Introduction
Head and neck squamous cell carcinoma (HNSCC)
represents one of the six most common types of cancer in the world,
with up to 500,000 novel cases annually; this includes malignancies
arising in the oral cavity, larynx and pharynx. The highest rates
of HNSCC are found in South-Central Asia, Central and Eastern
Europe; rates are lowest in Africa and Central America, with no
variation in prevalence between genders (1,2).
Significant risk factors for HNSCC carcinogenesis include tobacco
use, alcohol consumption and infection with human papilloma virus.
In Taiwan, a positive correlation has been reported between the
consumption of betel nuts and incidence of HNSCC (3). Furthermore, ~30% of HNSCC revealed
mutations in genes that regulate squamous differentiation,
including Notch1, interferon regulatory factor 6 and p63, therefore
implicating their dysregulation as a major driver of HNSCC
carcinogenesis (4). However, the
mechanisms underlying the malignant progression of HNSCC remains to
be fully elucidated.
Epidermal growth factor (EGF) receptor (EGFR) is
overexpressed in 40–90% of HNSCC and is associated with a poorer
outcome for HNSCC patients (5,6).
Ligand binding triggers the EGFR receptor to either homodimerize or
heretrodimerize with other members of the Erb family, both leading
to EGFR autophosphorylation. The activated receptor recruits
signaling complexes and activates Ras-mitogen-activated protein
kinase (MAPK), extracellular signal-regulated kinase (ERK),
phosphatidynositol-3-kinase (PI3K)-Akt, signal transducers and
activators of transcription and the phospholipase C gamma pathway
(7). These unique cascades
converge with the upregulation of cyclin D1, a key mediator of
mitosis and G1 to S-phase progression (8). Angiogenesis has recently been
recognized as a novel therapeutic strategy due to its important
role in the pathogenesis of HNSCC. Vascular endothelial growth
factors (VEGFs) and their receptors are expressed in the majority
of HNSCC tumors. Several preclinical studies indicated that these
markers are associated with tumor progression, changes in
microvessel density and the development of lymph node metastasis
(9,10).
Sprouty was first reported as an antagonistic
regulator of fibroblast growth factor (FGF) signaling during
tracheal branching in Drosophila (11). Four mammalian sprouty genes have
been defined, based on their sequence similarity with
Drosophila sprouty (12).
In analogy with Drosophila sprouty, mammalian sprouty
proteins were proven to antagonize FGF-stimulated organogenesis
(13). It was reported that the
overexpression of mammalian sprouty1 and sprouty2 inhibited FGF-,
VEGF- and EGF-induced proliferation and migration in human
umbilical vein endothelial cells and a mouse embryonic fibroblast
cell line, NIH3T3 (14,15). It has become evident that
expression of sprouty protein is dysregulated in numerous types of
human cancer. A decrease in Sprouty2 expression has been
demonstrated in hepatocellular carcinoma, lung cancer and other
cancer types, this is consistent with its inhibitory on ERK1/2
signaling and proliferation (16,17).
A previous study by our group, identified that sprouty2 was
downregulated in colon cancer and had a tumor suppresive role
(18). However, the
tumor-suppressing effect of sprouty2 in HNSCC has not yet been
investigated.
Cetuximab is a well-known targeted therapy in HNSCC,
which has FDA approval. In addition to EGFR targeted therapy,
angiogenesis-based cancer treatment is under extensive
investigation. Therapeutic strategies that have been developed in
treating cancer patients include monoclonal antibodies and kinase
inhibitors. VEGF activation has been demonstrated in HNSCC
(19), and high VEGF expression in
HNSCC tumor samples has been associated with progression of lymph
node spread and poor prognosis (20). Sorafenib, also known as NSC 724772,
BAY 43-9006 or Nexavar, is a multi-targeted agent that inhibits
VEGF receptor (VEGFR), platelet-derived growth factor receptor
(PDGFR), KIT, fetal liver tyrosine kinase 3 (FLT-3), and the
serine-threonine kinase Raf. Williamson et al (21) conducted a phase II trial of
sorafenib in chemotherapy-naïve patients with recurrent or
metastatic HNSCC. The median times to progression and overall
survival were four and nine months, respectively. The majority of
participants tolerated the sorafenib treatment well. The present
study aimed to explore potential factors that may influence the
response of HNSCC patients to sorafenib-based targeted
therapies.
Materials and methods
Clinical specimens and cell lines
Primary HNSCC tissues and normal-appearing tissue
from the head and neck, adjacent to the carcinomas, were resected
from 42 patients who underwent surgery for malignant tumors. These
samples were obtained from Biobank (Chi-Mei Hospital, Liouying,
China) without identification of patients, following the approval
of the Institutional Review Board of the Chi-Mei Hospital
(Liouying, China; IRB No. CLH-0120). Written informed consent was
obtained from all patients. The samples included 3 stage I, 11
stage II, 2 stage III and 26 stage IV carcinomas. Verrucous
carcinoma was not included. Paired samples of verrucous hyperplasia
and normal skin tissues were acquired from 19 patients who received
wide excision of skin lesions from the head and neck region. Human
cell lines of HNSCC were obtained from the Food Industry Research
and Development Institute (Hsinchu, Taiwan) and included SCC-4 and
SCC-25. Fadu cells were provided by the American Type Culture
Collection (ATCC; Rockville, MD, USA). HONE-1 and HSC-3 were from
Dr. Jenn-Ren Hsiao (Cheng Kung University, Tainan, Taiwan). Of
these, HONE-1 was originally obtained from the ATCC and HSC-3 was
obtained from the Food Industry Research and Development Institute.
These cell lines were cultured in complete medium containing
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10%
fetal bovine serum (FBS; both from Gibco Life Technologies,
Darmstadt, Germany).
RNA extraction and reverse
transcription
Total RNA was extracted from primary tumor tissue
and normal mucosa of patients using RNA extraction kits (RNeasy
Midi kit; Qiagen, Hilden, Germany). RNA was treated according to
the manufacturer’s instructions of the SuperScript First-Strand
Synthesis System (Invitrogen Life Technologies, Carlsbad, CA,
USA).
