Introduction
The fundamental aspect of interactions among
microbes and the host organism is the ability of the pathogen to
entrench itself and establish a persistent infection. Cystic
fibrosis (CF) is an autosomal recessive genetic disease (6); the opportunistic pathogen
Pseudomonas aeruginosa may cause chronic lung disease in CF
patients, depending on the genetic adaptation of the pathogen, and
is a prevalent pathogen in CF patients with pulmonary infection
(1–5). Worldwide, 80% of CF patients were
found to be infected with P. aeruginosa (7,8).
Pseudomonas colonization in the lungs of CF patients results
in tissue destruction and reduced respiratory function (9). Identification of CF isolates is
difficult due to the phenotypic diversity, including the formation
of mucoid colonies, loss of pigment and synthesis of rough
lipopolysaccharides (10).
However, identification using genotypic methods may evade this
problem of identifying the variable phenotypes.
A high level of resistance has been exhibited by
P. aeruginosa to numerous antimicrobials. Active efflux pump
systems are of great importance in P. aeruginosa resistance.
The MexA-mexB-oprM operon significantly contributes to the
increased resistance of opportunistic pathogens (11). The rapid increase in antimicrobial
resistance among Pseudomonas spp has resulted in extensive
investigations aimed at understanding the factors that promote the
emergence of antimicrobial resistance in Pseudomonas. Since
Pseudomonas is inherently resistant to a number of
antibiotics, infection with this bacterium is a serious problem in
the treatment of CF patients. Carbapenem antibiotics, including
imipenem and meropenem, are used in the treatment of infections
caused by P. aeruginosa (12); however, carbapenem resistance among
P. aeruginosa strains has been reported in recent years
(13). The production of
β-lactamase and metallo-β-lactamase (MBL), and reduced penetration
of the drugs are challenging factors in the therapeutic management
of these infections (13,14).
The MBLs are classified into three subgroups: B1, B2
and B3, according to their molecular structure. GIM, VIM, SPM and
IMP are genes in integrons, which integrate into chromosomes or
plasmids (15,16). The genes involved in MBL production
are either plasmid- or chromosome-mediated, and are transferred
horizontally. This horizontal transfer of genes poses a health
threat through spreading of resistance among other Gram-negative
bacteria (17). Knowledge of these
enzymes conferring resistance is required to prevent the spread of
the infection among clinical samples. Numerous nosocomial outbreaks
of P. aeruginosa producing metallo-lactamases have been
reported, with an urgent requirement to implement infection control
programs (18–21). Thus, the present study was
undertaken to detect the presence of MBL-producing P.
aeruginosa isolates from the sputa of CF patients by phenotypic
and genotypic methods.
Materials and methods
Bacterial strains
A total of 572 CF patients were included in the
study from Henan Hospital of Traditional Chinese Medicine
(Zhengzhou, China). Sputum samples from patients with CF were
collected and processed according to standard methods (Clinical and
Laboratory Standards Institute®; CLSI Guidelines, 2012).
All demographic details, including age, gender and history of
antibiotic usage were collected. P. aeruginosa was isolated
and characterized using biochemical methods. The present study was
approved by the ethical committee of Henan Hospital of Traditional
Chinese Medicine. Written informed consent was obrained from the
patient’s families.
Antimicrobial susceptibility
analysis
The following antibiotics (Oxoid Ltd., Basingstoke,
UK) were used for the antimicrobial susceptibility analysis by
Kirby Bauer’s disc diffusion method according to CLSI guidelines
(23): Amikacin (30 mg),
gentamicin (10 mg), netilmicin (30 mg), tobramycin (10 mg),
cefoperazone (75 mg), cefepime (30 mg), ceftazidime (30 mg),
ceftriaxone (30 mg), ceftizoxime (30 mg), ciprofloxacin (5 mg),
gatifloxacin (5 mg), imipenem (10 mg), meropenem (10 mg) and
piperacillin-tazobactam (100/10 mg). P. aeruginosa ATCC
27853, obtained from the Microbology Laboratory at Henan Hospital
of Traditional Chinese Medicine, served as a control.
Minimum inhibitory concentration
(MIC)
The MIC was determined on imipenem-resistant
isolates using the agar dilution method, with serial dilution of
the imipenem powder at a concentration range of 0.06–512 μg/ml. A
volume of 1 ml of the appropriate dilution of imipenem was added to
19 ml Muller Hinton agar, cooled to 55°C and subsequent to mixing
thoroughly, and the mixture was poured onto Petri dishes. The
culture grown overnight was collected and the turbidity was matched
to McFarland’s standard 0.5 (23).
A sample of 2 μl culture was delivered onto a Petri dish, which was
divided into quadrants, and the plate was incubated for 18–24 h at
37°C. Following incubation, the highest dilutions exhibiting no
visible growth were considered as the MIC of the particular strain
(CLSI 2012 guidelines) (23).
