Introduction
A glioma is a tumor that originates in the brain or
spine, arising from glial cells. The most common site of gliomas is
in the brain (1). Gliomas
attribute for ~30% of all brain and central nervous system tumors,
and ~80% of all malignant brain tumors. The anthracyclines, such as
adriamycin (ADM), are among the most effective anticancer
treatments, and are effective against more types of cancer than any
other class of chemotherapeutic agents (2–4).
However, the effect of chemotherapy on the survival rate in
patients with malignant glioma is low, giving an improvement in
survival rate of 6% after one year and 5% after two years (5). This poor response to therapy may be
due to multi-drug resistance (MDR) (6).
Mechanisms to explain drug resistance have been
reported to include the blood-brain barrier (BBB), efflux pumps,
DNA repair and cancer stem-like cells (7–9).
P-glycoprotein (Pgp), the MDR1 gene product in humans, is a
drug efflux pump which has a significant role in modulating MDR in
a wide variety of human cancers (10,11).
Pgp is highly expressed in the BBB, with weak expression in tumor
cells (12). MDR-associated
protein 1 (MRP1), expressed in glioma cell lines, has been
suggested to be involved in MDR both in vivo and in
vitro (13,14). Low density lipoprotein
receptor-related protein (LRP) is a member of the low density
lipoprotein (LDL) receptor (15).
A previous study showed that the association of statins, plus an
LDL receptor-targeted liposomal drug, may increase in vitro
drug delivery across the BBB (16). It has been previously observed that
the expression levels of prostaglandin-endoperoxide synthase 2
(COX-2), protein kinase C (PKC) and Activator Protein 1 were
significantly upregulated in drug resistant cell lines that
overexpress MDR1/Pgp170, suggesting that there is a potential link
between COX-2, PKC and MDR1/Pgp170 (17). Glutathione S transferase π (GSTπ)
is another protein that catalyzes conjugation reactions of
glutathione (GSH), linking to cytotoxic drugs and leading to their
inactivation (18). In addition,
the Fanconi anemia (FA) pathway is involved in glioma drug
resistance (19).
The pattern of methylation is an important line of
research in MDR (20–22). Recent research has demonstrated
that acquired gemcitabine resistance is associated with DNA
promoter methylation-independent human equilibrative nucleoside
transporter 1 and deoxycytidine kinase gene downregulation and
hyper-expression of G9A methyltransferase (21). The death-associated protein kinase
(DAPK) is hypermethylated in drug-resistant derivatives generated
from both non-small cell lung cancer (NSCLC) and head and neck
squamous cell carcinoma (HNSCC) cell lines (22). Expression of O6-methylguanine-DNA
methyltransferase is associated with resistance to radiotherapy in
gliomas (23). However, the
association between methylation and MDR in glioma has yet to be
evaluated.
In the present study, a stable MDR glioma cell line,
SGH-44/ADM, was generated and the association between DNA
methylation and MDR was analyzed using a methylation DNA
immunoprecipitation microarray chip (MeDIP-Chip). The findings from
this study may provide novel insight for the treatment of
gliomas.
Materials and methods
Cell culture and construction of an ADM
resistant SGH-44/ADM cell line
The human glioma cell line SHG-44 (Medicine College
of Suzhou University, Suzhou, China) was routinely cultured in RPMI
1640 medium (Gibco-BRL, Invitrogen Life Technologies, Carlsbad, CA,
USA) supplemented with 5 or 10% heat-inactivated fetal bovine serum
(FBS; HyClone Laboratories, Inc., Logan, Utah, USA), l00 U/ml
penicillin and 100 U/ml streptomycin (Qilu Pharmaceutical Co.,
Ltd., Jinan, China). An ADM-resistant cell line of SHG-44, named as
SHG-44/ADM, was obtained by culturing the cells in the presence of
gradually increasing doses of ADM (Shenzhen Main Luck
Pharmaceuticals Inc., Shenzhen, China). In brief, SHG-44 cells were
cultured in RPMI 1640 medium with 0.01 mg/ml ADM. The medium was
removed and replaced after 48 h, and the cells were cultured to
resume normal growth. The cells were then transferred and treated
with 0.1 mg/ml ADM. The above process was repeated to obtain
ADM-resistant cells. After eight months, cells cultured in 0.1 μM
ADM were harvested as the ADM-resistant cell line.
Antitumor drug resistance of SHG-44 and
SGH-44/ADM
The proliferation inhibition of simvastatin on
glioma cell lines was determined using a WST-8 Cell Counting Kit-8
(CCK-8; Beyotime Institute of Biotechnology, Haimen, China). The
cells were seeded in fresh RPMI 1640 medium containing 10% fetal
calf serum 24 h prior to the experiments. Cells were seeded at an
initial concentration of 1×105 cells/ml in 100 μl medium
in 96-well culture plates with various concentrations of ADM,
epinephrine (EPI; Harvest Pharmaceutical Co., Ltd., Shanghai,
China), vincristine (VCR; Shenzhen Main Luck Pharmaceuticals Inc.),
mitomycin C (MMC; Nanjing Duoyan Biochemistry Co., Ltd., Nanjing,
China), arabinofuranosyl cytidine (Ara-C; Nanjing Duoyan
Biochemistry Co., Ltd.,) and cisplatin (DDP; Qilu Pharmaceutical
Co., Ltd.) for 72 h. Then 20 μl of CCK-8 staining solution was
added to each well for 2 h at 37°C before the end of the
incubation. The absorbance at 450 nm was measured using a
microplate reader (Model 550; Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The half inhibitory concentration (IC50) and
resistance index (RI) were calculated.
Effect of cryopreservation, recovery and
withdrawal on the RI
SHG44/ADM cells were stored in liquid nitrogen,
recovered after three months, and then the RI was measured. In
addition, the RI of SHG44/ADM cells, which were cultured without
ADM for one month, was also measured.
Analysis of adherence rate, cellular
morphology, cell growth curve and doubling time
The cellular morphology was observed using an
inverted microscope (IX-71, Olympus Corporation, Tokyo, Japan).
Cells were seeded at an initial concentration of 1×105
cells/ml in 24-well culture plates. The pelagic cells were counted
every two hours in an eight-hour period. The adherence rate (AR)
was calculated as (1 - Cad/Ctotal) × 100%.
Cells were seeded at an initial concentration of
5×105cells/ml in 24-well culture plates, and the viable
cells were counted every day for one week. The cell growth curve
was constructed, and the doubling time was calculated using the
Patterson formula: Td = T × lg2/(1gN2-lgN1). Where Td is doubling
time; T is time interval; N2 is end point cell number; and N1 is
initial cell number.
Double layer soft agar detection for
colony formation
Cells were diluted to 125, 250 and 500 cells/ml with
culture medium, respectively. The diluted cells were then mixed 1:1
with 0.7% agar and poured on 1.2% concretionary agar. When the
upper layer was curdled, the cells were cultured for seven days at
37°C with 5% CO2. The colony with >50 cells was
counted.
Flow cytometry for cell cycle
analysis
The measurement of the cell cycle was performed by
flow cytometry (BD FACSCalibur™, BD Biosciences, Franklin Lakes,
NJ, USA). After synchronization, the cells were cultured in fresh
medium with 10% FBS to 90% confluency, harvested and suspended with
0.3 ml phosphate buffered saline (PBS) containing 5% FBS. A volume
of 0.7 ml ethanol was added for immobilization. The cells were
stored at −20°C for 24 h and washed with PBS. RNase A, 0.1 mg, was
added for RNA degradation, then the cells were placed in the dark
for 10 min after adding 0.2 μg propidium iodide (PI). The
percentages of cells in different phases were detected by flow
cytometry.
Methylated DNA immunoprecipitation
microarray chip (MeDIP-Chip) and analysis
The DNA of SHG-44 and SHG-44/ADM was extracted using
a DNA extraction kit (Qiagen, Hilden, Germany). The methylated DNA
was purified using EpiQuik Methylated DNA Immunoprecipitation Kit
(Qianchen Biotechnology Company, Shanghai, China). The target DNA
was amplified with primer A and digested with Udp and
ApeI restriction enzymes. The DNA was mixed with 12 μl TdT
buffer, 2 μl TdT and 1 μl DNA Labeling reagent. The mixture was
used for gel shift analysis. The data analysis was performed as
previously described (24).
