Introduction
Chronic pancreatitis is predominantly characterized
by extensive and permanent fibrosis in the basic structure of the
pancreas, which is a pathological process with major features of
ductal alterations, fibrosis surrounding the pancreatic lobule and
final scar formation in the pancreatic lobule (1–3).
Pathological and metabolic alterations co-exist and co-develop in
the disease progression of chronic pancreatitis. Detection of
characteristics of the metabolite levels may facilitate a greater
understanding of the pathophysiological events and aid in the
clinical treatment of the disease (4).
High-resolution magic-angle spinning nuclear
magnetic resonance spectroscopy (HR-MAS NMR) is a non-invasive
method which can be used to analyze the metabolic components of
in vitro tissues, and it exhibits high sensitivity and
accuracy in analyzing the components and concentrations of
different compounds in tissues (5–8).
In the current study, a rat model of CP was
established and the development of this disease was studied at
different time points. HR-MAS NMR of pancreatic tissues was
performed in vitro, in order to determine the metabolic
profiles of CP at different time periods in the rat model. In
addition, correlations between the observed pathological changes in
pancreatic tissues and changes in metabolite profiles were
assessed. The major aim of studying any observed correlation was to
identify a non-invasive and effective approach for determining the
major pathological characteristics of CP.
Materials and methods
Establishing a rat model for CP
The present study was approved by the local Animal
Ethics Committee of the Shanghai Changhai Hospital Ethics Committee
(Shanghai, China). A total of 36 male Wistar rats, each weighing
~200 g, were purchased from the Laboratory Animal Center of the
Second Military Medical University (Shanghai, China). The rats were
maintained in environmentally controlled conditions (temperature,
22±2°C; light/dark cycle, 12/12 h; relative humidity, 60–70%). All
rats were randomly assigned to 6 groups with 6 rats per group.
Dibutyltin dichloride (DBTC; Sigma-Aldrich, St Louis, MO, USA) was
dissolved in 100% ethanol, and mixed with glycerol (at a ratio of
~1:2:3). DBTC (~200 μl) was administered to each rat by tail vein
injection at a dose of 8 mg/kg (9)
to establish CP.
One group of rats was sacrificed on each of the
following days after establishing the disease model: 0 (immediate
sacrifice after establishing the model of CP), 7, 14, 21, 28 and
35. The rats were anesthetized with ~0.2 ml of pentobarbital
solution (Sigma-Aldrich) by intraperitoneal injection, and then the
abdomen was exposed. In total, 30 pancreatic tissue specimens were
obtained from the successful models. The pancreatic tissues were
observed, and carefully separated. Immediately following this
initial observation, a number of the pancreatic tissue specimens
were placed in 4% formaldehyde solution for pathological
examination, and other tissue specimens were snap-frozen in liquid
nitrogen and immediately stored at −80°C for HR-MAS NMR
analysis.
Pathological examination and scoring
Formaldehyde-fixed pancreatic tissues were embedded
in paraffin, sliced into sections and stained with hematoxylin and
eosin (HE; Beyotime Institute of Biotechnology, Haimen, China). The
pancreatic tissue sections were scored by a previously described
method (10–12). The sections were examined for
abnormal structures, tubular complex, gland atrophy, fibrosis,
edema and inflammatory cell infiltration, which collectively served
as basic indicators of disease pathology. The pathological sections
were scored (Table I) according to
the following scoring system: 0 (no abnormal structure); 1 (mild,
<10%); 2 (moderate, 10–50%); 3 (severe, >50%).
 | Table IScoring criteria of pathological
sections of pancreatic specimens in vitro. |
Table I
Scoring criteria of pathological
sections of pancreatic specimens in vitro.