Quantitative real-time polymerase chain
reaction (PCR)
For each PCR reaction, 2 μg of cDNA was used for PCR
amplification on each sample with 12.5 μl of TagMan PCR Master Mix
(Applied Biosystems, Foster City, CA, USA) and 1.25 μl of TagMan
Assay-on-Demand kit (Applied Biosystems) that included forward
primer, reverse primer and probe. Samples were run on an ABI HT7900
Sequence Detector (Applied Biosystems) according to the
manufacturer’s instructions. The following primers were used: human
Sprouty2, 5′-GCGATCACGGAGTTCAG-3′ (forward) and
5′-GTGGAGTCTCTCGTGT-3′ (reverse); and human GAPDH,
5′-GGTGTGAACCATGAGAAG-3′ (forward) and 5′-CCACAGTTTCCCGGAG-3′
(reverse). The crossing points that were calculated with SDS
software version 2.2 (Applied Biosystems) took into account the
difference in amplification efficiency of the target (Sprouty2) and
the reference genes. The delta-delta Ct assay was used for the
calculation of quantitative gene expression. Fold changes in gene
expression of sprouty2 between tumor and normal tissue were
calculated.
Immunoblot analysis
Cells were washed twice with phosphate-buffered
saline (PBS) and harvested for immunoblot analysis. Cell lysates
were prepared in the SDS sample buffer and proteins were separated
by SDS-PAGE. Proteins were transferred to a nitrocellulose membrane
and then blocked with 5% BSA in PBS-T (0.1% Tween-20 in PBS). The
primary antibodies were diluted according to the manufacturer’s
recommendation in 5% BSA in PBS-T and incubated with the membrane
at 4°C overnight. The antibodies used were: Rabbit anti-human
polyclonal antibodies to Sprouty2 (1:2,000; Upstate Biotechnology,
Temecula, CA, USA), rabbit polyclonal anti-human-actin (1:3,000; A
2668; Sigma Aldrich, St. Louis, MO, USA), rabbit anti-human
monoclonal antibodies to phosphatase and tensin homolog (PTEN,
1:1,000; Epitomics, Burlingame, CA, USA), mouse anti-human
monoclonal antibodies to phosphorylated Akt (1:1,000; Cell
Signaling Technology, Inc., Beverly, MA, USA) and rabbit anti-human
monoclonal antibodies to Akt (1:1,000; Cell Signaling Technology,
Inc.) as primary antibodies. Horseradish peroxidase-conjugated
anti-rabbit immunoglogulin G (IgG) (Santa Cruz Biotechnology, Santa
Cruz, CA, USA) and anti-mouse IgG (Santa Cruz Biotechnology) were
used as secondary antibodies where appropriate and protein-antibody
complexes were visualized using the enhanced chemoluminescence
(ECL) system (Amersham Biosciences, Piscataway, NJ, USA).
Plasmids and transfection
The pSprouty2-EGFP-N3 plasmid encoding Sprouty2-EGFP
fusion protein was derived from pEGFP-N3 (Clontech, Mountain View,
CA, USA). SCC-4 and HONE-1 cells were transiently transfected with
pSprouty2-EGFP-N3 with Lipofectamine 2000 (Invitrogen Life
Technologies). The control cells were transfected with empty
vector.
In vitro proliferation assay
Cell proliferation was measured by MTT assay to
assess the effect of sprouty2 and sorafenib on the growth of human
HNSCC cell lines. Cells transfected with pSprouty2-EGFP-N3 or
control cells were cultured in 96-well plates (Corning Costar,
Corning, NY, USA) at a density of 4,000 cells per well. Cell
proliferation was determined with the MTT assay 72 hours after cell
plating. The absorbance was measured at 570 nm using a microtiter
plate reader (MRX Revelation, DYNEX, Chantilly, VA, USA). To
evaluate the cytotoxic effect of sorafenib (Bayer HealthCare,
Whippany, NJ, USA) influenced by sprouty2 regulation, SCC-4 or
HONE-1 cells were plated at a density of 4,000 cells per well in
DMEM/F12 (Gibco Life Technologies) with 10% FBS. After 24 hours of
incubation, increasing doses of sorafenib (0, 0.125, 0.25, 0.5, 1,
2, 4, 8, 16 and 32 μM) were added to the culture medium. The MTT
assay was processed as mentioned above following incubation with
sorafenib for three days. The IC50 was calculated by
SigmaPlot 9.0 statistical software (Systat Software, Inc., San
Jose, CA, USA). Values are expressed as the ratio of cell numbers
relative to the controls. Each value represents the mean ± standard
deviation (SD) of six determinations.
Statistical analysis
The Kaplan-Meier method was used to analyze
time-to-event variables, and the 95% confidence interval (CI) for
the median time-to-event was computed. The Student’s t test and
paired t-test were used for the difference between groups. Any
P-value of <0.05 was considered to indicate statistical
significance. All statistical analyses were performed using
SigmaPlot 9.0 statistical software (Jandel Scientific).
