Introduction
Controlled ovarian hyperstimulation (COH) is an
effective method of assisted reproductive technology (ART) to
stimulate the generation of more oocytes than are produced during
natural cycles (1). Despite the
increase in the number of embryos generated using COH, pregnancy
rates following ART remain low at 20–30% per fresh cycle (2). Several studies and meta-analyses have
shown that the gonadotropin-releasing hormone analogues (GnRH-as),
including GnRH agonists and antagonists, which are used in COH, may
have negative effects on endometrial receptivity (3–5).
However, the mechanisms regulating endometrial receptivity
deficiency following GnRH-as treatment remain to be elucidated
(2,4).
Homeobox (HOX) A10/Hoxa10 (human/mouse),
respectively) is a homeobox-containing transcription factor that
regulates embryo uterine development and is essential for
endometrial development during each menstrual cycle in adults
(6–7). Hoxa10 targeted mutation Hoxa10 (− /−)
mice ovulate normally, but ~80% are sterile due to the low
expression of maternal Hoxa10 in the distal oviductal and uterine
epithelium, which may affect embryo implantation (8). Hoxa10 is a characteristic molecular
marker of endometrial receptivity with peak expression exhibited
during the window of implantation (9). Hoxa10 has been demonstrated to be
involved in the regulation of pinopode development and downstream
target genes, which are involved in implantation, including
integrin β3 (10–12).
Impaired endometrial receptivity is associated with
altered Hoxa10 methylation. Abnormal expression of Hoxa10 has been
found to be associated with disrupted endometrial receptivity in
several diseases, including endometrial carcinoma, endometriosis,
endometrial polyps, ovarian cancer, polycystic ovary syndrome and
in conditions associated with exposure to diethylstilbestrol and
bisphenol-A (13–18). However, few studies have
demonstrated the association between the uterine methylation status
and alterations in endometrial receptivity following GnRH-as
therapies. The present study aimed to investigate the uterine
methylation status of the Hoxa10 gene following GnRH-as treatment
in order to explore the potential mechanism underlying the
epigenetic effect of GnRH-as on endometrial receptivity.
Materials and methods
Animals
Animal care and use was conducted according to the
institutional guidelines established by the Animal Care and Use
Committee of Wuhan University (Wuhan, China) of 2010 Zhongnan
Hospital of Wuhan University Animal Care and Use 025. Female,
virgin BALB/c mice (7–9 weeks) were purchased from Hubei Medical
Laboratory Animal Center (Wuhan, China) and were housed under a
12/12 h light/dark cycle at 25±0.5°C and 50–60% humidity. Mice were
fed with a standard pellet diet and water. Smear samples of vaginal
discharge were observed daily in order to identify the estrus. Only
mice with more than two consecutive periods of regular 4-day estrus
cycles were used in the present study. Suitable mice [age, 8–12
weeks; body weight (bw), 20–23 g] were randomly divided into three
groups: The GnRH agonist treatment group (n=10), the GnRH
antagonist treatment group (n=10) and the control (natural cycle)
group (n=10).
Ovarian stimulation
Treatment procedures were performed as previously
described, but with minor revisions (19). In brief, mice in the GnRH agonist
group received intraperitoneal (IP) injection with the GnRH agonist
Decapeptyl® (Ferring Co., Kiel, Germany)) at 1.5 μg/100
g bw/day between days three and 9 of estrus. At 9 am of day 9, 20
IU/mouse human menopausal gonadotropin (HMG; Livzon Pharmaceutical
Group Inc., Shanghai, China) was injected IP, followed by IP
injection with 100 IU/100 g bw human chorionic gonadotrophin (HCG;
Pregnyl®; Organon International, Oss, Netherlands) at 28
h after the injection of HMG. Mice in the GnRH antagonist group
received IP injection of the GnRH antagonist Cetrotide®
(Serono Inc., Rockland, MA, USA) at 4 μg/100 g bw on day three of
estrus. HMG was then injected at 20 IU/mouse IP at 9 am of day 9,
followed by IP injection with 100 IU/100 g bw HCG 28 h after the
injection of HMG. The mice in the control (natural cycle) group
received IP injection with saline only at the same volume as the
injections received by the mice in the GnRH agonist and antagonist
groups, from day three of estrus onwards. The same injection
schedule was followed as described for the GnRH agonist and
antagonist groups.
Tissue collection and application
Fresh whole uterus samples were collected from the
mice in the three groups 48 h after the treatment. Fresh whole
uterus samples were quickly divided into four equal sections
subsequent to being washed in cold phosphate-buffered saline. One
uterus section was fixed in 2.5% glutaraldehyde at room temperature
for 30 min and at incubated at 4°C overnight, prior to being fixed
for ≥1 h in 1% osmium tetroxide in the dark for scanning electron
microscopy (SEM) analysis. The remaining three sections of each
sample were stored at −80°C until required for protein, DNA and
mRNA extraction.