| Degree | Abnormal
structure | Gland atrophy | Fibrosis | Edema | Inflammatory cell
infiltration | Tubular complex |
|---|
| None | 0 | 0 | 0 | 0 | 0 | 0 |
| Mild <10% | 0 | 1 | 1 | 1 | 1 | 1 |
| Moderate 10–50% | 0 | 2 | 2 | 2 | 2 | 2 |
| Severe >50% | 0 | 3 | 3 | 3 | 3 | 3 |
HR-MAS NMR
Snap-frozen pancreatic tissues were thawed at room
temperature, and the central specimens, each weighing ~15 mg, were
carefully cut on sterile dry culture dishes. The tissue specimens
were washed with deuterated water and transferred to 4-mm
ZrO2 tubes (Unipretec Ceramic Technology Co., Ltd.,
Xiamen, China) with a spherical cavity. The specimens were next
placed in a Bruker Avance III 600 spectrophotometer (Bruker
BioSpin, Rheinstetten, Germany) with a standard HR-MAS probe for
NMR measurements.
One-dimensional 1H NMR spectroscopy was
performed using Carr-Purcell-Meiboom-Gill (CPMG) pulse sequences
with pre-saturation of the water peak, with a total echo time of
180 msec. The signals of macromolecules and certain metabolites
with a short spin-spin (T2) relaxation time in the tissues were
suppressed. Other parameters included: 1H resonance
frequency, 600.13 MHz; magic-angle spinning speed, 5 kHz; test
temperature, 300.0 K; spectral width, 12.02 kHz; and recovery time,
2 sec. The spectral peaks of the major metabolites were identified
based on observations from previous studies (8,9).
A specific software program enabled Fourier
transform of the experimental data (TopSpin version 3.0; Bruker
BioSpin) and the corresponding phase and baseline correction were
also completed for this data set. The data obtained from
one-dimensional proton CPMG experiments were calibrated using the
fractional anisotropy peak at 1.31 ppm, with a range of 0.5–4.6
ppm. Principal component analysis (PCA) was employed to analyze the
experimental data set using the AMIX software program (version
3.9.7; Bruker BioSpin), and the relative concentrations of the
typical metabolites were calculated. The corresponding integral
areas in the CPMG spectra were obtained for statistical
analysis.
Statistical analysis
The integral areas corresponding to the major
metabolites in the CPMG spectrum of the three groups were entered
into an Excel spreadsheet (Microsoft Corporation, Redmond, WA,
USA). The integral areas of the major metabolites, which were
screened by PCA, were further studied by multiple rank order
logistic regression analysis in order to screen the metabolites
with the most discriminatory value. Spearman rank-order correlation
analysis of the scoring results of fibrosis with inflammatory cell
infiltration and the integral areas of the major metabolites was
also performed. All statistical analyses were performed with the
SPSS statistical software program, version 16.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Pathological scores
One day after establishing CP in the rat model, two
rats (one from the day 7 group and the other from the day 28 group)
died. Four additional rats died two days after establishing the CP
model (two from the day 21 group and two from the day 35
group).
The pathological scores of non-normal structures,
tubular complex, gland atrophy, fibrosis, edema, and inflammatory
cell infiltration are shown in Table
II. The extent of fibrosis and inflammatory cell infiltration
was aggravated according to the increased duration of CP. Based on
the examination of the pathological specimens, the CP model was
classified into three groups: A control group (n=10, six from the
day 1 group, three from the day 35 group and one from the day 28
group), a mild CP group (n=10, five from each of the day 7 and 14
groups) and a severe CP group (n=10, four from each of the day 21
and 28 groups, and one from each of the day 14 and 35 groups).
