Introduction
It is estimated that 21,980 novel ovarian cancer
(OC) cases will be diagnosed in 2014 in the United States. While
this number ranks OC as the ninth most common type of cancer in
females, it is the fifth leading cause of cancer-related mortality
in this group, with over 15,000 fatalities per year in the US
(1). The five-year survival rate
for early stage OC is ~92%, but it is difficult to detect OC at an
early stage due to the fact that it commonly presents with vague
and non-specific symptoms (2).
Unfortunately, the majority of patients are diagnosed with advanced
stage disease, for which the five-year survival rate is only ~30%.
Thus, early diagnosis of OC is likely to significantly improve
disease outcome.
Cathepsins are lysosomal proteases of various
classes. The largest group are the family of cysteine
endopeptidases, which share close structural and functional
characteristics with the plant cysteine endopeptidase, papain,
which represents a typical example of a C1A peptidase (3). Cathepsins participate in a number of
processes in healthy cells, but have also been reported to be
involved in certain pathological processes, including cancer
progression (4–7). In a number of cancer types, increased
expression of cysteine cathepsins and alterations in their
subcellular trafficking were reported (8–13).
A previous study demonstrated that CTSL, which is a
member of the cathepsins family, is overexpressed in OC tissues and
that its expression is inversely correlated with patient survival
(14). However, it remains unclear
whether inhibition of CTSL expression affects the biological
function of OC cells (15). The
current study aimed to analyze the effects CTSL on cultured OC
cells, in terms of the growth and invasion of these cells.
Materials and methods
Cell lines and reagents
The OV-90 and SKOV3 human OC cell lines were
obtained from the American Type Culture Collection (Manassas, VA,
USA), and the HO-8910 and 3AO human OC cell lines were purchased
from the Shanghai Institute of Cell Biology, Chinese Academy of
Science (Shanghai, China). Cells were cultured in Dulbecco’s
modified Eagle’s medium (GE Healthcare Life Sciences, Logan, UT,
USA) supplemented with 10% fetal calf serum (Invitrogen Life
Technologies, Grand Island, NY, USA), 100 U/ml penicillin, and 100
U/ml streptomycin (GE Healthcare Life Sciences). Cell culture was
performed at 37°C in humidified air with 5% CO2.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total cellular RNA was isolated from the four OC
cell lines using TRIzol® reagent (Invitrogen Life
Technologies) according to the manufacturer’s instructions and
quantified using a UV spectrophotometer (Beijing LabTech
Instruments Co., Ltd., Beijing, China). RNA (2 μg) was reverse
transcribed using an access reverse transcription system (Promega
Corporation, Madison, WI, USA) according to the manufacturer’s
instructions. In brief, reaction mixtures (total volume, 20 μl)
containing 500 ng cDNA were amplified to a final concentration of
250 nM using 10 μl of 2X Brilliant SYBR Green QPCR Master Mix kit
(Agilent Technologies, Inc., Santa Clara, CA, USA). The following
primers were used: Forward: 5′-GAATGGACG-GACTGCTAC-3′ and reverse:
5′-CCAAAGAATACTTGCCTCA-3′ (NM_006665.5; GenBank®,
International Nucleotide Sequence Database Collaboration) for CTSL
and forward: 5′-TAAGAAGCTGCTGTGCTACG-3′ and reverse:
5′-GACTCGTCATACTCCTGCTT-3′ (NM_001101; GenBank) for β-actin.
Thermal cycling conditions were as follows: 94°C for 5 min and then
45 cycles at 94°C for 30 sec, followed by 60°C for 30 sec and 72°C
for 45 sec. Experiments were performed in triplicate. Target genes
and β-actin were amplified in the same reaction. The relative
quantities were analyzed by comparison of 2−ΔΔCt.
Vector construction and transfection
A pcDNA3.0 vector [Cyagen Biosciences (Gunagzhou)
Inc., Guangzhou, China] was used to generate pcDNA-CTSL. The CTSL
small hairpin RNA (shRNA) plasmid was purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA; sc-40685-SH). Vector
transfection was performed according to the manufacturer’s
instructions, OV-90 cells were transfected with pcDNA expressing
either CTSL or empty vector, and CTSL-shRNA was used to knock down
the expression of CTSL in SKOV3 cells. OV-90 cells expressing
either CTSL or empty vector were selected for 14 days with the
antibiotic G418 following transfection. SKOV3 transfected with
CTSL-shRNA was selected for 14 days with puromycin following
transfection.