Results
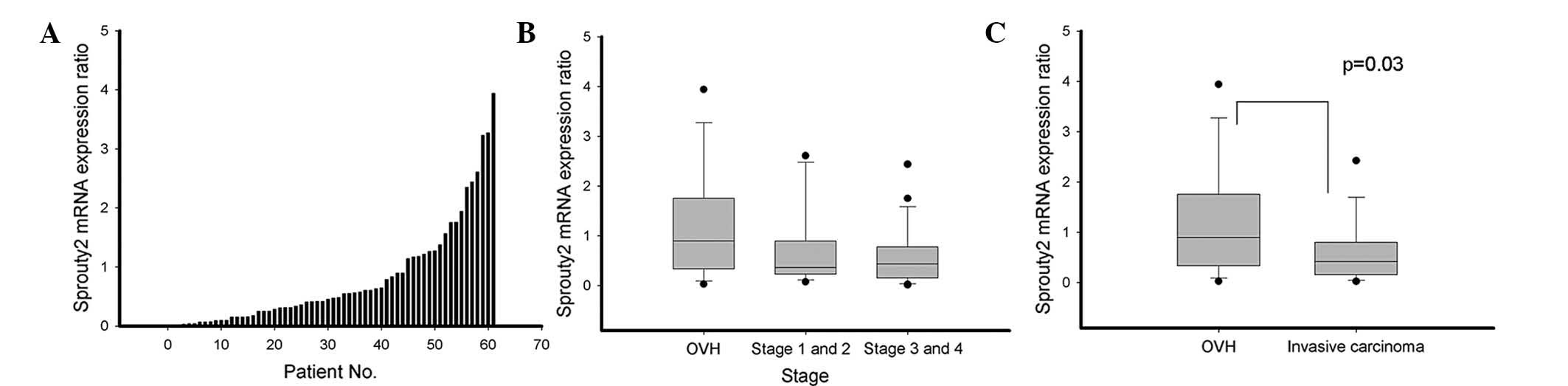
Sprouty2 expression is downregulated in
human HNSCC and oral verrucous hyperplasia (OVH) - the
downregulation was correlated with the severity of the disease
mRNA expression of sprouty2 was determined in tumor
tissues at varying disease stages and tissues from adjacent normal
mucosa from 19 OVH patients and 42 HNSCC patients by real-time
quantitative PCR. Expression of sprouty2 mRNA in human tumors
relative to that in their normal tissue counterparts from the same
patients was compared. Overall, the mRNA expression of sprouty2 was
decreased in 44 of 61 (72%) paired tumor samples compared with
normal mucosal tissues. Specifically, a decrease in sprouty2 mRNA
expression was found in 58% (11/19) of OVH samples, 79% (11/14) of
stage I–II HNSCC samples and 79% (22/28) of stage III–IV HNSCC
samples (Fig. 1A and B). The mean
sprouty2 mRNA expression levels relative to their adjacent normal
mucosa from three determinations were significantly lower in HNSCC
(mean=0.634; 95% CI=0.425–0.844) than in OVH (mean=1.164;
CI=0.599–1.730; P=0.029) (Fig.
1C). These findings showed that sprouty2 was downregulated in
OVH and HNSCC.
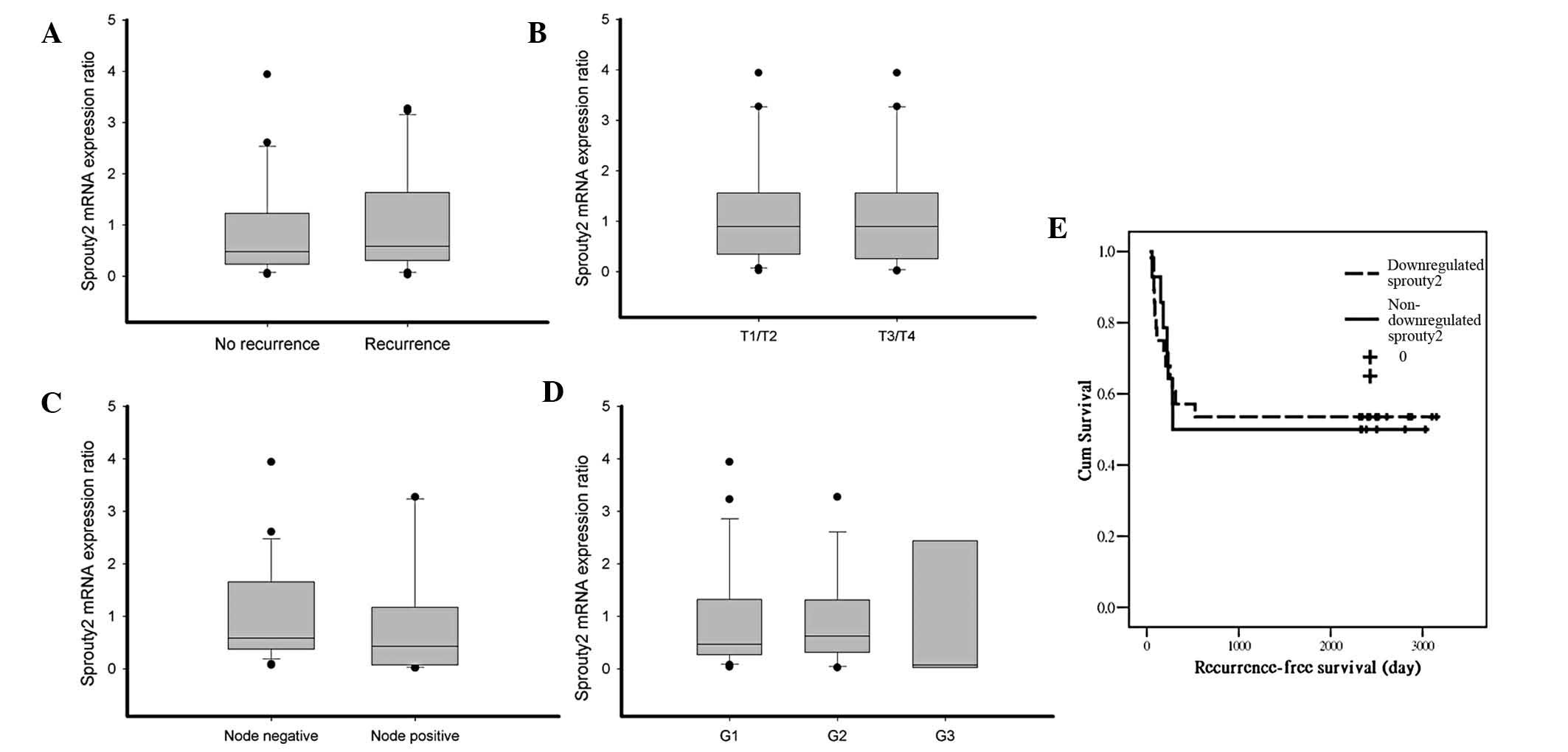
Sprouty2 expression is not associated
with features of HNSCC, including tumor size, differentiation
grading, nodal metastasis and recurrence - downregulation of
sprouty2 has no role in the recurrence-free survival of HNSCC
The downregulation of sprouty2 was correlated with
invasiveness of HNSCC. In prostate cancer, the mean expression of
sprouty2 was significantly decreased in patients experiencing
recurrences during the follow-up period. The expression of sprouty2
tended to be decreased in advanced-stage cancers and in cases with
lymph node metastasis in comparison with early stage cancer (T2 vs
>T2) (22). Among 42 cases of
invasive HNSCC samples in the present study, the expression of
sprouty2 did not correlate with recurrence, nodal metastasis, tumor
size or histological grading (Fig.