Pathological examination of the pancreatic tissues following HE
staining is shown in Fig. 1.
 | Table IIPathological scoring of the pancreatic
tissues after different time periods (mean ± standard
deviation). |
Table II
Pathological scoring of the pancreatic
tissues after different time periods (mean ± standard
deviation).
| Day |
|---|
|
|
|---|
| Pathology | 0 | 7 | 14 | 21 | 28 | 35 |
|---|
| Abnormal
structure | 0.00±0.00 | 1.00±0.63 | 1.20±0.44 | 2.00±1.00 | 2.33±0.58 | 2.75±0.50 |
| Tubular complex | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| Gland atrophy | 0.00±0.00 | 1.00±0.00 | 1.00±0.00 | 2.00±1.00 | 2.33±0.58 | 2.25±0.50 |
| Fibrosisa | 0.00±0.00 | 1.00±0.00 | 1.00±0.00 | 2.33±1.15 | 1.67±0.58 | 2.75±0.96 |
| Edema | 0.00±0.00 | 1.00±0.00 | 1.00±0.00 | 2.67±0.58 | 2.33±0.58 | 2.75±0.96 |
| Inflammatory cell
infiltrationa | 0.00±0.00 | 1.00±0.00 | 1.00±0.00 | 2.33±0.58 | 2.00±1.00 | 1.50±0.58 |
| Total score | 0.00±0.00 | 5.00±0.71 | 5.20±0.44 | 11.33±1.15 | 10.66±0.57 | 11.00±0.81 |
Pancreatic tissue analysis
Following the analysis of pancreatic tissue
specimens obtained from the control, mild and severe CP groups by
HR-MAS (Fig. 2), PCA was employed
to screen 8 metabolic biochemicals including betaine,
phosphocholine/glycerophosphocholine, choline, aspartate, lactate,
fatty acids, 3-hydroxybutyrate and isoleucine/leucine/valine
(Fig. 3), and the scores from each
of the models within the three CP groups formed three distinct
clusters (Fig. 3A).
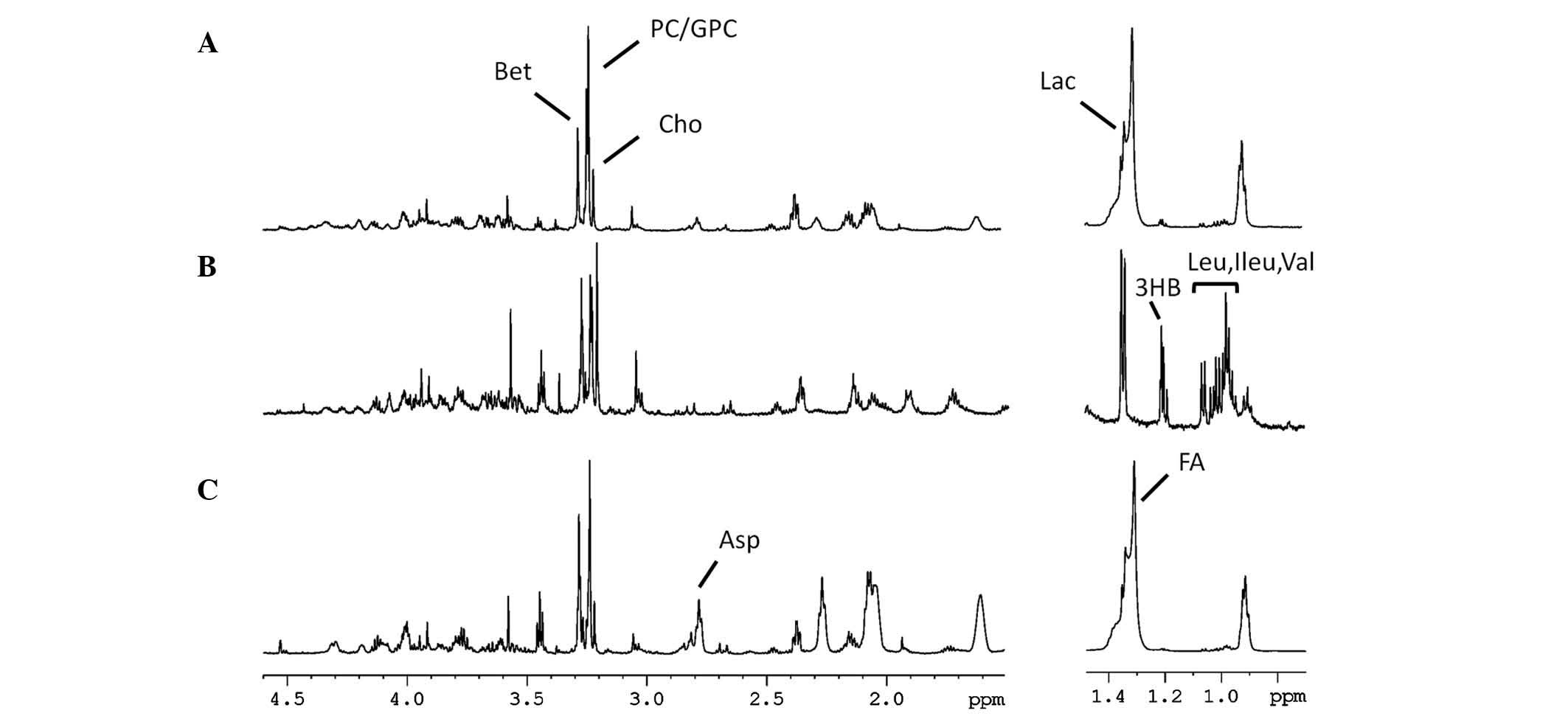 | Figure 2Representative 1H MAS NMR
spectra of the pancreatic tissues in the (A) control, (B) severe
chronic pancreatitis and (C) mild chronic pancreatitis groups. The
region of δ1.50–4.60 ppm is zoomed in 4 times of that in δ0.50–1.50
ppm (600 MHz, CPMG pulse sequence, temperature 300.0 K). Bet,
betaine; PC/GPC, phosphocholine/glycerophosphocholine; Cho,
choline; Lac, lactate; 3HB, 3-hydroxybutyrate; Leu, Ileu, val,
