting
cell invasion induced by GPC3. We silenced the GPC3 gene in
HepG2 cells with an RNA interference method using lentiviral
vectors, thereby reducing its expression at both the protein and
mRNA levels. In addition, we detected the expression of EMT-related
proteins by western blot analysis in order to investigate the
progress of EMT in the GPC3-silenced cells. Our results
reveal a potential link between GPC3-mediated invasion and the EMT
process. Taken together, our study provides evidence that GPC3 may
be an effective therapeutic target for treatment of HCC and may
play an important role in gene therapy of HCC.
Materials and methods
Cell lines and culture conditions
The human HCC cell line HepG2 and the 293T cell line
were purchased from the Cell Collection of the Chinese Academy of
Sciences (Shanghai, China). Both cell lines were cultured in
Dullbecco’s modified Eagle’s medium (DMEM), supplemented with 10%
fetal bovine serum (FBS) (both HyClone®, commercialized
by Thermo Fisher Scientific, Waltham, MA, USA) at 37°C in a
humidified atmosphere of 5% CO2 and 95% air.
Construction of a recombinant lentiviral
vector targeting GPC3
Small interfering RNA (siRNA) sequences targeting
the human GPC3 gene (GenBank accession no., NP_004475) were
designed following standard principles for the design of RNA
interference sequences using Designer 3.0 (GenePharma, Shanghai,
China). We selected the sequence 5′-GGCTCTGAATCTTGGAATT-3′ in order
to silence the expression of GPC3. The scrambled sequence
5′-TTCTCCGAACGTGTCACGT-3′ was used as a negative control. The
lentivirus PGLV3-green fluorescent protein (GFP) vector was
purchased from GenePharma (Shanghai, China). Short hairpin RNAs
(shRNAs) were generated based on the above siRNA sequences and were
cloned into the PGLV3-GFP vector. The resulting plasmids were
transfected into 293T cells using Invitrogen™ Lipofectamine 2000
(Thermo Fisher Scientific) according to the manufacturer’s
instructions. At 48 and 72 h following the transfection, GFP
expression was detected under a fluorescent microscope (Olympus
IX71, Olympus, Tokyo, Japan; Apogee Alta U2 Cooled Camera,
Sartorius Instrument System, Beijng, China) to determine the vector
titer. Then, viral products were aliquoted and stored at −80°C in
DMEM containing 2.5% FBS. Finally, HepG2 cells were transfected
with the appropriate titer, which was selected based on the
transfection efficiency, measured through the expression of GFP
under the fluorescent microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to determine the mRNA level of
GPC3 in HCC following transfection with the lentiviral
vector. Total RNA was isolated using the TRIzol reagent (Takara Bio
Inc., Dalian, China) and reverse transcribed to cDNA using the RT
Master Mix (Takara). The resulting cDNA was then used for
measurement of RNA abundance by qPCR. Amplification was performed
using the SYBR-Green PCR Master mix (Takara), with 100 ng of cDNA
in 20 μl of the final reaction mixture. We used the following
primer sequences (5′-3′): forward, GATGAGTGCATTGGAGGCTCTG, and
reverse, ATGAACGTTCCCGAGGTTGTG. The cycling conditions were the
following: one cycle at 95°C for 30 sec, 40 cycles at 95°C for 5
sec, and 60°C for 30 sec, and one cycle at 95°C for 15 sec, 60°C
for 30 sec, and 95°C for 15 sec. Three independent experiments were
performed for each sample. The data were analyzed by comparing the
2−ΔΔCt values (32).
Annexin V-phycoerythrin
(PE)/7-aminoactinomycin D (7-AAD) apoptosis assay
To study the effect of GPC3 silencing on
HepG2 cell apoptosis, cells were harvested after a 72 h
transfection by brief trypsinization and were washed in
phosphate-buffered saline (PBS) twice. Then, we used the Annexin
V-PE/7-AAD staining kit (KeyGen Biotech, Nanjing, China) to detect
the apoptotic rate of HepG2 cells. Cells were suspended in 500 μl
of binding buffer and incubated at room temperature in the dark for
15 min after labeling with 5 μl of Annexin V/7-AAD and 1 μl of
Annexin V-PE. The stained cells were then analyzed by flow
cytometry (FACSCalibur; Becton-Dickinson, Franklin Lakes, NJ, USA)
and CellQuest software (BD Biosciences, Franklin Lakes, NJ, USA).
The experiments were performed in triplicate.
Cell proliferation assay
To further study the effect of GPC3 silencing
on cell proliferation, we used the cell counting kit-8 (cck-8)
assay (Beyotime Institute of Biotechnology, Shanghai, China)
following the manufacturer’s instructions. Briefly,
1×104/well cells were seeded onto 96-well plates and
incubated at 37°C for 24, 48 and 72 h. Following cell attachment,
10 μl CCK-8 were added to each well, and cells were incubated at
37°C for 2 h. The optical density (OD) was measured at 450 nm using
a microplate reader (RT-6000; Rayto Life and Analytical Sciences
Co, Ltd., Shenzhen, China). Three independent experiments were
performed.
Transwell Matrigel invasion assay
To evaluate the effect of silencing of GPC3
on HepG2 cell invasion, we used 24-well Transwell chambers with 8.0
μm pore membranes (Corning, Inc., Corning, NY, USA). Following
transfection, cells were seeded into the upper chamber at a density
of 2×104 in 200 μl serum-free medium. The lower chamber
contained 600 μl medium supplemented with 10% FBS as a
chemoattractant. After incubation for 48 h, the remaining cells on
the upper surface of the filters were removed with cotton swabs,
and migrating cells were stained with crystal violet and observed
under a confocal microscope (DM77300B; Leica Microsystems,
Mannheim, Germany). Invading cells were quantified by counting
cells in 10 random fields at ×100 magnification. Three independent
experiments were performed.
Wound healing assay
To determine the effect of GPC3 silencing on
HepG2 cell migration, a wound healing assay was performed. Cells
(6×105) were seeded onto 6-well plates at 80%
confluence. The cells were treated with serum-free medium and then
wounded with a pipette tip of 10 μl. After washing with PBS three
times, images were acquired at 0 and 24 h of incubation after
wounding, using a microscope (DM77300B; Leica Microsystems). Images
of the same area were acquired to determine wound coverage due to
cellular motility. Data were quantified by measuring the scratch
area of every field of vision. The assay was performed in
triplicate.
