Introduction
Resveratrol (Res;
C14H12O3; PubChem, CID: 445154;
Fig. 1A) is primarily found in the
skin of grapes and has been demonstrated to exhibit
health-promoting benefits to the coronary, neurological, hepatic
and cardiovascular systems (1–3). In
addition, Res inhibits the proliferation of tumor cells of
different etiologies (4,5). The biological properties of Res have
been described in detail (6–9). The
predominant form of resveratrol in plants is the glycosylated
(3-O-β-D-glucosides) form (also termed the piceid form). It has
traditionally been used as an anti-inflammatory agent. Other
beneficial properties of resveratrol include antioxidant effects,
cardioprotection and increased longevity. The antitumor activities
of Res are mediated through several cell signaling pathways,
including cell cycle arrest, suppression of tumor cell
proliferation, induction of apoptosis and differentiation,
angiogenesis and the inhibition of invasion, adhesion and
metastasis (10). Skin cancer is
one of the major causes of cancer-associated mortality worldwide.
In addition, human cutaneous malignant melanoma is an aggressive
cause of mortality, which exhibits a rising trend every year
(9). In order to inhibit the
development of cancer, pharmacological or natural chemopreventive
and chemotherapeutic agents are commonly used (12).
p53 is a frequent target for mutation in various
types of human tumor. It functions as a cell nucleus phosphate
protein, which responds to various types and levels of stress
arising from apoptosis, cell cycle arrest, senescence, DNA repair
and cell metabolism (13–15). A previous study demonstrated that
gambogic acid, an efficient apoptosis inducer, was able to repress
the expression of B-cell lymphoma 2 (Bcl-2) via increasing the
level of p53 in MCF-7 cells (16).
Although previous studies have described intracellular changes
leading to cell cycle arrest or apoptosis in response to Res
treatment, the precise mechanisms underlying Res-regulated tumor
growth remain to be fully elucidated (20–22).
In the present study, the anti-cancer effects of Res in human
melanoma A375 and SK-MEL-31 cells were assessed. The results
demonstrated for the first time, to the best of our knowledge, that
the regulation of caspase and p53 proteins was involved in the
induction of apoptosis and cell cycle arrest by Res.
Materials and methods
Cell culture
Human melanoma A375 and SK-MEL-31 cells, obtained
from the Affiliated Hospital of Guangdong Medical College
(Guangdong, China), were grown as a suspension culture in
Dulbecco’s modified Eagle’s medium (Invitrogen Life Technologies,
Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum
(Gibco-BRL, Carlsbad, CA, USA) 100 U/ml penicillin and 100 U/ml
streptomycin. They were maintained in a humidified atmosphere of
95% air and 5% CO2 at 37°C.
Reagents
Res was purchased from Sigma-Aldrich (St. Louis, MO,
USA). Monoclonal human anti-rabbit antibodies to caspase-9,
caspase-3, Bcl-2, Bcl-2-associated X protein (Bax), p53 and GAPDH
were obtained from Cell Signaling Technology, Inc. (Beverly, MA,
USA) and goat anti-rabbit IgG-horseradish peroxidase (EarthOx Life
Sciences, Millbrae, CA, USA) was used as a secondary antibody.
Cell viability assays
The cell densities were adjusted to 2×104
cells/100 μl. The cells were seeded into a 96-well plate and
treated with 10, 50 and 100 μM Res for 24 h. The cell viability was
then assessed using a Cell Titer 96 AQueous One Solution Cell
Proliferation Assay kit (Promega Corporation, Madison, WI, USA)
according to the manufacturer’s instructions.
Analysis of cell apoptosis and cell cycle
arrest
Cells were pretreated with 10, 50 and 100 μM Res for
48 h. Cells were quantified using a Cell Cycle Analysis kit
(Beyotime Institute of Biotechnology) and a PE Annexin V Apoptosis
Detection kit I (BD Biosciences, Franklin Lakes, NJ, USA),
respectively. Analysis was preformed using flow cytometry
(FACSCalibur; Beckton Dickinson, Franklin Lakes, NJ, USA) and then
analyzed using ModFit and CellQuest software (BD Biosciences).