2A–D). Song et al (23)
showed that hepatoma patients negative for sprouty2 had poorer
survival. In the present study, the correlation of sprouty2
expression with recurrence-free survival was estimated by the
Kaplan-Meier method in HNSCC tumors. The expression of sprouty2 was
not associated with any significant changes in recurrence-free
survival (Fig. 2E).
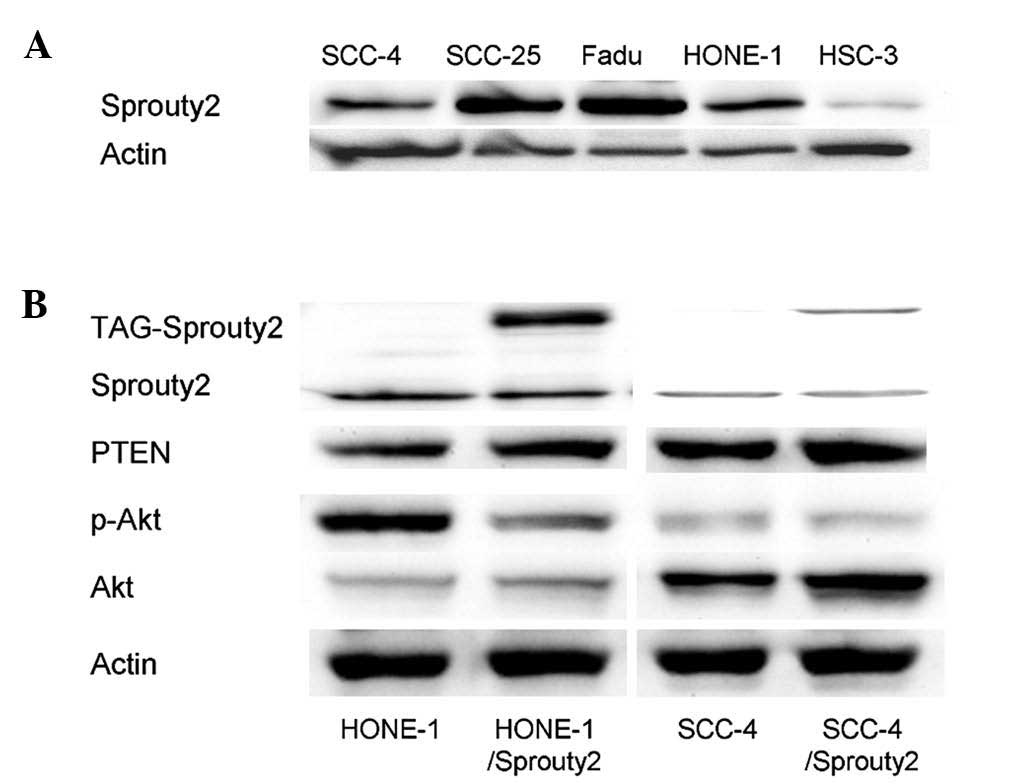
Sprouty2 enhances the expression of PTEN
and unexpectedly suppresses Akt phosphorylation, which indicates
the tumor suppressive role of sprouty2 in HNSCC
Western blot analysis showed that HSC-3, HONE-1, and
SCC-4, cells expressed lower levels of sprouty2 among the five
HNSCC cell lines examined (Fig.
3). To verify the effect of sprouty2 in signaling pathways in
HNSCC, HONE-1 and SCC-4, cells were transfected with
pSprouty2-EGFP-N3 plasmid to enhance the expression of sprouty2.