Western blot assay
Total cellular protein was extracted following
transfection, using the radio-immunoprecipitation assay (RIPA;
Beyotime Institute of Biotechnology, Shanghai, China). The protein
concentrations were determined by the bicinchoninic acid assay
(BCA; KeyGen Biotech Co, Ltd., Nanjing China). SDS-PAGE was
performed on 10% glycine gels to separate the proteins, which were
then transferred onto polyvinylidene difluoride (PVDF) membranes.
The PVDF membranes were blocked with 5% non-fat milk in
Tris-buffered saline and Tween-20 (TBST) buffer for 1 h, then
incubated with rabbit polyclonal anti-human anti-GPC3 (ProteinTech
Group, Hubei, China) and anti-β-actin (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) primary antibodies overnight at 4°C,
followed by incubation with a horseradish peroxidase-conjugated
anti-rabbit IgG (Santa Cruz Biotechnology, Inc.) as the secondary
antibody. Protein bands were detected using the enhanced
chemiluminescence (ECL) kit (CoWin Biotech, Beijing, China). To
further investigate the mechanism underlying the changes in
biological functions induced by GPC3 silencing, we also
assessed the expression of proteins using mouse monoclonal
anti-human anti-matrix metalloproteinase-2 (MMP-2), mouse
monoclonal anti-human anti-MMP-9, mouse monoclonal anti-human
anti-β-catenin, rabbit polyclonal anti-human anti-E-cadherin, mouse
monoclonal anti-human anti-Slug and rabbit polyclonal anti-human
anti-Snail using the same procedure. The antibodies were purchased
from Santa Cruz Biotechnology, Inc. The intensity of the bands on
the gels was quantified using Image J software (National Institutes
of Health, Bethesda, MA, USA) and the experiments were repeated
over five times.
Statistical analysis
Data were expressed as mean ± standard deviation
(SD) and subjected to one-way analysis of variance using the SPSS
17.0 software (SPSS Inc., Chicago, IL, USA). Differences between
groups were examined by Student’s t-tests. Statistical significance
was accepted at a p-value <0.05.
Results
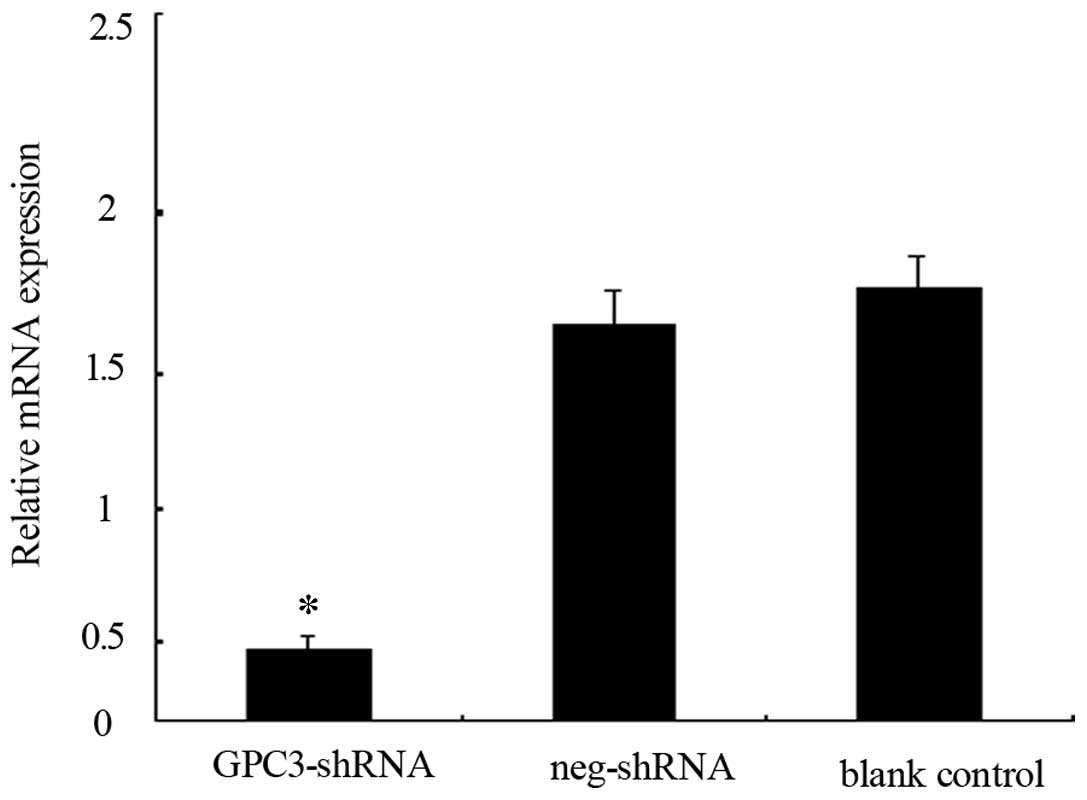
The expression of GPC3 is reduced upon
GPC3-shRNA transfection
Transfection with the GPC3-shRNA decreased the
expression of GPC3 in HepG2 cells at both the mRNA and protein
levels. RT-qPCR and western blot analysis were used to evaluate the
differences between the transfected group and the blank control
(non-transfected cells). The results showed that the expression
level of the GPC3 mRNA (Fig.
1) and protein (Fig. 5, upper
right panel) were significantly reduced following transfection of
HepG2 cells. These results indicated that the recombinant
lentiviral vector for GPC3 silencing was successfully
constructed.