Western blot analysis
Cell samples were lysed in a lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China) following collection
from a 100-mm dish and lysis. Proteins (20 μg) were resolved on a
10% SDS-PAGE gel and transferred to polyvinylidene fluoride
membranes (Sigma-Aldrich, St. Louis, MO, USA). Western blot
analysis was performed using a monoclonal anti-human CTSL antibody,
with monoclonal anti-human β-actin as a control. The blocking steps
and dilutions for the assessment of all proteins were conducted in
5% bovine serum albumin. Following incubation with horseradish
peroxidase-conjugated antibodies (GE Healthcare Life Sciences,
Chalfont, UK), labeled proteins were detected with an Enhanced
Chemiluminescence-Plus detection system (GE Healthcare Life
Sciences). The monoclonal anti-human CTSL antibody, anti-p38 and
anti-ERK antibodies were obtained from Abcam (Cambridge, UK) and
raised in rabbits. The monoclonal anti-human β-actin antibody was
purchased from Santa Cruz Biotechnology, Inc. and raised in mouse.
In addition, all secondary antibodies were monoclonal.
Colony forming assay
For the colony forming assay, cells were seeded
evenly in 6-well plates (2×102 cells/well) and cultured
for 14 days. Cells were fixed with methanol for 10 min and stained
with Giemsa dye for 1 min. The number of colonies which were <1
mm was counted using a CX31 microscope (Olympus Corporation). Plate
clone formation efficiency was calculated as follows: Clone
formation efficiency (%) = number of colonies/number of cells
inoculated. Each experiment was performed in triplicate.
3-(4, 5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide reduction (MTT) assay
Cells were seeded into 96-well plates at a density
of 2,000 cells/well. There were four samples from each group. Cells
were incubated with 0.2% MTT for 4 h at 37°C, 100 μl
dimethylsulfoxide was added to each well to dissolve the crystals
and cells were counted daily by reading the absorbance at 490 nm
using a microplate reader (Multiskan MK3; Thermo Fisher Scientific,
Waltham, MA, USA).
Cell invasion assay
The upper chambers of a 24-well transwell plate
(Corning Incorporated, Corning, NY, USA) were coated with 50%
Matrigel (BD Biosciences Franklin Lakes, NJ, USA) in
phosphate-buffered saline. Cells were incubated in the upper
chamber. Following 24 h incubation, invaded cells were stained with
0.5% crystal violet, examined by bright field microscopy (CX31;
Olympus Corporation, Tokyo, Japan) and photographed (DP21 Camera;
Olympus Corporation). The invasion rate was quantified by counting
the invaded cells in five randomly selected fields per chamber
under a fluorescence microscope (IX71; Olympus Corporation). Three
independent experiments were conducted for each group.
Tumorigenicity assay
Female/male athymic BALB/C nu/nu mice (4- to 6-week
old) were purchased from the Central Laboratory of Animal Science
at Southern Medical University (Guangzhou, China) and maintained in
laminar flow cabinets under specific pathogen-free conditions.
SKOV3/shCTSL and SKOV3/Con (5×106 cells) were injected
subcutaneously into the flanks of the nude mice. Tumor growth was
evaluated every 7 days for 3 weeks following inoculation. Tumor
volume (V) was determined by measuring the largest (a) and the
smallest (b) axis using calipers, and calculated as
V=0.5ab2. Mice handling and experimental procedures
followed institutional guidelines. All experimental procedures and
protocols were approved by the Institutional Animal Care and Use
Committee of the 458th Hospital of PLA (Guangzhou,
China).
Statistical analysis
Unless otherwise stated, all data are presented as
the mean ± standard error of the mean. Statistical significance was
determined by a t-test or analysis of variance followed by
assessment of differences using SPSS 16.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference
Results
Expression of CTSL in four OC cell
lines
To investigate the expression of CTSL in
nasopharyngeal carcinoma cells, RT-qPCR and western blotting was
conducted in HO-8910, 3AO, OV-90 and SKOV3 human OC cell lines. The
mRNA and protein levels of CTSL in the three cell lines are shown
in Fig. 1. The results showed that
SKOV3 and OV-90 expressed the highest and lowest levels of CTSL
mRNA and protein, respectively. Thus, these two cell lines were
selected for subsequent investigation into the biological function
of CTSL in OC.
