Introduction
Prostate cancer is one of the most common non-skin
malignancies in males, and the morbidity and mortality are
increasing significantly in China. However, the incidence of
prostate cancer in China remains low compared with incidences in
Western countries, including the USA and those in Europe (1,2).
Currently, digital rectal examination, prostate-specific antigen
testing and histopathological evaluation of prostate needle
biopsies are all used to detect and monitor prostate cancer
progression (3,4). The loss of normal glandular tissue is
measured by the prostate cancer scoring system called the Gleason
score, which aims to assist the evaluation of patient prognosis
(5). However, prostate cancer
progression is variable and occasional cases behave independently
of their Gleason scores (6).
Therefore, further studies of the molecular pathogenesis of
prostate cancer are required in order to develop novel and
effective biomarkers to improve the prediction of cancer
prognoses.
microRNAs (miRNAs) are a class of small (<22 nt),
non-coding RNA molecules that are essential in numerous biological
processes (7). Accumulating
evidence has demonstrated that miRNAs have the ability to regulate
the expression of a variety of genes involved in tumorigenesis and
are critical in the processes of cell proliferation,
differentiation and apoptosis (8,9). In
animals, miRNAs have been indicated to produce regulatory effects
through binding to the complementary sequences within the
3′-untranslated regions (3′UTRs) of their mRNA targets. A previous
study suggested that miRNAs may be responsible for the regulation
of more than one-third of all human genes (10). miR-155 is a particularly important
miRNA. It is produced from the processing of the B-cell integration
cluster, has been observed to be overexpressed in numerous types of
cancer, and is linked to the development of leukemia, breast and
lung cancer (11,12). However, the biological function and
specific mechanism of action of miR-155 in prostate cancer has yet
to be elucidated. Therefore, in the present study, the clinical
relevance of miR-155 in prostate cancer was examined.
Materials and methods
Cell culture and tissue samples
The PNT1B human prostate normal tissue cell line and
human prostate cancer cell lines DU145, LNCaP, 22RV1 and PC-3 were
obtained from the Chinese Academy of Sciences (Shanghai, China).
All cell lines used were cultured in RPMI 1640 medium (Gibco Life
Technologies, Beijing, China) supplemented with 10% fetal calf
serum, 100 IU/ml penicillin and 100 mg/ml streptomycin (Gibco Life
Technologies). A total of 35 paired human prostate cancer and
adjacent non-tumor tissues were collected from routine therapeutic
surgery at our department. Written informed consent was obtained
from all participants involved in the present study. The study was
performed in accordance with the Declaration of Helsinki and
approved by the Institutional Review Board of the Ninth People’s
Hospital, School of Medicine, Shanghai Jiaotong University
(Shanghai, China).
RNA extraction and quantitative
polymerase chain reaction (qPCR)
Cells were seeded onto 12-well plates and total RNA
was isolated from tissues or cells with TRIzol reagent (Invitrogen
Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. The quantity and concentration of RNA
were determined by ultraviolet (UV) measurement of the optical
density (OD) at 260/280 nm using the NanoDrop 2000 UV-Vis
spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). In
order to quantify the expression of miR-155, the isolated RNA was
reverse-transcribed and amplified using the Real Time-PCR miRNA
Detection kit (Ambion Life Technologies, Carlsbad, MA, USA)
according to the manufacturer’s instructions. PCR reactions were
performed with an Applied Biosystems 7500 Real-Time PCR system
(Foster City, CA, USA) using the following cycling conditions: 95°C
for 10 min, then 40 cycles of 95°C for 15 sec and 60°C for 1 min.
The U6 small nuclear RNA (Sigma-Aldrich, St. Louis, MO, USA) was
used as a loading control. GAPDH was used as a control in the PCR
reactions. The miRNA-specific primers were designed using Primer
Premier 5.0 (Premier Biosoft International, Palo Alto, CA, USA),
and the primer sequences were as follows: miR-155 sense,
5′-ACACTCCAGCTGGGTAGCTTATCAGACT-3′ and antisense,
5′-CTCAACTGGTGTCGTGGAGTCGGCAA-3′; U6 sense, 5′-CTCGCTTCGGCAGCACA-3
and antisense, 5′-AACGCTTCACGAATTTGCGT-3′.
MTT assay
Proliferation of cells was determined by MTT assay.
Briefly, the prostate cancer cell lines LNCaP and PC-3 transfected
with oligonucleotides were seeded into 96-well plates at a density
of 3×104 cells/well. Next, 10 ml MTT (5 mg/ml) was added
and incubated in the dark at 37°C for 2 h. The absorbance was
determined at a wavelength of 492 nm.