HSC-3 cells did not demonstrate ectopic expression of sprouty2 due
to inadequate transfection efficacy by lipofectamine. As shown in
Fig. 3, the overexpression of
sprouty2 reduced the phosphorylation of Akt and increased the
expression PTEN. Of note, PTEN is known to be a tumor suppressor
gene, implicating that sprouty2 may synergize with PTEN to suppress
HNSCC cell growth (18,24). Furthermore, the effect of sprouty2
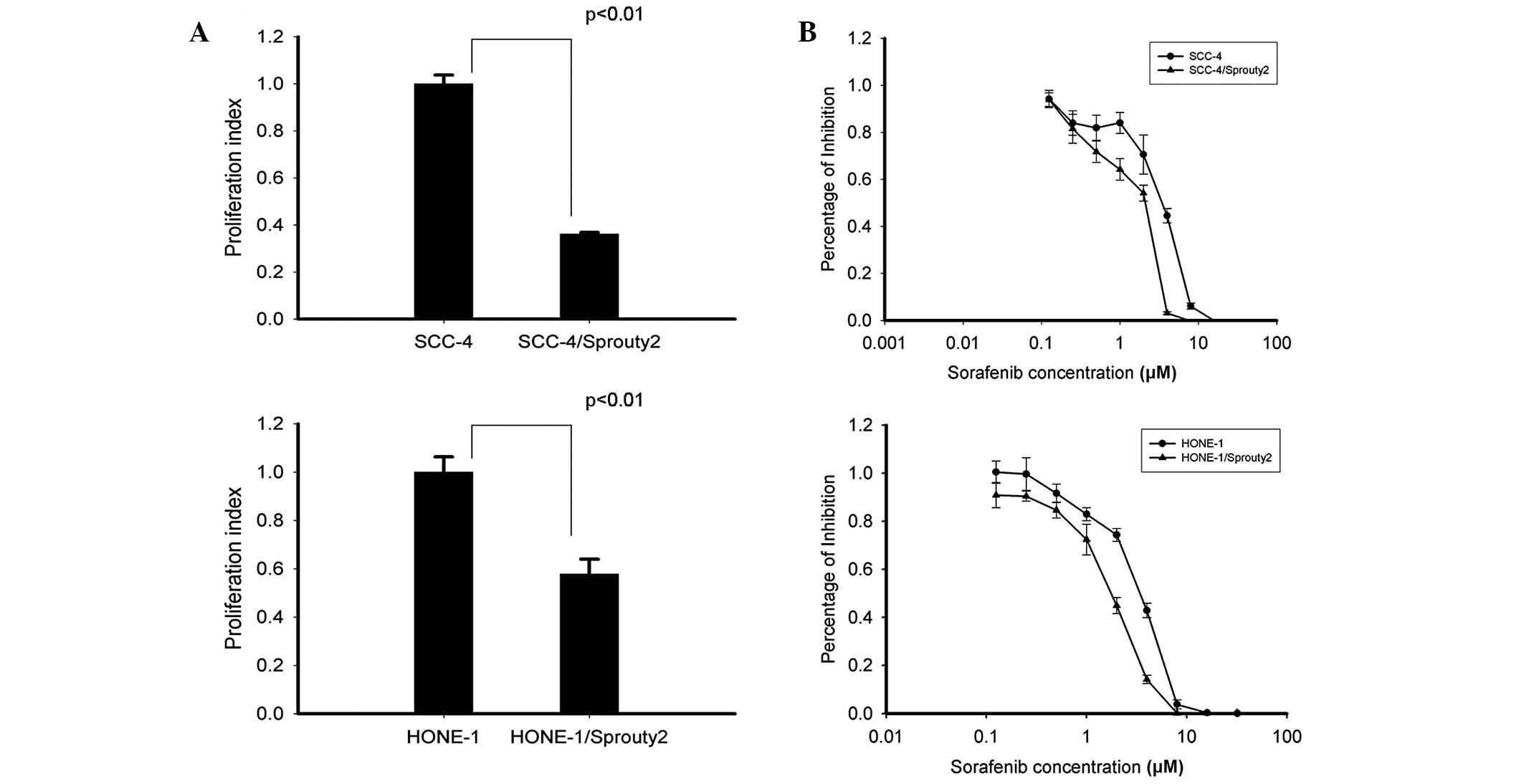
on the proliferation of HONE-1 and SCC-4 cells was investigated.
Fig. 4A shows that cell
proliferation, examined by the MTT assay, was decreased in both
HONE-1 and SCC-4 cells overexpressing sprouty2 compared with their
control counterparts, which were transfected with empty vector
(P<0.01).
Sprouty2 enhances the sensitivity of
sorafenib in HNSCC cells, SCC-4 and HONE-1
The influence of sprouty2 expression on the effect
of sorafenib was determined. SCC-4 and HONE-1 cells were
transfected with pSprouty2-EGFP-N3 plasmid to express sprouty2
protein. Overexpression of sprouty2 enhanced the sensitivity of
both SCC-4 and HONE-1 cells to sorafenib compared with control
cells. The half maximal inhibitory concentration (IC50)
values of sorafenib were in 3.54±0.24 μM in SCC-4 control cells and
1.78±0.18 μM in SCC-4/sprouty2 cells. In HONE-1 cells, the
IC50 values of sorafenib were 3.30±0.15 μM in control
cells and 1.86±0.08 μM in sprouty2-transfected cells.
Discussion
Sprouty proteins have been identified as modulators
of receptor tyrosine kinase signaling in normal development and
disease states (25). Evidence
suggested that the expression of sprouty proteins is dysregulated
in a number of cancers; in most types of cancer it is
downregulated, with the exception of colon cancer and melanoma,
where it is upregulated (Table I)
(16–18,22,23,26–37).
In prostate cancer, downregulated sprouty2 expression in invasive
prostate cancer cell lines and high-grade clinical prostate cancer
is similar to that of benign prostatic hyperplasia and
well-differentiated tumors (32).
A previous study by our group disclosed that sprouty2 was
deregulated in human colon cancer and associated with inverse
regulation by microRNA 21 (18).
It implied that deregulation of sprouty2 expression may promote
tumorigenesis. To date, it has remained elusive whether sprouty2
acts as a negative inhibitor of growth factors in HNSCC. The
present study confirmed that sprouty2 was downregulated in
preneoplasia and HNSCC. Importantly, it was identified that the
decrease in sprouty2 expression in HNSCC was significant compared
with OVH, a precursor of verrucous carcinoma. It is noteworthy that
sprouty2 expression decreased in 58% of OVH samples, but was
significantly decreased in invasive carcinoma. This result
suggested that the decrease of sprouty2 expression may be an early
event in the carcinogenesis of HNSCC.
 | Table ISprouty2 expression in human cancer
disease. |
Table I
Sprouty2 expression in human cancer
disease.
