Introduction
Ovarian cancer is the predominant gynecological
malignant tumor that poses a serious and under-recognized threat to
the health of females. Ovarian cancer is insidious, and advances
gradually, therefore resulting in a low five-year survival rate
(1,2). Ovarian cancer has a higher mortality
rate in females than all other gynecological cancers combined;
therefore, development of novel treatments is required.
Cyclooxygenase-2 (COX-2) is an inducible enzyme that is either
lowly expressed or absent in normal cells. Under certain
conditions, including inflammation, tumors and other pathological
conditions, COX-2 is highly expressed, which is associated with
upregulation of prostaglandins. Recent studies have identified that
COX-2 is closely associated with the development of various types
of cancer (3–7). Currently available evidence indicates
that COX-2 expression is significantly higher in ovarian cancer
tissues compared with benign tumors (8–11).
High expression of COX-2 in ovarian cancer tissues is associated
with poorer prognosis, shorter survival time and decreased
sensitivity to treatment. This suggests that COX-2 may act as an
oncogene involved in the development of ovarian cancer. However,
the precise role of COX-2 in the development and progression of
ovarian cancer, as well as the molecular mechanisms involved in
these processes, remain to be elucidated. The present study
employed small hairpin (sh)RNA interference technology to observe
the effects of COX-2 gene silencing in vitro and in
vivo on the biological behavior of SKOV3 human ovarian cancer
cells, to explore the role of COX-2 gene in ovarian cancer
development. The present study provided a theoretical basis for
COX-2-targeted therapy of ovarian cancer.
Materials and methods
Cell lines and experimental animals
The SKOV3 human ovarian cancer cell line and E.
coli DH5 alpha strain were purchased from the Shanghai
Biological Cell Bank, Chinese Academy of Sciences (Shanghai,
China). Female SPF BALB/C nude mice (n=18) (4–5 weeks of age; 17–20
g body weight) were purchased from the Shanghai Slack Laboratory
Animal Center (Shanghai, China). The mice were housed in a
temperature-controlled and closed aseptic environment (at a
constant temperature of 18–22°C and humidity of 50–80%) under a
12-h light/dark cycle, and provided free access to sterile water
and food. The experiments were carried out according to the
guidelines and practices established by the ethics committee of The
Second Hospital, Jilin University (Changchun, China).
Plasmids
Plasmid pGPU6/GFP/Neo was purchased from Shanghai
GenePharma Co., Ltd. (Shanghai, China).
Primer sequences
The following primers were used: COX-2 upstream,
5′-TCAAGTCCCTGAGCATCTAC-3′ and downstream,
5′-CATTCCTACCACCAGCAACC-3′; GAPDH upstream,
5′-GCACCGTCAAGGCTGAGAAC-3′ and downstream,
5′-TGGTGAAGACGCCAGTGGA-3′. The primers were synthesized by Dalian
Takara Bio Company Limited (Shiga, Japan).
Selection of COX-2 RNA target sequence,
shRNA design and synthesis
Human COX-2 siRNA target sequence was designed
according to literature searches (12) as follows:
5′-GGACTTATGGGTAATGTTA-3′. Considering the BbsI and
BamHI restriction sites in the plasmid, ‘CACC’ was added to
the 5′ cohesive end on the sense strand and ‘GATC’ was added to the
5′ cohesive end on the antisense strand. ‘TTTTTT’ was added as the
transcription termination sequence. A ‘G’ nucleotide was inserted
into the plasmid in order to preserve the BamHI restriction
site. The LOOP structure was designed as TTCAAGAGA. The final COX-2
shRNA sequence was designed as follows: sense,
5′-CACCGGACTTATGGGTAATGTTATTCAAGAGATAACATTACCCATAAGTCCTTTTTTG-3′
and antisense,
5′-GATCCAAAAAAGGACTTATGGGTAATGTTATCTCTTGAATAACATTACCCATAAGTCC-3′.
COX-2 shRNA was synthesized by Shanghai Gemma Bio-tech, Inc.
Reagents
A quantitative polymerase chain reaction (qPCR) kit
was purchased from Takara. A Total RNA Extraction TRIzol™ Reagent
kit was from Fermentas (Pittsburgh, PA, USA); Lipofectamine 2000
was purchased from Invitrogen (Invitrogen Life Technologies,
Carlsbad, CA, USA); G418 was from Shanghai PuFei Co.; MTT Kit was
purchased from Sigma (Sigma, St Louis, MO, USA); Propidium iodide
was purchased from Nanjing Keygen Biotechnology Company; Cell
invasion Chamber was obtained from Corning (Corning Inc., Corning,
NY, USA).
Cell culture and transfection
SKOV-3 cells were maintained as monolayer cultures
in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal
bovine serum and 1% antibiotics in a humidified chamber with 5%
CO2 at 37°C. COX-2 shRNA double-stranded template was
inserted into the pGPU6/GFP/Neo plasmid using an annealing and
digestion technique, and the recombinant plasmid pGPU6-COX-2-shRNA
was constructed. The plasmid was transformed into Escherichia
coli competent cells and the positive recombinant colonies were
selected and amplified. The extracted recombinant plasmids were
digested and subjected to DNA sequencing. The recombinant plasmids
were transiently transfected into SKOV3 cells using the
Lipofectamine™ 2000 Transfection kit, according to manufacturer’s
instructions. Following selection with medium containing G418 for
neomycin selection, the resistant clones that stably expressed
human COX-2 shRNA were obtained. The resistant clones were
gradually amplified followed by routine culture and sub-culture.
Following transfection, the fluorescence expression levels were
observed under an inverted fluorescence microscope. The
experimental groups of plasmid transfection were established as
follows: i) Control group (CON), normal SKOV3 cells without plasmid
transfection; ii) negative control group (NC), SKOV3 cells
transfected with the recombinant negative control plasmid; iii)
interference group (KD), SKOV3 cells transfected with
pGPU6-COX-2-shRNA recombinant plasmid.