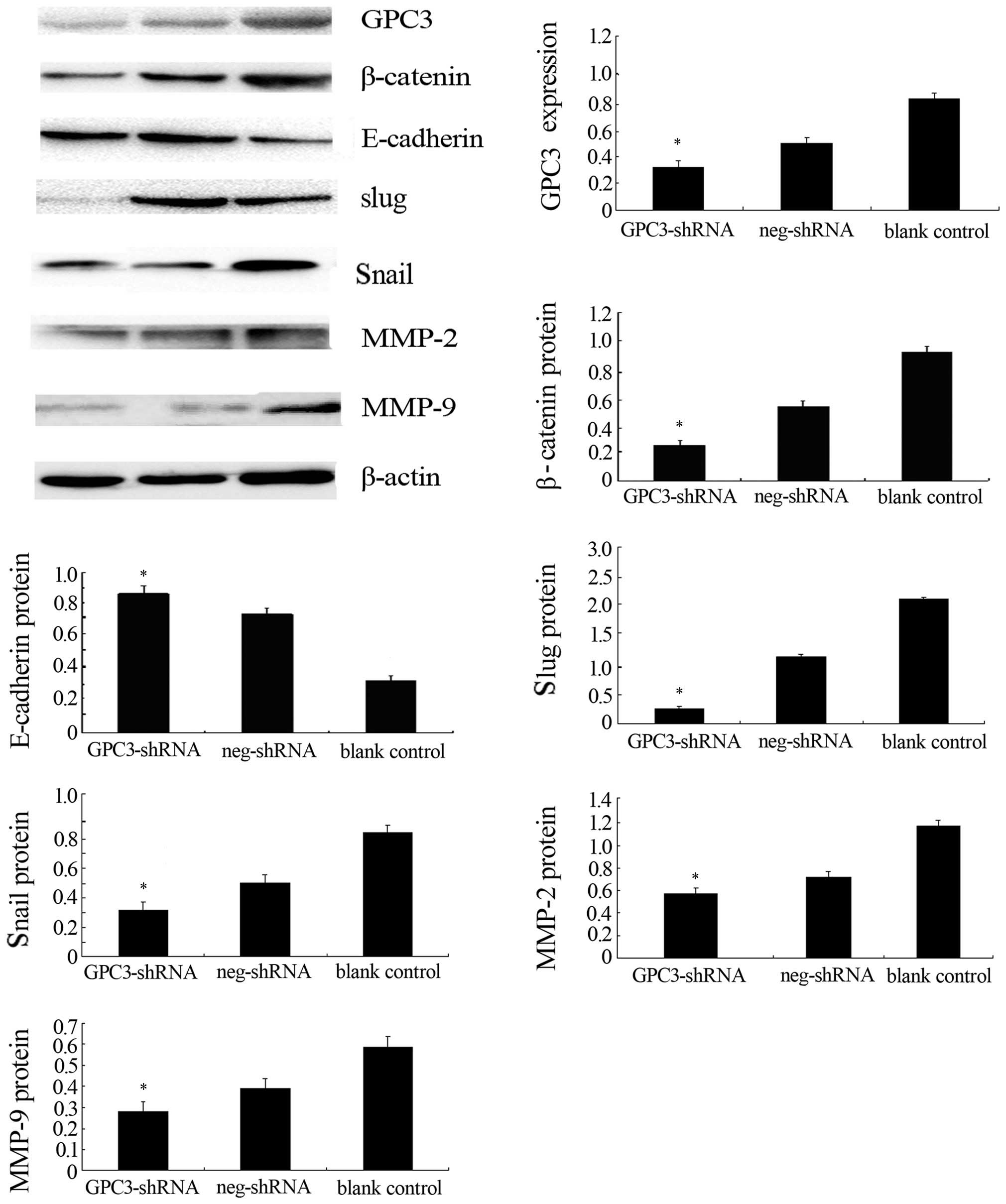 | Figure 5The protein expression of glypican-3
(GPC3), E-cadherin, β-catenin, Snail, Slug, matrix
metalloproteinase-2 (MMP-2), and MMP-9 in HepG2 cells transfected
with the GPC3-shRNA was detected by western blotting. At 72 h after
transfection with the GPC3-shRNA, the expression level of GPC3,
β-catenin, Snail, Slug, MMP-2 and MMP-9 is reduced in HepG2 cells;
by contrast, the level of E-cadherin is increased. β-actin was used
as an internal normalization control.*P<0.05 vs.
control groups. The experiments were repeated at least five
times. |
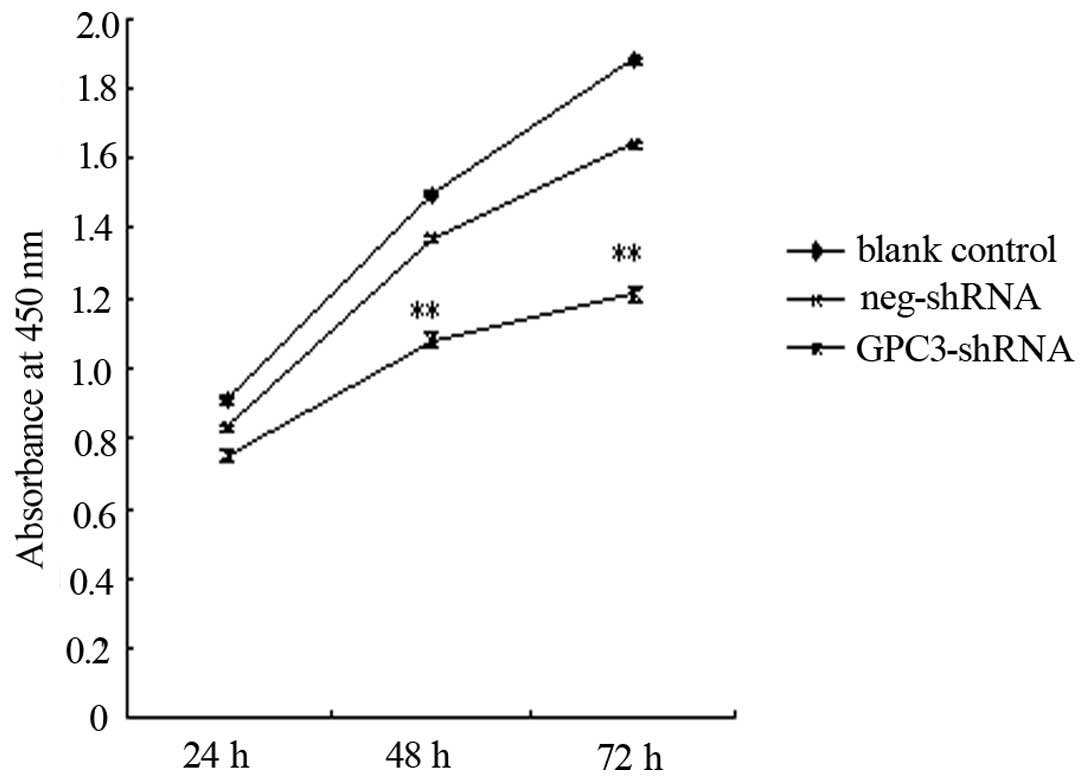
GPC3 gene silencing decreases cell
proliferation in HepG2 cells
The CCK-8 assay, a sensitive and specific method for
the assessment of cell proliferation, was carried out to analyze
the effects of GPC3 silencing on HepG2 cell proliferation.