| Cancer type | Sample origin | First author, year
(reference) |
|---|
| Sprouty2
downregulated cancer |
| Breast cancer | Clinical human
tissue | Lo et al,
2004 (24) |
| Colon cancer | Clinical human
tissue | Feng et al,
2011 (18) |
| Endometrial
cancer | Clinical human
tissue | Velasco et
al, 2011 (25) |
| Glioma | Clinical human
tissue and cell lines | Kwak et al,
2011 (26) |
| Squamous cell
carcinoma of the head and neck | Clinical human
tissue | Present study |
| Hepatocellular
carcinoma | Clinical human
tissue and cell lines | Fong et al,
2006 (17)
Lee et al, 2008 (27)
Lee et al, 2010 (28)
Song et al, 2012 (22) |
| Lymphoma | Cell lines | Frank et al,
2009 (29) |
| Non-small cell
lung cancer | Clinical human
tissue and cell lines | Sutterlüty et
al, 2007 (16) |
| Neuroblastoma | Cell lines | Ishida et
al, 2007 (30) |
| Pancreatic
cancer | Clinical human
tissue and cell lines | Ma et al,
2010 (31) |
| Prostate
cancer | Clinical human
tissue | McKie et al,
2005 (32)
Fritzsche et al, 2006 (21) |
| Sprouty2
upregulated cancer |
| Colon cancer | Clinical human
tissue and cell lines | Holgren et
al, 2010 (33)
Barbáchano et al, 2010 (34) |
| Melanoma | Cell lines | Bloethner et
al, 2005 (35) |
Previous studies on human cancers suggested the
prognostic impact of sprouty2. In a hepatoma study, Lee et
al (30) found that
significant downregulation of sprouty2 protein and sprouty2 mRNA
expression characterized most hepatomas with poorer outcomes.
Differentiation grading is a strong predictor for prognosis in most
types of cancers. Sprouty2 protein has been found to be associated
with high-grade cancers. In human glioma, sprouty2 protein levels
were significantly decreased in 79% of invasive World Health
Organization (WHO) grade II–IV tumors, but not in non-invasive WHO
grade I tumors and normal tissues (28). Furthermore, by assessing the
expression of sprouty2 mRNA in resected prostate cancer, McKie
et al (34) reported a
trend between downregulated sprouty2 mRNA expression and increasing
Gleason score (less differentiated) tumors. The present study
focused on invasive HNSCC and did not suggest sprouty2 was a
prognostic predictor, in part due to the limited number of stage I
and stage II samples. Only fourteen tumor samples of stage I and
stage II disease were analyzed in the present study. To verify the
conclusion, the inclusion of a larger number of stage I and stage
II disease samples would be required.
Sprouty proteins have been demonstrated to inhibit
signaling by a diverse range of stimuli, including VEGF, PDGFR,
hepatocyte growth factor, glial-derived growth factor and nerve
growth factor (38,39). The inhibitory function of sprouty
is mainly associated with the control of the Ras/Raf/MAPK/ERK
pathway at multiple levels (25).
Sprouty2 interacts with different components of the EGF signaling
pathway. Sprouty2 interacts with E3 ubiquitin ligase/c-Casitas
B-lineage lymphoma (c-Cbl), which leads to the reduction of EGFR
endocytosis and degradation (40).
Thus, sprouty2 serves as a negative regulator of FGF and other
growth factors but as a positive enhancer of EGF signaling.
Enhanced sprouty2 protein expression has been shown to suppress Akt
phosphorylation and promote PTEN expression in cancer cells
(18,24). In HNSCC cell lines, HONE-1 and
SCC-4, ectopic expression of sprouty2 exerted a similar regulation
pattern of signaling pathways. The present study showed that the
expression of sprouty2 protein suppressed Akt phosphorylation and
enhanced PTEN expression. This correlated with the
tumor-suppressive effect of sprouty2 in HNSCC.
Several mechanisms have been proposed to define the
decrease of sprouty2 in cancer cells, including the methylation of
the sprouty2 promoter, increasing degradation of sprouty2 by neural
precursor cell expressed developmentally down-regulated 4 (NEDD4)
and upregulation of microRNA-21. McKie et al (34) found the suppression of sprouty2
expression correlated with methylation of the CpG region in
clinical samples of prostate cancer. Sprouty2 inactivation by
promoter methylation in human B-cell lymphoma was suggested by
Frank et al (31). Sprouty2
can be proteolytically degraded by c-Cbl, seven in absentia
homolog-2 (SIAH-2), or NEDD4 (30,40).
In a hepatoma study, all 13 hepatoma samples exhibiting NEDD4
upregulation displayed low sprouty2 protein expression (30). Furthermore, upregulated microRNA-21
was associated with a decrease in sprouty2 protein levels in glioma
cells (28) and reduced sprouty2
mRNA expression in colon cancer (18), implying that the deregulation of
sprouty2 is triggered differently depending on cancer biology.
Further studies are warranted to address the mechanisms of
deregulated sprouty2 in HNSCC.
Sorafenib is an oral inhibitor of serine/threonine
kinases C-Raf and B-Raf and the receptor tyrosine kinases VEGFR-2,
VEGFR-3, PDGFR, FLT-3 and KIT. It inhibits cancer cell activity via
two major pathways: The EGFR/Ras/Raf/MEK/ERK signaling pathway,
which is a key regulator in cancer biology, and the VEGF/VEGFR
pathway, which is essential for angiogenesis (41,42).
Sorafenib has been applied clinically for the treatment of patients
with advanced hepatocellular carcinoma. Combining antiangiogenics
to overcome resistance of anticancer therapy poses a promising
strategy in the future of cancer medicine. In HNSCC, antiangiogenic
agents, including bevacizumab and sorafenib, have been integrated
into the treatment strategies but none of these agents have reached
approval in clinical practice, despite extensive clinical studies.
The proposed mechanisms of sorafenib resistance include EGFR
activation (43), multidrug
resistance protein 2 expression (44) and the activation of the PI3K/Akt
signaling pathway in hepatocellular carcinoma (45). Accurate predictors of patient
response to anticancer therapy would minimize the use of
ineffective and expensive treatment with possible toxic side
effects. Previous research efforts have focused on
‘forward-signaling’ mechanisms of pathway activation and have
implied that the loss of negative feedback control can also lead to
aberrant pathway activation. PTEN, a well-known tumor suppressor
gene, has been extensively studied in EGFR inhibition of colon
cancer therapy as well as trastuzumab treatment in breast cancer.