Genomic DNA extraction and bisulfite
sequencing polymerase chain reaction (BSP)
Genomic DNA was extracted from the frozen tissue
samples from the three groups using the DNeasy® Blood
and Tissue kit (Qiagen, Valencia, CA, USA). The genomic DNA (500
ng) was then bisulfite-modified using the EZ DNA Methylation-Gold™
kit (Qiagen) according to the manufacturer’s instructions.
Bisulfite-modified DNA was dissolved in 20 μl water and stored at
−80°C.
Quantification of Hoxa10 promoter
methylation in mice using BSP
A total of 200 ng bisulfite-treated DNA was used in
a 50 μl reaction system containing 1.5 μl forward and reverse
primers (Table I), 1.25 mmol/l
deoxynucleotide triphosphates, 25 mM Mg2+ and 0.5 μl
HotStarTaq DNA polymerase (Qiagen). The amplification conditions
were as follows: 10 min at 95°C followed by 40 cycles of 95°C for
30 sec, 53°C for 30 sec and 72°C for 40 sec, then a final extension
at 72°C for 10 min. Polymerase chain reaction (PCR) products were
resolved using electrophoresis on a 2% agarose gel and stained with
GoldView (SBS Genetech Co., Ltd., Beijing, China). The
appropriate-sized product bands were then isolated and excised from
the gel and purified using a Gel Extraction kit (Axygen
Biotechnology, Hangzhou, China) according to the manufacturer’s
instructions. The resultant products were sequenced using MicoRead
Biotechnology (Beijing Microread Genetics Co., Ltd., Beijing,
China).
 | Table IPrimer sequences used for BSP and
qPCR. |
Table I
Primer sequences used for BSP and
qPCR.
| Gene | Sense primer
(5′-3′) | Antisense primer
(5′-3′) |
|---|
| Hoxa10 (BSP) |
TATTTTGAGGTAGTTTTTATAGTTT |
CAAATAACCCTTTCTAACTAACATTTC |
| Hoxa10 (qPCR) |
CCTTCCGAGAGCAGCAAA |
GTCTGGTGCTTCGTGTAGGG |
| Integrin β3
(qPCR) |
GCCTTCGTGGACAAGCCTGTA |
GGACAATGCCTGCCAGTCTTC |
| β-actin (qPCR) |
TTCCAGCCTTCCTTCCTGG |
TTGCGCTCAGGAGGAGCAAT |
Quantitative polymerase chain reaction
(qPCR) analysis
Total RNA was extracted from the tissue samples
using the REzol RNA extraction kit (SBS Genetech Co., Ltd.)
according to the manufacturer’s instructions. Total RNA (100 ng)
from each sample was treated with DNase and converted to
complementary (c)DNA using the PrimeScript™ RT reagent kit (Takara
Bio Inc., Dalian, China). mRNA levels were analyzed using an iQ5
Real-Time PCR detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) with SYBR® Premix Ex Taq™ II (Takara
Bio Inc.). The primer sequences for Hoxa10, integrin β3 and β-actin
are listed in Table I. All primers
were obtained from Servicebio (Wuhan, China).
The qPCR amplification conditions for Hoxa10 were as
follows: 40 cycles of 95°C for 10 sec, 62°C for 15 sec and 72°C for
15 sec. The qPCR amplification conditions for integrin β3 and
β-actin were as follows: 40 cycles of 95°C for 10 sec, 60°C for 15
sec and 72°C for 15 sec. The increasing fluorescence of the PCR
products during amplification was monitored to create a
quantitative standard curve. Quantification of the target gene
expression in the samples was assessed and adjusted to the
quantitative expression of β-actin in the same samples. Melting
curve analysis was conducted to determine the specificity of the
amplified products and to ensure the absence of primer-dimer
formation. All products obtained yielded the predicted melting
temperature. Relative gene expression was calculated using the
2−ΔΔCt method.
Western blot analysis
Samples were lysed in radio-immunoprecipitation
assay buffer (Beyotime, Shanghai, China) with protease inhibitors
(Sigma-Aldrich, St. Louis, MO, USA) to extract whole-cell proteins.
Protein levels were measured using the Micro Bicinchoninic Acid™
Protein Assay kit (Beyotime). Proteins were run on a precast 7.5%
acrylamide gel (Beyotime) and transferred onto polyvinylidene
fluoride membranes (Millipore Corporation, Billerica, MA, USA).