Apoptosis and cell cycle analysis
At 48 h post-transfection with the miR-155
precursor/inhibitor or control, LNCaP and PC-3 cells were washed
with phosphate-buffered saline (PBS), detached with trypsin and
harvested. The cells were resuspended in 1 ml Hoechst 33258 stain
(BD Biosciences, San Diego, CA, USA) for 5 min and then washed with
PBS three times. Apoptotic cells were evaluated using the annexin
V-fluorescein isothiocyanate/propidium iodide (PI) cell Apoptosis
Detection kit (BD Biosciences, Franklin Lakes, NJ, USA) following
the manufacturer’s instructions. For cell cycle analysis, cells
transfected with oligonucleotides were washed twice with PBS and
collected by centrifugation, followed by fixation in ice-cold 70%
ethanol at −20°C overnight. Cells were collected and stained with
100 μl PI staining solution for 30 min in the dark prior to cell
cycle analysis.
Western blot
Cultured cells were lysed using
radioimmunoprecipitation assay buffer (Sigma-Aldrich) and tissue
samples were lysed using the T-PER Tissue Protein Extraction
reagent (Pierce, Rockford, IL, USA) in the presence of Halt
Protease Inhibitor Cocktail (Pierce). The tissue and cell lysates
were then subjected to SDS-PAGE. For immunoblotting, the membranes
were blocked with 5% non-fat milk in Tris-buffered saline
(Sigma-Aldrich) and then incubated with mouse anti-human annexin
(ANX)7 monoclonal antibody (Abcam, Cambridge, MA, USA) followed by
horseradish peroxidase-conjugated secondary antibody (Abcam).
Signals with Immobilon immunoreactive bands were detected with an
Enhanced Chemiluminescence (ECL) Western Blot kit (Sigma-Aldrich)
using a Chemigenius Bioimaging system (Syngene, Frederick MD, USA).
GAPDH contents were measured as a loading control.
Plasmid construction and luciferase
activity assay
Luciferase reporters were generated based on the
firefly luciferase-expressing vector pMIR-Report (Ambion, USA). The
wild-type and mutant ANX7-3′UTRs were amplified using the following
primers, designed by Primer Premier 5.0: Wild-type forward,
5′-ATAGCATCGCAGGTAACGCTACGCGGGACG-3′ and reverse,
5′-GCAGGTAGCCGACATCGTACGCGATATACT-3′; mutant type forward,
5′-ATGCCGATGCTCGATCATACTACAGTAGCT-3′ and reverse,
5′-GACGCCGTACTCGACAGCGTCGTATGAGTG-3′. Cells were seeded into
24-well plates at a density of 5×104 cells/well one day
prior to transfection. Luciferase reporter (500 ng), miRNA-155
precursor or negative control (NC) (50 pmol) and pRL-TK (40 ng)
were added to each well. Cells were collected 48 h after
transfection and analyzed using the Dual-Luciferase Reporter Assay
system (Promega, Madison, WI, USA).
Statistical analysis
All values are presented as the mean ± standard
error and analyzed by SPSS version 13.0 (IBM, Armonk, NY, USA). For
comparisons between two different groups, statistical significance
was determined using Student’s t-test. Comparisons among groups
were performed using analysis of variance. P<0.05 was considered
to indicate a statistically significant difference.
Results
MiR-155 is upregulated in prostate cancer
tissue and cell lines
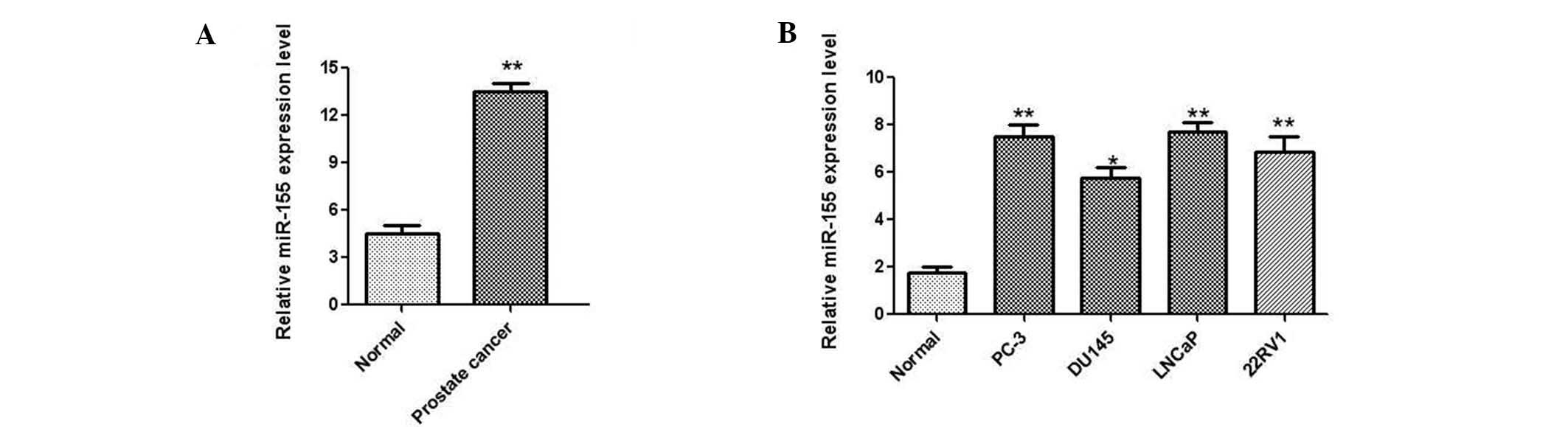
To investigate the biological role of miR-155 in
prostate cancer, the expression levels of miR-155 were determined
in 35 paired prostate cancer and adjacent non-tumor tissues by
qPCR. The results demonstrated that the expression of miR-155 was
significantly upregulated in cancer tissues compared with the
matched non-tumor samples (Fig.