Western blot analysis
Cells were lysed in lysis buffer (100 mM
Tris-hydrochloride, 4% pH 6.8 (m/v) sodium dodecyl sulfonate, 20%
(v/v), glycerol, 200 mM mercaptoethanol, 1 mM phenylmethyl
sulfonylfluoride and 1 g/ml aprotinin). The total protein
concentration in the supernatants was detected using a BCA Protein
assay kit (Beyotime Institute of Biotechnology). Proteins were then
transferred onto nitrocellulose membranes (Millipore, Billerica,
MA, USA). Detection was performed using an Odyssey Infrared Imaging
System (LI-COR Biosciences, Lincoln, NE, USA).
Statistical analysis
The data were analyzed using GraphPad Prism 5
software (GraphPad Software, Inc., San Diego, CA, USA). All results
are expressed as the mean ± standard deviation from triplicate
experiments performed in a parallel manner, unless otherwise
indicated.
Results
Res induces growth inhibition,
proliferation and cell apoptosis in A375 and SK-MEL-31 cells
In the Res-treated cells, certain cells became round
and floating and a marked reduction in cell viability was observed
over the experimental period in a concentration-dependent manner
(Fig. 1B and C). Following
treatment with 50 and 100 μM Res, inhibition of the cell cycle was
observed in the A375 and SK-MEL-31 cells (Fig. 2). The population of cells in the G1
phase increased in A375 (78–93%) and SK-MEL-31 (78–94%) cells,