Membranes were blocked with 5% fat-free milk in Tris-buffered
saline with 0.1% Tween-20 followed by incubation with primary
antibodies against Hoxa10 (AV100932; Sigma-Aldrich) and integrin β3
(ab33171; Abcam PLC, Cambridge, UK). Membranes were then incubated
with secondary antibodies rabbit anti-goat polyclonal antibody
(ZDR-5308; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.,
Beijing, China) or goat anti-rabbit polyclonal antibody (ZDR-5306;
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) in
blocking buffer, respectively. Immunoreactive bands were detected
using a chemiluminescent detection kit (Beyotime). Densitometry
measurements were analyzed using Quantity One v4.4.0 (Bio-Rad
Laboratories, Inc.). Target protein expression levels were
normalized to that of β-actin.
SEM analysis
SEM was performed for morphological analysis and to
confirm the presence of pinopodes in the endometrium of mice during
the implantation window in the natural cycle or following GnRH
agonist or antagonist treatment. For SEM preparation, endometrial
tissues were fixed in 2.5% glutaraldehyde at room temperature for
30 min then at 4°C overnight, prior to being fixed for at least 1 h
in 1% osmium tetroxide in the dark. Samples were then dehydrated in
a graded series of ethanol, critical-point-dried, mounted and
coated with gold in a sputter coater (JFC-1300 Auto Fine Coater;
Jeol Ltd., Tokyo, Japan). Samples were observed using a scanning
electron microscope (JSM-5600LV SEM, Jeol Ltd.). SEM was performed
in order to observe the morphology of the pinopodes in the samples
from the different groups.
Statistical analysis
Statistical analyses for western blot analysis, BSP
and qPCR values were performed using SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA). χ2 and analysis of variance