Detection of MBLs
Phenotypic detection of MBLs was conducted by the
zone enhancement method using ceftazidime discs (Oxoid Ltd) with
EDTA (22). Muller Hinton agar
plates were seeded with the test organism matched to 0.5
McFarland’s standard, according to the CLSI 2012 guidelines
(23). A 0.5 M EDTA solution was
prepared with 186.1 g disodium EDTA dissolved in 1.0 ml distilled
water at pH 8.0 using NaOH. Subsequent to sterilization by
autoclaving, EDTA solution was added to 750-μg ceftazidime discs.
The discs impregnated with EDTA were dried in the incubator and
stored in airtight vials at −20°C. The ceftazidime (30 μg) discs
and ceftazidime-EDTA discs (750 μg) were placed upon the agar
surface and incubated for 16–18 h at 35°C. The zone of enhancement
surrounding the ceftazidime EDTA disc was considered to be positive
for MBL production.
Genotypic characterization of the MBL
gene
DNA was extracted from the P. aeruginosa
isolates by the boiling method (24). Cultures of P. aeruginosa
were grown overnight in Trypticase soy broth (Difco Laboratories,
Inc., Detroit, MI, USA). A sample of 1.5 ml overnight culture was
transferred to an Eppendorf tube and centrifuged at 17,310 × g in
an Eppendorf cooling centrifuge for 5 min. Following
centrifugation, the supernatant was decanted and the pellet was
suspended in 500 μl MilliQ water (Millipore Corp., Billerica, MA,
USA). The suspension was boiled at 95°C for 10 min and cell debris
was removed by centrifugation at 17,310 × g for 5 min. The
supernatant served as a template for amplification. Duplex
polymerase chain reaction (PCR) was performed to detect the
presence of blaIMP and blaVIM β-lactamase in a
Thermal Cycler 9600 instrument (Applied Biosystems, Norwalk, CT,
USA). The reaction was prepared in a final volume of 50 μl,
containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2.5 mM
MgCl2, 0.25 mM deoxyribonucleotide triphosphate, 0.75 mM
deoxyuridine triphosphate (Roche Diagnostics, Quebec, Canada),
0.125 U uracil-DNA glycosylase and 2 U Taq polymerase (Roche
Diagnostics). Concentrations of 0.16 mM of each primer were used in
PCR. The primer sequences were as follows: IMP-A, forward
5′-GAAGGYGTTTATGTTCATAC-3′ and IMP-B, reverse,
5′-GTAMGTTTCAAGAGTGATGC-3′ with a product size of 587 bp (33) and VIM2004A, forward
5′-GTTTGGTCGCATATCGCAAC-3′ and VIM2004B, reverse 5′-AATGCGCAGCACCAG
GATAG-3′ with a 382 bp amplicon size (Roche Diagnostics) A 2-μl
sample served as a template. The following thermocycling conditions
were used for amplification: Initial denaturation step at 94°C for
5 min, for 30 cycles followed by denaturation at 94°C for 1 min,
annealing at 54°C for 1 min and primer extension at 72°C for 1.5
min. Subsequent to amplification, the amplicons were visualized on
1.5% agarose gel in TAE buffer [containing 0.04 M Tris-acetate and
0.002 M EDTA (pH 8.5)] to detect the presence of bands and the gels
were scanned under ultraviolet illumination, visualized and
digitized with a Bio-Rad Gel Doc imaging system (Bio-Rad, Sydney,
Australia). SPSS 11 software (SPSS, Inc., Chicage, IL, USA) was
used for statistical analysis of data.
Results
Patient characteristics
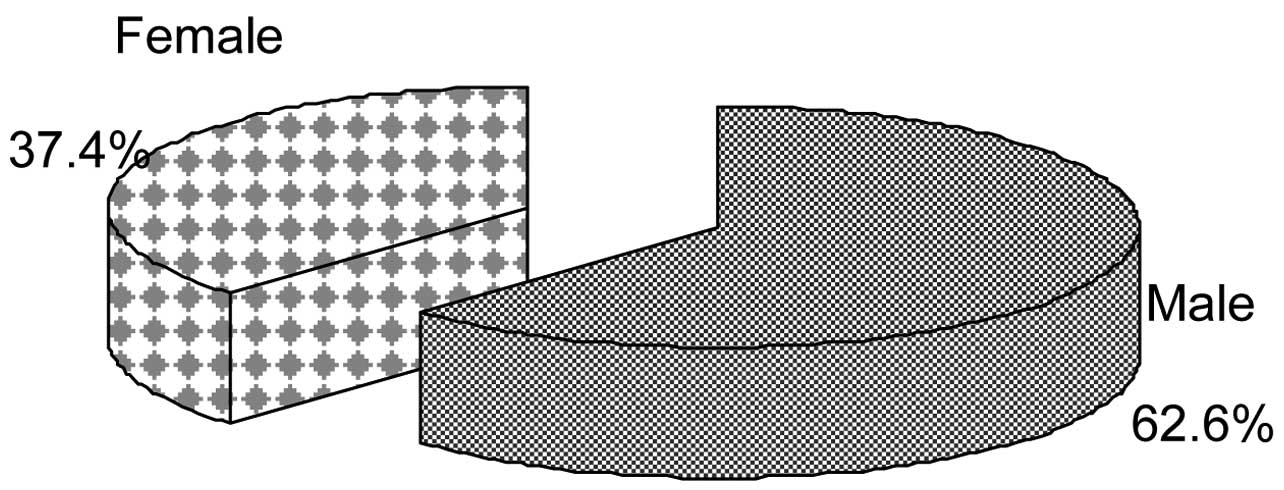
Of the 572 CF patients, 358 (62.6%) were male and
214 (37.4%) were female (Fig. 1).