1A). Additionally, the human prostate normal tissue cell line
PNT1B and several prostate cancer cell lines, including LNCaP,
DU145, PC-3 and 22RV1, were analyzed by qPCR. As shown in Fig. 1B, a significant upregulation of
miR-155 was also observed in the four prostate cancer cell lines
compared with the PNT1B cells. Taken together, these results
suggested that miR-155 was significantly upregulated in prostate
cancer tissues and cell lines.
Effects of miR-155 on prostate cancer
cell proliferation
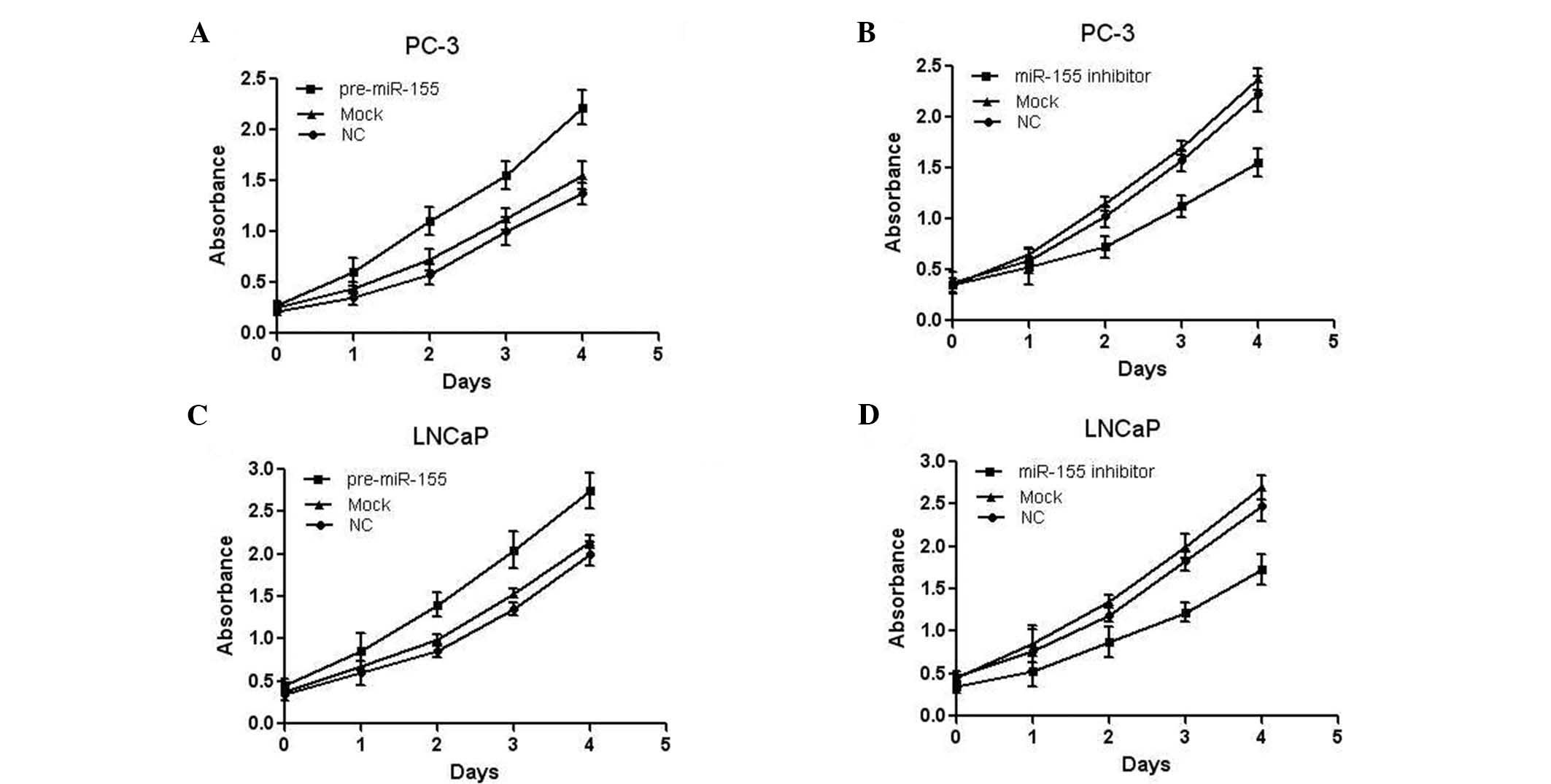
As the expression of miR-155 was markedly increased
in prostate cancer, it was reasonable to hypothesize that it may
function as a tumor promoter. Therefore, the effect of
overexpression/inhibition of miR-155 on the cell proliferation of
the prostate cancer cell lines was assessed. PC-3 cells were
transfected with miR-155 precursor and the cell proliferation was
measured by MTT assay. As presented in Fig. 2A, miR-155 overexpression