tests were performed to compare the groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Hoxa10 promoter methylation status and
GnRH-as
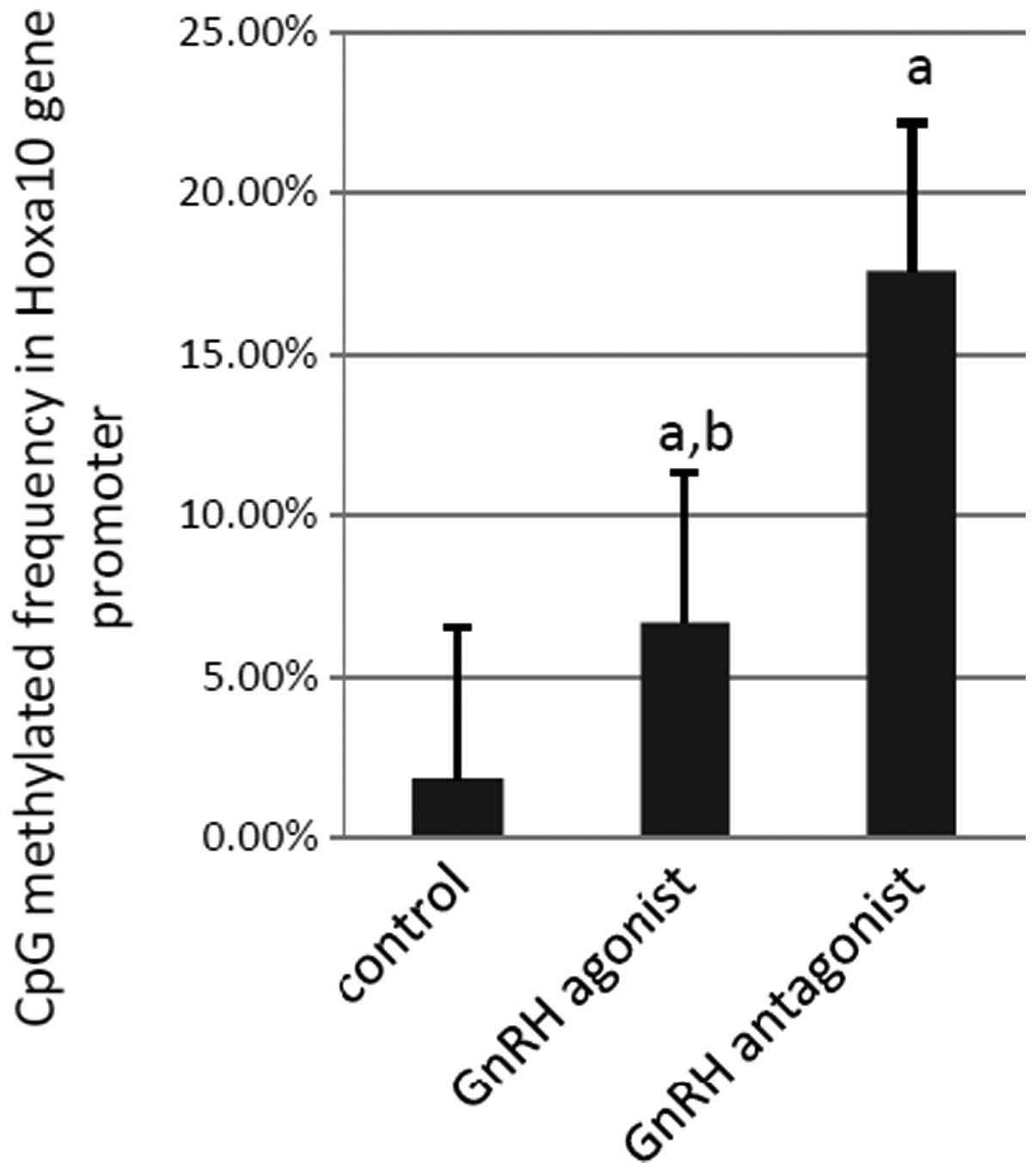
BSP was performed to examine the methylation status
of the 21 CpG sites in the Hoxa10 promoter in each sample. In
total, 210 CpG sites were analyzed in each experimental group. A
total of 37 (17.6%) methylated CpG sites were observed in the
samples from the GnRH antagonist group, compared with 14 (6.7%)
methylated sites in the samples from the GnRH agonist group and
four (1.9%) methylated sites in the samples from the control group
(GnRH antagonist vs. control, P<0.001; GnRH agonist vs. control,
P=0.006 and GnRH agonist vs. GnRH antagonist, P=0.008; Fig. 1). Thus, compared with the mice in
the control group, the levels of methylation within the Hoxa10
promoter sequence were found to be higher in the mice that had
received GnRH-as treatment, particularly in those treated with the
GnRH antagonist.
Endometrial Hoxa10 and integrin β3 mRNA
expression during the implantation window following GnRH-as
treatment
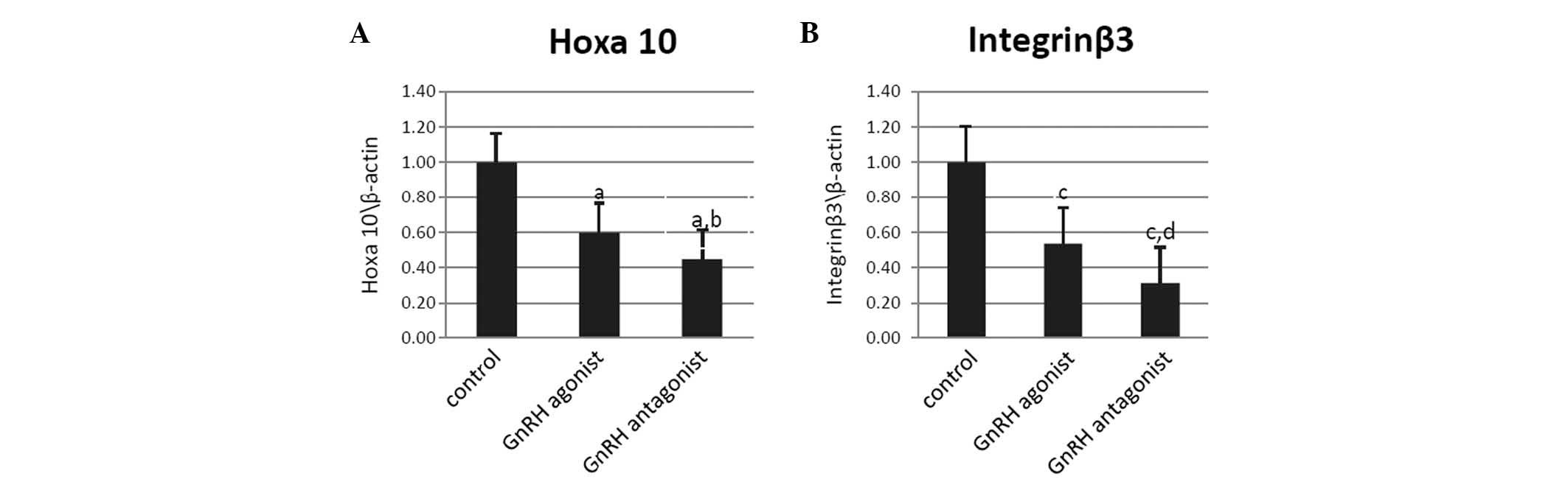
Hoxa10 and integrin β3 mRNA were expressed during
the implantation window in the uteri of the mice in all three
groups. Following normalization to β-actin expression, Hoxa10 mRNA
expression was observed to be significantly decreased in the mice
in the GnRH antagonist and agonist groups compared with the control
group (P<0.001 and P=0.004, respectively; Fig. 2A). Compared with the control group,
integrin β3 mRNA expression was also found to be reduced in the
mice in the GnRH antagonist and agonist groups (P<0.001 and
P=0.002, respectively; Fig. 2B).