coinciding with a reduction in cells in the S phase, when compared
with the vehicle-treated control. This result demonstrated that the
primary growth inhibitory effect of Res was due to inhibition of
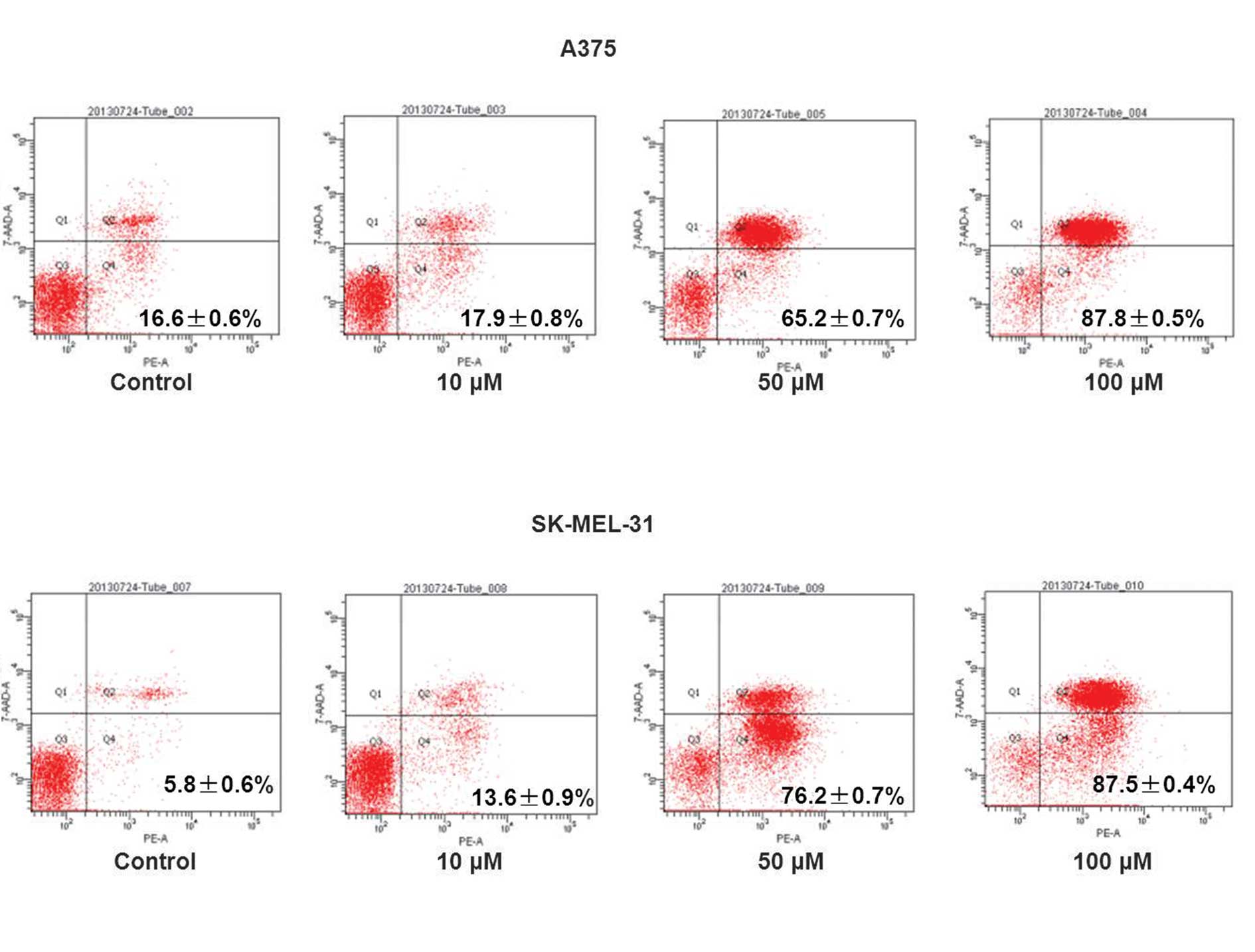
the cell cycle at the G1 phase. Cell apoptosis was detected using
flow cytometry. Data revealed that Res induced cell apoptosis in a
concentration-dependent manner (Fig.
3). In addition, the Q2 and Q4 cell population in A375
(16.6–87.8%) and SK-MEL-31 (5.8–87.5%) cells increased compared
with the vehicle-treated control (Fig.
3).
Res induces the expression of cell cycle
and apoptotic-related proteins in A375 and SK-MEL-31 cells
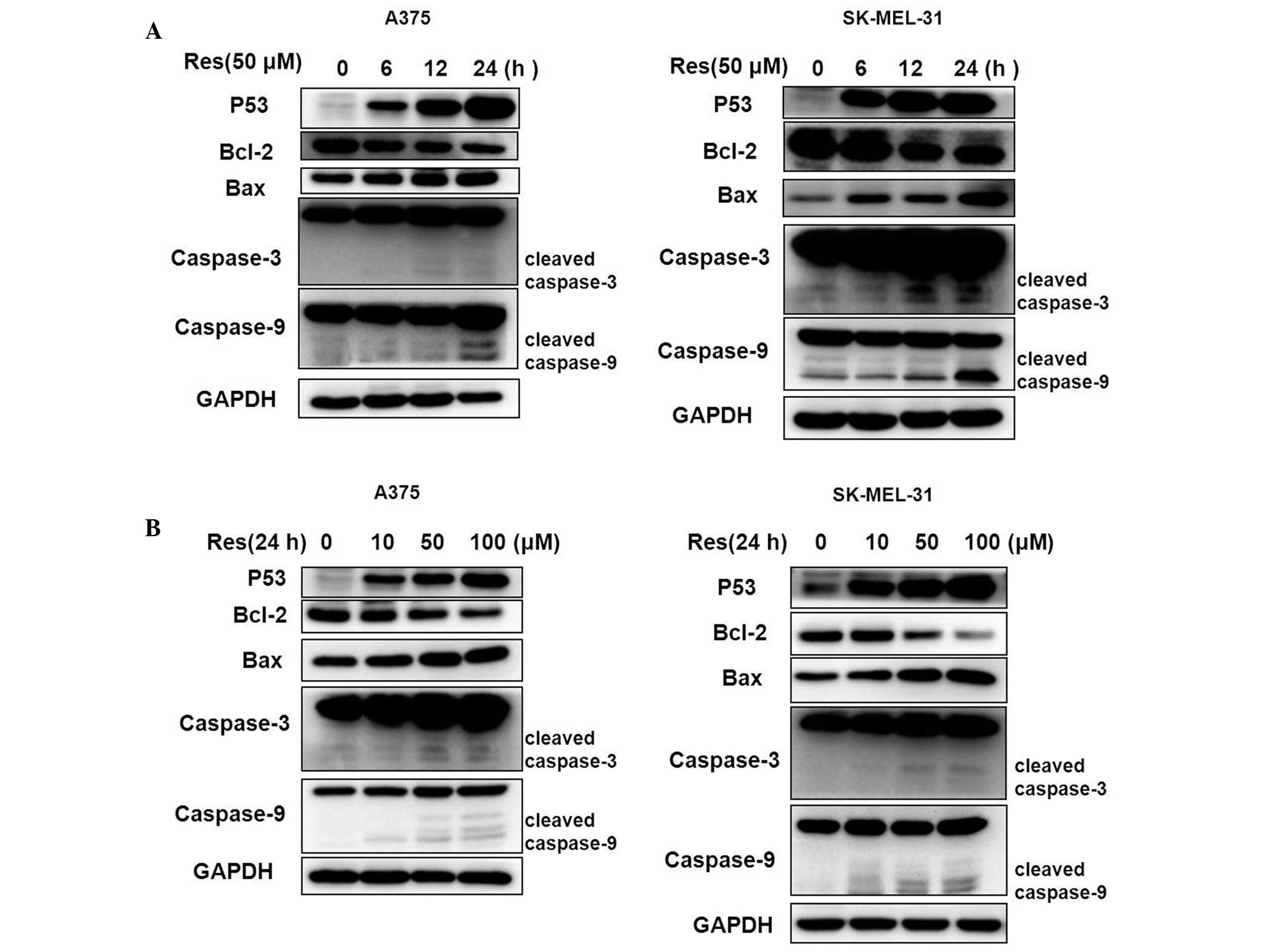
Western blot analysis demonstrated that cell cycle
and apoptotic-related proteins altered following treatment with Res
(Fig. 4). The Bcl-2 protein was
downregulated, however, the apoptotic proteins of p53, Bax,
caspase-3 and caspase-9 were markedly upregulated in a
concentration- and time-dependent manner.
Discussion
Res, a natural plant polyphenol compound, has been
extensively investigated for several years due to its various
potential health-promoting benefits (8,20–23).
In the present study, the effects of Res in human melanoma A375 and
SK-MEL-31 cell lines was investigated. The inhibitory and