significantly promoted the proliferation of PC-3 cells. PC-3 cells
were also transfected with miR-155 inhibitor, and an MTT assay
indicated reduced proliferation of the cells (Fig. 2B). Similar results were also
observed in LNCaP cells (Fig. 2C and
D). Collectively, these results demonstrated that miR-155 was
able to promote the proliferation of prostate cancer cell lines
in vitro.
Inhibition of miR-155 promotes apoptosis
of prostate cancer cells
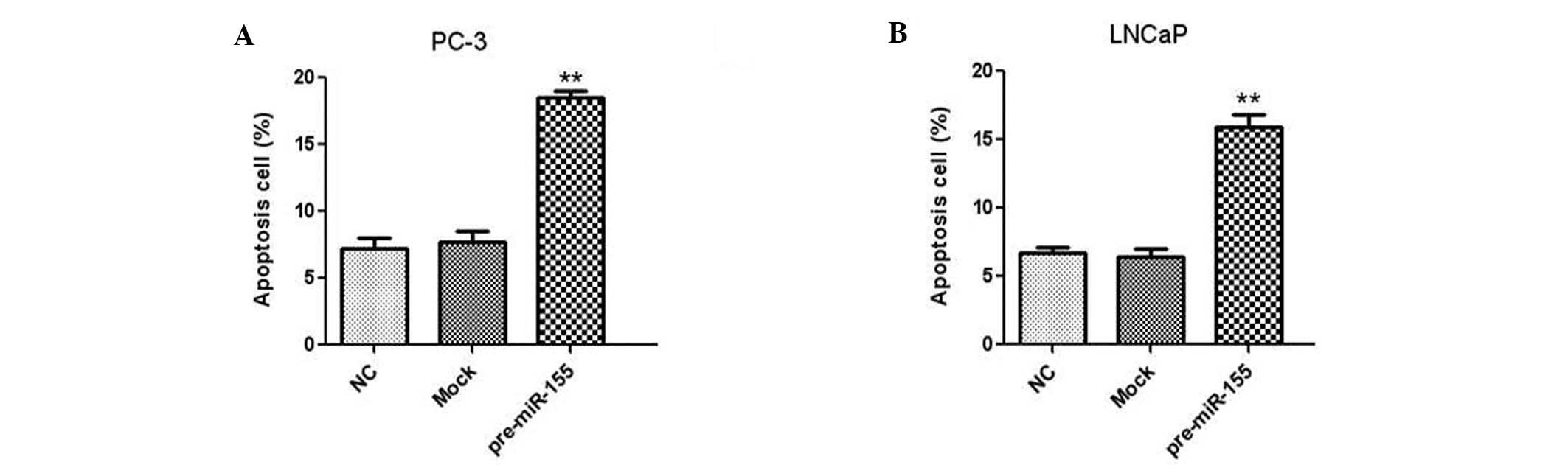
The effect of miR-155 on the apoptosis of human
prostate cancer cells was investigated by flow cytometry. Two
prostate cancer cell lines, PC-3 and LNCaP, were transfected with
miR-155 inhibitor and the apoptotic rate was analyzed using Annexin
V and PI staining. The results indicated that downregulation of
miR-155 led to a significant increase in the apoptotic rates in
PC-3 (Fig. 3A) and LNCaP (Fig. 3B) prostate cancer cells.