P. aeruginosa infection among the CF patients was more
prevalent in the 0–5 year-old group compared with the >15
year-old group. Among the 572 patients, 298 (52.1%) were
hospitalized and were aged <12 years and found to be culture
negative for P. aeruginosa. Out of 572 patients recruited,
217 (37.9%) were infected with P. aeruginosa. No significant
correlation was observed between Pseudomonas infection and
CF.
Resistance pattern
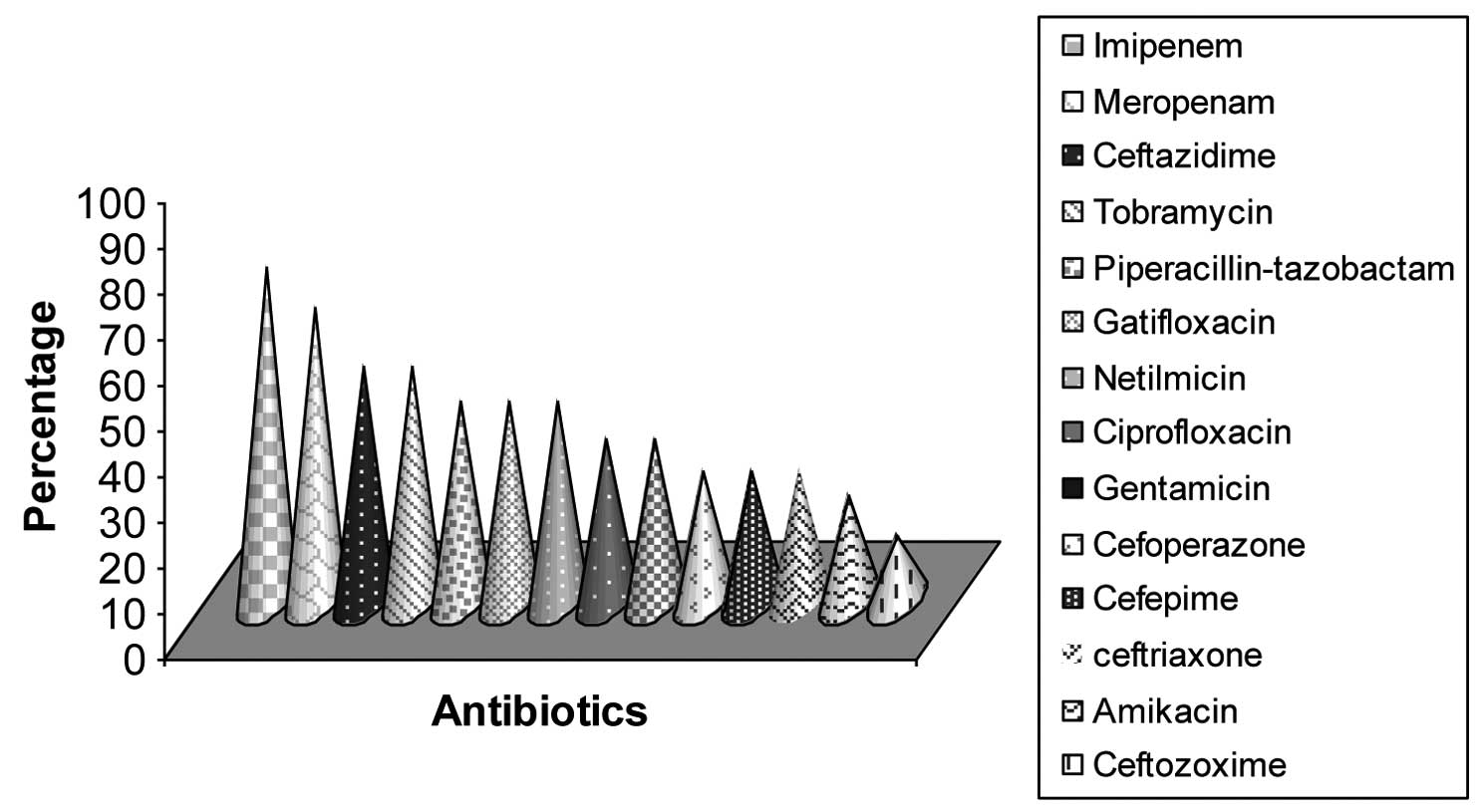
Of the 217 P. aeruginosa isolates, 159
(73.3%) were resistant to imipenem and 141 (64.9%) to meropenem.