Sprouty2 protein has been shown to be involved in trastuzumab
resistance in breast cancer (46)
and gefitinib resistance in colon cancer cells (47). The ability of sprouty2 to suppress
Akt phosphorylation and modulate EGFR activation has encouraged
researchers to explore the role of sprouty2 in altering the
effectiveness of sorafenib in HNSCC. The present study indicated
that the overexpression of sprouty2 protein suppressed HNSCC
proliferation and enhanced sensitivity to sorafenib. Therefore,
sprouty2 provides a potential target to increase sorafenib
effectiveness in clinical practice. However, further investigations
and validation through clinical trials are required prior to
application of sprouty2 as a novel treatment for cancer patients in
the clinic.
In conclusion, the present study suggested that
sprouty2 was downregulated in a high proportion of OVH and HNSCC.
Sprouty2 had a negative feedback role in the suppression of Akt
phosphorylation and upregulation of PTEN, which had an inhibitory
effect on cell proliferation. Sprouty2 may serve as a potential
target in HNSCC treatment and a predictor of response to sorafenib
therapy.
Acknowledgements
This work was supported by grants from Chi-Mei
Medical Center (nos. CMNCKU10004, CMFHR10006, CLFHR9905 and
CLFHR0014) and Chung Hwa University of Medical Technology (no.
HWAI10003001). Thanks for the samples provided by Biobank of
Chi-Mei Medical Center, Liouying. The results have been reported at
the annual meeting of the American Society of Oncology, 2013.
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
3
|
Ko YC, Huang YL, Lee CH, Chen MJ, Lin LM
and Tsai CC: Betel quid chewing, cigarette smoking and alcohol
consumption related to oral cancer in Taiwan. J Oral Pathol Med.
24:450–453. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stransky N, Egloff AM, Tward AD, et al:
The mutational landscape of head and neck squamous cell carcinoma.
Science. 333:1157–1160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ang KK, Berkey BA, Tu X, et al: Impact of
epidermal growth factor receptor expression on survival and pattern
of relapse in patients with advanced head and neck carcinoma.
Cancer Res. 62:7350–7356. 2002.PubMed/NCBI
|
|
6
|
Rubin Grandis J, Melhem MF, Gooding WE, et
al: Levels of TGF-alpha and EGFR protein in head and neck squamous
cell carcinoma and patient survival. J Natl Cancer Inst.
90:824–832. 1998.
|
|
7
|
Kalyankrishna S and Grandis JR: Epidermal
growth factor receptor biology in head and neck cancer. J Clin
Oncol. 24:2666–2672. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sharafinski ME, Ferris RL, Ferrone S and
Grandis JR: Epidermal growth factor receptor targeted therapy of
squamous cell carcinoma of the head and neck. Head Neck.
32:1412–1421. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lalla RV, Boisoneau DS, Spiro JD and
Kreutzer DL: Expression of vascular endothelial growth factor
receptors on tumor cells in head and neck squamous cell carcinoma.
Arch Otolaryngol Head Neck Surg. 129:882–888. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Neuchrist C, Erovic BM, Handisurya A, et
al: Vascular endothelial growth factor C and vascular endothelial
growth factor receptor 3 expression in squamous cell carcinomas of
the head and neck. Head Neck. 25:464–474. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hacohen N, Kramer S, Sutherland D, Hiromi
Y and Krasnow MA: Sprouty encodes a novel antagonist of FGF
signaling that patterns apical branching of the Drosophila
airways. Cell. 92:253–263. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tefft JD, Lee M, Smith S, et al: Conserved
function of mSpry-2, a murine homolog of Drosophila sprouty,
which negatively modulates respiratory organogenesis. Curr Biol.
9:219–222. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Minowada G, Jarvis LA, Chi CL, et al:
Vertebrate Sprouty genes are induced by FGF signaling and can cause
chondrodysplasia when overexpressed. Development. 126:4465–4475.
1999.PubMed/NCBI
|
|
14
|
Impagnatiello MA, Weitzer S, Gannon G,
Compagni A, Cotten M and Christofori G: Mammalian sprouty-1 and -2
are membrane-anchored phosphoprotein inhibitors of growth factor
signaling in endothelial cells. J Cell Biol. 152:1087–1098. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gross I, Bassit B, Benezra M and Licht JD:
Mammalian sprouty proteins inhibit cell growth and differentiation
by preventing ras activation. J Biol Chem. 276:46460–46468. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sutterlüty H, Mayer CE, Setinek U, et al:
Down-regulation of Sprouty2 in non-small cell lung cancer
contributes to tumor malignancy via extracellular signal-regulated
kinase pathway-dependent and -independent mechanisms. Mol Cancer
Res. 5:509–520. 2007.PubMed/NCBI
|
|
17
|
Fong CW, Chua MS, McKie AB, et al: Sprouty
2, an inhibitor of mitogen-activated protein kinase signaling, is
down-regulated in hepatocellular carcinoma. Cancer Res.
66:2048–2058. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feng YH, Wu CL, Tsao CJ, et al:
Deregulated expression of sprouty2 and microRNA-21 in human colon
cancer: Correlation with the clinical stage of the disease. Cancer
Biol Ther. 11:111–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bancroft CC, Chen Z, Dong G, et al:
Coexpression of proangiogenic factors IL-8 and VEGF by human head
and neck squamous cell carcinoma involves coactivation by MEK-MAPK
and IKK-NF-kappaB signal pathways. Clin Cancer Res. 7:435–442.
2001.PubMed/NCBI
|
|
20
|
Mineta H, Miura K, Ogino T, et al:
Prognostic value of vascular endothelial growth factor (VEGF) in
head and neck squamous cell carcinomas. Br J Cancer. 83:775–781.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Williamson SK, Moon J, Huang CH,
Guaglianone PP, LeBlanc M, Wolf GT, et al: Phase II evaluation of
sorafenib in advanced and metastatic squamous cell carcinoma of the
head and neck: Southwest Oncology Group Study S0420. J Clin Oncol.