Pathway analysis of MeDIP-Chip
Pathway analysis was performed according to the
Kyoto Encyclopedia of Genes and Genomes (KEGG; www.genome.jp/kegg/), BioCarta (www.biocarta.com) and Reactome (www.reactome.org). In addition, Fisher’s exact test
and χ2 test was performed to select the significant
pathways, and the threshold of significance was defined by P-value
and false discovery rate (FDR). The enrichment (Re) was calculated
as Re = (nf/n)/(Nf/N), where nf
was the number of flagged genes within the particular category, n
was the total number of genes within the same category,
Nf was the number of flagged genes in the entire
microarray and N was the total number of genes in the microarray
(25–27).
Gene ontology (GO) analysis of
MeDIP-Chip
GO analysis was applied to analyze the main
functions of the differentially expressed genes according to the
GO, which was the key functional classification of the National
Center for Biotechnology Information (NCBI), which can organize
genes into hierarchical categories and uncover the gene regulatory
network on the basis of biological processes and molecular function
(28,29). Specifically, a two-tailed Fisher’s
exact test and χ2 test was used to classify the GO
category, and the FDR (30) was
calculated to correct the P-value. The smaller the FDR, the smaller
the error in judging the P-value. The FDR was defined as FDR
= 1 - Nk/T, where
Nk referred to the number of Fisher’s test
P-values < χ2 test P-values. The P-values were
computed for the GOs of all the differential genes. Enrichment
provided a measure of the significance of the function. As the
enrichment increased, the corresponding function was more specific.
This helped identify those GOs with a conclusive function
description in the experiment. Within the significant category, the
enrichment Re was given by: Re =
(nf/n)/(Nf/N)
with the variables defined as stated above (31).
Expression analysis of MDR1, MRP1, LRP1,
PKCα and COX-2
Total RNA of the lymph node was isolated using
TRIzol™ reagent (Invitrogen Life Technologies). The reverse
transcription reaction was conducted using random primers and the
SuperScript III first-strand synthesis system (Invitrogen Life
Technologies). The quantitative polymerase chain reaction (qPCR)
was conducted using the following conditions: 95°C for 30 sec
followed by 40 cycles of 94°C for 5 sec and 60°C for 30 sec, and
dissociation curve analysis of the amplification products was
performed at the end of each PCR reaction to confirm that only one
product was amplified and detected. Each sample was run in
triplicate together with the internal control gene, GAPDH.
Data analysis of the qPCR was performed with Rotor Gene 6000 Series
Software (Corbett Life Sciences, Sydney, Australia). The specific
primer sequences (Invitrogen, Shnaghai, China) are listed in
Table I.
 | Table ISpecific primers for quantitative
polymerase chain reaction. |
Table I
Specific primers for quantitative
polymerase chain reaction.
| Genes | Primer
sequence |
|---|
| PRPS1 | F:
5′-ATCTTCTCCGGTCCTGCTATT-3′
R: 5′-TGGTGACTACTACTGCCTCAAA-3′ |
| ARRB2 | F:
5′-TCCATGCTCCGTCACACTG-3′
R: 5′-ACAGAAGGCTCGAATCTCAAAG-3′ |
| GBA | F:
5′-CATCCGCACCTACACCTATGC-3′
R: 5′-TGAGCTTGGTATCTTCCTCTGG-3′ |
| GAPDH | F:
5′-GGAGCGAGATCCCTCCAAAAT-3′
R: 5′-GGCTGTTGTCATACTTCTCATGG-3′ |
The SGH-44/ADM and SGH-44 cell lines were washed
three times with PBS and cell lysates were prepared in lysis
buffer. Protein concentration was estimated using the bicinchoninic
acid assay (Pierce Biotechnology, Inc., Rockford, IL, USA) and 20
mg of protein was loaded in each lane. The proteins were resolved
by 10% SDS-PAGE. The gel was transferred to a nitrocellulose
membrane and blocked with 5% non-fat dry milk (NFDM) for one hour
at room temperature. The blot was then incubated with primary
antibodies for overnight at 4°C in 2.5% NFDM. Blots were washed
with Tris-buffered saline containing 0.1% Tween 20 three times and
subsequently incubated with horseradish peroxidase-conjugated
goat-anti-rabbit immunoglobulin G antibody (Pierce Biotechnology,
Inc.). Enhanced Chemiluminescence substrate (Pierce Biotechnology,
Inc.) was used to detect the signal. The blots were stripped and
re-probed with mouse monoclonal β-actin antibody, which served as
an internal control. All the antibodies were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA).
Rhodamine-123 (Rh123) ingestion and
exuding of SGH-44/ADM
The SGH-44 and SGH-44/ADM cells were diluted to
1×106 cells/ml with medium. Rh123 (Sigma-Aldrich) was
added to the cells to a final concentration of 1 μg/ml. The cells
were incubated at 37°C for 1 h and washed three times with PBS. The
relative fluorescence intensity of Rh123 accumulated in the cells
was measured by flow cytometry at 4°C. To detect the Rh123
exudation, the cells were diluted with RPMI 1640 medium and
incubated for 2 h. Then the cells were washed three times with PBS
and the relative fluorescence activity of Rh123 was measured
again.
Apoptosis of SGH-44 and SGH-44/ADM
SGH-44 and SGH-44/ADM cells were collected and
diluted in PBS. Annexin V-fluorescein isothiocyanate (FITC; Nanjing
KeyGen Biotech. Co., Ltd., Nanjing, China), 195 μl, was added to
5×104 cells and the solution (1 μg/ml) was incubated in
the dark for 10 min and centrifuged. Following this, 190 μl Annexin
V-FITC and 10 μl propidium iodide (PI; Molecular Probes®
Life Technologies, Carlsbad, CA, USA) was added. The solution was
analyzed by flow cytometry. To evaluate the effect of MDR1 on the
MDR of SGH-44/ADM, cyclosporin A (CsA; Sandoz, Holzkirchen,
Germany), an MDR1 inhibitor, was added to the SGH-44/ADM cells. A
fluorometric assay was performed as previously described (32).
Results
Antitumor drug sensitivities and
stability of SGH-44/ADM
The IC50 to ADM of wild-type SGH-44 was
1.67 μg/ml, while the IC50 to ADM of SGH-44/ADM was
17.83 μg/ml. The RI of SGH-44/ADM was 10.7, and following freezing
and recovery of the cells, the RI was 10.5. If the ADM was
withdrawn, the RI of SGH-44/ADM was reduced to 9.3. The resistance
of SGH-44/ADM to other antitumor drugs is shown in Table II.
 | Table IIResistance of SGH-44 and SGH-44/ADM
to different drugs. |
Table II
Resistance of SGH-44 and SGH-44/ADM
to different drugs.