Following transfection with the GPC3-shRNA, proliferation was
significantly decreased compared to the control group (Fig. 2). This result indicated that
silencing of GPC3 may decrease the growth and survival of
the HepG2 cells.
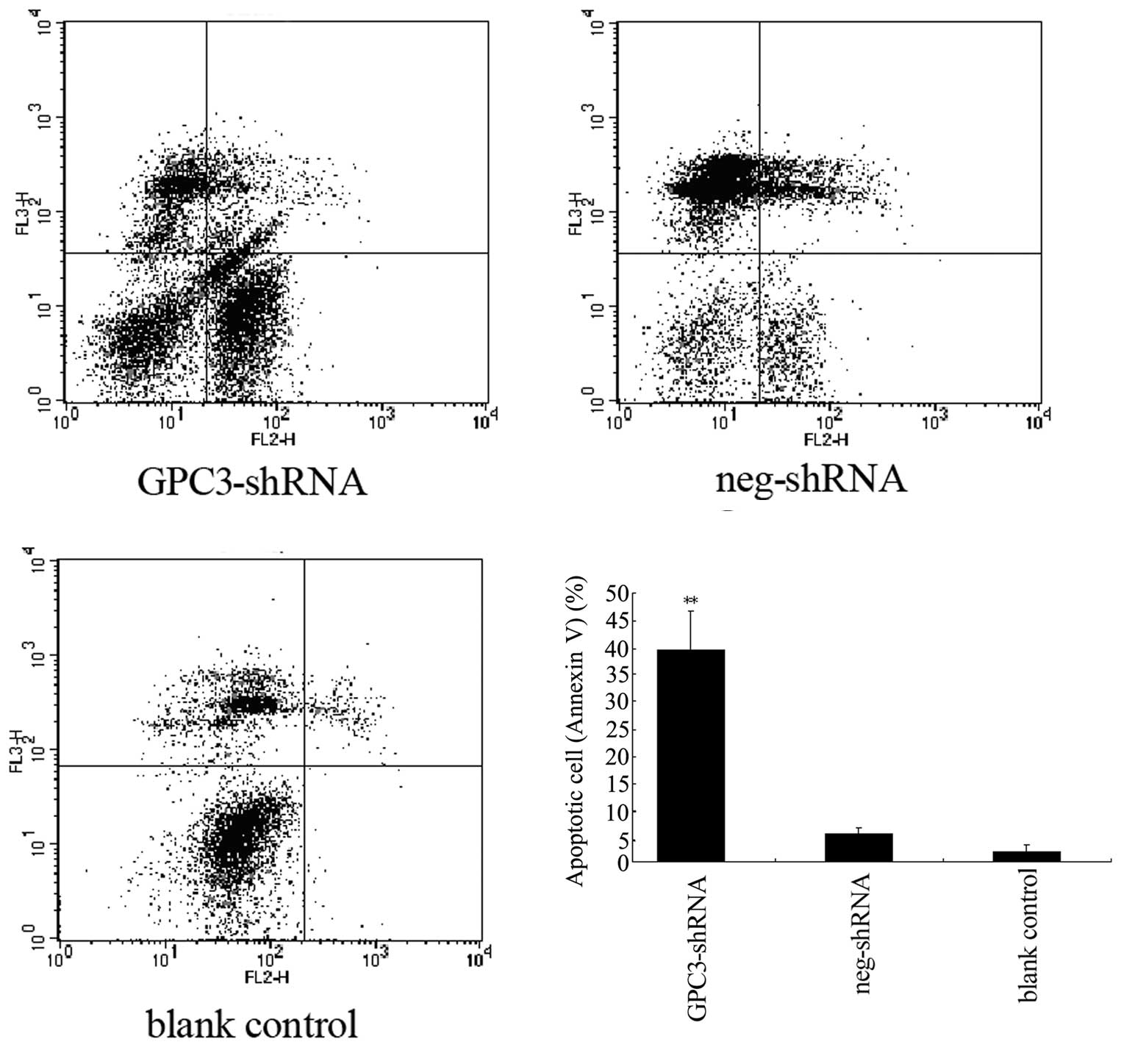
Silencing of the GPC3 gene increases the
apoptotic rate of HepG2 cells
Resistance to apoptosis is a characteristic feature
of tumor cells, and we thus investigated whether GPC3 is associated
with cell apoptosis. We used the Annexin V PE/7-AAD assay to
analyze the effect of GPC3 silencing on cell apoptosis. The
data shown in Fig. 3 reveal that,
compared to the control group, the apoptotic rate of HepG2 cells
that were GPC3-silenced was notably increased (P<0.05). We
conclude that GPC3 may affect cell apoptosis, since the inhibition
of its expression markedly increases HepG2 cell apoptosis.