MiR-155 downregulation induces prostate
cancer cell cycle arrest
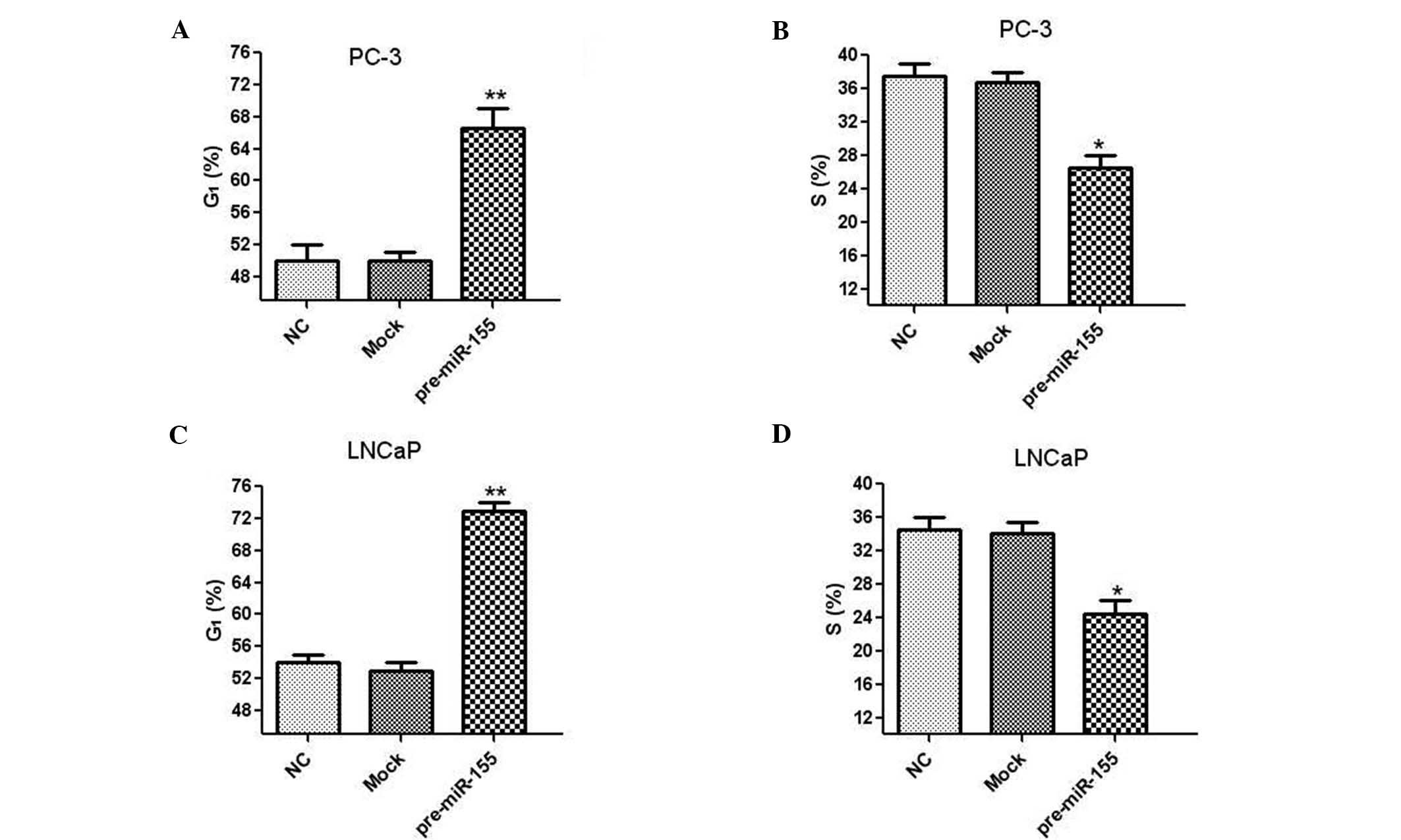
Human prostate cancer cells were transfected with
miR-155 inhibitor to investigate the effect of miR-155 on cell
cycle distribution. In PC-3 cells, treatment with miR-155 inhibitor
significantly increased the proportion of cells in G1
phase and reduced the number of cells in S phase (Fig. 4A and B). Similar results were also
observed in LNCaP cells following transfection with the miR-155
inhibitor (Fig. 4C and D). These
results demonstrated that inhibition of miR-155 induced cell cycle
arrest in G1 phase in human prostate cancer cells.