Vector stably expressing CTSL-shRNA
causes effective and specific downregulation of cathepsin L
expression
The knock down efficiencies of three CTSL specific
shRNAs in SKOV3 cells were evaluated using RT-qPCR. Relative CTSL
mRNA levels in individual stably transfected cells were normalized
against mRNA levels of a reference gene, β-actin, which were
measured in the same run. As shown in Fig. 2A and B, cells transfected with
CTSL-shRNA had a significantly reduced level of CTSL protein
compared with that of parental SKOV3 cells and Con-transfected
cells. The above results demonstrated that expression of CTSL was
downregulated specifically and effectively using specific CTSL
shRNA. SKOV3 cells transfected with CTSL-shRNA were termed
SKOV3/shCTSL, and SKOV3 cells transfected with Con were termed
SKOV3/Con.
CTSL overexpression in OC cells
OV-90 cells transfected with pc.DNA3.0-CTSL plasmid
exhibited a significant increase in the expression level of CTSL
compared with the vector control group (Fig. 2C and D). The overexpression of CTSL
was confirmed by western blot analysis and RT-qPCR. The OV-90 cells
overexpressing CTSL were termed OV-90/CTSL.
Effect of CTSL on cell proliferation of
OC cells
The proliferative activity of tumor cells is
important in the invasion and metastasis of tumors. Having
established SKOV3/shRNA cells and OV-90/CTSL cells, cell
proliferation activity of the transfected cells was measured using
an MTT assay. As shown in Fig. 3A and
B, the growth of SKOV3 cells in vitro was markedly
inhibited following the transfection of CTSL-shRNA (P<0.05),
whereas overexpression of CTSL promoted OV-90 cell proliferation
(P<0.05). This indicates a positive correlation between the
expression of CTSL and the rate of OC cell growth.
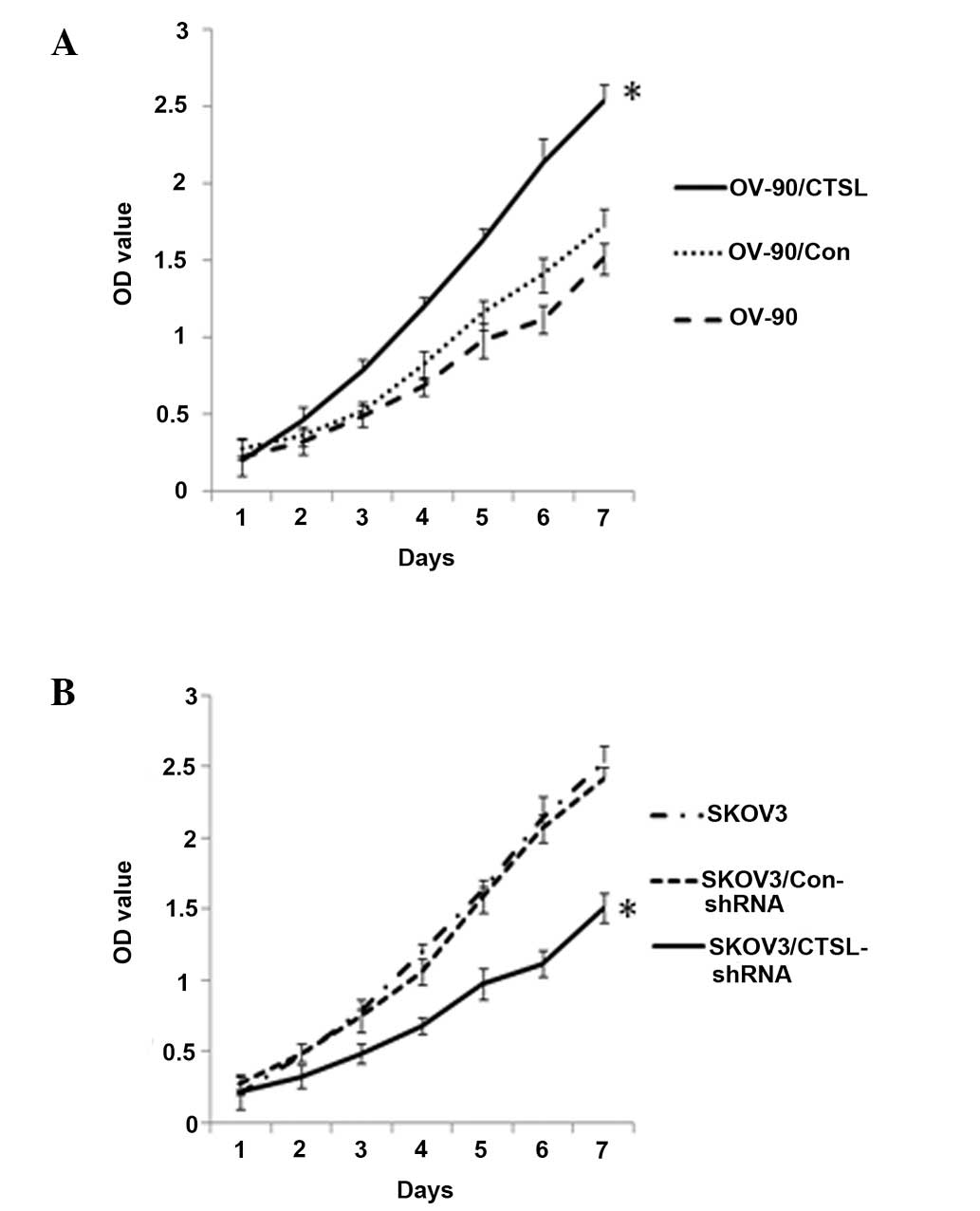 | Figure 3Effect of CTSL on cell proliferation
in vitro. (A) MTT assay of OV-90 cells overexpressing CTSL.