Furthermore, Hoxa10 and integrin β3 mRNA expression were observed
to be higher in the GnRH agonist group compared with the GnRH
antagonist group (P=0.044 and P=0.032, respectively; Fig. 2A and B)
Endometrial Hoxa10 and integrin β3
protein expression during the implantation window following GnRH-as
treatment
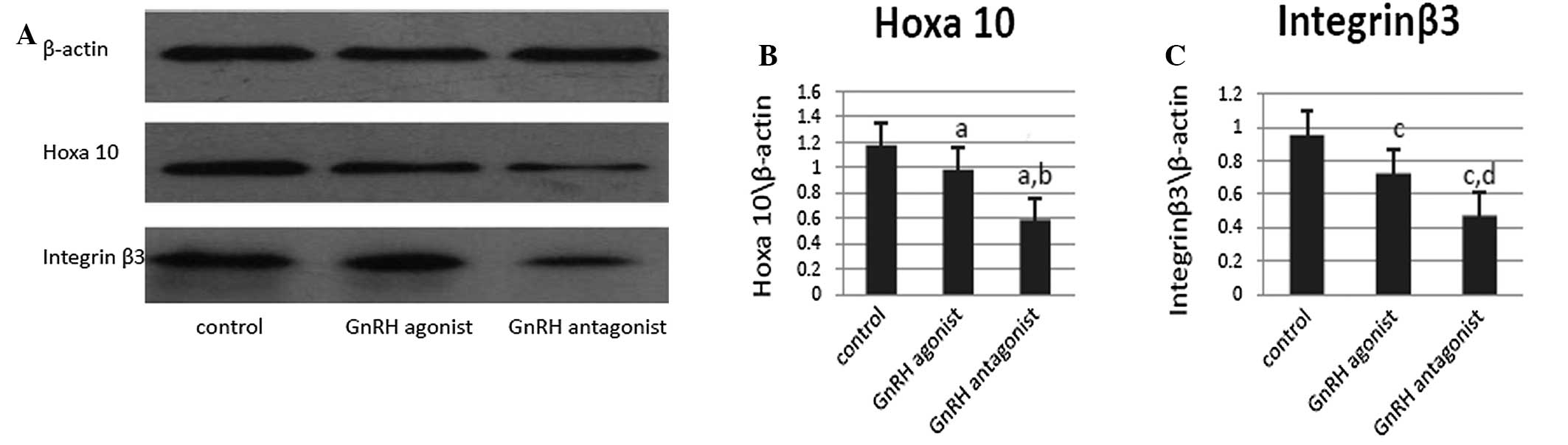
The protein expression of endometrial Hoxa10 and
integrin β3 were detected using western blot analysis during the
implantation window in the uteri of the mice in the three groups
(Fig. 3A). In accordance with the
mRNA expression findings, following normalization to β-actin
expression, Hoxa10 protein expression was found to be lowest in the
mice in the GnRH antagonist treatment group and highest in the mice
in the control group (GnRH agonist vs. control, P=0.032; GnRH
antagonist vs. control, P=0.047 and GnRH agonist vs. GnRH
antagonist, P=0.005). Integrin β3 expression was also observed to
be lowest in the mice in the GnRH antagonist group and highest in
those in the control group (GnRH agonist vs. control, P=0.006; GnRH
antagonist vs. control P=0.004 and GnRH agonist vs. GnRH
antagonist, P=0.0041; Fig.
3C).