| IC50
(μg/ml) | |
|---|
|
| |
|---|
| Drugs | SGH-44 | SGH-44/ADM | RI |
|---|
| ADM | 1.67±0.05 | 17.83±0.52 | 10.7 |
| EPI | 0.87±0.06 | 7.22±0.14 | 8.3 |
| VCR | 2.95±0.09 | 21.42±0.67 | 7.2 |
| MMC | 8.74±0.33 | 47.73±1.12 | 5.4 |
| Ara-C | 8.38±0.42 | 10.27±0.39 | 1.2 |
| DDP | 0.78±0.07 | 2.46±0.11 | 3.1 |
Adherence rate, cellular morphology, cell
growth curve and doubling time
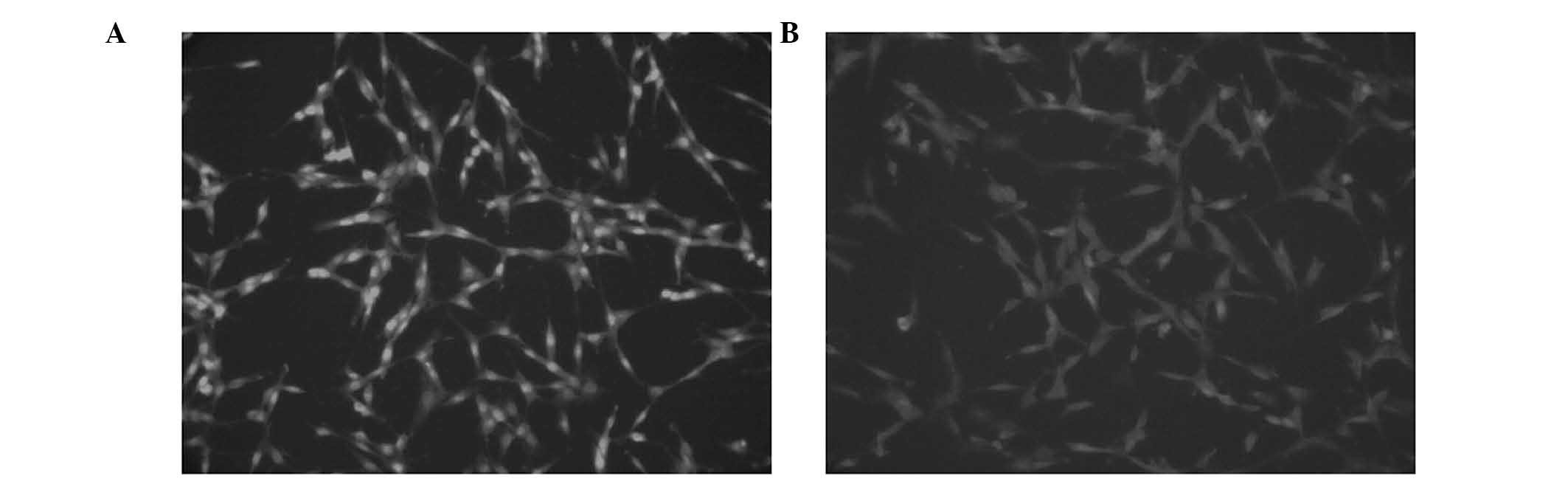
The cell shape of SGH-44 and SGH-44/ADM cells is
shown in Fig. 1. In the drug
resistance formation process, the SGH-44 cells were shaped as
elongated fibers, uniform in size, tightly packed, adherent to the
plate and grown with a clear boundary. Upon administration of the
drugs, the surviving cells were not uniform in size, weakly
adherent to the plate and shaped with a clear boundary. The most
significant difference between the SGH-44 and SGH-44/ADM cells was
that the SGH-44 cells had larger nuclei as compared with SGH-44/ADM
cells. With extension of the incubation time and number of
passages, this emergent abnormal state was reduced. Finally,
SGH-44/ADM cells showed minimal differences as compared with
parental cells, apart from SGH-44/ADM cells being bigger in size,
and with a wizened nucleus.
The adherence rate was calculated in order to
evaluate the viability of SGH-44/ADM cells. The adherence rate of
SGH-44 cells at 0, 2, 4, 6 and 8 h was 0, 68.5, 86.6, 93.5 and
96.8%, respectively. The adherence rate of SGH-44/ADM cells at 0,
2, 4, 6 and 8 h was 0, 62.3, 81.5, 91.6 and 94.7%, respectively.
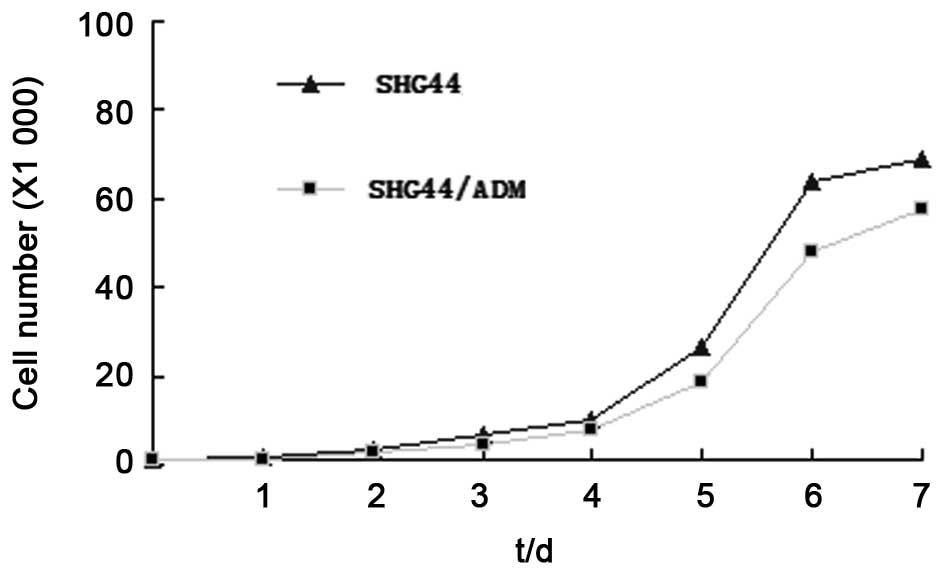
The growth curve is shown in Fig.
2. SGH-44/ADM cells showed a slower growth rate as compared
with parental cells. The doubling time of SGH-44/ADM was 32.22 h as
compared with 26.67 h for SGH-44 cells.
Improvement of the rate of colony
formation in SGH-44/ADM cells
The colony formation rate was calculated as the
percentage of cells which could form small groups when dispersed
into single cells. Double-layer soft agar experiments showed that
the colony formation rate of SGH-44 cells was 20%, whereas the
colony formation rate of SGH-44/ADM cells was significantly
improved to 52% (P<0.01).
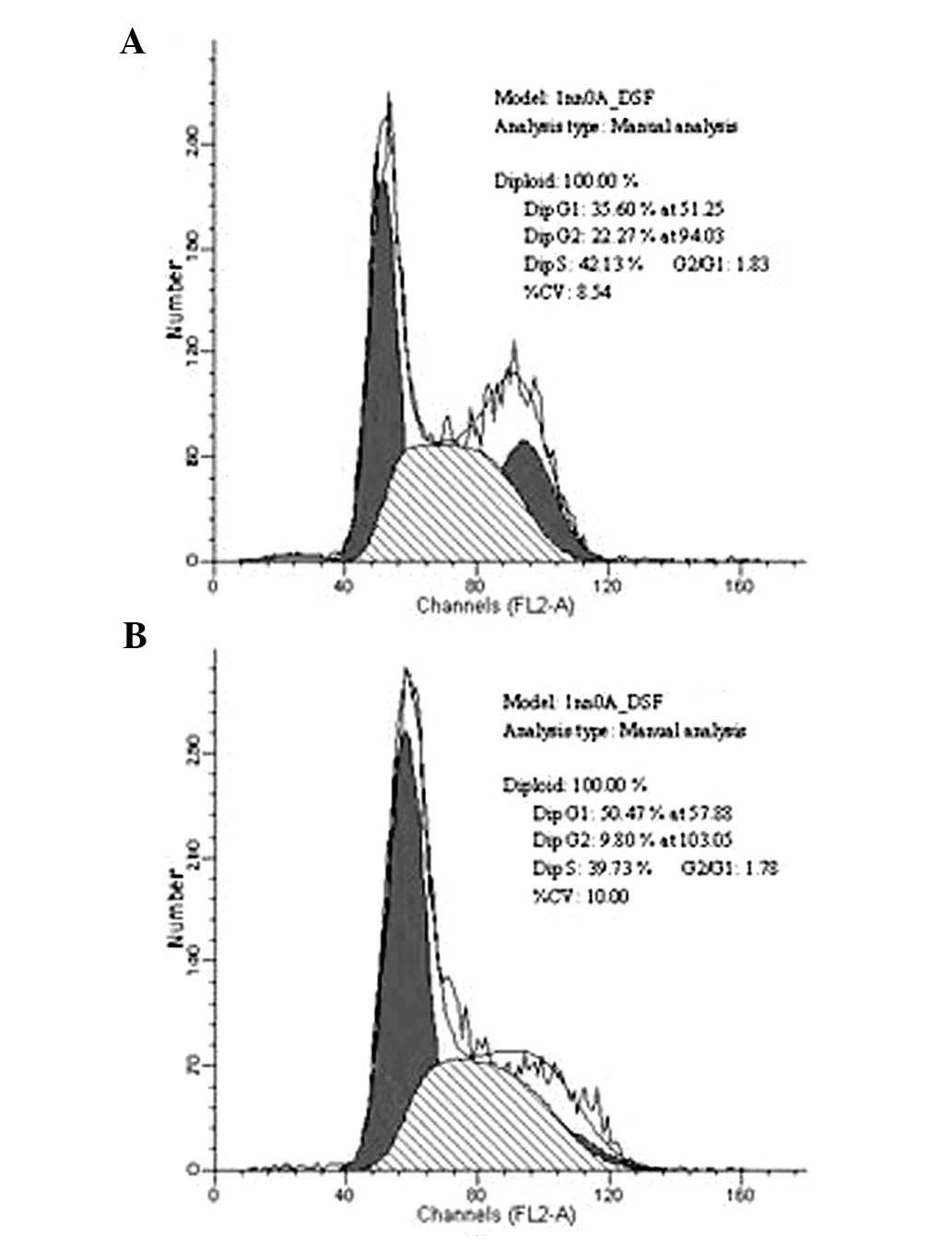
Analysis of the cell cycle by flow
cytometry
Flow cytometry was used to evaluate the percentage
of SGH-44 and SGH-44/ADM cells in different phases of the cell
cycle. The percentage of cells in G1 phase was increased from 35.6
to 50.4% when SGH-44 acquired ADM resistance. The percentage of
cells in S phase was 39.7% in SGH-44/ADM cells (Fig. 3).