The number of viable cells was assessed using an MTT assay at 1, 2,
3, 4, 5, 6 and 7 days. Each sample was tested in triplicate and the
results are reported as optical density readings. (B) MTT assay of
SKOV3 cells with downregulation of CTSL. Values represent the mean
± standard deviation of at least three independent experiments.
*P<0.05. CTSL, cathepsin L; MTT,
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bormide
reduction; OV-90/CTSL, OV-90 cells transfected with CTSL vector;
OV-90/Con, OV-90 cells transfected with empty vector;
SKOV3/Con-shRNA, SKOV3 cells transfected with control shRNA;
SKOV3/CTSL-shRNA, SKOV3 cells transfected with CTSL shRNA; shRNA,
small hairpin RNA. |
Effect of CTSL on the colony forming
potential of OC cells
In order to examine the ability of SKOV3/CTSL-shRNA
cells and OV-90/CTSL to form colonies, single-cell suspensions were
plated at a density of 100 cells in 30-mm culture dishes. As shown
in Fig. 4, following 12 days of
culture, SKOV3/shRNA cells, compared with parental SKOV3 and
SKOV3/Con cells, had a significant reduction in the ability to form
colonies (P<0.05), whereas OV-90/CTSL showed an increase in this
ability compared with parental OV-90 and OV-90/Con cells
(P<0.05).
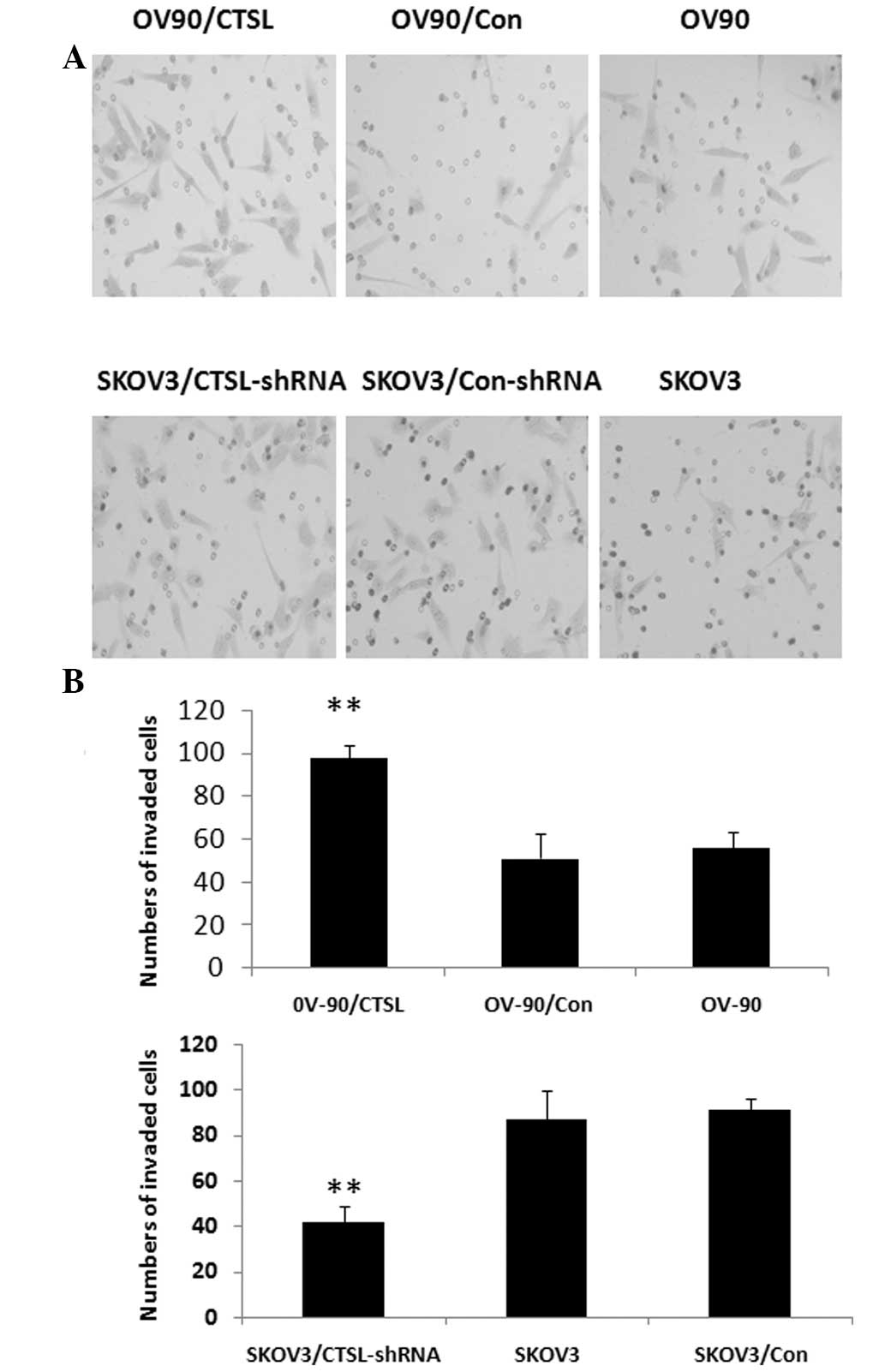
Effect of CTSL on invasiveness of OC
cells
OV-90/CTSL cells showed higher levels of
invasiveness than either parental OV-90 or OV-90/Con cells