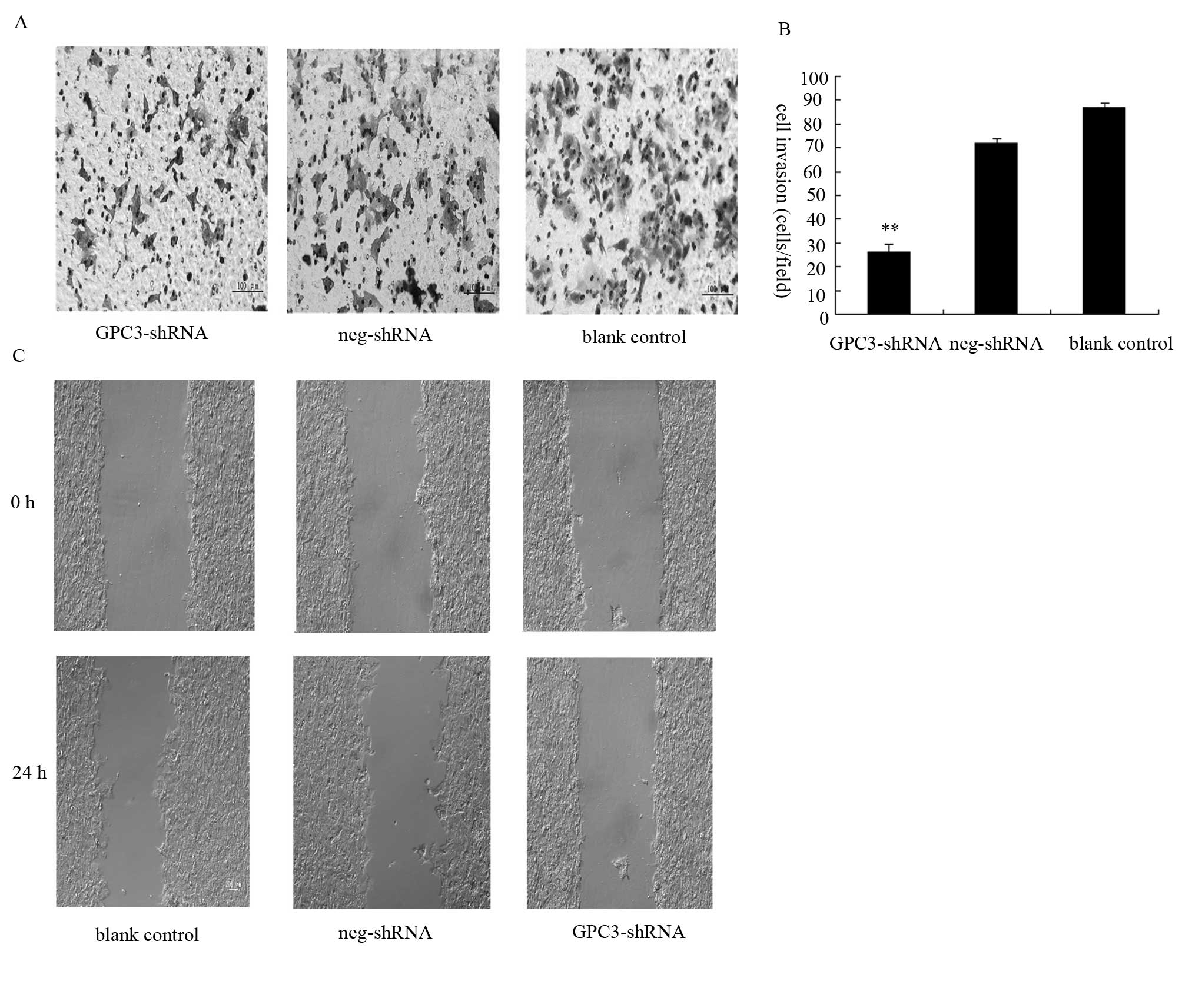
Alterations in HepG2 cell invasiveness
and migration after transfection
The effects of transfection with the GPC3-shRNA on
cell invasion were investigated with the Transwell assay. The
results of this assay (Fig. 4A)
showed that HepG2 cell invasion is considerably inhibited by
GPC3 silencing. Forty-eight hours after transfection with
the GPC3-shRNA, the number of cells that successfully invaded
through the Matrigel was significantly decreased compared to the
blank control (Fig. 4B). To
further investigate the GPC3-mediated effects on migration, a
scratch wound-healing assay was performed. Wound healing was
observed and measured at different time-points. At 24 h after
wounding, the blank control cells had covered >50% the cell-free
area, while the cells transfected with the GPC3-shRNA changed
subtly compared with 0 h after wounding, as shown in Fig. 4C. Overall, these data indicate that
the migration rate of GPC3-shRNA-transfected cells was lower than
that of the blank control cells at the indicated time-points. We
therefore conclude that the migratory and invasive ability of HepG2
cells is tightly linked to the expression of GPC3.
Silencing of GPC3 downregulates the
expression of EMT-related proteins in HepG2 cells
To further investigate the mechanism underlying the
GPC3 silencing-induced changes in cell invasion and
migration, we examined the expression of several invasion-related
proteins by western blot analysis. It was previously demonstrated
that GPC3 is associated with the Wnt signaling pathway, which is
associated with the EMT process (33,34).
Here, we examined the expression of the EMT-related proteins
E-cadherin, Snail and Slug, of the Wnt signaling-associated protein
β-catenin, and of the migration-related proteins MMP-2 and MMP-9.
The results are shown in Fig. 5.
The expression of the EMT-related proteins Snail and Slug decreased
and that of E-cadherin increased compared to the blank and negative
controls (P<0.05). The expression of β-catenin was markedly
decreased after GPC3-shRNA transfection (P<0.05) and the
expression of migrated associated proteins MMP-2 and MMP-9 were
decreased in the HCC cells compared with the blank and negative
controls (P<0.05). Therefore, GPC3 had an effect on the
migration-associated proteins and the EMT-associated proteins in
the HCC cell line. Combined with the data previously mentioned,
these results suggested that GPC3 affects the invasion and
metastatic abilities in the HCC cell line and had an association
with the EMT program, which is important in cell invasion and
migration.
Discussion
The GPC3 protein plays a critical role in HCC
oncogenesis. It is associated with cell growth, apoptosis, adhesion
and invasion. It may be a valuable diagnostic marker and a
potential therapeutic target in HCC. Numerous studies have shown
that GPC3 is associated with a number of tumor-related signaling
pathways, including Wnt, Hedgehog, SULF, FGF-2, IGF-2, TGF-β and
BMP-4 (35–44). Among these, the most well studied
pathway related to the biological functions of GPC3 is the Wnt
pathway. Glypicans are cell surface-anchored heparan sulfate
proteoglycans that regulate the activity of Wnts (45,46).