ANX7 is a target of miRNA-155 in prostate
cancer cells
To elucidate the molecular mechanism by which
miRNA-155 enhanced prostate cancer cell proliferation, putative
miRNA-155 targets were identified using a number of programs,
including miRanda (http://www.microrna.org/microrna/home.do), TargetScan
(http://www.targetscan.org/)and PicTar
(http://pictar.mdc-berlin.de/). A 3′UTR
of ANX7 containing the conserved putative miRNA-155 binding sites
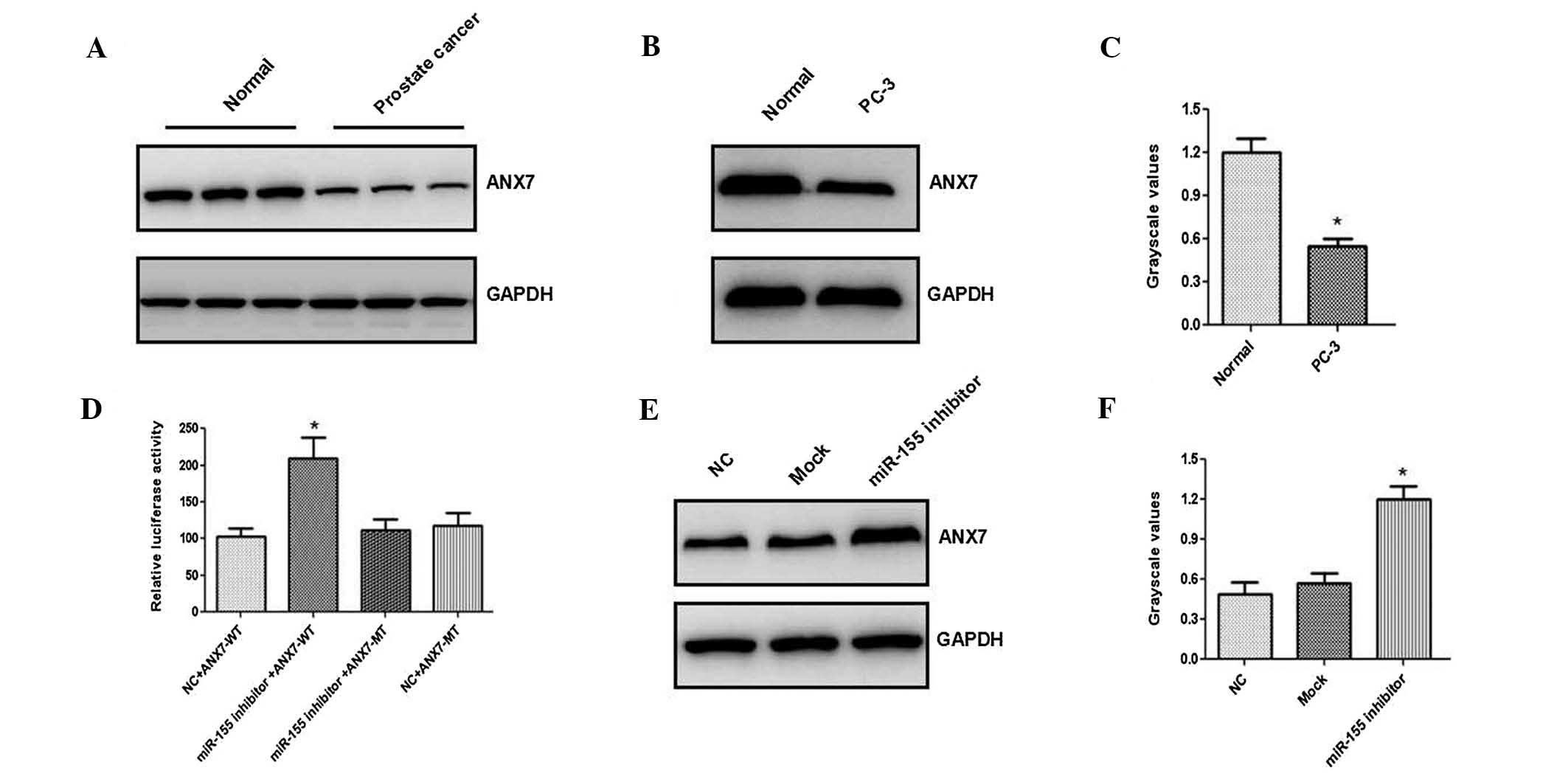
was identified. Western blot analysis indicated that the ANX7
expression levels were significantly reduced in prostate tissues
(Fig. 5A) and cancer cells
(Fig. 5B and C). Firefly
luciferase activity was significantly increased in PC-3 cells
following transfection with miR-155 inhibitor (Fig. 5D). In addition, treatment of PC-3
cells with miR-155 inhibitor led to a significant increase in the
ANX7 expression (Fig. 5E and F).
Together, these results suggested that the ANX7-3′-UTR is located
at direct binding sites of miR-155.