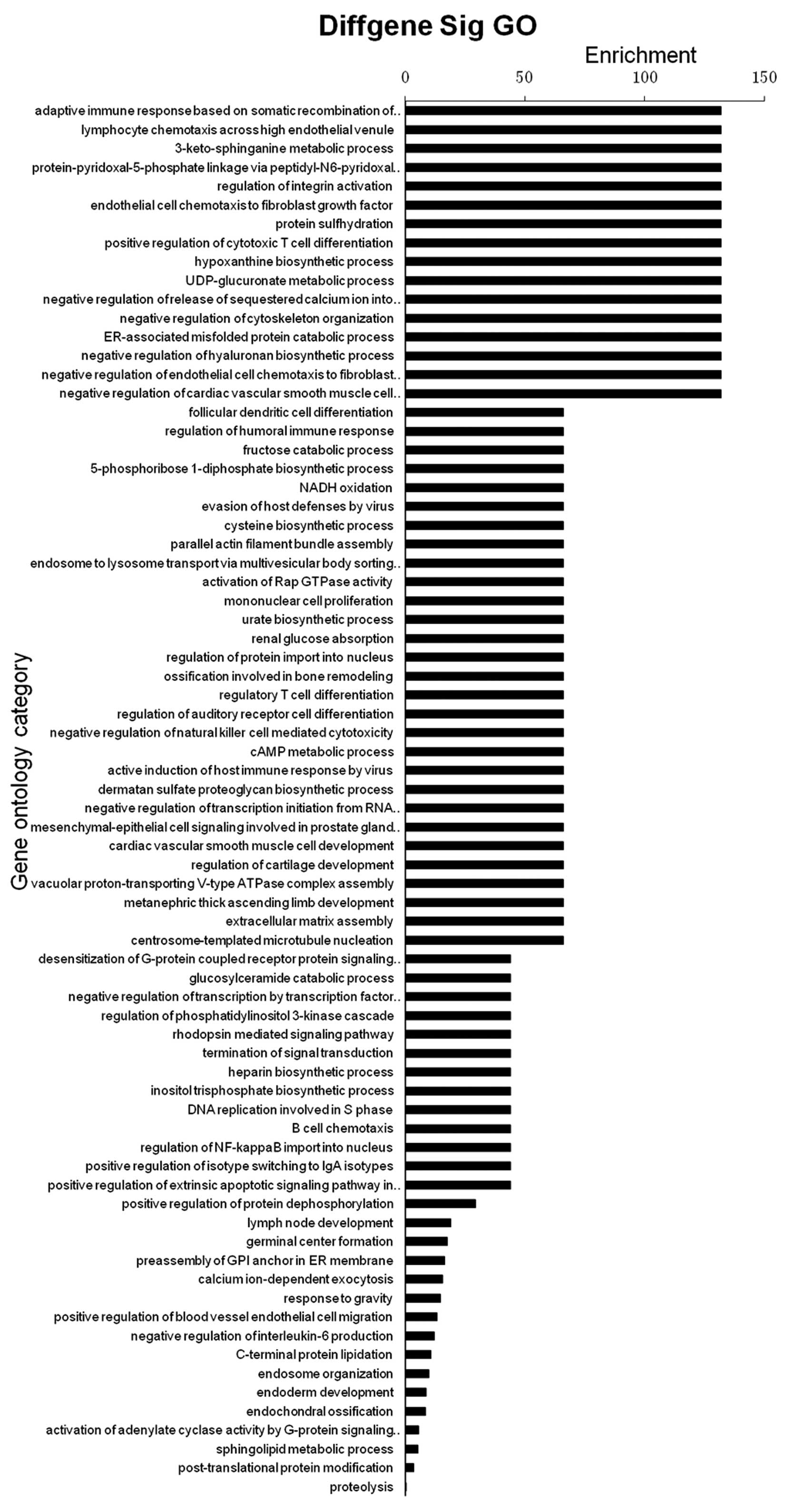
GO analysis by MeDIP-Chip
A functional enrichment analysis was performed using
the Web-based Gene Set Analysis Toolkit (33,34)
and 74 significantly enhanced functions were identified (Fig. 4; FDR < 0.05). Immune-associated
reactions, including the adaptive immune response based on somatic
recombination of immune receptors built from immunoglobulin
superfamily domains, lymphocyte chemotaxis across the high
endothelial venule and positive regulation of cytotoxic T-cell
differentiation were significantly enriched (enrichment = 131;
P<0.05).
Metabolic processes, including the
3-keto-sphinganine metabolic, hypoxanthine biosynthetic,
UDP-glucuronate metabolic, endoplasmic reticulum-associated
misfolded protein catabolic and negative regulation of hyaluronan
biosynthetic processes were enriched (enrichment = 131;
P<0.05).
Pathway enrichment analysis by
MeDIP-Chip
Pathway enrichment analysis of all genes involved in
MeDIP-Chip was performed using the KEGG automatic annotation
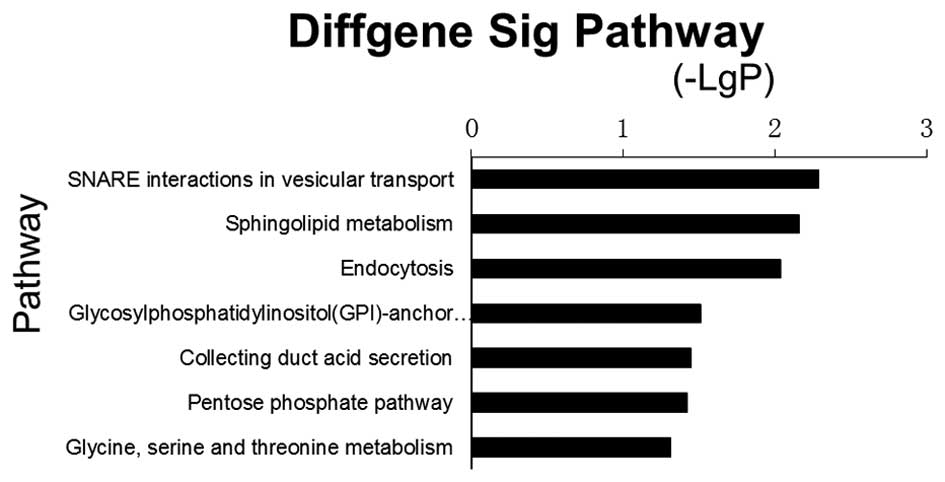
server. A total of seven significant pathways with P<0.05 were
enriched (Fig. 5). The most
significant pathway was synaptosomal-associated protein (SNAP)
receptor (SNARE) interaction in vesicular transport (path_id=4130),
where P=0.005. Three genes were involved in this pathway, including
SNAP47, vesicle associated membrane protein
(VAMP)4 and VAMP3. The other significantly
enriched pathways included sphingolipid metabolism, endocytosis,
glycosylphosphatidylinositol (GPI)-anchor biosynthesis, collecting
duct acid secretion, pentose phosphate pathway and glycine, serine
and threonine metabolism. Genes associated with endocytosis
included charged multivesicular body protein 1b (CHMP1B),
arrestin β2 (ARRB2), par-6 family cell polarity regulator β
(PARD6B), transforming growth factor β1 (TGFB1),
vacuolar protein sorting 4 homolog B (VPS4B) and cbl
proto-oncogene, E3 ubiquitin protein ligase B (CBLB).