apoptotic-promoting effects of Res on the growth of two human
melanoma cells were determined, these cells have different genetic
aberrations and acquired growth aggressiveness. The results from
the cell viability and apoptotic rate assay demonstrated that the
anticancer properties of Res in A375 and SK-MEL-31 cells were
almost identical. The cell viability, G1 phase cell-cycle arrest
and apoptotic rates increased in a concentration-dependent manner.
Subsequently, the cell cycle and apoptotic-related proteins p53,
Bcl-2, Bax, caspase-3 and caspase-9 were analyzed. The protein
expression of Bcl-2 reduced, however, the protein expression of
p53, Bax, caspase-3 and caspase-9 were markedly enhanced compared
with the control. Cleaved caspase-9, cleaved caspase-3 and the
ratio of Bax and Bcl-2 increased gradually in a concentration- and
time-dependent manner. Caspase-9 and caspase-3 activation is a
crucial step in apoptotic cell death (24,25)
and the increased expression of cleaved caspase-9 and cleaved
caspase-3 may be considered as a marker of apoptosis. The
apoptosis-inducing effect is more dependent on the balance of Bcl-2
and Bax than on the quantity of Bcl-2 alone, which is important in
cell proliferation. The steady state of cell survival is decided by
the balance of Bcl-2 and Bax expression (26,27).