Ceftazidime and tobramycin resistance was detected in 112 (51.6%)
respective samples, and 96 (44.2%) isolates were resistant to
piperacillin-tazobactam, gatifloxacin and netilmicin, respectively.
A total of 78 (35.9%) colonies were resistant to ciprofloxacin and
gentamicin, and 62 (28.6%) were resistant to cefoperazone, cefepime
and ceftriaxone. The least resistance was observed for amikacin,
with 51 (23.5%) resistant isolates, followed by ceftizoxime with 32
(14.7%) resistant samples (Fig.
2).
MIC
The MIC for the antibiotics was determined by the
agar dilution method according to the CLSI 2012 guidelines
(23). Among the 159
imipenem-resistant isolates examined, 72 exhibited a four-fold
reduction in MIC values (Table
I).
 | Table INumber of Pseudomonas
aeruginosa imipenem-resistant isolates at different
concentrations of MIC analyzed using the agar dilution method for
imipenem. |
Table I
Number of Pseudomonas
aeruginosa imipenem-resistant isolates at different
concentrations of MIC analyzed using the agar dilution method for
imipenem.
| MIC (μg) | 512 | 256 | 128 | 64.0 | 3.02 | 16.0 | 8.00 | 4.00 | 2.00 | 1.00 | 0.500 | 0.250 | 0.125 | 0.0600 |
|---|
| Imipenem- resistant
isolates (n=159) | 22 | 16 | 6 | 2 | 8 | 2 | 1 | 5 | 2 | 3 | 1 | 1 | 1 | 2 |
Detection of MBLs
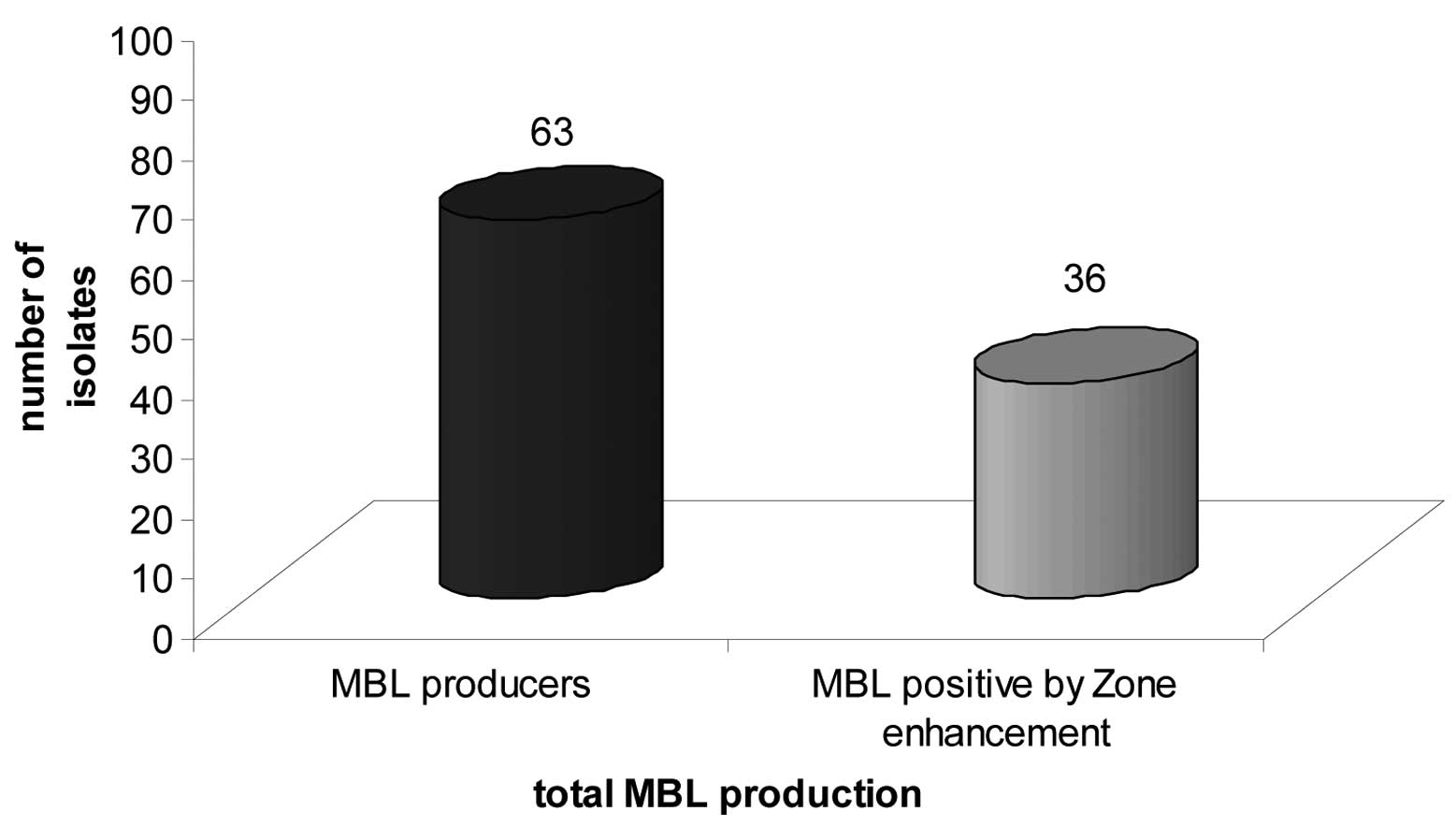
Of the 112 isolates resistant to ceftazidime, 63
(56.25%) were found to be positive for MBL production. A total of
36 (57.1%) showed enhancement of the zone surrounding the
ceftazidime-EDTA discs (Fig. 3).