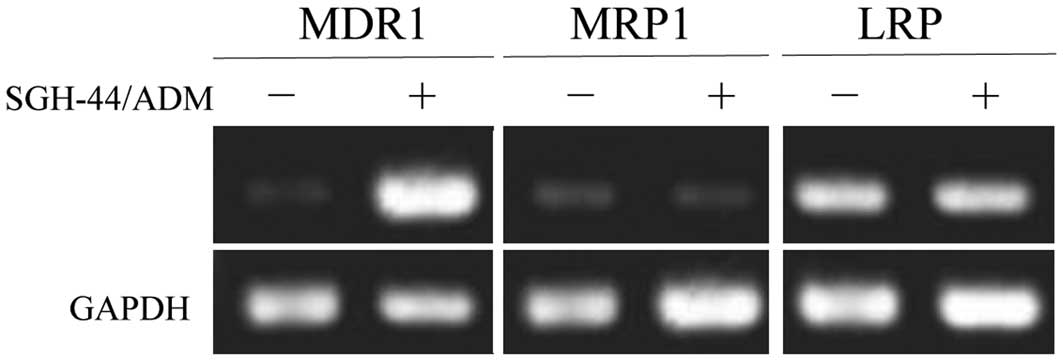
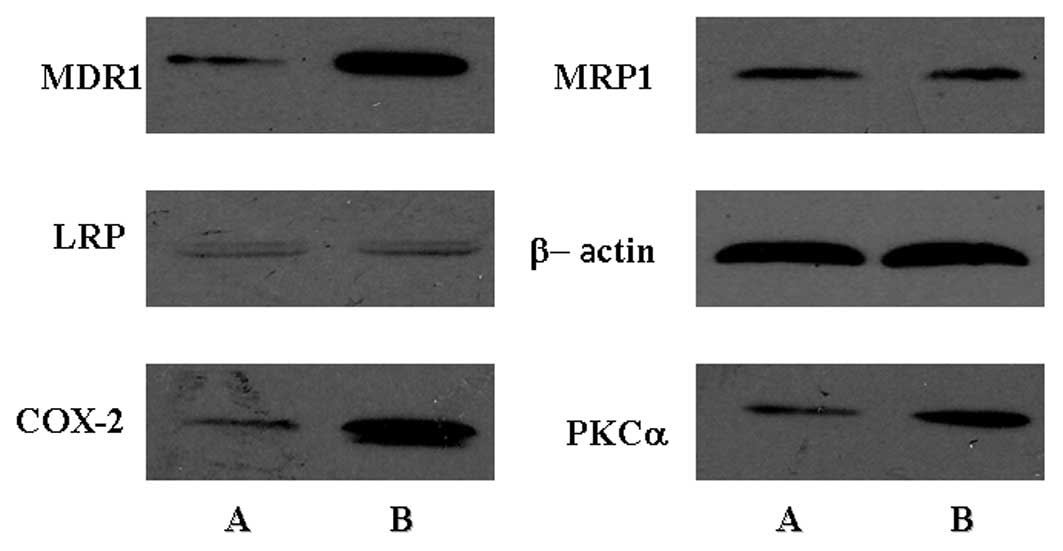
mRNA and protein expression levels of
MDR1, MRP1 and LRP
The expression of MDR1, MRP1 and
LRP at the mRNA levels are shown in Fig. 6. The expression of MDR1 at
the mRNA level were significantly increased in SGH-44/ADM cells as
compared with SGH-44 cells. The expression of MRP1 and LRP remained
unchanged in the two cell lines at the mRNA and protein level. The
expression of MDR1 at the protein level was increased in SGH-44/ADM
cells as compared with SGH-44 cells, which was consistent with the
mRNA expression levels. In addition, the expression of COX-2 and
PKCα was increased in SGH-44/ADM cells. The protein expression
levels are shown in Fig. 7.
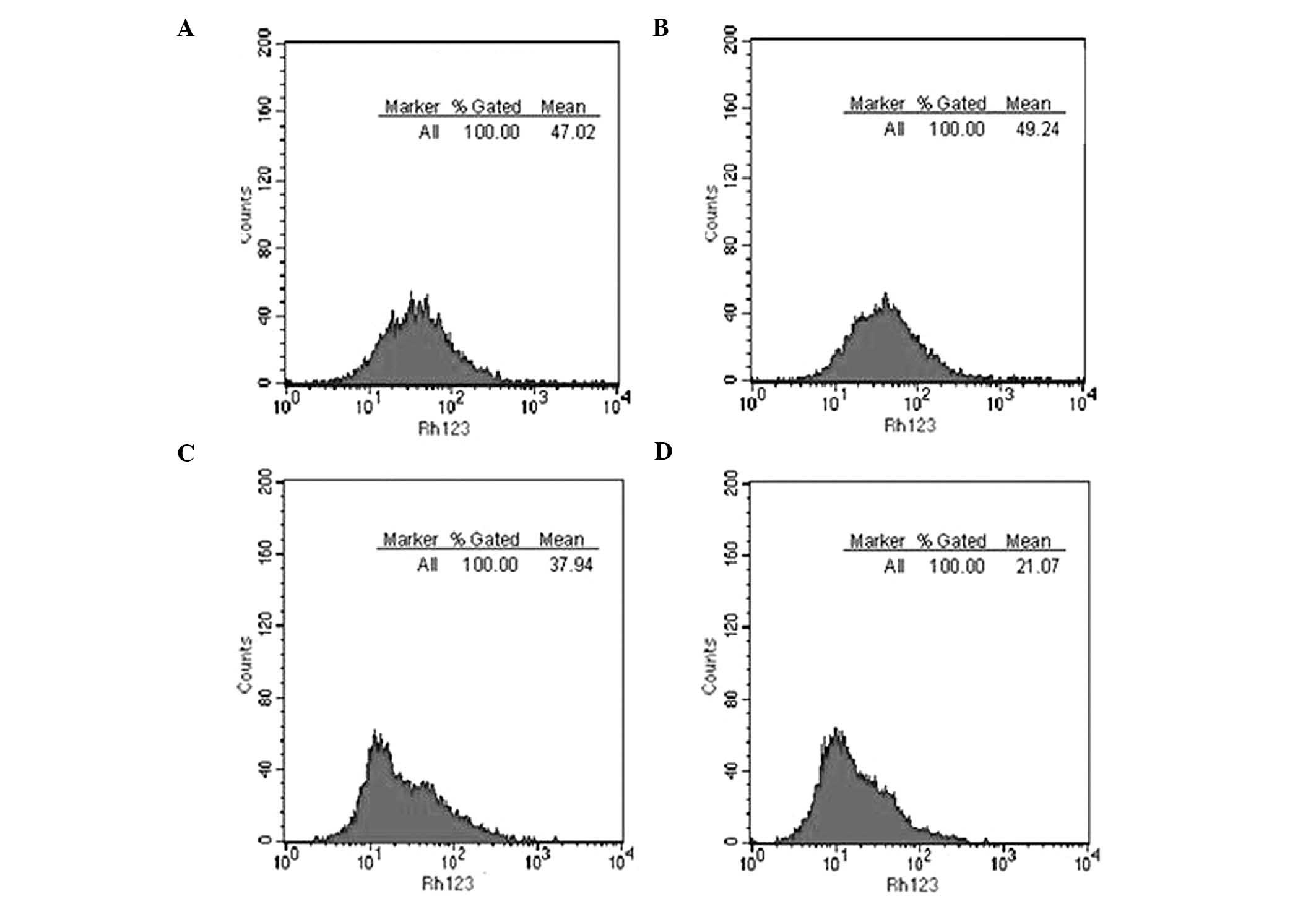
Rh123 ingestion and exudation in
SGH-44/ADM cells
An Rh123 ingestion and exudation experiment was
performed by flow cytometry (Fig.