(P<0.01, respectively; Fig.
5A). The percentage of invading cells in the OV-90/CTSL, OV-90
and OV-90/Con groups was 100, 95 and 37.4% respectively. The
results indicate that CTSL is associated with the invasiveness of
OC cells. In addition, SKOV3/shRNA cells displayed a significant
reduction in invasion ability compared with SKOV3/Con or parental
SKOV3 cells (P<0.01). As shown in Fig. 5B, the percentage of migrating cells
in the SKOV3/shRNA group was 45.9%, while in the SKOV3 and
SKOV3/Con groups it was 98 and 100%, respectively. Inhibition of
CTSL caused significantly attenuated migration in SKOV3 cells.
Effects of CTSL on expression of
mitogen-activated protein kinase (MAPK) transduction
As shown in Fig. 6,
western blotting was conducted to detect the expression of
components of MAPK transduction in OC cell lines. Inhibition of
CTSL resulted in significant reduction in the expression of
phosphorylated MEK, RAF and extracellular signal-regulated kinase
(ERK) (p-MEK, p-RAF and p-ERK, respectively) in SKOV3 cells,
whereas overexpression of CTSL resulted in a significant increase
in p-MEK, p-RAF and p-ERK expression in OV-90 cells. Protein levels
of p-MEK, p-RAF and p-ERK in SKOV3/shRNA were lower than those in
the SKOV3 and SKOV3/Con groups, and in the OV-90/CTSL groups these
levels were higher than those in the OV-90 and OV-90/Con
groups.