One notable feature among the 63 MBL-producing P. aeruginosa
samples was that all strains were found to be resistant to
meropenem and ceftazidime. Out of the 63 MBL-producing isolates, 38
(60.3%) isolates were from male patients and 25 (39.7%) were from
female patients; no statistical significance (P<0.05) was
identified between gender and MBL production. However, statistical
significance (P<0.05) was detected in the association between
ceftazidime resistance and MBL production in the isolates.
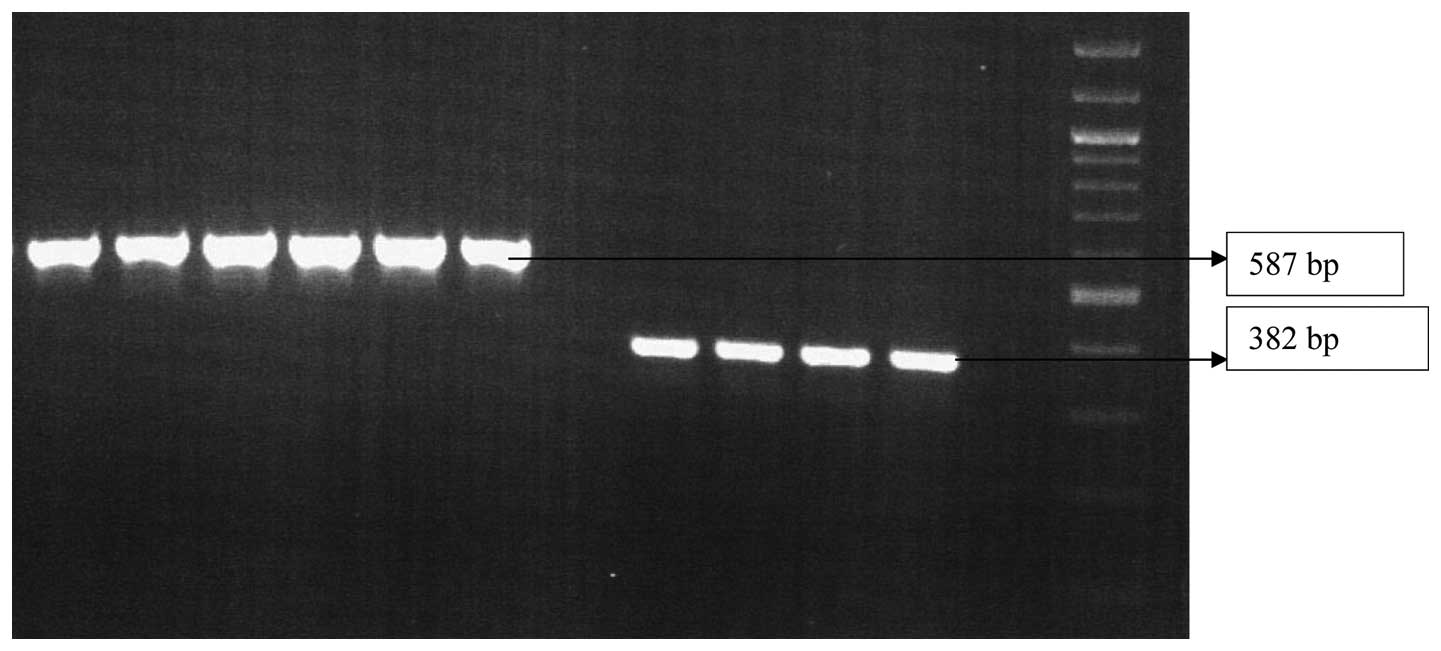
Genotypic detection of MBL genes
Primers were designed to detect the presence of the
β-lactamase genes blaVIM and blaIMP. Of the 217 P.
aeruginosa strains screened, only 63 isolates were found to
produce MBL. These 63 strains were then analyzed by PCR. Out of 63
isolates, 53 (84.1%) exhibited the presence of blaVIM genes
and 48 (76.1%) exhibited the presence of blaIMP genes
(Fig. 4).