Analysis of COX-2 mRNA expression by
qPCR
Following stable transfection, total RNA was
isolated with TRIzol® reagent according to the
manufacturer’s instructions. The qPCR cycling conditions were 95°C
for 5 min; 94°C for 30 sec, 55°C for 30 sec, 72°C for 30 sec, for
30 cycles; 72°C for 10 min; 4°C for 5 min. COX-2 mRNA content was
analyzed using a gel image analysis system (BioCapMW software
11.01; Microsoft, Redmond, USA).
Western blot analysis
COX-2 protein expression was analyzed by western
blotting. Briefly, total cellular protein (30–50 μg) was subjected
to 7.5% SDS-PAGE and electrotransferred onto a polyvinylidene
fluoride membrane (Bio-Rad, Hercules, CA, USA). Following blocking
with 5% nonfat dry milk in 10 mM Tris, pH 7.5, containing 0.15 M
NaCl and 0.05% Triton X-100, the membranes were probed with a mouse
monoclonal anti-COX-2 antibody (1:500 in 5% milk; Cayman Chemical,
USA). The membranes were then washed and incubated with
horseradish-peroxidase conjugated secondary antibody (goat
anti-mouse; 1:2000 in 5% milk; Bio-Rad). Protein bands were
visualized using a chemiluminescent detection system. The relative
expression of COX-2 protein was analyzed using Quantity One v.4.62
software (Bio-Rad).
MTT assay
The proliferation status of SKOV3 cells was
determined by MTT assay. SKOV3 cells were seeded at a density of
1×104 cells per well in a flat-bottomed 96-well
microplate. The cells were incubated in 5% CO2 at 37°C
for 12 h. After 1, 2, 3, or 4 days incubation, 10 μl MTT was added
to each well and further incubated for 4 h. The supernatant was
then removed and 100 μl dimethyl sulfoxide was added. It was
agitated for 10 min until the crystals dissolved. The optical
density (OD) at 490 nm was measured using an ELISA reader
(Molecular Devices Corp, Sunnyvale, CA, USA). The negative control
well contained no cells, and its OD was subtracted from that of the
other samples. Each well was read three times in triplicate.
Colony forming assay
SKOV cells were seeded in triplicate at 1,000
cells/well in six-well plates in complete medium. After 2–3 weeks
of growth in 5% CO2 at 37°C, the cells were fixed with 5
ml paraformaldehyde for 15 min and stained with the appropriate
amount of Giemsa staining solution for 15 min, and the grossly
visible colonies were counted. All experiments were repeated at
least three times. Groups that consisted of >50 cells were
counted as a clone. The plating efficiency was determined as the
number of colonies formed divided by the total number of cells
plated.
Cell cycle analysis by flow
cytometry
The total number of cells collected was
>104. Propidium iodide (PI) was added to the cells,
which were analyzed by flow cytometry (EPICS XL; Beckman Coulter,
Miami, FL, USA). The proliferation index was calculated using the
following formula: Proliferation index = (S + G2/M) / (G0/G1 + S +
G2/M).
Transwell cell culture chamber assay
An invasion assay was employed to assess the ability
of SKOV3 cells to invade a synthetic basement membrane. Transwell
chambers (Corning, Inc.) with a polycarbonate filter (6.5-mm pore
size), separating the upper and lower chambers, were used. SKOV3
cells in the exponential growth phase were digested using trypsin,
and a single cell suspension was prepared. The cell density was
adjusted to 2.5×106/ml. The assay was performed in
accordance with the Transwell Chamber instructions of Corning
Company. The non-invading cells on the top surface of the filter
membrane were removed using a cotton swab. Cells on the bottom
surface of the filter were counted, and the mean number of cells
was determined from five high-power fields under a light microscope
(Olympus FP50; Olympus Tokyo, Japan). Each experiment was performed
three times in triplicate.
Xenograft model
Eighteen nude mice were randomly divided into three
groups (six mice/group), which were marked as CON, NC and KD,
respectively. The three groups of nude mice were inoculated with
SKOV3 cells, SKOV3 cells transfected with negative control
recombinant plasmid and SKOV3 cells transfected with
pGPU6-COX-2-shRNA recombinant plasmid, respectively. Each group of
nude mice was transferred to a clean bench and underwent strong
iodine disinfection. The cell suspension (0.2 ml, ~5×107
cells) was subcutaneously injected into the left armpit of BALB/C
nude mice using a 1-ml syringe. Following inoculation, the nude
mice were maintained in a pathogen-free sterile environment with
appropriate temperature and free access to sterile water and food.
Following inoculation with different SKOV3 cells as indicated, the
subcutaneous tumor growth in nude mice was observed daily. The
tumor inoculation time and the survival conditions of the nude mice
bearing a tumor were recorded. Tumors exceeding 3 mm in diameter
were recorded as positive (tumorigenic standard). Following tumor
development, the long and short radius of the implanted tumors was
measured every seven days. In addition, the volume of the tumor was
calculated (volume = 1/2 long radius × short radius2).
Following 28 days of tumor development, the nude mice were
sacrificed by cervical vertebrae dislocation. The tumors were
separated, the tumor volume and weight were measured, and images of
the tumors were captured (Olympus E-M10; Olympus, Tokyo, Japan).
The tumor inhibition rate was calculated using the following
formula: Tumor inhibition rate = (tumor weight of control group -
tumor weight of experimental group)/tumor weight of control group
×100%.
Statistical analysis
All statistical analyses were performed using SPSS
13.0 software (SPSS, Chicago, IL, USA). Values are expressed as the
mean ± standard deviation. Statistical comparisons were performed
using an independent Student’s t-test and one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference between values.