GPC3 serves as a selective regulator of Wnt signaling, modulating
both the canonical and the non-canonical pathways (38,39,42).
It was previously reported that the stimulatory activity of
glypicans is based on their ability to act as facilitators of the
interaction between Wnts and their receptors (29). The activation of the canonical Wnt
signaling pathway has been found to be one of the most frequent
events associated with malignant transformation of liver cells
(47–49). We found that GPC3-silenced
cells exhibit alterations in the Wnt signaling pathway, which is
associated with the regulation of cell invasion. We therefore
hypothesize that at least in some cell types, GPC3 serves as a
selective regulator of Wnt signaling by inhibiting the canonical
Wnt signaling pathway. Therefore, GPC3 may stimulate the
Wnt/β-catenin pathway to induce HCC cell invasion and
migration.
Invasion and migration are the main biological
characteristics of malignant tumors that cause treatment failure,
poor diagnosis and prognosis (3).
Therefore, it is of great interest to study the molecular mechanism
underlying HCC cell invasiveness. Differentiated epithelial cells
can be transformed into mesenchymal cells through a cellular
program named epithelial-mesenchymal transition (50). EMT is a key factor in tumor
invasion, metastasis and chemotherapy resistance (51,52).
It plays a crucial role in local advancement and metastasis of
tumors (53). A number of studies
have reported that EMT is associated with the invasive and
migratory ability of malignant tumors, including esophageal
carcinoma, gastric carcinoma, HCC, colorectal cancer and pancreatic
cancer (54–59). There are various factors associated
with EMT, among which the repression of E-cadherin is an important
hallmark of EMT (54). E-cadherin
acts as a regulator of cell adhesion in epithelial cells.
Downregulation of E-cadherin results in the migration of primary
malignant epithelial cells out of their site of origin, where they
degrade the surrounding extracellular matrix, migrate into the
blood vessels and invade secondary organs (60). Other factors that are involved in
the EMT program, such as Snail and Slug, may inhibit the expression
of E-cadherin, consequently impairing cell-cell adhesion. The EMT
is a complex process that involves crosstalk with several pathways
such as TGF, Wnt, PI3K/AKT, Ras-MAPK, Notch, and Hedgehog. Among
these pathways, the Wnt/β-catenin one plays a crucial role in
tumorigenesis (61,62). Recent studies elaborated the role
of β-catenin in cancer metastasis, showing that β-catenin
facilitates EMT in tumor cells (63–64).
A proposed mechanism for this involves the complex formed by
E-cadherin and β-catenin; when the E-cadherin level is reduced in
the adherens junctions, its partner β-catenin is released into the
cytosol, where it can activate LEF/TCF-mediated transcription and
drive the expression of important cell-cycle proteins and
oncogenes; via this mechanism, β-catenin signaling may contribute
to EMT and eventually result in tumor invasion and metastasis
(65). On the other hand, several
studies also suggested that β-catenin-mediated transcription can
induce the expression of Slug and Snail, thereby
contributing to the EMT program (66–67).
Extensive studies in various developmental EMT systems provide
convincing evidence that Wnt signaling is a key event of EMT
(33,34,51,57,58,63,68),
although the precise signaling pathway activated by individual
family members may differ among EMT events in different
systems.
In this study, we hypothesized that silencing of
GPC3 may decrease HCC cell invasion and migration, and
provided experimental evidence supporting this hypothesis.
EMT-related proteins were detected in HCC cells by western blot
analysis, and the invasive ability of these cell lines was assessed
following transfection using the Matrigel Transwell and the wound
healing assays. Furthermore, the expression of certain critical
proteins in the Wnt/β-catenin signaling pathway was also analyzed
by western blot analysis. In this study, the main focus was the
potential relationship between EMT, β-catenin and GPC3. Our
findings demonstrated that cell invasion and migration are
inhibited following transfection (Figs. 4 and 5). Silencing of GPC3 reduced the
protein level of Snail and Slug and increased the level of
E-cadherin, potentially contributing in inhibition of the EMT
program, which also inhibited the HCC invasive and metastatic
activities, in agreement with the reduced expression of β-catenin.
Based on the marked cellular changes observed in our study, we
propose that the cell invasive ability that is associated with the
EMT process in HCC may be regulated, at least in part, by the
expression of GPC3.
In summary, this study demonstrated that GPC3
induces HCC invasiveness and metastasis, potentially via induction
of EMT. These data are novel and important for the understanding of
the role of GPC3 in HCC and the future development of gene therapy
for this type of tumor. However, further studies are needed to
elucidate the relationship between GPC3-mediated cell invasion and
the EMT program.
Acknowledgements
This study was supported by the Project of Science
and Technology of Liaoning (2011415052-3).
References
|
1
|
Mazzanti R, Gramantieri L and Bolondi L:
Hepatocellular carcinoma: epidemiology and clinical aspects. Mol
Aspects Med. 29:130–143. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kawano Y, Sasaki A, Kai S, et al: Short-
and long-term outcomes after hepatic resection for hepatocellular
carcinoma with concomitant esophageal varices in patients with
cirrhosis. Ann Surg Oncol. 15:1670–1676. 2008. View Article : Google Scholar
|
|
3
|
Poon RT, Fan ST and Wong J: Risk factors,
prevention and management of postoperative recurrence after
resection of hepatocellular carcinoma. Ann Surg. 232:10–24. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mischak H, Allmaier G, Apweiler R, et al:
Recommendations for biomarker identification and qualification in
clinical proteomics. Sci Transl Med. 2:42–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mann CD, Neal CP, Garcea G, et al:
Prognostic molecular markers in hepatocellular carcinoma: a
systematic review. Eur J Cancer. 43:979–992. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Shen Z, Zhu Z, et al: Clinical
values of AFP, GPC3 mRNA in peripheral blood for prediction of
hepatocellular carcinoma recurrence following OLT:AFP, GPC3 mRNA
for prediction of HCC. Hepat Mon. 11:195–199. 2011.PubMed/NCBI
|
|
7
|
Huynh H: Molecularly targeted therapy in
hepatocellular carcinoma. Biochem Pharmacol. 80:550–560. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marra M, Sordelli IM, Lombardi A, et al:
Molecular targets and oxidative stress biomarkers in hepatocellular
carcinoma: an overview. J Transl Med. 9:171–185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gonzalez SA and Keeffe EB: Diagnosis of
hepatocellular carcinoma: role of tumor markers and liver biopsy.