leucine/isoleucine/valine; Asp, aspartate; FA, fatty acids; MAS,
magic-angle spinning; NMR, nuclear magnetic resonance; CPMG,
Carr-Purcell-Meiboom-Gill. |
Multiple rank-order logistic regression analysis of
integral areas within the major metabolites is shown in Table III. According to the descending
order of the regression coefficient values (estimated values) of
the major metabolites obtained from logistic regression analysis,
the most important metabolites to discriminate the severity in CP
were aspartate, betaine and fatty acids.
 | Table IIIMultiple rank order logistic
regression analysis of the major metabolites in the control, mild
and severe chronic pancreatitis groups. |
Table III
Multiple rank order logistic
regression analysis of the major metabolites in the control, mild
and severe chronic pancreatitis groups.
| Betaine |
Phosphocholine/Glycerophosphocholine | Choline | Aspartate | Lactate | Fatty acid |
3-Hydroxybutyrate |
Isoleucine/Leucine/Valine |
|---|
| Estimatea | −3.477 | −0.372 | −0.296 | 3.773 | 0.401 | −0.983 | −1.401 | −0.629 |
| P-value | 0.017 | 0.564 | 0.854 | 0.001 | 0.248 | 0.004 | 0.060 | 0.274 |
Correlation analysis
The correlation analysis between the pathology
grading scores of fibrosis and inflammatory cell infiltration and
the related integral area of main metabolites in CP are shown in
Table IV. The content of betaine
shows positive correlation with fibrosis (correlation coefficient,
0.454; P=0.044) and inflammatory cell infiltration (correlation
coefficient, 0.716; P=0.0001). The content of aspartate shows
negative correlation with fibrosis (correlation coefficient,
−0.768; P=0.0001). No significant correlation between the content
of aspartate and inflammatory cell infiltration was found
(correlation coefficient, −0.394; P=0.085). In addition, the
content of fatty acids shows negative correlation with fibrosis
(correlation coefficient, −0.764; P=0.0001) and with inflammatory
cell infiltration (correlation coefficient, −0.619; P=0.004).