Results
Identification of PGPU6-COX-2-shRNA
recombinant plasmid by DNA sequencing
The positive recombinant plasmids were identified by
Shanghai Yingjun Biotechnology Co., Ltd. (Shanghai, China). The
obtained sequence was compared to the previously synthesized
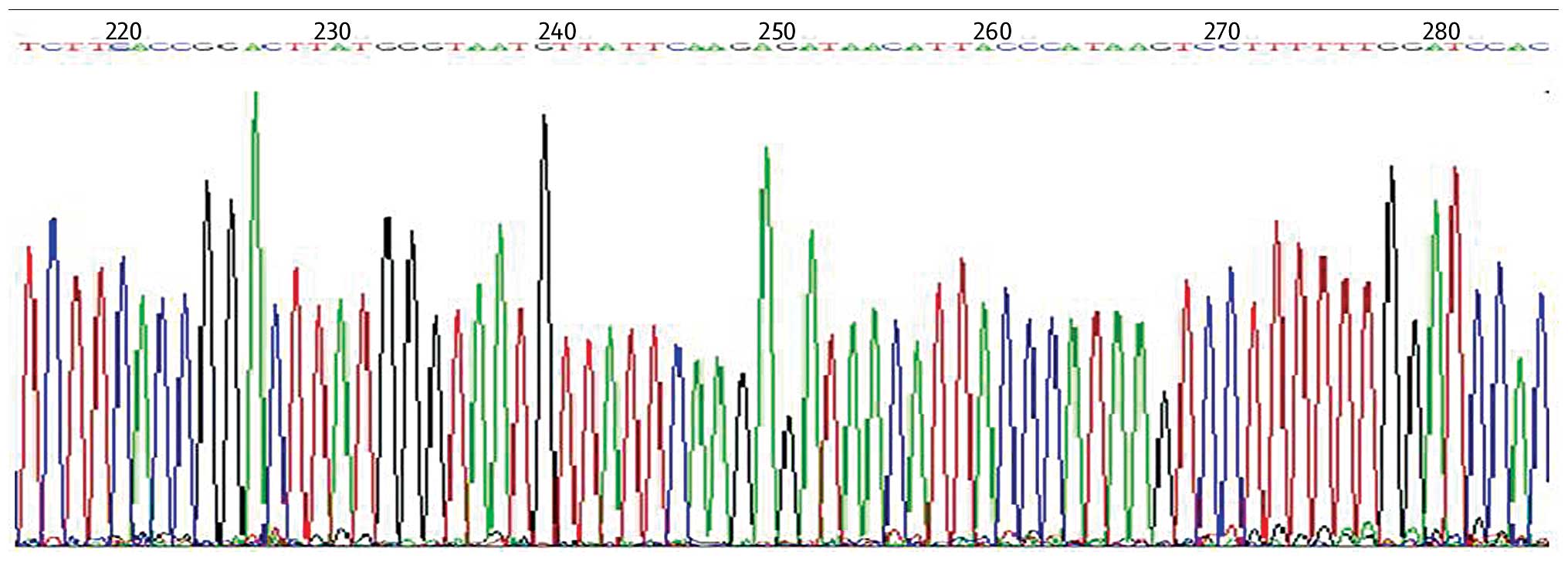
sequence. As indicated in Fig. 1,
the sequencing results confirmed that the pGPU6-COX-2-shRNA
recombinant plasmid matched the expected DNA sequences and the
COX-2 shRNA sequence was inserted correctly, suggesting a
successful construction of recombinant plasmid.
Stable transfection of SKOV3 cells with
recombinant plasmid
Following antibiotic selection with G418, SKOV3
cells that stably expressed the recombinant plasmid were obtained.
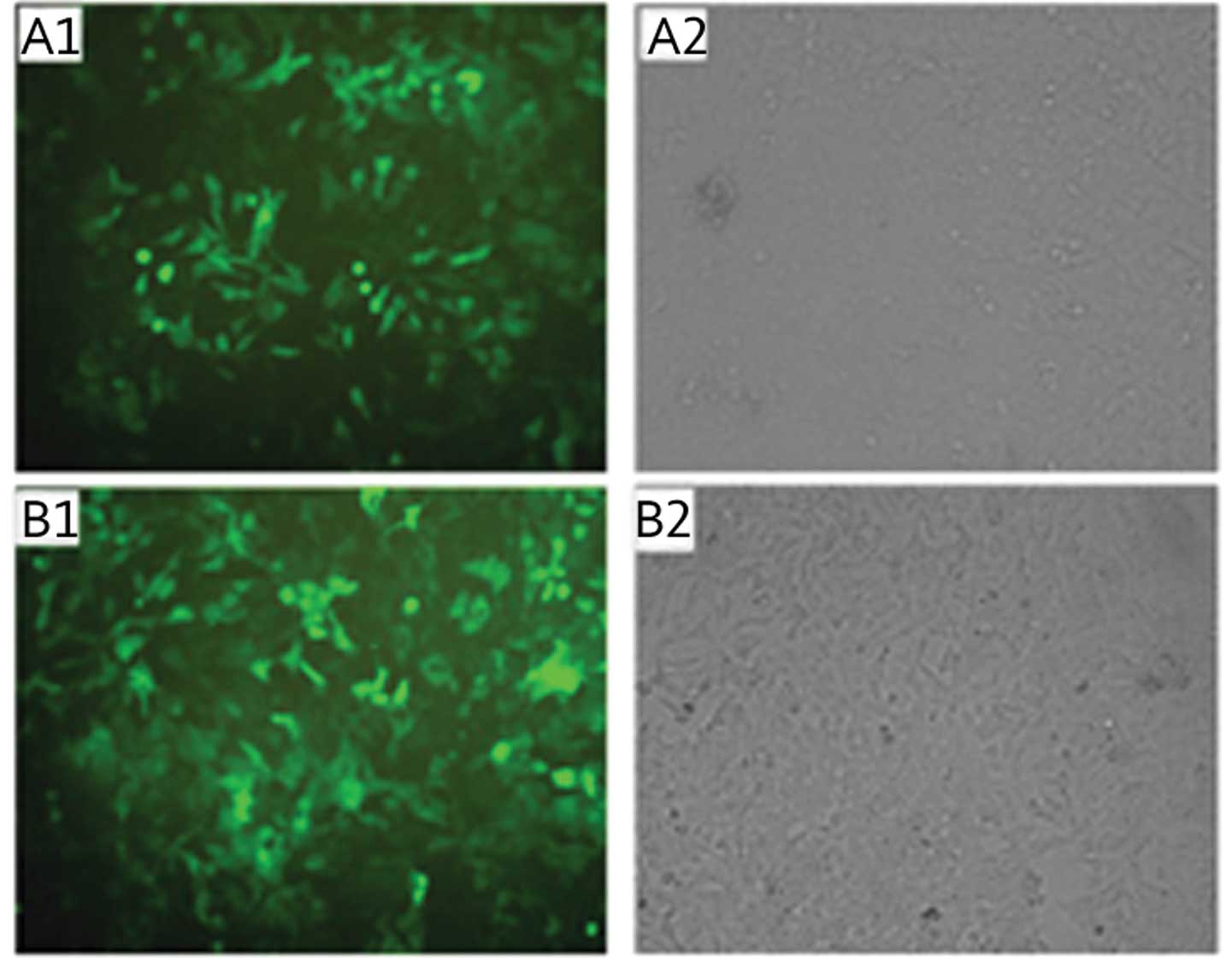
Fluorescence microscopy identified that the majority of SKOV3 cells
expressed green fluorescent protein for different lengths of time
(10, 20, 30 and 40 d). Comparison with the transmitted light images
showed that the transfection efficiency of the different groups was
>90%, which was suitable for the subsequent experiments
(Fig. 2).