8). The amount of Rh123 intake by SHG-44 and SHG-44/ADM cells
was 43.76±3.65 and 45.87±3.36 (relative fluorescence intensity),
respectively. Following effusion, the residual amount of Rh123 in
SGH-44 and SGH-44/ADM cells was 35.43±2.17 and 18.49±3.54,
respectively. SHG44/ADM cells showed no difference in Rh123
ingestion as compared with SGH-44 cells, but significantly exuded
more Rh123 than SGH-44 cells (P<0.01).
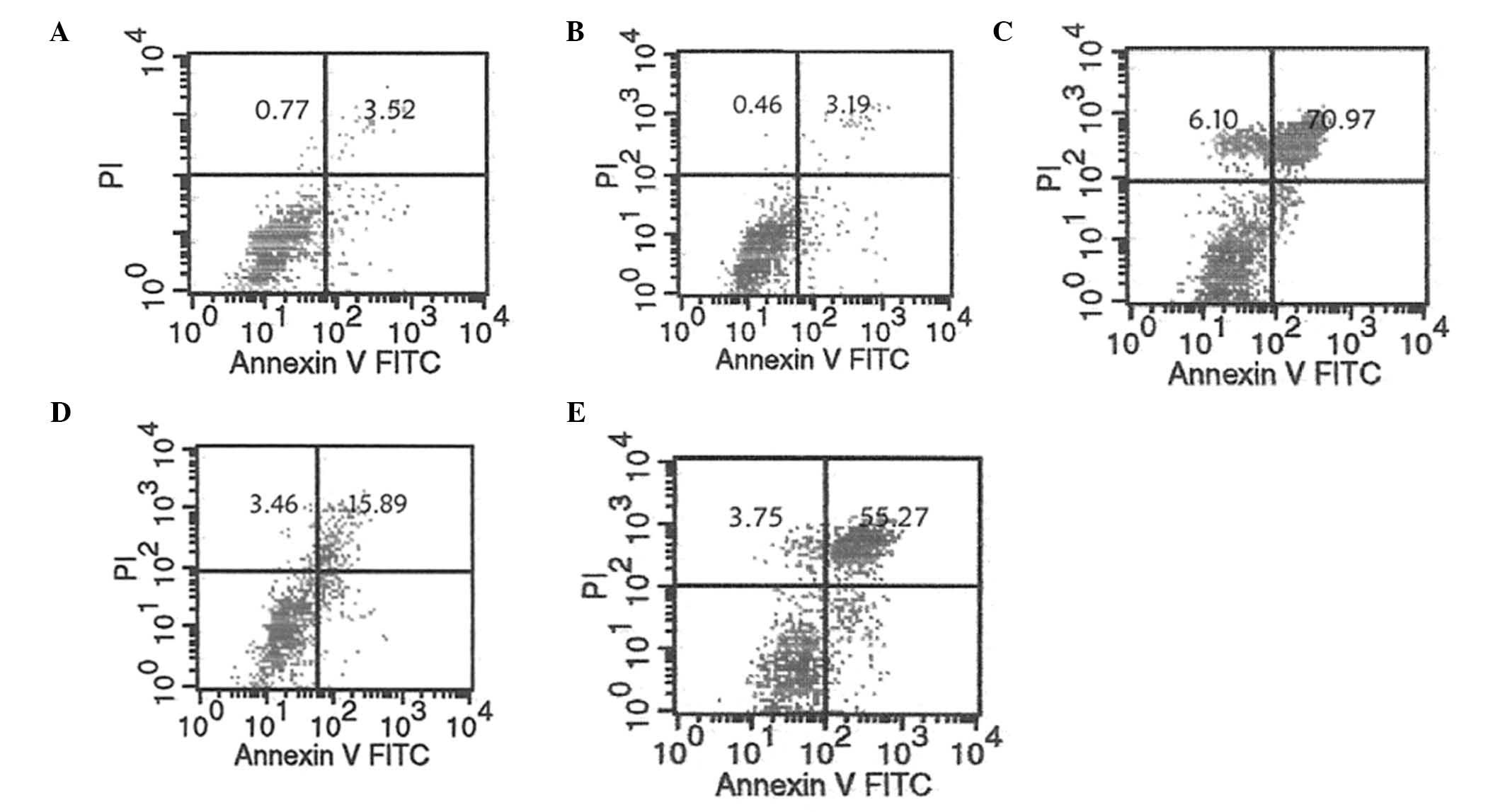
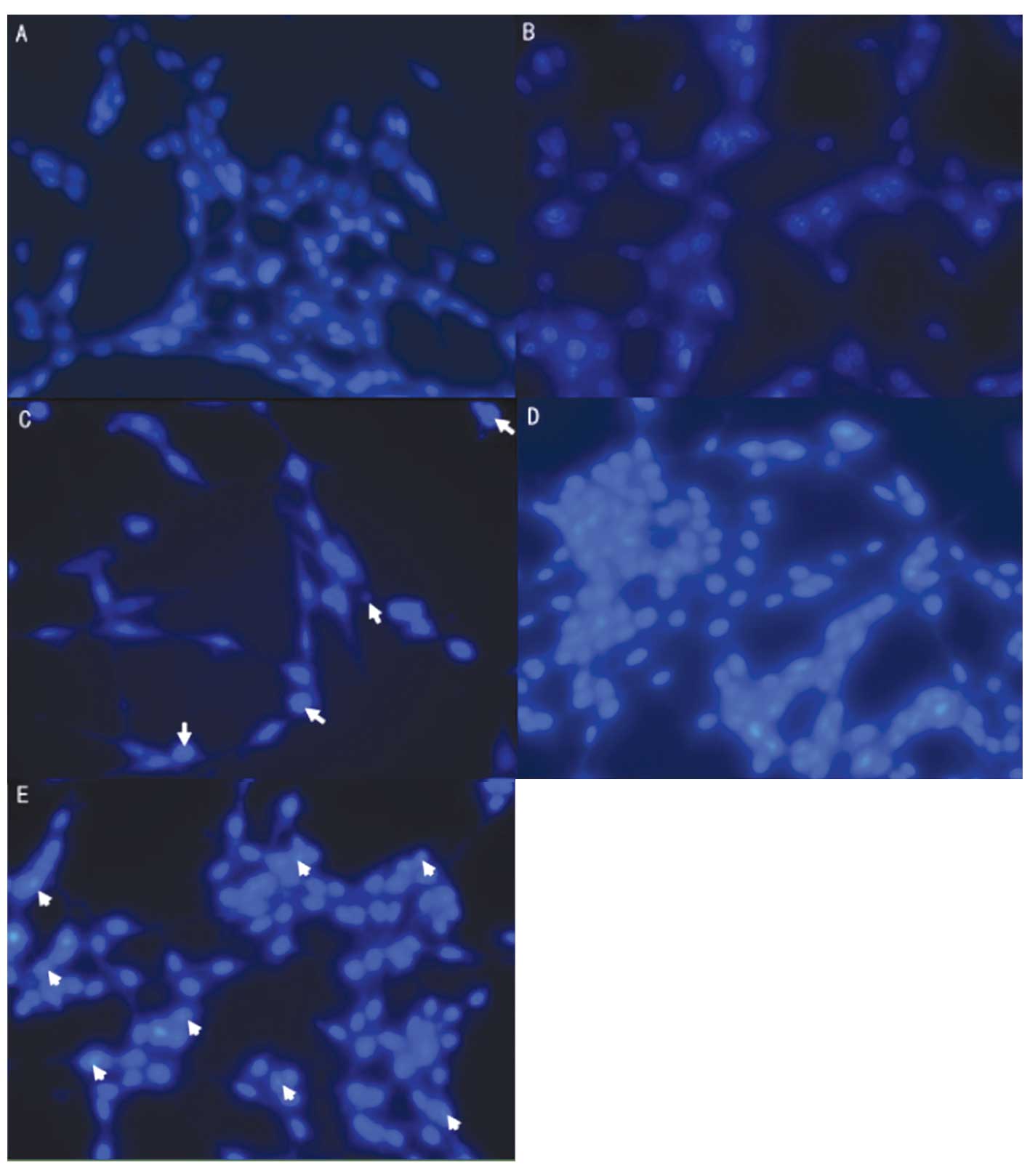
Analysis of apoptosis in SGH-44/ADM cells
by flow cytometry and fluorescence microscopy
The apoptotic rate of SGH-44 cells increased from
3.52 to 70.97% when treated with ADM (Fig. 9). SGH-44/ADM cells were more
resistant to ADM than SGH-44 cells, as the apoptotic rate showed a
smaller increase from 3.19 to 15.89% when treated with ADM.