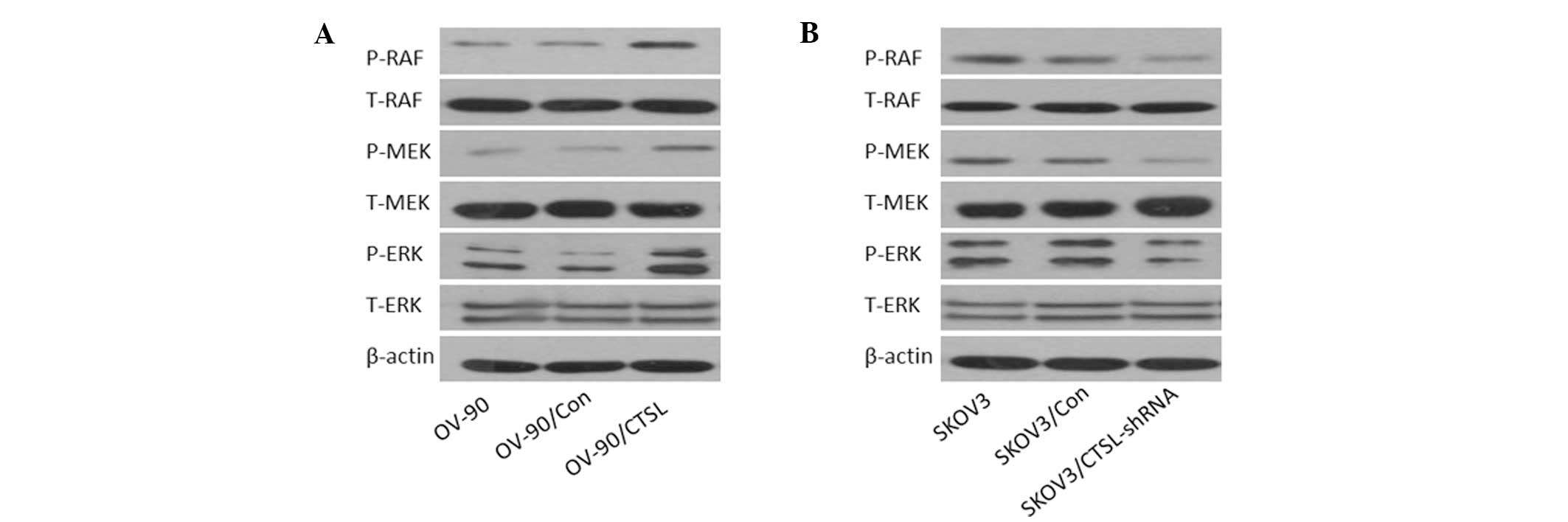 | Figure 6Effect of CTSL on the Raf/MEK/ERK
pathway. Total cellular protein was collected and subjected to
western blot analysis. (A) Stable overexpression of CTSL increased
the level of P-RAF, P-MEK and P-ERK expression. (B) Downregulation
of CTSL led to opposite effects. CTSL, cathepsin L; ERK,
extracellular signal-regulated kinase; P-, phosphorylated; T-,
total; OV-90/Con, OV-90 cells transfected with empty vector;
OV-90/CTSL, OV-90 cells transfected with CTSL vector; SKOV3/Con,
SKOV3 cells transfected with control shRNA; SKOV3/CTSL-shRNA, SKOV3
cells transfected with CTSL shRNA; shRNA, short hairpin RNA. |
CTSL gene silencing suppresses cell
proliferation in vivo
As shown in Fig. 7,
the effect of CTSL on in vivo tumor growth was assessed by
subcutaneous injection of SKOV3/shRNA and SKOV3/Con cells into nude
mice for a period of 21 days. A marked reduction in tumor size in
the SKOV3/shRNA group was observed compared with that in the
control group (P<0.05). On day 21 following cell injection, the
average tumor weight (n=4) of the SKOV3/shRNA and SKOV3/Con groups
was 1.58±0.23 and 3.41±0.15 g, respectively (P<0.01), indicating
that knockdown of CTSL in OC cells reduced their tumorigenic
potential.
Discussion
The present study showed that overexpression of CTSL
is important in the progression of OC. These findings suggest that
CTSL is a significant contributor to the proliferation, invasion
and migration of OC cells.
Cells require interactions with extracellular matrix
(ECM) components in order to undergo normal morphogenesis with
respect to organogenesis. ECM is involved in regulating cell shape
and numerous cellular functions, including adhesion, migration,
proliferation, polarity, differentiation and apoptosis. In
pathological conditions, such as cancer, the increased synthesis of
certain ECM components, in addition to the increased breakdown of a
number of these molecules, with consequent generation of ECM
cleavage products, can contribute to cancer growth and progression.
A number of growth factors, including fibroblast growth factor and
vascular endothelial growth factor are stored in the ECM
environment and are released upon protease-dependent cleavage of
ECM components, which further indicates the importance of the ECM
in the regulation of cellular functions. Tumor metastasis remains
the principal cause of treatment failure and poor prognosis in
patients with OC. Metastasis is a multistage process, which
includes the proteolysis, motility and migration of cells,
proliferation at a new site, and neoangiogenesis. A crucial step in
the process of intra- and extravasation of cancer cells is the
activation of proteolytic enzymes capable of degrading the ECM.
CTSL degrades ECM and basement membrane components, and this enzyme
is involved in tumor metastasis and angiogenesis. Elevated
expression of CTSL mRNA and protein in tumors has been demonstrated
in tissue specimens derived from gliomas (16), oral squamous cell carcinoma
(13), hepatocellular carcinoma
(17), prostate carcinoma
(18), bladder carcinoma (19), colon carcinoma (20), stomach carcinoma (21), breast carcinoma (22), pancreatic adenocarcinoma (23), esophageal carcinoma (24) and chronic myeloid leukemia
(25). Notably, increased CTSL
levels were often found to be associated with a reduction in
patient survival rate, increased carcinoma metastasis and high
microvessel density.