Confirmation of COX-2 silencing
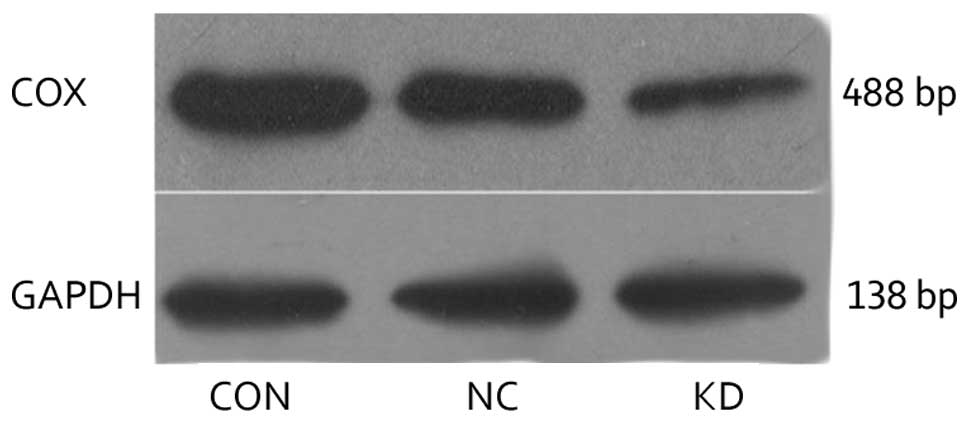
SKOV3 cells were transfected with pGPU6-COX-2-shRNA
and recombinant negative control plasmid, respectively. Total RNA
was extracted and COX-2 mRNA was detected by qPCR. As shown in
Fig. 3, as compared with the CON
and NC cells, COX-2 mRNA expression levels in the KD cell group
were significantly decreased (P<0.05). There was no
statistically significant difference in the mRNA expression levels
between the CON and NC groups (P>0.05). Furthermore, the total
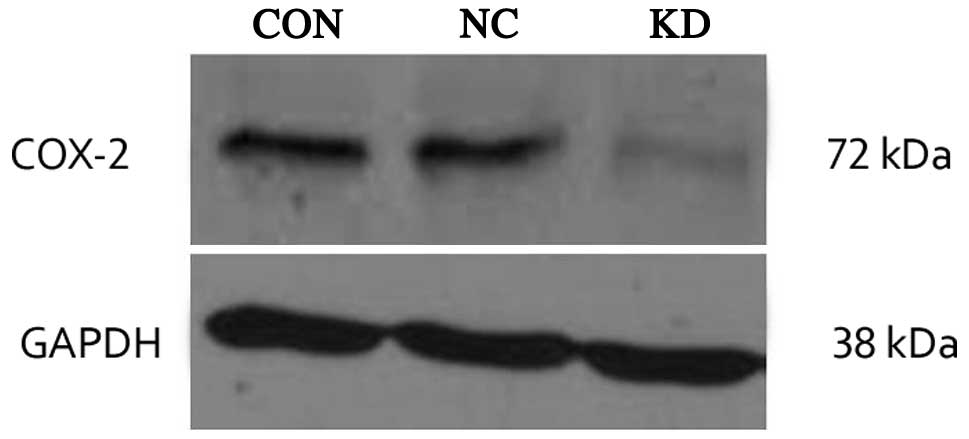
protein was extracted from the SKOV3 cells and levels of COX-2 were
detected by western blot analysis. As shown in Fig. 4, as compared with the CON and NC
cells, the COX-2 protein expression levels in the KD cell group
were significantly decreased (P<0.05). There was no
statistically significant difference in protein expression levels
between CON and NC groups (P>0.05).