Previous studies have demonstrated that the apoptotic inducer, p53,
induces cell growth arrest, apoptosis and senescence in response to
different stimuli and this was associated with cancer cell
metastasis (28–31). The increased expression level of
the Bax/Bcl-2 protein ratio in cells treated with Res suggested
that the p53 and Bax/Bcl-2 proteins are important in Res-induced
apoptosis.
Acknowledgements
This study was supported, in part, by the following
grants: The National Natural Science Fund (no. 81041099) and the
Guangdong Province Natural Science Fund (no. S2011010003750),
China. This study was also supported by the Institute of Neurology,
Affiliated Hospital of Guangdong Medical College and National
Natural Science Foundation of China (no. 81102066), the Natural
Science Foundation of Guangdong Province of China (no.
S2011040003129) and the Medical Research Foundation of Guangdong
Province of China (no. A2011441).
References
|
1
|
Chan V, Fenning A, Iver A, et al:
Resveratol improves cardiovascular function in DOCA-salt
hypertensive rats. Curr Pharm Biotechnol. 12:429–436. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang L, Gu Y, Ye J, Liu F, et al:
Resveratrol prevents hepatic steatosis induced by hepatitis C virus
core protein. Biotechnol Lett. 34:2205–2212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang F, Liu J and Shi JS:
Anti-inflammatory activities of resveratrol in the brain: role of
resveratrol in microglial activation. Eur J Pharmacol. 25:1–7.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin JN, Lin VC, Rau KM, et al: Resveratrol
modulates tumor cell proliferation and protein translation via
SIRT1-dependent AMPK activation. J Agric Food Chem. 58:1584–92.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang F, Wu XN, Chen J, et al: Resveratrol
reverses multidrug resistance in human breast cancer
doxorubicin-resistant cells. Exp Ther Med. 7:1611–1616.
2014.PubMed/NCBI
|
|
6
|
Fulda S: Resveratrol and derivatives for
the prevention and treatment of cancer. Drug Discov Today.
15:757–765. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calabrese EJ, Mattson MP and Calabrese V:
Dose response biology: the case of resveratrol. Hum Exp Toxicol.
29:1034–1037. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park SJ, Ahmad F, Philp A, et al:
Resveratrol ameliorates aging-related metabolic phenotypes by
inhibiting cAMP phosphodiesterases. Cell. 148:421–433. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bishayee A, Politis T and Darvesh AS:
Resveratrol in the chemoprevention and treatment of hepatocellular
carcinoma. Cancer Treat Rev. 36:43–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bishayee A: Cancer prevention and
treatment with resveratrol: from rodent studies to clinical trials.
Cancer Prev Res (Philia). 2:409–418. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsiao YP, Yu CS, Yu CC, et al: Triggering
apoptotic death of human malignant melanoma a375.s2 cells by
bufalin: Involvement of caspase cascade-dependent and independent
mitochondrial signaling pathways. Evi Based Complemen Alternat Med.
2012:5912412012.
|
|
12
|
Kuo JH, Chu YL, Yang JS, et al:
Cantharidin induces apoptosis in human bladder cancer TSGH 8301
cells through mitochondria-dependent signal pathways. Int J Oncol.
37:1243–1250. 2010.PubMed/NCBI
|
|
13
|
Vazquez A, Bond EE, Levine AJ and Bond GL:
The genetics of the p53 pathway, apoptosis and cancer therapy. Nat
Rev Drug Discov. 7:979–987. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brady CA, Jiang D, Mello SS, et al:
Distinct p53 transcriptional programs dictate acute DNA-damage
responses and tumor suppression. Cell. 145:571–583. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chee JL, Saidin S, Lane DP, et al:
Wild-type and mutant p53 mediate cisplatin resistance through
interaction and inhibition of active caspase-9. Cell Cycle.