Clin Liver Dis. 15:297–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zou ZQ, Ding YP, Long B, et al: Gpc-3 is a
notable diagnostic, prognostic and a latent targeted therapy marker
in hepatocellular carcinoma. Hepatogastroenterology. 57:1285–1290.
2010.
|
|
11
|
Capurro M, Wanless IR, Sherman M, et al:
Glypican-3: a novel serum and histochemical marker for
hepatocellular carcinoma. Gastroenterology. 125:89–97. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamauchi N, Watanabe A, Hishinuma M, et
al: The glypican 3 oncofetal protein is a promising diagnostic
marker for hepatocellular carcinoma. Mod Pathol. 18:1591–1598.
2005.PubMed/NCBI
|
|
13
|
Libbrecht L, Severi T, Cassiman D, et al:
Glypican-3 expression distinguishes small hepatocellular carcinomas
from cirrhosis, dysplastic nodules, and focal nodular
hyperplasia-like nodules. Am J Surg Pathol. 30:1405–1411. 2006.
View Article : Google Scholar
|
|
14
|
Liovet JM, Chen Y, Wurmbach E, et al: A
molecular signature to discriminate dysplastic nodules from early
hepatocellular carcinoma in HCV-cirrhosis. Gastroenterology.
131:1758–1767. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakatsura T, Kageshita T, Ito S, et al:
Identification of glypican-3 as a novel tumor marker for melanoma.
Clin Cancer Res. 10:6612–6621. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stadlmann S, Gueth U, Baumhoer D, et al:
Glypican-3 expression in primary and recurrent ovarian carcinomas.
Int J Gynecol Pathol. 26:341–344. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zynger DL, Dimov ND, Luan C, et al:
Glypican 3: a novel marker in testicular germ cell tumors. Am J
Surg Pathol. 30:1570–1575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baumhoer D, Tornillo L, Stadlmann S, et
al: Glypican 3 expression in human nonneoplastic, preneoplastic,
and neoplastic tissues: a tissue microarray analysis of 4,387
tissue samples. Am J Clin Pathol. 129:899–906. 2008. View Article : Google Scholar
|
|
19
|
Saikali Z and Sinnett D: Expression of
glypican 3 (GPC3) in embryonal tumors. Int J Cancer. 89:418–422.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim H, Xu GL, Borczuk AC, et al: The
heparan sulfate proteoglycan GPC3 is a potential lung tumor
suppressor. Am J Respir Cell Mol Biol. 29:694–701. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murthy SS, Shen T, De Rienzo A, et al:
Expression of GPC3, an X-linked recessive overgrowth gene, is
silenced in malignant mesothelioma. Oncogene. 19:410–416. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiang YY, Ladeda V and Filmus J:
Glypican-3 expression is silenced in human breast cancer. Oncogene.
20:7408–7412. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu ZW, Friess H, Wang L, et al: Enhanced
glypican-3 expression differentiates the majority of hepatocellular
carcinomas from benign hepatic disorders. Gut. 48:558–564. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Filmus J and Capurro M: The role of
glypican-3 in the regulation of body size and cancer. Cell Cycle.
7:2787–2790. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stigliano I, Puricelli L, Filmus J, et al:
Glypican-3 regulates migration, adhesion and actin cytoskeleton
organization in mammary tumor cells through Wnt signaling
modulation. Breast Cancer Res Treat. 114:251–262. 2009. View Article : Google Scholar
|
|
26
|
Filmus J, Church JG and Buick RN:
Isolation of a cDNA corresponding to a developmentally regulated
transcript in rat intestine. Mol Cell Biol. 8:4243–4249.
1998.PubMed/NCBI
|
|
27
|
Pilia G, Hughes-Benzie RM, MacKenzie A, et
al: Mutations in GPC3, a glypican gene, cause the
Simpson-Golabi-Behmel overgrowth syndrome. Nat Genet. 12:241–247.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
De Cat B and David G: Developmental roles
of the glypicans. Semin Cell Dev Biol. 12:117–125. 2001.
|
|
29
|
Capurro MI, Xiang YY, Lobe C and Filmus J:
Glypican-3 promotes the growth of hepatocellular carcinoma by
stimulating canonical Wnt signaling. Cancer Res. 65:6245–6254.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ruan J, Liu F, Chen X, et al: Inhibition
of glypican-3 expression via RNA interference influences the growth
and invasive ability of the MHCC97-H human hepatocellular carcinoma
cell line. International journal of molecular medicine. 28:497–503.
2011.PubMed/NCBI
|
|
31
|
Yang J, Mani SA, Donaher JL, et al: Twist,
a master regulator of morphogenesis, plays an essential role in
tumor metastasis. Cell. 117:927–939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(T)(-Delta Delta C) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Howard S, Deroo T, Fujita Y, et al: A
positive role of cadherin in Wnt/β-catenin signalling during
epithelial-mesenchymal transition. PloS one. 6:e238992011.
|
|
34
|
Yook JI, Li XY, Ota I, et al:
Wnt-dependent regulation of the E-cadherin repressor snail. Journal
of Biological Chemistry. 280:11740–11748. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baeg GH and Perrimon N: Functional binding
of secreted molecules to heparan sulfate proteoglycans in
Drosophila. Curr Opin Cell Biol. 12:575–580. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jackson SM, Nakato H, Sugiura M, et al:
dally, a Drosophila glypican, controls cellular responses to
the TGF-β-related morphogen Dpp. Development. 124:4113–4120.
1997.PubMed/NCBI
|
|
37
|
Perrimon N and Bernfield M: Specificities
of heparin sulphate proteoglycans in developmental processes.
Nature. 404:725–728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Topczewsky J, Sepich DS, Myers DC, et al:
The zebrafish glypican knypek controls cell polarity during
gastrulation movements of convergent extension. Dev Cell.