Discussion
miRNAs are a group of small non-coding RNAs that
modulate gene expression by targeting mRNAs for translational
repression (13,14). The expression of miR-155 has been
demonstrated to be upregulated in numerous types of cancer, and is
involved in the development of leukemia, breast and lung cancer
(11,12). However, the biological role of
miR-155 in prostate cancer has not been investigated, to the best
of our knowledge. In the present study the expression patterns of
miR-155 were investigated in prostate cancer for the first time.
The results indicated that miR-155 was significantly upregulated in
prostate cancer tissues and four different cell lines (DU145,
LNCaP, 22RV1 and PC-3).
The aberrant expression of miRNAs is important in
tumor cell proliferation, apoptosis and cell cycle arrest. A recent
study indicated that miR-125b, which is overexpressed in prostate
cancer tissues and cells, promoted the growth of a prostate cancer
xenograft by downregulation of the key pro-apoptotic genes,
including p53, p35 upregulated modulator of apoptosis and B-cell
lymphoma 2 homologous antagonist killer (15). Another study reported that miR-155
promoted the proliferation of human breast cancer MCF-7 cells by
targeting p53-induced nuclear protein 1, and therefore created a
novel therapeutic strategy for breast cancer (16). In the present study, an MTT assay
revealed that overexpression of miR-155 significantly promoted cell
proliferation of PC-3 and LNCaP cells. Additionally, human prostate
cancer cells transfected with miR-155 inhibitor exhibited reduced
proliferation, suggesting that miR-155 promoted prostate cancer
cell proliferation in vitro.
The effect of miR-155 on levels of cell apoptosis
and cell cycle distribution in prostate cancer cells was also
examined. Apoptosis is the process of programmed cell death that is
crucial for cell growth and the maintenance of cellular
homeostasis, and is regulated by numerous signaling pathways
(17). Cell cycle processes are
precise, and controlled by cell cycle checkpoints which guarantee
the fidelity of cell division. However, cell cycle arrest is
triggered by various factors that lead to cell death and apoptosis
(18). Petrocca et al
(19) demonstrated that the
miR-106b-25 cluster is indispensible in the development of
transforming growth factor-β-dependent cell-cycle arrest and
apoptosis in gastric cancer. Another study demonstrated that
miR-155 downregulation induced cell apoptosis by targeting many
anti-apoptotic factors, leading to cell cycle arrest (20). The present study revealed that
inhibition of miR-155 promoted apoptosis of PC-3 and LNCaP prostate
cancer cell lines. Transfection with an miR-155 inhibitor
significantly increased the proportion of cells in G1
phase and reduced that in S phase, demonstrating that low
expression of miR-155 induced cell cycle arrest in G1
phase.
miRNAs control a number of biological functions of
the cell by targeting various genes and altering their expression
levels; therefore, it is critical to elucidate the functional
targeted genes. The annexin family consists of a group of proteins
with a common structure that contains two distinct regions, an
annexin core and an amino (N)-terminus (21). Annexins are involved in the
regulation of membrane trafficking, cellular adhesion and
tumorigenesis (22,23). Annexin 7 (ANX7) is a substrate for
protein kinase C and other kinases associated with cell survival,
proliferation, differentiation and cell migration (24). Srivastava et al (25) have demonstrated that reduced
expression of ANX7 may be associated with the progression of
prostate cancer, indicating that ANX7 functions as a tumor
suppressor. Consistent with previous studies, the present study
observed that ANX7 expression levels were significantly reduced in
prostate cancer samples and cell lines. In addition, transfection
with an miR-155 inhibitor led to increased luciferase activity and
ANX7 expression in prostate cancer cells, indicating that ANX7 is a
target gene of miR-155.
In conclusion, the present study demonstrated that
miRNA-155 was significantly upregulated in prostate cancer tissues
and cell lines. In addition, miRNA-155 was indicated to promote the
proliferation of prostate cancer cells by regulating ANX7
expression levels, indicating the potential for the therapeutic use
of miRNA-155 in the treatment of prostate cancer.
Acknowledgements
The present study was supported by a project of the
Science and Technology Commission of Shanghai Municipality (no.
134119a9800) and the National Natural Science Foundation of China
(nos. 81172450 and 81202008).