Discussion
Knowledge of the susceptibility of P.
aeruginosa to antimicrobial agents is urgently required, since
understanding of the pattern of antibiotic resistance may aid in
treatment of this infection, particularly in CF patients. The
prevalence of resistant strains among CF patients may be elucidated
by testing these antibiotics on isolates collected from patients
(25). Resistance to carbapenem is
of clinical concern (26,27). P. aeruginosa is an
opportunistic multidrug-resistant pathogen, which is an increasing
problem worldwide (28,29). Kulczycki et al (2) revealed a 76.6% prevalence rate, which
is high compared with that of the present study (37.9%). Various
studies of P. aeruginosa infection worldwide have observed
percentages of resistance to imipenem and meropenem of 4–70%
(5). In the present study, 73.3%
isolates were resistant to imipenem and 64.9% to meropenem. These
are higher values than usual, which reveals that there is
increasing resistance of P. aeruginosa towards antimicrobial
drugs. MBL expression among P. aeruginosa samples was found
to be 10–65% across the country from varying clinical samples
(15). In the present study,
56.25% P. aeruginosa isolates produced MBL, which was
a lower percentage than that previously identified in a study group
of severe acute respiratory infection, defined by the World Health
Organisation as an acute respiratory illness of recent onset
(within 7 days) manifested by fever (≥38°C), cough and dypnea
requiring overnight hospitalization) (30) and a study by Behara et al
(31), which observed that 62.5%
P. aeruginosa isolates produced MBL. The higher rate
of MBL production among P. aeruginosa suggested that
carbapenem resistance in P. aeruginosa is mediated by MBL
production. Higher morbidity and mortality are associated
with P. aeruginosa producing MBL (32). In the present study, 36 (57.1%)
isolates showed enhancement of the zone surrounding the
ceftazidime-EDTA disc, which is lower than the percentage
identified by Hemlatha et al (33) (87.5%), who also observed a lower
percentage of isolates producing MBL.
In the present study, the majority of CF patients
recruited were male (62.6%). Among the 217 isolates of P.
aeruginosa examined, the antibiogram analysis revealed high
resistance to ceftazidime (51.6%), which was marginally less
compared with that reported by Mayank et al (34), who had detected ceftazidime
resistance in 63% of isolates. In other studies by Obritsch et al
(35) and Arya et al
(36), 55.4% ceftazidime
resistance was observed, which is concordant with the present
study. The present study also identified higher resistance to other
antibiotics, including tobramycin (51.6%), piperacillin-tazobactam,
gatifloxacin, netilmicin (44.2%), ciprofloxacin and gentamicin
(35.9%). However, resistance to cefoperazone and cefepime was
detected in 28.9% of samples, which is similar to other studies
demonstrating reduced susceptibility to commonly used antibiotics
(3,4,11,12).
The 159 isolates resistant to imipenem were examined
for MIC by agar dilution. A significant four-fold reduction was
observed in the MIC of 72 (45.3%) of these samples. In total, MIC
ranges of 0.06- to 512-fold were observed, which is in concordance
with previous studies by Migliavacca et al (37) in 2002, Hemlatha et al
(33) in 2005, Aggarwal et
al (38) in 2008 and Jakumar
et al (39) in 2007.
It is important to confirm the presence of the
blaVIM and blaIMP β-lactamase genes by PCR. In the
present study, out of the 217 P. aeruginosa strains, the 63
isolates positive for MBL production were selected for PCR
analysis. Of the 63 isolates, 53 (84.1%) exhibited the presence of
blaVIM genes and 48 (76.1%) exhibited the presence of
blaIMP genes, which corresponds with studies by Mayank et al
(34) and Sader et al
(40).
In conclusion, the present study emphasizes the
requirement for clinical microbiology laboratories to analyze MBL
production in carbapenem-resistant P. aeruginosa strains. As
an increase in multi-drug resistance has been identified among
Gram-negative bacteria, an uncontrolled increase in MBL production
may result in therapeutic complications, which may in turn raise
mortality and morbidity. Early and accurate detection of MBLs may
control the spread of MDR pathogens in the future. The use of
molecular techniques aids in MBL detection in regional
laboratories, to provide the appropriate diagnosis and
identification of outbreaks by MBL-producing MDR pathogens,
particularly in cystic fibrosis patients. Thus, regular
surveillance of MBL-producing P. aeruginosa, along with
judicious use of antibiotics, may prevent the spread of drug
resistance.
Acknowledgements
Financial support was provided by the Scientific and
technological project of Henan Province (no. 132102310244) and the
Henan University of Traditional Chinese Medicine Graduate
Innovation Fund Project (no. 201210).