Pinopode development following GnRH-as
treatment
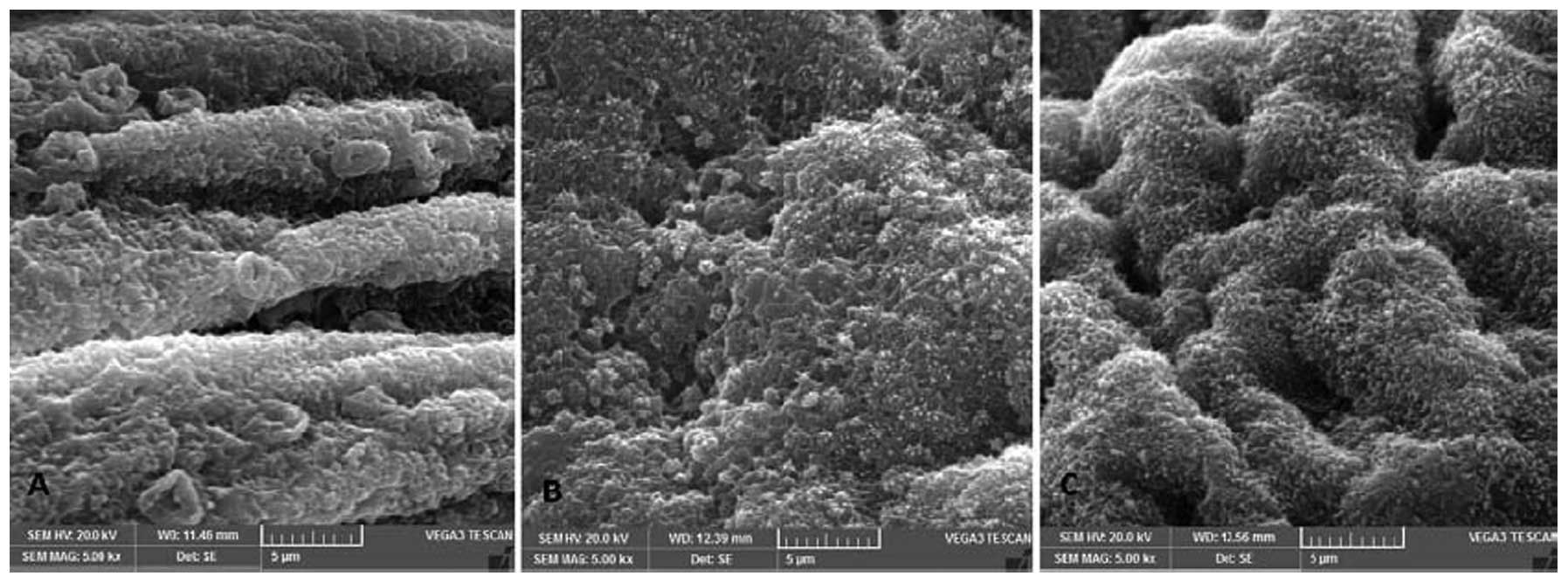
SEM photomicrographs of the endometrial luminal
surface of the uterus during the implantation window were captured
to identify the developmental status of the pinopodes (Fig. 4). Pinopode development was observed
to be repressed in the GnRH agonist and GnRH antagonist groups
compared with the control group. The mice in the control group
exhibited fully developed pinopodes on the apical pole of the
majority of the non-ciliated epithelial cells (Fig. 4A). However, in the mice in the GnRH
agonist group, the pinopodes were less developed and the luminal
uterine epithelium exhibited a villous surface (Fig. 4B). Pinopode development in the mice
in the GnRH antagonist group was repressed and delayed, with the
luminal uterine epithelium being ciliated and microvillous and
exhibiting few pinopodes (Fig.
4C).
Discussion
The lower pregnancy rates in COH cycles during ART
treatment may be a consequence of the negative effects of GnRH-as
on endometrial receptivity; however, the specific mechanism has yet
to be elucidated (1–2,20).
The present study investigated Hoxa10 DNA methylation patterns and
expression, as well as the effect of GnRH agonist and antagonist
treatment on endometrial receptivity during the implantation window
in mice.
In the present study, methylation in the promoter
region of Hoxa10 was found to increase following GnRH agonist and
antagonist treatment, with such increases being most evident under
GnRH antagonist treatment. Hoax10 is a well established biomarker
for endometrial receptivity and alterations in its methylation have
been demonstrated to be associated with disturbances in endometrial
receptivity in several pathological endometrial conditions,
including reproductive system diseases and exposure to
environmental endocrine disruptors (12–17).
Several studies have shown that methylation regulation may be
involved in endometrial development during the adult menstrual
cycle and endometrial decidualization (21–23).
In addition, increasing evidence has suggested that epigenetic
mechanisms may regulate numerous aspects of pregnancy and the
outcome of ART, with roles in implantation, placentation and foetal
growth (24–26). Therefore, Hoxa10 methylation may be
involved in endometrial receptivity following GnRH-as treatment. To
the best of our knowledge, this is the first study to show the
methylation pattern of Hoxa10 following GnRH agonist and antagonist
treatment.
In the present study, compared with the natural
cycle control mice, Hoxa10 mRNA and protein expression was observed
to decrease following GnRH-as treatment, particularly following
GnRH antagonist treatment. This finding is supported by several
studies, which have reported that despite there being no evidence
to suggest that GnRH-as negatively effects oocyte quality,
fertilization rates or embryo quality, GnRH-as treatment is
associated with a statistically significant reduction in pregnancy
rates (1–2). Thus, this reduction in pregnancy
rates associated with GnRH-as treatment may be due to the negative
effects associated with GnRH-as, particularly GnRH antagonists, on
the endometrium and the repression of endometrial receptivity
(27–29). Altered HOXA10 expression may be
caused by aberrant methylation of the gene, with promoter
hypermethylation often correlated with suppressed gene expression
(9,30). Therefore, the aberrant expression
of Hoxa10 in the mouse endometrium following GnRH-as treatment may
result from altered Hoxa10 DNA methylation.