1:251–264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ohkarawa B, Yamamoto TS, Tada M and Ueno
N: Role of glypican 4 in the regulation of convergent extension
movements during gastrulation in Xenopus laevis.
Development. 130:2129–2138. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
De Cat B, Muyldermans SY, Coomans C, et
al: Processing by proprotein convertases is required for glypican-3
modulation of cell survival, Wnt signaling, and gastrulation
movements. J Cell Biol. 163:625–635. 2003.PubMed/NCBI
|
|
41
|
Kramer KL and Yost HJ: Heparan sulfate
core proteins in cell-cell signaling. Annu Rev Genet. 37:461–484.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baeg GH, Lin X, Khare N, et al: Heparan
sulfate proteoglycans are critical for the organization of the
extracellular distribution of Wingless. Development. 128:87–94.
2001.PubMed/NCBI
|
|
43
|
Han C, Belenkaya TY, Wang B and Lin X:
Drosophila glypicans control the cell-to-cell movement of
hedgehog by a dynamin-independent process. Development.
131:601–611. 2004. View Article : Google Scholar
|
|
44
|
Lum L, Yao S, Mozer B, et al:
Identification of Hedgehog pathway components by RNAi in
Drosophila cultured cells. Science. 299:2039–2045. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Filmus J, Capurro M and Rast J: Glypicans.
Genome Biol. 9:2242008. View Article : Google Scholar
|
|
46
|
Song HH and Filmus J: The role of
glypicans in mammalian development. Biochim Biophys Acta.
1573:241–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Feitelson MA, Sun B, Satiroglu Tufan NL,
et al: Genetic mechanisms of hepatocarcinogenesis. Oncogene.
21:2593–2604. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kern MA, Breuhahn K and Schirmacher P:
Molecular pathogenesis of human hepatocellular carcinoma. Adv
Cancer Res. 86:67–112. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Satoh S, Daigo Y, Furukawa Y, et al: AXIN1
mutations in hepatocellular carcinomas, and growth suppression in
cancer cells by virus-mediated transfer of AXIN1. Nat Genet.
24:245–250. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: at the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li X, Xu Y, Chen Y, et al: SOX2 promotes
tumor metastasis by stimulating epithelial-to-mesenchymal
transition via regulation of WNT/β-catenin signal network. Cancer
Lett. 336:379–389. 2013.PubMed/NCBI
|
|
52
|
van Zijl F, Zulehner G, Petz M, et al:
Epithelial-mesenchymal transition in hepatocellular carcinoma.
Future Oncol. 5:1169–1179. 2009.
|
|
53
|
Weinberg RA: Mechanisms of malignant
progression. Carcinogenesis. 29:1092–1095. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Thiery JP: Epithelial-mesenchymal
transitions in development and pathologies. Curr Opin Cell Biol.
15:740–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Thompson EW and Newgreen DF: Carcinoma
invasion and metastasis: a role for epithelial-mesenchymal
transition? Cancer Res. 65:5991–5995. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Vincan E and Barker N: The upstream
components of the Wnt signalling pathway in the dynamic EMT and MET
associated with colorectal cancer progression. Clinical Exp
Metastasis. 25:657–663. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Prasad CP, Mirza S, Sharma G, et al:
Epigenetic alterations of CDH1 and APC genes: Relationship with
activation of Wnt/β-catenin Pathway in invasive ductal carcinoma of
breast. Life Sci. 83:318–325. 2008.PubMed/NCBI
|
|
58
|
Zhao JH, Luo Y, Jiang YG, et al: Knockdown
of β-Catenin through shRNA cause a reversal of EMT and metastatic
phenotypes induced by HIF-1α. Cancer Invest. 29:377–382. 2011.
|
|
59
|
Miyoshi A, Kitajima Y, Kido S, et al:
Snail accelerates cancer invasion by upregulating MMP expression
and is associated with poor prognosis of hepatocellular carcinoma.
Br J Cancer. 92:252–258. 2005.PubMed/NCBI
|
|
60
|
Liotta LA: Tumor invasion and metastases -
role of the extracellular matrix: Rhoads Memorial Award lecture.
Cancer Res. 46:1–7. 1986.PubMed/NCBI
|
|
61
|
Ban KC, Singh H, Krishnan R and Seow HF:
GSK-3beta phosphorylation and alteration of beta-catenin in
hepatocellular carcinoma. Cancer Lett. 199:201–208. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lucero OM, Dawson DW, Moon RT and Chien
AJ: A reevaluation of the ‘oncogenic’ nature of Wnt/beta-catenin
signaling in melanoma and other cancers. Curr Oncol Rep.
12:314–318. 2010.
|
|
63
|
Sánchez-Tilló E, de Barrios O, Siles L, et
al: β-catenin/TCF4 complex induces the epithelial-to-mesenchymal
transition (EMT)-activator ZEB1 to regulate tumor invasiveness.
Proc Natl Acad Sci USA. 108:19204–19209. 2011.
|
|
64
|
Schmalhofer O, Brabletz S and Brabletz T:
E-cadherin, β-catenin, and ZEB1 in malignant progression of cancer.
Cancer Metastasis Rev. 28:151–166. 2009.
|
|
65
|
Kim K, Daniels KJ and Hay ED:
Tissue-specific expression of beta-catenin in normal mesenchyme and
uveal melanomas and its effect on invasiveness. Exp Cell Res.
245:79–90. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Conacci-Sorrell M, Simcha I, Ben-Yedidia
T, et al: Autoregulation of E-cadherin expression by
cadherin-cadherin interactions: the roles of beta-catenin
signaling, Slug, and MAPK. J Cell Biol. 163:847–857. 2003.
View Article : Google Scholar
|
|
67
|
Onder TT, Gupta PB, Mani S, et al: Loss of
E-cadherin promotes metastasis via multiple downstream
transcriptional pathways. Cancer Res. 68:3645–3654. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Oh SJ, Shin JH, Kim TH, et al: β-Catenin
activation contributes to the pathogenesis of adenomyosis through
epithelial–mesenchymal transition. J Path. 231:210–222. 2013.
|