2010.28(20): 3330–5
|
|
22
|
Fritzsche S, Kenzelmann M, Hoffmann MJ, et
al: Concomitant down-regulation of SPRY1 and SPRY2 in prostate
carcinoma. Endocr Relat Cancer. 13:839–849. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song K, Gao Q, Zhou J, et al: Prognostic
significance and clinical relevance of Sprouty 2 protein expression
in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int.
11:177–184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Edwin F, Singh R, Endersby R, Baker SJ and
Patel TB: The tumor suppressor PTEN is necessary for human Sprouty
2-mediated inhibition of cell proliferation. J Biol Chem.
281:4816–4822. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Edwin F, Anderson K, Ying C and Patel TB:
Intermolecular interactions of Sprouty proteins and their
implications in development and disease. Mol Pharmacol. 76:679–691.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lo TL, Yusoff P, Fong CW, et al: The
ras/mitogen-activated protein kinase pathway inhibitor and likely
tumor suppressor proteins, sprouty 1 and sprouty 2 are deregulated
in breast cancer. Cancer Res. 64:6127–6136. 2004. View Article : Google Scholar
|
|
27
|
Velasco A, Pallares J, Santacana M, et al:
Promoter hypermethylation and expression of sprouty 2 in
endometrial carcinoma. Hum Pathol. 42:185–193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kwak HJ, Kim YJ, Chun KR, et al:
Downregulation of Spry2 by miR-21 triggers malignancy in human
gliomas. Oncogene. 30:2433–2442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee SA, Ho C, Roy R, et al: Integration of
genomic analysis and in vivo transfection to identify sprouty 2 as
a candidate tumor suppressor in liver cancer. Hepatology.
47:1200–1210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee SA, Ladu S, Evert M, et al:
Synergistic role of Sprouty2 inactivation and c-Met up-regulation
in mouse and human hepatocarcinogenesis. Hepatology. 52:506–517.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Frank MJ, Dawson DW, Bensinger SJ, et al:
Expression of sprouty2 inhibits B-cell proliferation and is
epigenetically silenced in mouse and human B-cell lymphomas. Blood.
113:2478–2487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ishida M, Ichihara M, Mii S, et al:
Sprouty2 regulates growth and differentiation of human
neuroblastoma cells through RET tyrosine kinase. Cancer Sci.
98:815–821. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma Y, Yu S, Zhao W, Lu Z and Chen J:
miR-27a regulates the growth, colony formation and migration of
pancreatic cancer cells by targeting Sprouty2. Cancer Lett.
298:150–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McKie AB, Douglas DA, Olijslagers S, et
al: Epigenetic inactivation of the human sprouty2 (hSPRY2)
homologue in prostate cancer. Oncogene. 24:2166–2174. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Holgren C, Dougherty U, Edwin F, et al:
Sprouty-2 controls c-Met expression and metastatic potential of
colon cancer cells: sprouty/c-Met upregulation in human colonic
adenocarcinomas. Oncogene. 29:5241–5253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Barbáchano A, Ordóñez-Morán P, García JM,
et al: SPROUTY-2 and E-cadherin regulate reciprocally and dictate
colon cancer cell tumourigenicity. Oncogene. 29:4800–4813.
2010.PubMed/NCBI
|
|
37
|
Bloethner S, Chen B, Hemminki K, et al:
Effect of common B-RAF and N-RAS mutations on global gene
expression in melanoma cell lines. Carcinogenesis. 26:1224–1232.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sasaki A, Taketomi T, Kato R, et al:
Mammalian Sprouty4 suppresses Ras-independent ERK activation by
binding to Raf1. Nat Cell Biol. 5:427–432. 2003. View Article : Google Scholar
|
|
39
|
Mason JM, Morrison DJ, Basson MA and Licht
JD: Sprouty proteins: multifaceted negative-feedback regulators of
receptor tyrosine kinase signaling. Trends Cell Biol. 16:45–54.
2006. View Article : Google Scholar
|
|
40
|
Wong ES, Fong CW, Lim J, et al: Sprouty2
attenuates epidermal growth factor receptor ubiquitylation and
endocytosis, and consequently enhances Ras/ERK signalling. EMBO J.
21:4796–4808. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wilhelm SM, Carter C, Tang L, et al: BAY
43–9006 exhibits broad spectrum oral antitumor activity and targets
the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in
tumor progression and angiogenesis. Cancer Res. 64:7099–7109.
2004.
|
|
42
|
Chang YS, Adnane J, Trail PA, et al:
Sorafenib (BAY 43–9006) inhibits tumor growth and vascularization
and induces tumor apoptosis and hypoxia in RCC xenograft models.
Cancer Chemother Pharmacol. 59:561–574. 2007.
|
|
43
|
Ezzoukhry Z, Louandre C, Trécherel E, et
al: EGFR activation is a potential determinant of primary
resistance of hepatocellular carcinoma cells to sorafenib. Int J
Cancer. 131:2961–2969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shibayama Y, Nakano K, Maeda H, et al:
Multidrug resistance protein 2 implicates anticancer
drug-resistance to sorafenib. Biol Pharm Bull. 34:433–435. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen KF, Chen HL, Tai WT, et al:
Activation of phosphatidylinositol 3-kinase/Akt signaling pathway
mediates acquired resistance to sorafenib in hepatocellular
carcinoma cells. J Pharmacol Exp Ther. 337:155–161. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Faratian D, Sims AH, Mullen P, et al:
Sprouty 2 is an independent prognostic factor in breast cancer and
may be useful in stratifying patients for trastuzumab therapy. PLoS
One. 6:e237722011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Feng YH, Tsao CJ, Wu CL, et al: Sprouty2
protein enhances the response to gefitinib through epidermal growth
factor receptor in colon cancer cells. Cancer Sci. 101:2033–2038.
2010. View Article : Google Scholar : PubMed/NCBI
|