Like Hoxa10 expression, following GnRH-as treatment
the expression of integrin β3 and pinopode development were also
reduced in the present study. Furthermore, Quinn and Casper
(31) also reported that
downregulation of Hoxa10 caused a reduced number of pinopodes,
while overexpression of Hoxa10 resulted in an increase in pinopode
numbers (31). Endometrial
integrin β3 and pinopodes are characteristic biomarkers closely
associated with endometrial development and maturation and they
peak in expression during the implantation window (31–33).
Therefore, the repressed endometrial receptivity observed following
GnRH-as treatment may be due to altered Hoxa10 expression. The
findings of the present study are supported by previous studies
reporting decreased integrin β3 subunit expression following
GnRH-as intervention (1,19,32).
Further investigations are required to identify the
mechanism by which GnRH agonists and antagonists cause Hoxa10 gene
promoter hypermethylation. The methylation pattern in humans may be
different to that in mice; therefore, investigations on endometrial
biopsies from patients undergoing COH should be performed in the
future.
In conclusion, the present study has shown that
GnRH-as may influence the methylation status of the Hoxa10 gene in
mice, which may affect uterine receptivity and repress the
expression of endometrial integrin β3 and pinopode development.
These findings present a potential epigenetic mechanism by which
GnRH-as, particularly GnRH antagonists, may negatively affect
endometrial receptivity. These findings may explain the low
implantation rate associated with COH treatments, which involve
GnRH-as in human in vitro fertilization clinics. However,
additional studies are required to analyze the impact of
methylation regulation, particularly epigenetic regulation by
GnRH-as, on the endometrium.
Acknowledgements
The present study was supported by grants from the
National Key Basic Research Program of China (grant no.
2012CB526600), the Key Research Program from the Health Department
of Hubei Province (grant no. JX5A05), National Natural Science
Foundation of China (grant no. 81100411) and the Fundamental
Research Funds for the Central Universities of China (grant no.
2012303020204).
References
|
1
|
Rackow BW, Kliman HJ and Taylor HS: GnRH
antagonists may affect endometrial receptivity. Fertil Steril.
89:1234–1239. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Al-Inany HG, Abou-Setta AM and Aboulghar
M: Gonadotrophin-releasing hormone antagonists for assisted
conception: a Cochrane review. Reprod Biomed Online. 14:640–649.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bourgain C and Devroey P: The endometrium
in stimulated cycles for IVF. Hum Reprod Update. 9:515–522. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Devroey P, Bourgain C, Macklon NS and
Fauser BC: Reproductive biology and IVF: ovarian stimulation and
endometrial receptivity. Trends Endocrinol Metab. 15:84–90. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kolibianakis E, Bourgain C, Albano C, et
al: Effect of ovarian stimulation with recombinant
follicle-stimulating hormone, gonadotropin releasing hormone
antagonists, and human chorionic gonadotropin on endometrial
maturation on the day of oocyte pick-up. Fertil Steril.
78:1025–1029. 2002. View Article : Google Scholar
|
|
6
|
Taylor HS: The role of HOX genes in human
implantation. Hum Reprod Update. 6:75–79. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taylor HS, Vanden Heuvel GB and Igarashi
P: A conserved HOX axis in the mouse and human female reproductive
system: late establishment and persistent adult expression of the
HOXA cluster genes. Biol Reprod. 57:1338–1345. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Satokata I, Benson G and Maas R: Sexually
dimorphic sterility phenotypes in HOXA10-deficient mice. Nature.
374:460–463. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu Y, Halverson G, Basir Z, et al:
Aberrant methylation at HOXA10 may be responsible for its aberrant
expression in the endometrium of patients with endometriosis. Am J
Obstet Gynecol. 193:371–380. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Daftary GS, Troy PJ, Bagot CN, et al:
Direct regulation of beta3-integrin subunit gene expression by
HOXA10 in endometrial cells. Mol Endocrinol. 16:571–579.
2002.PubMed/NCBI
|
|
11
|
Taylor HS, Daftary GS and Selam B:
Endometrial HOXA10 expression after controlled ovarian
hyperstimulation with recombinant follicle-stimulating hormone.