COX-2 gene silencing reduces SKOV3
cellular proliferation
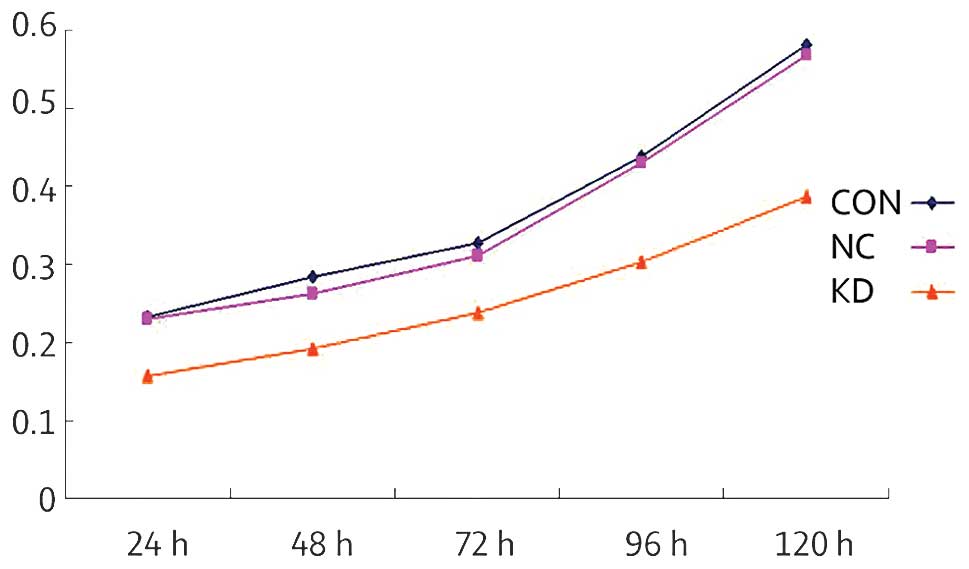
The MTT method was used to assess the cellular
proliferation ability following COX-2 gene silencing. As indicated
in Fig. 5, as compared with the
CON and NC groups, the number of live cells in the KD group was
significantly decreased, suggesting that following the inhibition
of COX-2 expression, SKOV3 cellular proliferation ability and
growth were significantly reduced. The cell proliferation ability
in the KD group following 24 h showed significant differences as
compared with the NC and CON groups (P<0.05). There was no
statistically significant difference in the cell proliferation
ability between the CON and NC groups (P>0.05).
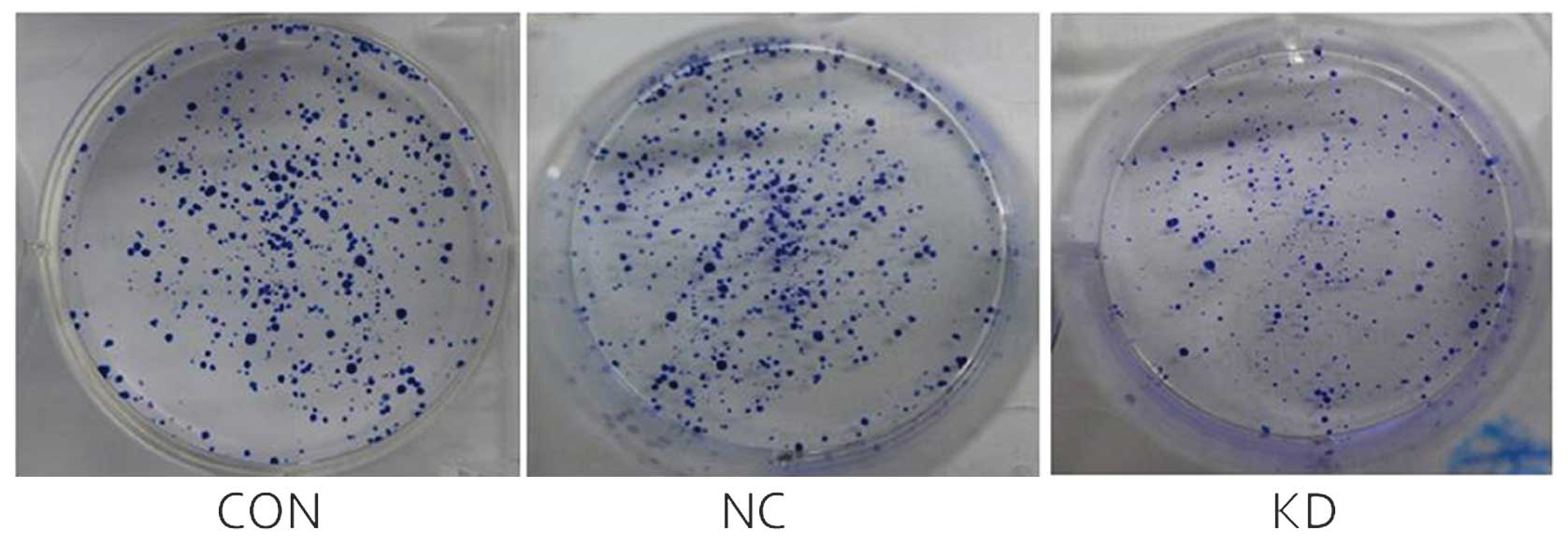
COX-2 gene silencing reduces colony
formation ability of SKOV3 cells
As indicated in Fig.
6, the colony size in the KD group was significantly reduced as
compared with the CON and NC groups. The colony-forming number in
the KD group was 131±14, whereas that of the CON and NC groups was
240±20 and 210±30, respectively. The number of colonies in the KD
group was significantly reduced as compared with that in the CON
and NC groups (P<0.05). There was no statistically significant
difference between the CON and NC groups (P>0.05).
COX-2 gene silencing causes G1 phase
arrest and reduces the proliferative index of SKOV3 cells
The effect of COX-2 gene silencing on the SKOV3 cell
cycle was detected PI staining and flow cytometric quantification.