 | Table IVSpearman’s rank correlation tests
between the pathology and metabolites of the pancreatic tissues
(n=20). |
Table IV
Spearman’s rank correlation tests
between the pathology and metabolites of the pancreatic tissues
(n=20).
| Score of
fibrosis | Score of inflammatory
cell infiltration |
|---|
|
|
|
|---|
| Metabolite | Correlation
coefficient | P-value | Correlation
coefficient | P-value |
|---|
| Betaine | 0.454 | 0.0440 | 0.716a | 0.0001 |
| Aspartate | −0.768a | 0.0001 | −0.394 | 0.0851 |
| Fatty acid | −0.764a | 0.0001 | −0.619a | 0.0040 |
Discussion
In the present study, rat models of CP with
different severity were established by tail vein injection of DBTC
solution. It was found that inflammatory cell infiltration and
fibrosis exhibit vital roles in the progression of CP. According to
the standard scoring criteria of pathological sections, thirty rats
were divided into three groups, with 10 rats in each group. For the
mild CP group, all of the pathology specimens were collected from
the day 7 and 14 groups, while 9 specimens were obtained from the
day 21, 28 and 35 groups for the severe CP group. According to the
pathological results, it is reasonable to conclude that the extent
of fibrosis and inflammatory cell infiltration causes an increase
with the duration of CP.
In current approaches for scoring the pathology of
CP, the extent of inflammatory cell infiltration is commonly
identified by counting the mean number of inflammatory cells in
five high-powered fields of view (13,14).
Inflammatory cell infiltration and fibrosis exhibit vital roles in
the development of CP. Pancreatic fibrosis is an important
pathophysiological process, which affects the prognosis of CP
(15). Thus, an assessment of the
occurrence, extent and development of fibrosis can identify the
duration and prognosis of CP. The most commonly used method in the
pathological evaluation of fibrosis in CP is van Gieson staining of
pancreatic collagen fibers, followed by scoring the mean area of
collagen fiber staining in five high-powered fields of view
(16). Pathological examination is
the most fundamental method for diagnosis of numerous diseases.
However, biopsy of the pancreas is difficult to perform and carries
a certain risk. Pathological examination is challenging to perform
for atypical early-stage CP. Therefore, there remains an urgent
requirement for a simple and effective method of assessing fibrosis
and inflammatory cell infiltration.
Magnetic resonance spectroscopy (MRS) has high
sensitivity and resolution, allows in vitro testing of
metabolites, and has been widely used in the field of metabolomics
(17). With the rapid development
of MRS techniques, high field-strength equipment gradually emerged,
and with it the sensitivity of MRS continuously improved. This
enabled a marked increase in the application of MRS in clinical
diagnostics and basic laboratory studies (18). Currently, the clinical application
of the 1.5T or 3.0T magnetic resonance imaging (MRI) scanner can
directly perform MRS analyses of pancreatic tissues and lesions
in vivo. However, this approach suffers from the drawbacks
of low magnetic field strength and low spatial resolution, and as a
consequence, the interference of respiratory and motion artifacts
of other abdominal organs, which collectively dampen the available
information of the metabolites studied. Thus, it is necessary to
conduct a study in rat models, so that the metabolic profiling of
in vitro tissues is undertaken prior to any clinical
application or intervention (19).
In the current study, HR-MAS NMR was used to investigate pancreatic
tissues obtained from rats with CP, and separate the various
components of different amino acids. In the use of this approach,
physical or chemical treatment of the samples is not necessary,
small specimen sizes are all that is required (10–20 mg) and
complete placement (100%) of the sample in the detection coils is
possible. Consequently, HR-MAS NMR ensures even magnetic exposure
of the sample and improved sensitivity and resolution. This study
in the field of metabolomics is auxiliary to proteomics, and goes a
step further in the study of pancreatopathy.
Spearman’s rank correlation, a rank correlation
test, is a statistical approach used to measure bivariate data sets
and rank that data for linear correlation analysis. The Spearman’s
rank correlation between fibrosis and inflammatory cell infiltrates
in 20 cases of CP and their metabolites aspartate, betaine and
fatty acids in the present study demonstrated the utility of this
approach.