Fertil Steril. 80(Suppl 2): 839–843. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim JJ, Taylor HS, Lu Z, et al: Altered
expression of HOXA10 in endometriosis: potential role in
decidualization. Mol Hum Reprod. 13:323–332. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nasu K, Kawano Y, Tsukamoto Y, et al:
Aberrant DNA methylation status of endometriosis: epigenetics as
the pathogenesis, biomarker and therapeutic target. J Obstet
Gynaecol Res. 37:683–695. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rackow BW, Jorgensen E and Taylor HS:
Endometrial polyps affect uterine receptivity. Fertil Steril.
95:2690–2692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Widschwendter M, Apostolidou S, Jones AA,
et al: HOXA methylation in normal endometrium from premenopausal
women is associated with the presence of ovarian cancer: a proof of
principle study. Int J Cancer. 125:2214–2218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bromer JG, Wu J, Zhou Y and Taylor HS:
Hypermethylation of homeobox A10 by in utero diethylstilbestrol
exposure: an epigenetic mechanism for altered developmental
programming. Endocrinology. 150:3376–3382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Varayoud J, Ramos JG, Bosquiazzo VL,
Muñoz-de-Toro M and Luque EH: Developmental exposure to Bisphenol a
impairs the uterine response to ovarian steroids in the adult.
Endocrinology. 149:5848–5860. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hiyama M, Choi EK, Wakitani S, et al:
Bisphenol-A (BPA) affects reproductive formation across generations
in mice. J Vet Med Sci. 73:1211–1215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ruan HC, Zhu XM, Luo Q, et al: Ovarian
stimulation with GnRH agonist, but not GnRH antagonist, partially
restores the expression of endometrial integrin beta3 and
leukaemia-inhibitory factor and improves uterine receptivity in
mice. Hum Reprod. 21:2521–2529. 2006. View Article : Google Scholar
|
|
20
|
Shapiro BS, Daneshmand ST, Garner FC, et
al: Evidence of impaired endometrial receptivity after ovarian
stimulation for in vitro fertilization: a prospective randomized
trial comparing fresh and frozen-thawed embryo transfer. Fertil
Steril. 96:344–348. 2011. View Article : Google Scholar
|
|
21
|
Yamagata Y, Asada H, Tamura I, et al: DNA
methyltransferase expression in the human endometrium:
down-regulation by progesterone and estrogen. Hum Reprod.
24:1126–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Munro SK, Farquhar CM, Mitchell MD and
Ponnampalam AP: Epigenetic regulation of endometrium during the
menstrual cycle. Mol Hum Reprod. 16:297–310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vincent ZL, Farquhar CM, Mitchell MD and
Ponnampalam AP: Expression and regulation of DNA methyltransferases
in human endometrium. Fertil Steril. 95:1522–1525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Logan PC, Ponnampalam AP, Rahnama F, et
al: The effect of DNA methylation inhibitor 5-Aza-2′-deoxycytidine
on human endometrial stromal cells. Hum Reprod. 25:2859–2869.
2010.
|
|
25
|
van Montfoort AP, Hanssen LL, de Sutter P,
et al: Assisted reproduction treatment and epigenetic inheritance.
Hum Reprod Update. 18:171–197. 2012.
|
|
26
|
Horsthemke B and Ludwig M: Assisted
reproduction: the epigenetic perspective. Hum Reprod Update.
11:473–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tarlatzis BC and Bili HN:
Gonadotropin-releasing hormone antagonists: im-pact of IVF practice
and potential nonassisted reproductive technologyapplications. Curr
Opin Obstet Gynecol. 15:259–264. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ludwig M, Katalinic A and Diedrich K: Use
of GnRH antagonists in ovarian stimulation for assisted
reproductive technologies compared to the long protocol.
Meta-analysis. Arch Gynecol Obstet. 265:175–182. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Olivennes F, Cunha-Filho JS, Fanchin R, et
al: The use of GnRH antagonists in ovarian stimulation. Hum Reprod
Update. 8:279–290. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grimaldi G, Christian M, Quenby S and
Brosens JJ: Expression of epigenetic effectors in decidualizing
human endometrial stromal cells. Mol Hum Reprod. 18:451–458. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Quinn CE and Casper RF: Pinopodes: a
questionable role in endometrial receptivity. Hum Reprod Update.
15:229–236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Creus M, Ordi J, Fábregues F, et al: αvβ3
integrin expression and pinopod formation in normal and
out-of-phase endometria of fertile and infertile women. Hum Reprod.
17:2279–2286. 2002.
|
|
33
|
Creus M, Ordi J, Fábregues F, et al: The
effect of different hormone therapies on integrin expression and
pinopode formation in the human endometrium: a controlled study.
Hum Reprod. 18:683–693. 2003. View Article : Google Scholar : PubMed/NCBI
|