Regardless of the potential significance of CTSL in
OC, the functional role of CTSL in this disease has not been
clearly defined and evidence of its oncogenic activity in OC is
lacking. To investigate the role of CTSL in OC, endogenous CTSL
expression in an OC cell line (SKOV3) was silenced using shRNA.
Properties of the CTSL-depleted cells were then analyzed and
compared with control cells in various functional assays. The
results showed that CTSL knockdown led to reduced cell
proliferation and anchorage-independent growth. Furthermore, the
motility and invasiveness of cells were significantly impeded with
CTSL depletion. In addition, overexpression of CTSL promoted these
characteristics of OV-90 cells. To the best of our knowledge, the
current study provides the first validation of the effect of the
functional loss of CTSL expression in vivo. SKOV3 cells with
high levels of CTSL expression displayed an increased ability to
form tumors in nude mice. These results affirmed the findings from
the other experiments conducted in this study, that CTSL exerts an
oncogenic effect on SKOV3 cells. The results suggest a possible
explanation for the poorer outcomes observed in OC patients with
CTSL overexpression.
The MAPK signaling pathway is a major determinant in
the control of diverse cellular processes, such as proliferation,
survival, differentiation and motility, and is associated with
tumor development (26). The
results from the present study indicate that the antiproliferative
effects of CTSL in OC cells are partially mediated by MAPK
signaling. Suppression of CTSL in OC cells inhibited cell
proliferation in association with a decrease in phosphorylation of
MEK, RAF and ERK. Furthermore, overexpression of CTSL enhanced the
growth of OV-90 cells by increasing phosphorylation of MEK, RAF and
ERK. However, it was also observed that even following knock down
of CTSL, OC cells nonetheless possessed capabilities of invasion
and metastasis. It is postulated that other factors, including
urokinase and matrix metalloproteinases, also influence these
characteristics of OC (27,28),
which requires further investigation.
In the present study, downregulation of CTSL by
shRNA was shown to inhibit OC cell growth and migration, and
overexpression of CTSL by cDNA transfection was demonstrated to
promote OC cell proliferation and motility. Previous studies have
shown that interference with CTSL expression additionally regulates
at least two major signaling pathways (Wnt/β-catenin and
transforming growth factor-β) in cancers. It is therefore possible
that interfering with CTSL expression and function exerts
broad-spectrum biological effects that may be beneficial for the
treatment of OC. Further studies of the mechanisms by which CTSL
regulates multiple signaling pathways may reveal other points of
intervention for this important target.
Acknowledgements
The authors would like to thank Dr Chengwei Lv from
the Cancer Biotherapy Center for the collection and maintenance of
the HO-8910, 3AO, OV-90 and SKOV3 cells used in this research. This
study was supported by the National Natural Science Foundation of
China (grant no. 81001212), the China Postdoctoral Science
Foundation (grant no. 20080431074), the Foundation of Zhejiang
Provincial Educational Committee (grant no. Y201019175), the
Zhejiang Provincial Health Bureau Foundation (grant no. 2010KYB036)
and the Natural Science Foundation of Guangdong (grant no.
s2012010008209).
References
|
1
|
American Cancer Society. Cancer Facts and
Figures 2014. Atlanta, Ga: American Cancer Society; 2014 Last
accessed May 21, 2014
|
|
2
|
Su Z, Graybill WS and Zhu Y: Detection and
monitoring of ovarian cancer. Clin Chim Acta. 415:341–345. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Turk V, Stoka V, Vasiljeva O, et al:
Cysteine cathepsins: from structure, function and regulation to new
frontiers. Biochim Biophys Acta. 1824:68–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gocheva V and Joyce JA: Cysteine
cathepsins and the cutting edge of cancer invasion. Cell Cycle.
6:60–64. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mason SD and Joyce JA: Proteolytic
networks in cancer. Trends Cell Biol. 21:228–237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Conus S and Simon HU: Cathepsins and their
involvement in immune responses. Swiss Med Wkly.
140:w130422010.PubMed/NCBI
|
|
7
|
Krepela E: Cysteine proteinases in tumor
cell growth and apoptosis. Neoplasma. 48:332–349. 2001.PubMed/NCBI
|
|
8
|
Mohamed MM and Sloane BF: Cysteine
cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer.