As indicated in Table I, compared
with the CON and NC groups, the number of cells in G1 phase in the
KD group was considerably increased and the cell number in S and G2
phase was significantly reduced (P<0.05). There was no
statistically significant difference in the cell cycle distribution
between the CON and NC groups (P>0.05). The proliferation index
in the KD group was significantly lower than that in the CON and NC
groups. The apoptotic peak was not identified in all three
groups.
 | Table ICell cycle analysis across the
experimental groups. |
Table I
Cell cycle analysis across the
experimental groups.
| Cell cycle |
|---|
|
|
|---|
| Groups | G0/G1 (%) | S (%) | G2/M (%) | Proliferation index
(%) |
|---|
| CON | 36.34±5.22 | 30.50±5.12 | 33.16±2.54 | 63.66±4.21 |
| NC | 37.50±4.54b | 27.68±4.53b | 34.82±5.21b | 62.50±4.63b |
| KD | 62.45±5.23a | 23.53±2.21a | 14.02±1.93a | 37.55±3.62a |
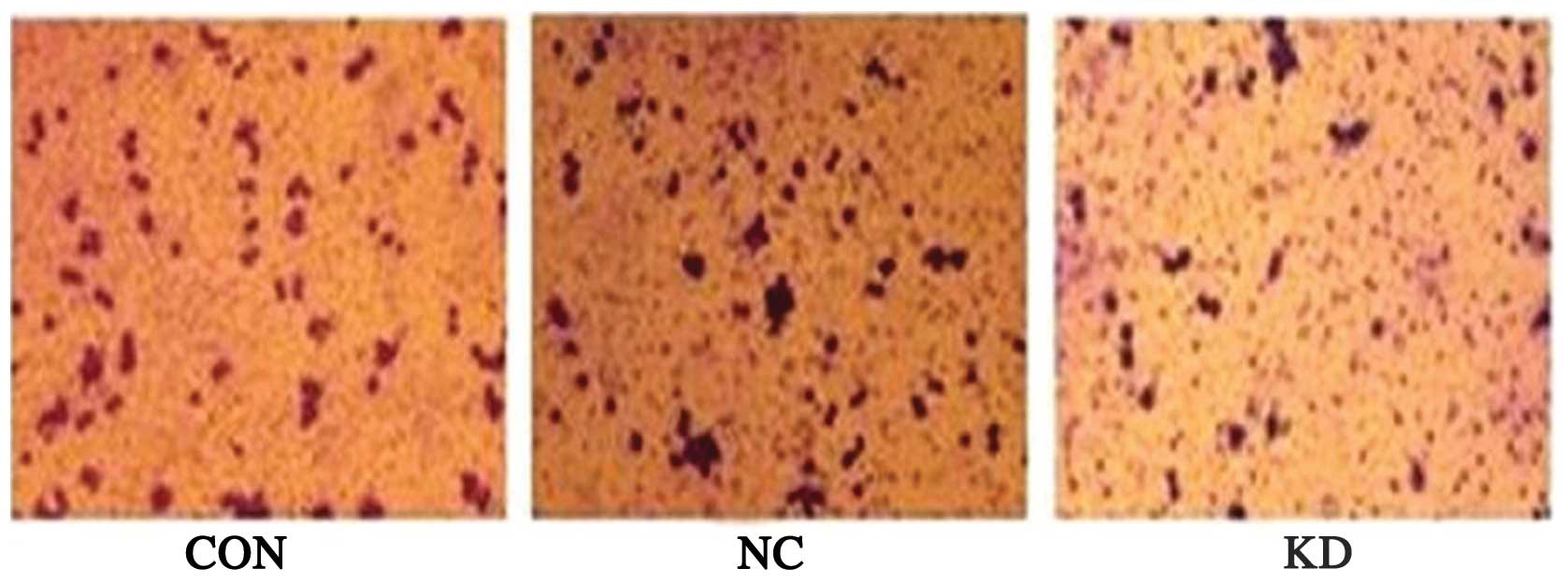
COX-2 gene silencing attenuates SKOV3
cell invasiveness
In the Transwell assay, the number of cells
penetrating the membrane in the CON, NC and KD groups was 85±9,
79±7 and 38±5, respectively. The number of cells penetrating the
membrane in the KD group was significantly reduced as compared with
that in the NC and CON groups (P<0.05). There was no
statistically significant difference between the CON and NC groups
(P>0.05) (Fig. 7). These
results suggested that inhibition of COX-2 expression significantly
attenuated SKOV3 cell invasiveness.
Establishment of a SKOV3 xenograft
model
In the present study, the success rate of
subcutaneous implantation tumors in 18 nude mice was 100%. During
the experiment, the growth conditions of the tumor-bearing nude
mice and their diets were normal. There were no mortalities or
abnormal behavior. The tumors appeared ~7 days following
inoculation of the cells in the CON and NC groups, and 10 days
following inoculation in the KD group.
Effects of COX-2 gene silencing on the
growth of SKOV3 xenograft tumors in nude mice
From day 7 of inoculation, the long and short radius
of the xenograft tumors in tumor-bearing nude mice was measured
every seven days, and the volume of the tumor was calculated. As
indicated in Table II, the tumor
volume in the KD group was significantly lower as compared with
that in the CON and NC groups (P<0.05). There was no
statistically significant difference between the CON and NC groups.