References
|
1
|
Friend PA: Pulmonary infection in cystic
fibrosis. J Infect. 13:55–72. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kulczycki LL, Murphy TM and Bellanti JA:
Pseudomonas colonization in cystic fibrosis. A study of 160
patients. JAMA. 240:30–34. 1978. View Article : Google Scholar
|
|
3
|
May JR, Herrick NC and Thompson D:
Bacterial infection in cystic fibrosis. Arch Dis Child. 47:908–913.
1972. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mearns MB, Hunt GH and Rushworth R:
Bacterial flora of respiratory tract in patients with cystic
fibrosis, 1950–1971. Arch Dis Child. 47:902–907. 1972.PubMed/NCBI
|
|
5
|
Thomassen MJ, Demko CA, Boxerbaum B, et
al: Multiple isolates of Pseudomonas aeruginosa with
differing antimicrobial susceptibility patterns from patients with
cystic fibrosis. J Infect Dis. 140:873–880. 1979.
|
|
6
|
Arancibia F, Bauer TT, Ewig S, et al:
Community-acquired pneumonia due to gram-negative bacteria and
Pseudomonas aeruginosa: incidence, risk, and prognosis. Arch
Intern Med. 162:1849–1858. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
West SE, Zeng L, Lee BL, et al:
Respiratory infections with Pseudomonas aeruginosa in
children with cystic fibrosis: early detection by serology and
assessment of risk factors. JAMA. 287:2958–2967. 2002.PubMed/NCBI
|
|
8
|
Spilker T, Coenye T, Vandamme P and LiPuma
JJ: PCR-based assay for differentiation of Pseudomonas
aeruginosa from other Pseudomonas species recovered from
cystic fibrosis patients. J Clin Microbiol. 42:2074–2079.
2004.PubMed/NCBI
|
|
9
|
Gillespie SH and Hawkey PM: Principles and
Practice of Clinical Bacteriology. 2nd edition. Wiley; Chichester,
UK: 2006, View Article : Google Scholar
|
|
10
|
Lyczak JB, Cannon CL and Pier GB: Lung
infections associated with cystic fibrosis. Clin Microbiol Rev.
15:194–222. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nikaido H: Multidrug efflux pumps of
gram-negative bacteria. J Bacteriol. 178:5853–5859. 1996.PubMed/NCBI
|
|
12
|
Sonnesyn SW and Gerding DN: Antimicrobials
for the treatment of respiratory infections. Respiratory
Infections: A Scientific Basis for Management. Niederman MS, Sarosi
GA and Glassroth J: Saunders; Philadelphia: pp. 511–537. 1994
|
|
13
|
Lee K, Lim YS, Yong D, et al: Evaluation
of the Hodge test and the imipenem-EDTA double-disk synergy test
for differentiating metallo-beta-lactamase-producing isolates of
Pseudomonas spp. and Acinetobacter spp. J Clin
Microbiol. 41:4623–4629. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Varaiya A, Kulkarni N, Kulkarni M, et al:
Incidence of metallo beta lactamase producing Pseudomonas
aeruginosa in ICU patients. Indian J Med Res. 127:398–402.
2008.PubMed/NCBI
|
|
15
|
Oh EJ, Lee S, Park YJ, et al: Prevalence
of metallo-beta-lactamase among Pseudomonas aeruginosa and
Acinetobacter baumannii in a Korean university hospital and
comparison of screening methods for detecting
metallo-beta-lactamase. J Microbiol Methods. 54:411–418.
2003.PubMed/NCBI
|
|
16
|
Pitout JD, Gregson DB, Poirel L, et al:
Detection of Pseudomonas aeruginosa producing
metallo-beta-lactamases in a large centralized laboratory. J Clin
Microbiol. 43:3129–3135. 2005.
|
|
17
|
Gladstone P, Rajendran P and Brahmadathan
KN: Incidence of carbapenem resistant nonfermenting gram negative
bacilli from patients with respiratory infections in the intensive
care units. Indian J Med Microbiol. 23:189–191. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cornaglia G, Mazzariol A, Lauretti L, et
al: Hospital outbreak of carbapenem-resistant Pseudomonas
aeruginosa producing VIM-1, a novel transferable
metallo-beta-lactamase. Clin Infect Dis. 31:1119–1125.
2000.PubMed/NCBI
|
|
19
|
Crespo MP, Woodford N, Sinclair A, et al:
Outbreak of carbapenem-resistant Pseudomonas aeruginosa
producing VIM-8, a novel metallo-beta-lactamase, in a tertiary care
center in Cali, Colombia. J Clin Microbiol. 42:5094–5101.
2004.PubMed/NCBI
|
|
20
|
Pournaras S, Maniati M, Petinaki E, et al:
Hospital outbreak of multiple clones of Pseudomonas
aeruginosa carrying the unrelated metallo-beta-lactamase gene
variants blaVIM-2 and blaVIM-4.
J Antimicrob Chemother. 51:1409–1414. 2003.
|
|
21
|
Tsakris AS, Pournaras S, Woodford M, et
al: Outbreak of infections caused by Pseudomonas aeruginosa
producing VIM-1 carbapenemase in Greece. J Clin Microbiol.