References
|
1
|
Dunn MW and Kazer MW: Prostate cancer
overview. Semin Oncol Nurs. 27:241–250. 2011. View Article : Google Scholar
|
|
2
|
Ha HK, Lee W, Park HJ, et al: Clinical
significance of CXCL16/CXCR6 expression in patients with prostate
cancer. Mol Med Rep. 4:419–424. 2011.PubMed/NCBI
|
|
3
|
Gosselaar C, Roobol MJ, Roemeling S, van
der Kwast TH and Schröder FH: Screening for prostate cancer at low
PSA range: the impact of digital rectal examination on tumor
incidence and tumor characteristics. Prostate. 67:154–161. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katahira K, Takahara T, Kwee TC, et al:
Ultra-high-b-value diffusion-weighted MR imaging for the detection
of prostate cancer: evaluation in 201 cases with histopathological
correlation. Eur Radiol. 21:188–196. 2011. View Article : Google Scholar
|
|
5
|
Stewart GD, Gray K, Pennington CJ, et al:
Analysis of hypoxia-associated gene expression in prostate cancer:
lysyl oxidase and glucose transporter-1 expression correlate with
Gleason score. Oncol Rep. 20:1561–1567. 2008.PubMed/NCBI
|
|
6
|
Arora R, Koch MO, Eble JN, et al:
Heterogeneity of Gleason grade in multifocal adenocarcinoma of the
prostate. Cancer. 100:2362–2366. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang YW and Chen LA: microRNAs as tumor
inhibitors, oncogenes, biomarkers for drug efficacy and outcome
predictors in lung cancer (review). Mol Med Rep. 5:890–894.
2012.PubMed/NCBI
|
|
8
|
Fang YX and Gao WQ: Roles of microRNAs
during prostatic tumorigenesis and tumor progression. Oncogene.
2013.PubMed/NCBI
|
|
9
|
Hamamoto J, Soejima K, Yoda S, et al:
Identification of microRNAs differentially expressed between lung
squamous cell carcinoma and lung adenocarcinoma. Mol Med Rep.
8:456–462. 2013.PubMed/NCBI
|
|
10
|
Nazarov PV, Reinsbach SE, Muller A, et al:
Interplay of microRNAs, transcription factors and target genes:
linking dynamic expression changes to function. Nucleic Acids Res.
41:2817–2831. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marcucci G, Maharry KS, Metzeler KH, et
al: Clinical role of microRNAs in cytogenetically normal acute
myeloid leukemia: miR-155 upregulation independently identifies
high-risk patients. J Clin Oncol. 31:2086–2093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang M, Shen H, Qiu C, et al: High
expression of miR-21 and miR-155 predicts recurrence and
unfavourable survival in non-small cell lung cancer. Eur J Cancer.
49:604–615. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang X, Taeb S, Jahangiri S, et al:
MiRNA-95 mediates radioresistance in tumors by targeting the
sphingolipid phosphatase SGPP1. Cancer Res. 2013.PubMed/NCBI
|
|
14
|
Huang X and Jia Z: Construction of
HCC-targeting artificial miRNAs using natural miRNA precursors. Exp
Ther Med. 6:209–215. 2013.PubMed/NCBI
|
|
15
|
Shi XB, Xue L, Ma AH, et al: MiR-125b
promotes growth of prostate cancer xenograft tumor through
targeting pro-apoptotic genes. Prostate. 71:538–549. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang CM, Zhao J and Deng HY: MiR-155
promotes proliferation of human breast cancer MCF-7 cells through
targeting tumor protein 53-induced nuclear protein 1. J Biomed Sci.
20:792013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Powell-Coffman JA and Coffman CR:
Apoptosis: Lack of oxygen aids cell survival. Nature. 465:554–555.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li T, Kon N, Jiang L, et al: Tumor
suppression in the absence of p53-mediated cell-cycle arrest,
apoptosis, and senescence. Cell. 149:1269–1283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Petrocca F, Visone R, Onelli MR, et al:
E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest
and apoptosis in gastric cancer. Cancer Cell. 13:272–286. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Higgs G and Slack F: The multiple roles of
microRNA-155 in Oncogenesis. J Clin Bioinforma. 3:172013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Streicher WW, Lopez MM and Makhatadze GI:
Annexin I and annexin II N-terminal peptides binding to S100
protein family members: specificity and thermodynamic
characterization. Biochemistry. 48:2788–2798. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lokman NA, Ween MP, Oehler MK and
Ricciardelli C: The role of annexin A2 in tumorigenesis and cancer
progression. Cancer Microenviron. 4:199–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paweletz CP, Ornstein DK, Roth MJ, et al:
Loss of annexin 1 correlates with early onset of tumorigenesis in
esophageal and prostate carcinoma. Cancer Res. 60:6293–6297.
2000.PubMed/NCBI
|
|
24
|
Jin Y, Wang S, Chen W, et al: Annexin A7
suppresses lymph node metastasis of hepatocarcinoma cells in a
mouse model. BMC Cancer. 13:5222013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Srivastava M, Bubendorf L, Srikantan V, et
al: ANX7, a candidate tumor suppressor gene for prostate cancer.
Proc Natl Acad Sci USA. 98:4575–4580. 2001. View Article : Google Scholar : PubMed/NCBI
|