6:764–775. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duffy MJ: The role of proteolytic enzymes
in cancer invasion and metastasis. Clin Exp Metastasis. 10:145–155.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mullins SR, Sameni M, Blum G, Bogyo M,
Sloane BF and Moin K: Three-dimensional cultures modeling
premalignant progression of human breast epithelial cells: role of
cysteine cathepsins. Biol Chem. 393:1405–1416. 2012. View Article : Google Scholar
|
|
11
|
Rao Q, Cheng L, Xia QY, et al: Cathepsin K
expression in a wide spectrum of perivascular epithelioid cell
neoplasms (PEComas): a clinicopathological study emphasizing
extrarenal PEComas. Histopathology. 62:642–650. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jevnikar Z, Rojnik M, Jamnik P, Doljak B,
Fonovic UP and Kos J: Cathepsin H mediates the processing of talin
and regulates migration of prostate cancer cells. J Biol Chem.
288:2201–2209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakashima T, Yasumatsu R, Masuda M,
Clayman GL and Komune S: Prognostic value of cathepsin L and its
inhibitor headpin in oral squamous cell carcinoma. J Laryngol Otol.
126:1134–1137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bar-Sela G, Kaplan-Cohen V, Ilan N,
Vlodavsky I and Ben-Izhak O: Heparanase expression in
nasopharyngeal carcinoma inversely correlates with patient
survival. Histopathology. 49:188–193. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang W, Hu XX, Yang XZ, et al: Combined
detection of serum matrix metalloproteinase 9, acetyl heparinase
and cathepsin L in diagnosis of ovarian cancer. Chin J Cancer Res.
24:67–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sivaparvathi M, Yamamoto M, Nicolson GL,
et al: Expression and immunohistochemical localization of cathepsin
L during the progression of human gliomas. Clin Exp Metastasis.
14:27–34. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tumminello FM, Leto G, Pizzolanti G, et
al: Cathepsin D, B and L circulating levels as prognostic markers
of malignant progression. Anticancer Res. 16:2315–2319.
1996.PubMed/NCBI
|
|
18
|
Friedrich B, Jung K, Lein M, et al:
Cathepsins B, H, L and cysteine protease inhibitors in malignant
prostate cell lines, primary cultured prostatic cells and prostatic
tissue. Eur J Cancer. 35:138–144. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Svatek RS, Karam J, Karakiewicz PI, et al:
Role of urinary cathepsin B and L in the detection of bladder
urothelial cell carcinoma. J Urol. 179:478–484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sheahan K, Shuja S and Murnane MJ:
Cysteine protease activities and tumor development in human
colorectal carcinoma. Cancer Res. 49:3809–3814. 1989.PubMed/NCBI
|
|
21
|
Watanabe M, Higashi T, Hashimoto M, et al:
Elevation of tissue cathepsin B and L activities in gastric cancer.
Hepatogastroenterology. 34:120–122. 1987.PubMed/NCBI
|
|
22
|
Gabrijelcic D, Svetic B, Spaić D, et al:
Cathepsins B, H and L in human breast carcinoma. Eur J Clin Chem
Clin Biochem. 30:69–74. 1992.PubMed/NCBI
|
|
23
|
Niedergethmann M, Wostbrock B, Sturm JW,
Willeke F, Post S and Hildenbrand R: Prognostic impact of cysteine
proteases cathepsin B and cathepsin L in pancreatic adenocarcinoma.
Pancreas. 29:204–211. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Casson AG, Wilson SM, McCart JA, et al:
ras mutation and expression of the ras-regulated genes osteopontin
and cathepsin L in human esophageal cancer. Int J Cancer.
72:739–745. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Samaiya M, Bakhshi S, Shukla AA, Kumar L
and Chauhan SS: Epigenetic regulation of cathepsin L expression in
chronic myeloid leukaemia. J Cell Mol Med. 15:2189–2199. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Luca A, Maiello MR, D’Alessio A,
Pergameno M and Normanno N: The RAS/RAF/MEK/ERK and the PI3K/AKT
signalling pathways: role in cancer pathogenesis and implications
for therapeutic approaches. Expert Opin Ther Targets. 16(Suppl 2):
S17–S27. 2012.PubMed/NCBI
|
|
27
|
Zhang W, Ling D, Tan J, Zhang J and Li L:
Expression of urokinase plasminogen activator and plasminogen
activator inhibitor type-1 in ovarian cancer and its clinical
significance. Oncol Rep. 29:637–645. 2013.PubMed/NCBI
|
|
28
|
Goldman S and Shalev E: MMPS and TIMPS in
ovarian physiology and pathophysiology. Front Biosci. 9:2474–2483.
2004. View Article : Google Scholar : PubMed/NCBI
|