38:1290–1292. 2000.
|
|
22
|
Yong D, Lee K, Yum JH, et al:
Imipenem-EDTA disk method for differentiation of
metallo-beta-lactamase-producing clinical isolates of
Pseudomonas spp. and Acinetobacter spp. J Clin
Microbiol. 40:3798–3801. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Clinical and Laboratory Standards
Institute (CLSI). Performance standards for antimicrobial
susceptibility testing. 11th informational supplement. CLSI; Wayne,
PA, USA: pp. M100–S23. 2013
|
|
24
|
Hall BG and Barlow M: Revised Ambler
classification of β-lactamases. J Antimicrob Chemother.
55:1050–1051. 2005.PubMed/NCBI
|
|
25
|
Pitt TL, Sparrow M, Warner M and
Stefanidou M: Survey of resistance of Pseudomonas aeruginosa
from UK patients with cystic fibrosis to six commonly prescribed
antimicrobial agents. Thorax. 58:794–796. 2003.PubMed/NCBI
|
|
26
|
Cheng K, Smyth RL, Govan JR, et al: Spread
of beta-lactam-resistant Pseudomonas aeruginosa in a cystic
fibrosis clinic. Lancet. 348:639–642. 1996.PubMed/NCBI
|
|
27
|
Gales AC, Jones RN, Turnidge J, et al:
Characterization of Pseudomonas aeruginosa isolates:
occurrence rates, antimicrobial susceptibility patterns, and
molecular typing in the global SENTRY antimicrobial surveillance
program, 1997–1999. Clin Infect Dis. 32(Suppl 2): S146–S155.
2001.
|
|
28
|
Neuhauser MM, Weinstein RA, Rydman R, et
al: Antibiotic resistance among gram-negative bacilli in US
intensive care units: implications for fluoroquinolone use. JAMA.
289:885–888. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cardoso O, Alves AF and Leitão R:
Metallo-beta-lactamase VIM-2 in Pseudomonas aeruginosa
isolates from a cystic fibrosis patient. Int J Antimicrob Agents.
31:375–379. 2008.PubMed/NCBI
|
|
30
|
Manoharan A, Chatterjee S and Mathai D;
SARI Study Group. Detection and characterization of metallo beta
lactamases producing Pseudomonas aeruginosa. Indian J Med
Microbiol. 28:241–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Behera B, Mathur P, Das A, et al: An
evaluation of four different phenotypic techniques for detection of
metallo-beta-lactamase producing Pseudomonas aeruginosa.
Indian J Med Microbiol. 26:233–237. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hemlatha V, Sekar U and Kamat V: Detection
of metallo betalactamase producing Pseudomonas aeruginosa in
hospitalized patients. Indian J Med Res. 122:148–152.
2005.PubMed/NCBI
|
|
33
|
Hemlatha V, Sekar U and Kamat V: Detection
of metallo betalactamase producing Pseudomonas aeruginosa in
hospitalized patients. Indian J Med Res. 122:148–152.
2005.PubMed/NCBI
|
|
34
|
Mayank D, Anshuman M, Singh RK, et al:
Nosocomial cross-transmission of Pseudomonas aeruginosa
between patients in a tertiary intensive care unit. Indian J Pathol
Microbiol. 52:509–513. 2009.
|
|
35
|
Obritsch MD, Fish DN, MacLaren R and Jung
R: National surveillance of antimicrobial resistance in
Pseudomonas aeruginosa isolates obtained from intensive care
unit patients from 1993 to 2002. Antimicrob Agents Chemother.
48:4606–4610. 2004.PubMed/NCBI
|
|
36
|
Arya M, Arya PK, Biswas D and Prasad R:
Antimicrobial susceptibility pattern of bacterial isolates from
post-operative wound infections. Indian J Pathol Microbiol.
48:266–269. 2005.PubMed/NCBI
|
|
37
|
Migliavacca R, Docquier JD, Mugnaioli C,
et al: Simple microdilution test for detection of
metallo-β-lactamase production in Pseudomonas aeruginosa. J
Clin Microbiol. 40:4388–4390. 2002.
|
|
38
|
Aggarwal R, Chaudhary U and Bala K:
Detection of extended-spectrum beta lactamase in Pseudomonas
aeruginosa. Indian J Pathol Microbiol. 51:222–224. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jayakumar S and Appalaraju B: Prevalence
of multi and pan drug resistant Pseudomonas aeruginosa with
respect to ESBL and MBL in a tertiary care hospital. Indian J
Pathol Microbiol. 50:922–925. 2007.PubMed/NCBI
|
|
40
|
Sader HS, Reis AO, Silbert S and Gales AC:
IMPs, VIMs and SPMs: the diversity of metallo-β-lactamases produced
by carbapenem-resistant Pseudomonas aeruginosa in a
Brazilian hospital. Clin Microbiol Infect. 11:73–76.
2005.PubMed/NCBI
|