12:278–288. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gu H, Rao S, Zhao J, et al: Gambogic acid
reduced bcl-2 expression via p53 in human breast MCF-7 cancer
cells. J Cancer Res Clin Oncol. 135:1777–1782. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu B, Zhou Z, Zhou W, et al: Resveratrol
inhibits proliferation in human colorectal carcinoma cells by
inducing G1/S-phase cell cycle arrest and apoptosis through
caspase/cyclin-CDK pathways. Mol Med Rep. 10:1697–1702.
2014.PubMed/NCBI
|
|
18
|
Li P, Yang S, Dou M, et al: Synergic
effects of artemisinin and resveratrol in cancer cells. J Cancer
Res Clin Oncol. 2014:Epub ahead of print. 2014.
|
|
19
|
Zhang P, Li H, Yang B, et al: Biological
significance and therapeutic implication of resveratrol-inhibited
Wnt, Notch and STAT3 signaling in cervical cancer cells. Genes
Cancer. 5:154–64. 2014.PubMed/NCBI
|
|
20
|
Szekeres T, Saiko P, Fritzer-Szekeres M,
Djavan B and Jäger W: Chemopreventive effects of resveratrol and
resveratrol derivatives. Ann NY Acad Sci. 1215:89–95. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nutakul W, Sobers HS, Qiu P, et al:
Inhibitory effects of resveratrol and pterostilbene on human colon
cancer cells: a side-by-side comparison. J Agric Food Chem.
59:10964–10970. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Piotrowska H, Kucinska M and Murias M:
Biological activity of piceatannol: Leaving the shadow of
resveratrol. Mutat Res. 750:60–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Um JH, Park SJ, Kang H, et al:
AMP-activated protein kinase-deficient mice are resistant to the
metabolic effects of resveratrol. Diabetes. 59:554–563. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y, Liu H, Jin J, Zhu X, Lu L and
Jiang H: The role of endogenous reactive oxygen species in
oxymatrine-induced caspase-3-dependent apoptosis in human melanoma
A375 cells. Anticancer Drugs. 21:494–501. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyata M, Kambe M, Tajima O, et al:
Membrane sialidase NEU3 is highly expressed in human melanoma cells
promoting cell growth with minimal changes in the composition of
gangliosides. Cancer Sci. 102:2139–2149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tasyriq M, Najmuldeen IA, In LL, Mohamad
K, Awang K and Hasima N: 7 alpha-Hydroxy-beta-Sitosterol from
Chisocheton tomentosus induces apoptosis via dysregulation
of cellular Bax/Bcl-2 ratio and cell cycle arrest by downregulating
ERK1/2 activation. Evid Based Complement Alternat Med.
2012:7653162012.PubMed/NCBI
|
|
27
|
Wu S, Liu B, Zhang Q, et al:
Dihydromyricetin reduced Bcl-2 expression via p53 in human hepatoma
HepG2 cells. PloS One. 8:e768862013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu HB, Yang K, Xie YQ, Lin YW, Mao QQ and
Xie LP: Silencing of mutant p53 by siRNA induces cell cycle arrest
and apoptosis in human bladder cancer cells. World J Surg Oncology.
11:222013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yeudall WA, Wrighton KH and Deb S: Mutant
p53 in cell adhesion and motility. Methods Mol Biol. 962:135–146.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Voskamp P, Bodmann CA, Koehl GE, et al:
Dietary immunosuppressants do not enhance UV-induced skin
carcinogenesis, and reveal discordance between p53-mutant early
clones and carcinomas. Cancer Prev Res. 6:129–138. 2013. View Article : Google Scholar
|
|
31
|
Lee JH, Gaddameedhi S, Ozturk N, Ye R and
Sancar A: DNA damage-specific control of cell death by cryptochrome
in p53-mutant ras-transformed cells. Cancer Res. 73:785–791. 2013.
View Article : Google Scholar : PubMed/NCBI
|