Betaine is a highly efficient active methyl donor.
During the process of tumor occurrence, low overall methylation and
locally high levels of methylation of DNA in tumor cells are
observed. It has been found that the level of betaine in pancreatic
cancer tissues is markedly reduced as compared with that in normal
pancreatic tissues. In the current study, it was shown that betaine
positively correlated with both fibrosis and inflammatory cell
infiltration of CP. The common form of aspartate is L-aspartate,
which can provoke disorders in protein synthesis through
hydrolyzing asparagines and thereby inhibit the growth and
proliferation of tumor cells. L-aspartate rarely induces acute
pancreatitis, which is the most severe adverse reaction associated
with its use. However, once acute pancreatitis occurs, it
progresses rapidly and the condition is severe, with morbidity
occurring quite rapidly due to the occurrence and consequences of
shock (20). It is thought that
L-aspartate directly damages pancreatic acini, which leads to
effusion, activation and autodigestion of pancreatic cells, the
corollary of which promotes the emergence of acute pancreatitis
(21).
Determination of serum unsaturated fatty acid levels
reveals that fatty acids may be important components in the complex
network of the core systemic inflammatory response syndrome
associated with the onset of acute and severe CP. However, the role
of fatty acids in CP remains unclear. The present study showed that
fatty acids were negatively correlated with the fibrosis and
inflammatory cell infiltration observed in CP.
The extent of fibrosis and inflammatory cell
infiltration exhibits an aggravated tendency to increase with the
duration of CP. Discrimination screening of the metabolites using
HR-NMR indicated the importance of betaine,
phosphocholine/glycerophosphocholine, choline, aspartate,
3-hydroxybutyrate, lactate, fatty acids, and
isoleucine/leucine/valine. From these metabolites, the three
identified as possessing the greatest discriminatory significance
were found to be aspartate, betaine and fatty acids. The metabolite
betaine positively correlated with fibrosis and inflammatory cell
infiltration in CP. In addition, aspartate negatively correlated
with fibrosis in CP, but exhibited no significant correlation with
inflammatory cell infiltration in CP. Furthermore, the presence of
fatty acids negatively correlates with fibrosis and inflammatory
cell infiltration in CP. HR-MAS NMR may be used to analyze
metabolic characteristics in a rat model of different degrees of
chronic pancreatitis.
References
|
1
|
Wakabayashi T, Kawaura Y, Satomura Y, et
al: Clinical study of chronic pancreatitis with focal irregular
narrowing of the main pancreatic duct and mass formation:
comparison with chronic pancreatitis showing diffuse irregular
narrowing of the main pancreatic duct. Pancreas. 25:283–289. 2002.
View Article : Google Scholar
|
|
2
|
Spanier BW, Dijkgraaf MG and Bruno MJ:
Trends and forecasts of hospital admissions for acute and chronic
pancreatitis in the Netherlands. Eur J Gasroenterol Hepatol.
20:653–658. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mitchell RM, Byrne MF and Baillie J:
Pancreatitis. Lancet. 361:1447–1455. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Ma C, Liao Z, Tian B and Lu JP:
Study on chronic pancreatitis and pancreatic cancer using MRS and
pancreatic juice samples. World J Gastroenterol. 17:2126–2130.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beckonert O, Keun HC, Ebbels TM, Bundy J,
Holmes E, Lindon JC and Nicholson JK: Metabolic profiling,
metabolomic and metabonomic procedures for NMR spectroscopy of
urine, plasma, serum and tissue extracts. Nat Protoc. 2:2692–2703.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moestue S, Sitter B, Bathen TF, et al: HR
MAS MR spectroscopy in metabolic characterization of cancer. Curr
Top Med Chem. 11:2–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Righi V, Constantinou C, Kesarwani M, et
al: Live-cell high resolution magic angle spinning magnetic
resonance spectroscopy for in vivo analysis of Pseudomonas
aeruginosa metabolomics. Bio Med Rep. 1:702–712.