However, the apoptotic rate of SGH-44/ADM cells was increased to
55.27% when treated with CsA, a MDR1 inhibitor. The apoptotic rate,
determined by fluorescence microscopy, was accordant with that
determined by flow cytometry (Fig.
10).
Discussion
In the present study, a stable MDR glioma cell line,
SGH-44/ADM, was generated by impulse ADM treatment. The effect of
cryopreservation, recovery and withdrawal on SGH-44/ADM MDR cells
was poor. SGH-44/ADM cells showed minimal differences as compared
with parental cells, except that SGH-44/ADM cells were bigger in
size, and with a wizened nucleus. However, SGH-44/ADM cells
exhibited a slower growth rate and stronger excretion ability. As
compared with SGH-44 cells, an increased number of SGH-44/ADM cells
remained in G1 and S phase, as measured by flow cytometry. The MDR
ability was associated with the upregulation of MDR1, PKCα and
COX-2, but the expression of these genes was not associated with
DNA methylation.
The cell model of MDR glioma has been described
previously. Denecke et al (35) established a subline of the rat
glioma cell line C6, named C6, 5×10(−7) Dox, by exposure to
increasing doses of doxorubicin over five months. In addition to
the typical cross-resistance to doxorubicin, daunorubicin,
vincristine and etoposide, it was observed that a significant
resistance of the C6, 5×10(−7) Dox cell line to irradiation was
developed, which cannot be explained by Pgp expression. As well as
the C6, 5×10(−7) Dox cell line, several other stable MDR human
glioma cell lines had been previously established (36–38).
All these previous studies failed to measure the resistance to ADM.
Kang and Kang (38) established a
dissociated cell system of human glioblastoma multiforme (GBM)
cells, A172. GBM2 cells were established with resistance to
1,3-bis(2-chloroethyl)-1-nitrosourea and expressed CD133, CD117,
CD90, CD71 and CD45 cell-surface markers.
In the present study, Pgp was shown to be
upregulated in the MDR cell line SGH-44/ADM, which was concordant
to a previous study (35). COX-2
is an inducible isoform enzyme that functions to generate
prostaglandins from arachidonic acid. It has been previously
observed that enforced expression of COX-2 results in enhancement
in MDR1 expression and functional activity, which suggests the
existence of a causal link between COX-2 activity and MDR1
expression (39). The protein
kinase C (PKC) family functions in regulating cell proliferation
and death, and is a key protein in the signaling pathway linking
epidermal growth factor receptor to mammalian target of rapamycin
(40,41). It had been observed that ribozymal
inhibition of PKCα may trigger apoptosis in glioma cells (42). In the present study, PKCα and COX-2
were upregulated in SGH-44/ADM cells, which is consistent with
previous studies (39,42).
Methylation of MDR1, PKCα and
COX-2 was not observed in the present study. The most
significantly enriched pathway identified by MeDIP-Chip was SNARE
interactions in vesicular transport (P=0.005). Three genes were
involved in this pathway, including SNAP47, VAMP4 and
VAMP3. The other significant pathways were sphingolipid
metabolism, endocytosis, GPI-anchor biosynthesis, collecting duct
acid secretion, pentose phosphate pathway and glycine, serine and
threonine metabolism. Enriched genes associated with endocytosis
were CHMP1B, ARRB2, PARD6B, TGFB1,
VPS4B and CBLB.
The human VPS4B gene is a homolog of yeast
VPS4, and shares a high degree of similarity with mouse
suppressor of K+ transport defect 1, which is associated
with transmembrane transport (43). VAMPs, NSF, SNAP and Annexins are
the main components of the protein complex involved in the docking
and fusion of synaptic vesicles with the presynaptic membrane
(44), and may function in
trans-Golgi network-to-endosome transport (45). CHMP1B is a peripherally-associated
component of endosomal sorting. CHMP1B is required for transport
complex III (ESCRT-III), which is involved in the formation and
sorting of endosomal cargo proteins into multivesicular bodies
(46). VPS4B, VAMPs and CHMPs are
components of the ESCRT-III (46),
which suggests that the major mechanism of MDR in glioma is
exocytosis.
In conclusion, SGH-44/ADM cells were shown to have
minimal differences from parental cells, except for a larger cell
size and a wizened nucleus. A larger proportion of SGH-44/ADM cells
remained in G1 and S phase, as compared with SGH-44 cells. The MDR
ability of SGH-44/ADM cells was associated with the upregulation of
MDR1, PKCα and COX-2. The expression of these genes was not
associated with DNA methylation, which suggested that the mechanism
of MDR in SGH-44/ADM is by active efflux rather than DNA
methylation. In addition, certain components of immune-associated
reactions, including positive regulation of cytotoxic T-cell
differentiation, were significantly enriched. These findings may
provide a novel insight into the MDR of human gliomas.
Acknowledgements
This study was supported by the Chongqing Natural
Science Foundation of China (no. CSTC, 2009BB5262).
References
|
1
|
Mamelak AN and Jacoby DB: Targeted
delivery of antitumoral therapy to glioma and other malignancies
with synthetic chlorotoxin (TM-601). Expert Opin Drug Deliv.
4:175–186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weiss RB: The anthracyclines: will we ever
find a better doxorubicin? Semin Oncol. 19:670–686. 1992.PubMed/NCBI
|
|
3
|
Minotti G, Menna P, Salvatorelli E, Cairo
G and Gianni L: Anthracyclines: molecular advances and
pharmacologic developments in antitumor activity and
cardiotoxicity. Pharmacol Rev. 56:185–229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peng X, Chen B, Lim CC and Sawyer DB: The
cardiotoxicology of anthracycline chemotherapeutics: translating
molecular mechanism into preventative medicine. Mol Interv.
5:163–171. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stewart LA: Chemotherapy in adult
high-grade glioma: a systematic review and meta-analysis of
individual patient data from 12 randomised trials. Lancet.