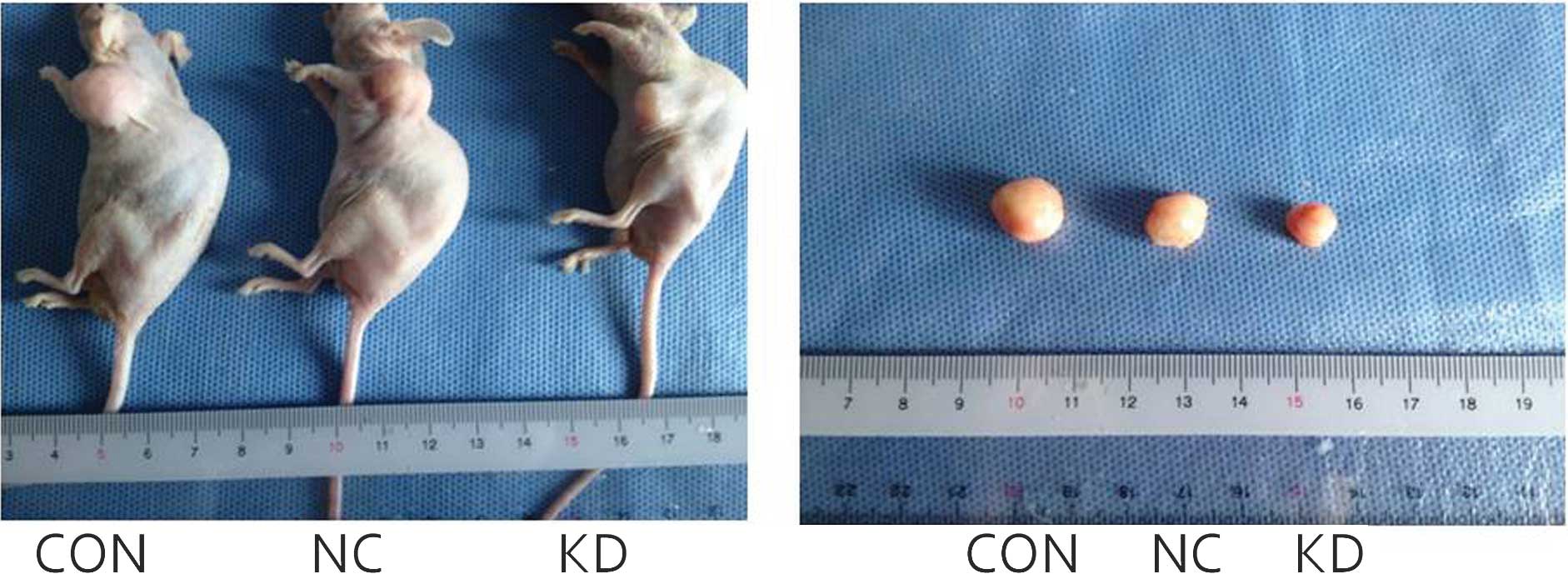
Twenty-eight days following tumor development, the nude mice were
sacrificed by cervical dislocation and the tumors were extracted.
The pink tumors presented round or oval-shaped and had a complete
capsule, medium texture and a clear boundary (Fig. 8). The average tumor weight in the
KD group was significantly lower as compared with the CON and NC
groups (P<0.05). When compared with the blank control group, the
tumor inhibition rate was 41.45%. As compared with the negative
control group, the tumor inhibition rate was 43.62%. There was no
statistically significant difference between the CON and NC
groups.
 | Table IIEffect of cyclooxygenase-2 gene
silencing on the volume of xenograft tumors. |
Table II
Effect of cyclooxygenase-2 gene
silencing on the volume of xenograft tumors.
| Days |
|---|
|
|
|---|
| Group | 7 | 14 | 21 | 28 | 35 |
|---|
| KD | 25.2±3.1a | 37.3±8.1a | 74.4±10.6a | 120.9±14.3a | 204.8±20.8a |
| NC | 30.2±4.5b | 62.7±10.2b | 128.2±11.5b | 260.4±21.3b | 386.5±49.7b |
| CON | 31.8±5.2 | 64.5±13.5 | 121.3±21.2 | 271.8±40.2 | 390.5±45.1 |
Discussion
In the present study, a COX-2 shRNA sequence was
designed and synthesized according to the COX-2 gene sequence, and
a pGPU6-COX-2-shRNA recombinant plasmid vector was constructed. The
recombinant plasmid pGPU6-COX-2-shRNA was transiently transfected
into SKOV3 cells. In addition, it was shown through in vitro
and in vivo experiments that COX-2 expression may have an
important role in the proliferation, growth, invasion and
metastasis of ovarian cancer cells.
The results of the MTT and colony formation assays
demonstrated that COX-2 gene silencing significantly inhibited the
proliferation and viability of ovarian cancer cells, suggesting
that COX-2 promoted the proliferation and viability of ovarian
cancer cells.
Cells that are growing and dividing go through a
repeated series of events leading to division and duplication
(replication), known as the cell cycle or cell-division cycle. In
this process, the genetic material of the cells is copied and
equally distributed to both daughter cells. As the cellular DNA
content in each period of the cell cycle is different, using the
DNA-binding dye, propidium iodide (PI), the cell cycle and cell
proliferation was detected using a flow cytometer. The results of
the present study demonstrated that following COX-2 gene silencing
in SKOV3 cells, the cell cycle was blocked in G1 phase and the
number of cells in the DNA synthesis phase was significantly
reduced, resulting in reduced DNA synthesis as well as cell
proliferation inhibition. This data implies that COX-2 promoted
tumor cell proliferation through modulation of the cell cycle. The
mechanism by which COX-2 regulates cell cycle progression still
requires further in-depth studies.
Among the invasive and metastatic processes of
malignant tumors, the critical step is to degrade the extracellular
matrix (ECM) and basement membrane and break the natural barrier,
thus allowing for tumor cell spread (13). The Transwell invasion assay is a
commonly used test for the effective analysis of the cell invasion
ability. The results of the present study suggested that COX-2 gene
silencing decreased the number of cells penetrating the membrane
and therefore inhibited the invasion ability of SKOV3 cells,
implying that COX-2 promoted the invasion ability of ovarian cancer
cells and was conducive to the spread and metastasis of ovarian
cancer. The possible underlying mechanism involves matrix
metalloproteinases (MMPs), vascular endothelial growth factor
(VEGF) and other cytokines (14).
MMPs are Ca2+- and Zn2+-dependent
endopeptidases that function in the degradation of various ECM
components (13). MMPs are enzymes
implicated in normal and pathological tissue remodeling processes,
wound healing, angiogenesis and tumor invasion (13–15).