2013.PubMed/NCBI
|
|
8
|
Fang F, He X, Deng H, Chen Q, Lu JP,
Spraul M and Yu Y: Discrimination of metabolic profiles of
pancreatic cancer from chronic pancreatitis by high-resolution
magic angle spinning 1H nuclear magnetic resonance and principal
components analysis. Cancer Sci. 98:1678–1682. 2007. View Article : Google Scholar
|
|
9
|
Merkord J, Weber H, Sparmann G, Jonas L
and Hennighausen G: The course of pancreatic fibrosis induced by
dibutyltin dichloride (DBTC). Ann NY Acad Sci. 880:231–237. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Westerloo DJ, Florquin S, de Boer AM,
Daalhuisen J, de Vos AF, Bruno MJ and van der Poll T: Therapeutic
effects of troglitazone in experimental chronic pancreatitis in
mice. Am J Pathol. 166:721–728. 2005.PubMed/NCBI
|
|
11
|
Bhatia M, Saluja AK, Singh VP, et al:
Complement factor C5a exerts an anti-inflammatory effect in acute
pancreatitis and associated lung injury. Am J Physiol Gastrointest
Liver Physiol. 280:G974–G978. 2001.PubMed/NCBI
|
|
12
|
Ma C, Tian B, Wang J, Yang GJ, Pan CS and
Lu JP: Metabolic characteristics of acute necrotizing pancreatitis
and chronic pancreatitis. Mol Med Rep. 6:57–62. 2012.PubMed/NCBI
|
|
13
|
Zhao HF, Ito T, Gibo J, et al:
Anti-monocyte chemoattractant protein-1 gene therapy attenuates
experimental chronic pancreatitis induced by dibutyltin dichloride
in rats. Gut. 54:1759–1767. 2005. View Article : Google Scholar
|
|
14
|
Emori Y, Mizushima T, Matsumura N, et al:
Camostat, an oral trypsin inhibitor, reduces pancreatic fibrosis
induced by repeated administration of a superoxide dismutase
inhibitor in rats. J Gastroenterol Hepatol. 20:895–899. 2005.
View Article : Google Scholar
|
|
15
|
Matsushita K, Mizushima T, Shirahige A, et
al: Effect of taurine on acinar cell apoptosis and pancreatic
fibrosis in dibutyltin dichloride-induced chronic pancreatitis.
Acta Med Okayama. 66:329–334. 2012.PubMed/NCBI
|
|
16
|
Köninger J, Giese NA, Bartel M, et al: The
ECM proteoglycan decorin links desmoplasia and inflammation in
chronic pancreatitis. J Clin Pathol. 59:21–27. 2006.PubMed/NCBI
|
|
17
|
Targosz-Gajniak MG, Siuda JS, Wicher MM,
et al: Magnetic resonance spectroscopy as a predictor of conversion
of mild cognitive impairment to dementia. J Neurol Sci. 355:58–63.
2013. View Article : Google Scholar
|
|
18
|
Chávez FV and Halle B: Molecular basis of
water proton relaxation in gels and tissue. Magn Reson Med.
56:73–81. 2006.PubMed/NCBI
|
|
19
|
Lei H and Gruetter R: Effect of chronic
hypoglycemia on glucose concentration and glycogen content in rat
brain: A localized 13C NMR study. J Neurochem. 99:260–268. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Trivedi CD and Pitchumoni CS: Drug-induced
pancreatitis: an update. J Clin Gastroenterol. 39:709–716. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schrader H, Menge BA, Belyaev O, Uhl W,
Schmidt WE and Meier JJ: Amino acid malnutrition in patients with
chronic pancreatitis and pancreatic carcinoma. Pancreas.
38:416–421. 2009. View Article : Google Scholar : PubMed/NCBI
|

