359:1011–1018. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lawson HC, Sampath P, Bohan E, et al:
Interstitial chemotherapy for malignant gliomas: the Johns Hopkins
experience. J Neurooncol. 83:61–70. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johannessen T-CA, Bjerkvig R and Tysnes
BB: DNA repair and cancer stem-like cells - potential partners in
glioma drug resistance? Cancer Treat Rev. 34:558–567. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bronger H, König J, Kopplow K, et al: ABCC
drug efflux pumps and organic anion uptake transporters in human
gliomas and the blood-tumor barrier. Cancer Res. 65:11419–11428.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calatozzolo C, Gelati M, Ciusani E, et al:
Expression of drug resistance proteins Pgp, MRP1, MRP3, MRP5 and
GST-pi in human glioma. J Neurooncol. 74:113–121. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dalton WS, Grogan TM, Meltzer PS, et al:
Drug-resistance in multiple myeloma and non-Hodgkin’s lymphoma:
detection of P-glycoprotein and potential circumvention by addition
of verapamil to chemotherapy. J Clin Oncol. 7:415–424. 1989.
|
|
11
|
Miller TP, Grogan TM, Dalton WS, et al:
P-glycoprotein expression in malignant lymphoma and reversal of
clinical drug resistance with chemotherapy plus high-dose
verapamil. J Clin Oncol. 9:17–24. 1991.PubMed/NCBI
|
|
12
|
Régina A, Demeule M, Laplante A, et al:
Multidrug resistance in brain tumors: roles of the blood-brain
barrier. Cancer Metastasis Rev. 20:13–25. 2001.PubMed/NCBI
|
|
13
|
Mohri M, Nitta H and Yamashita J:
Expression of multidrug resistance-associated protein (MRP) in
human gliomas. J Neurooncol. 49:105–115. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spiegl-Kreinecker S, Buchroithner J,
Elbling L, et al: Expression and functional activity of the
ABC-transporter proteins P-glycoprotein and multidrug-resistance
protein 1 in human brain tumor cells and astrocytes. J Neurooncol.
57:27–36. 2002. View Article : Google Scholar
|
|
15
|
Herz J, Kowal RC, Goldstein JL and Brown
MS: Proteolytic processing of the 600 kd low density lipoprotein
receptor-related protein (LRP) occurs in a trans-Golgi compartment.
EMBO J. 9:1769–1776. 1990.PubMed/NCBI
|
|
16
|
Pinzón-Daza M, Garzón R, Courard P, et al:
The association of statins plus LDL receptor-targeted
liposome-encapsulated doxorubicin increases in vitro drug delivery
across blood-brain barrier cells. Br J Pharmacol. 167:1431–1447.
2012.
|
|
17
|
Ratnasinghe D, Daschner PJ, Anver MR, et
al: Cyclooxygenase-2, P-glycoprotein-170 and drug resistance; is
chemoprevention against multidrug resistance possible? Anticancer
Res. 21:2141–2147. 2001.PubMed/NCBI
|
|
18
|
Ali-Osman F, Caughlan J and Gray GS:
Decreased DNA interstrand cross-linking and cytotoxicity induced in
human brain tumor cells by 1,3-bis (2-chloroethyl)-1-nitrosourea
after in vitro reaction with glutathione. Cancer Res.
49:5954–5958. 1989.PubMed/NCBI
|
|
19
|
Chen C, Taniguchi T and D’Andrea A: The
Fanconi anemia (FA) pathway confers glioma resistance to DNA
alkylating agents. J Mol Med. 85:497–509. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu XG, Peng SB and Huang Q:
Transcriptional regulation of breast cancer resistance protein. Yi
Chuan. 34:1529–1536. 2012.(In Chinese).
|
|
21
|
Candelaria M, de la Cruz-Hernandez E,
Taja-Chayeb L, et al: DNA methylation-independent reversion of
gemcitabine resistance by hydralazine in cervical cancer cells.
PLoS ONE. 7:e291812012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ogawa T, Liggett TE, Melnikov AA, et al:
Methylation of death-associated protein kinase is associated with
cetuximab and erlotinib resistance. Cell Cycle. 11:1656–1663. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He J: Expression of glioma stem cell
marker CD133 and O6-methylguanine-DNA methyltransferase is
associated with resistance to radiotherapy in gliomas. Oncol Rep.
26:1305–1313. 2011.PubMed/NCBI
|
|
24
|
Wang B, Li Y, Tan Y, et al: Low-dose Cd
induces hepatic gene hypermethylation, along with the persistent
reduction of cell death and increase of cell proliferation in rats
and mice. PLoS One. 7:e338532012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kanehisa M, Goto S, Kawashima S, Okuno Y
and Hattori M: The KEGG resource for deciphering the genome.
Nucleic Acids Res. 32:D277–D280. 2004.PubMed/NCBI
|
|
26
|
Yi M, Horton JD, Cohen JC, Hobbs HH and
Stephens RM: WholePathwayScope: a comprehensive pathway-based
analysis tool for high-throughput data. BMC Bioinformatics.
7:302006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Draghici S, Khatri P, Tarca AL, et al: A
systems biology approach for pathway level analysis. Genome res.
17:1537–1545. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gene Ontology Consortium. The Gene
Ontology (GO) project in 2006. Nucleic Acids Res. 34:D322–D326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ashburner M, Ball CA, Blake JA, et al:
Gene ontology: tool for the unification of biology. The Gene
Ontology Consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dupuy D, Bertin N, Hidalgo CA, et al:
Genome-scale analysis of in vivo spatiotemporal promoter activity
in Caenorhabditis elegans. Nat Biotechnol. 25:663–668. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schlitt T, Palin K, Rung J, et al: From
gene networks to gene function. Genome Res. 13:2568–2576. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim Y-J, Sah RLY, Doong J-YH and
Grodzinsky AJ: Fluorometric assay of DNA in cartilage explants
using Hoechst 33258. Anal Biochem. 174:168–176. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang B, Kirov S and Snoddy J: WebGestalt:
an integrated system for exploring gene sets in various biological
contexts. Nucleic Acids Res. 33:W741–W7748. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang J, Duncan D, Shi Z and Zhang B:
WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): update 2013.
Nucleic Acids Res. 41:W77–W7783. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Denecke J, Fiedler K, Hacker-Klom U, et
al: Multiple drug-resistant C6 glioma cells cross-resistant to
irradiation. Anticancer Res. 17:4531–4534. 1997.PubMed/NCBI
|
|
36
|
Shi L, Chen J, Yang J, et al: MiR-21
protected human glioblastoma U87MG cells from chemotherapeutic drug
temozolomide induced apoptosis by decreasing Bax/Bcl-2 ratio and
caspase-3 activity. Brain Res. 1352:255–264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang L, Xiong Y, Sun Y, et al: HLungDB: an
integrated database of human lung cancer research. Nucleic Acids
Res. 38:D665–D669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kang MK and Kang SK: Tumorigenesis of
chemotherapeutic drug-resistant cancer stem-like cells in brain
glioma. Stem Cells Dev. 16:837–847. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sorokin A: Cyclooxygenase-2: potential
role in regulation of drug efflux and multidrug resistance
phenotype. Current Pharmaceutical Design. 10:647–657. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fan Q-W, Cheng C, Knight ZA, et al: EGFR
signals to mTOR through PKC and independently of Akt in glioma. Sci
Signal. 2:ra42009.PubMed/NCBI
|
|
41
|
Basu A: PKC and resistance to
chemotherapeutic agents. Protein Kinase C in Cancer Signaling and
Therapy. Kazanietz MG: Humana Press; pp. 409–429. 2010, View Article : Google Scholar
|
|
42
|
Leirdal M and Sioud M: Ribozyme inhibition
of the protein kinase C alpha triggers apoptosis in glioma cells.
Br J Cancer. 80:1558–1564. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fujita H, Yamanaka M, Imamura K, et al: A
dominant negative form of the AAA ATPase SKD1/VPS4 impairs membrane
trafficking out of endosomal/lysosomal compartments: class E vps
phenotype in mammalian cells. J Cell Sci. 116:401–414. 2003.
View Article : Google Scholar
|
|
44
|
Schnitzer JE, Liu J and Oh P: Endothelial
caveolae have the molecular transport machinery for vesicle
budding, docking, and fusion including VAMP, NSF, SNAP, annexins,
and GTPases. J Biol Chem. 270:14399–14404. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kawano M, Kumagai K, Nishijima M and
Hanada K: Efficient trafficking of ceramide from the endoplasmic
reticulum to the Golgi apparatus requires a VAMP-associated
protein-interacting FFAT motif of CERT. J Biol Chem.
281:30279–30288. 2006. View Article : Google Scholar
|
|
46
|
Guizetti J and Gerlich DW: ESCRT-III
polymers in membrane neck constriction. Trends Cell Biol.
22:133–140. 2012. View Article : Google Scholar : PubMed/NCBI
|