Using immunohistochemical technology, previous research has
detected COX-2, MMP-9 and VEGF expression in epithelial ovarian
cancer, borderline ovarian tumors and normal ovarian tissue
(16). It was shown that COX-2
protein expression was positively correlated with expression of
MMP-9 and VEGF in epithelial ovarian cancer. It is speculated that
COX-2 affects tumor invasiveness through MMP-9 and VEGF. Symowicz
et al (17) suggested that
COX-2 inhibitors may reduce the expression and activity of MMP-2
precursors in ovarian cancer cells. Furthermore, Leung et al
(18) and Pan et al
(19) confirmed that COX-2
inhibitors reduced the expression of MMP-2 in colorectal cancer and
lung cancer cells.
The present study showed that COX-2 gene silencing
decreased tumor development in nude mice and that the tumor volume
and weight were significantly lower than those of the blank and
negative control groups, indicating that the inhibition of COX-2
gene expression suppressed the growth of ovarian cancer. Therefore,
COX-2 may be used as an effective target of gene therapy for
ovarian cancer. Lowering the target gene expression in vivo
is likely to inhibit the growth of ovarian cancer cells, achieving
successful ovarian cancer treatment.
RNAi technology has been successfully used to
silence the COX-2 protein in different in vitro and in
vivo models. Previous investigation demonstrated that the
silencing of COX-2 mediated by LV-COX-2-siRNA significantly
inhibited growth and induced cell cycle arrest in non-squamous cell
lung carcinoma cell lines. In addition, the decreased expression of
COX-2 modulated the expression of cell cycle-regulatory genes,
up-regulated p21 and down-regulated cyclin D1 (22). The gene knock down of COX-2 in
human osteosarcoma cells significantly inhibits the growth and
decreases the migration ability of SaOS2 cells.
Furthermore, it also reduces VEGF, EGF and basic fibroblast growth
factor mRNA and protein expression (23). COX-2 siRNA treatment also inhibits
cell proliferation and induces apoptosis in esophageal squamous
carcinoma EC109 cells (24).
In conclusion, the present study effectively
silenced COX-2 gene expression in SKOV3 cells by using plasmid
vector-mediated RNA interference technology. In addition, in
vitro and in vivo studies demonstrated that COX-2 gene
silencing had important implications in proliferation, cell cycle,
colony formation and invasion of ovarian cancer cells. These
findings provided a theoretical basis on understanding the role of
COX-2 in the development of ovarian cancer and additionally show
that COX-2 may be an effective target for gene therapy, which has
prospects in clinical applications.
Acknowledgements
This study was supported by the Young Scholars
Program of Norman Bethune Health Science Center of Jilin
University, Changchun, China (no. 2013206037).
References
|
1
|
Deraco M, Baratti D, Laterza B, et al:
Advanced cytoreduction as surgical standard of care and
hyperthermic intraperitoneal chemotherapy as promising treatment in
epithelial ovarian cancer. Eur J Surg Oncol. 37:4–9. 2011.
View Article : Google Scholar
|
|
2
|
Gómez-Raposo C, Mendiola M, Barriuso J,
Hardisson D and Redondo A: Molecular characterization of ovarian
cancer by gene-expression profiling. Gynecol Oncol. 118:88–92.
2010.PubMed/NCBI
|
|
3
|
Ji B, Liu Y, Zhang P, et al: COX-2
expression and tumor angiogenesis in thyroid carcinoma patients
among northeast Chinese population-result of a single-center study.
Int J Med Sci. 9:237–242. 2012. View Article : Google Scholar
|
|
4
|
Holmes MD, Chen WY, Schnitt SJ, et al:
COX-2 expression predicts worse breast cancer prognosis and does
not modify the association with aspirin. Breast Cancer Res Treat.
130:657–662. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yao L, Liu F, Hong L, et al: The function
and mechanism of COX-2 in angiogenesis of gastric cancer cells. J
Exp Clin Cancer Res. 30:132011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kwagyan J, Apprey V and Ashktorab H:
Linkage disequilibrium and haplotype analysis of COX-2 and risk of
colorectal adenoma development. Clin Transl Sci. 5:60–64. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen YF, Luo RZ, Li Y, et al: High
expression levels of COX-2 and P300 are associated with unfavorable
survival in laryngeal squamous cell carcinoma. Eur Arch
Otorhinolaryngol. 270:1009–1017. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thill M, Fischer D, Kelling K, et al:
Expression of vitamin D receptor (VDR), cyclooxygenase-2 (COX-2)
and 15-hydroxyprostaglandin dehydrogenase (15-PGDH) in benign and
malignant ovarian tissue and 25-hydroxycholecalciferol (25(OH2)D3)
and prostaglandin E2 (PGE2) serum level in ovarian cancer patients.
J Steroid Biochem Mol Biol. 121:387–390. 2010.
|
|
9
|
Raspollini MR, Amunni G, Villanucci A, et
al: COX-2 and preoperative CA-125 level are strongly correlated
with survival and clinical responsiveness to chemotherapy in
ovarian cancer. Acta Obstet Gynecol Scand. 85:493–498. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JY, Myung SK and Song YS: Prognostic
role of cyclooxygenase-2 in epithelial ovarian cancer: a
meta-analysis of observational studies. Gynecol Oncol. 129(3):
613–619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raspollini MR, Amunni G, Villanucci A, et
al: Increased cyclooxygenase-2 (COX-2) and P-glycoprotein-170
(MDR1) expression is associated with chemotherapy resistance and
poor prognosis. Analysis in ovarian carcinoma patients with low and
high survival. Int J Gynecol Cancer. 15:255–260. 2005. View Article : Google Scholar
|
|
12
|
Wang L, Chen W and Yang J: Effects of
cyclooxygenase-2 siRNA-mediated gene silencing on proliferation and
apoptosis of human colon cancer cells. Suzhou Univ J Med Sci.
2:16–19. 2008.(In Chinese).
|
|
13
|
Juncker-Jensen A, Deryugina EI, Rimann I,
et al: Tumor MMP-1 activates endothelial PAR1 to facilitate
vascular intravasation and metastatic dissemination. Cancer Res.
73:4196–4211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JS, Choi YD, Lee JH, et al: Expression
of cyclooxygenase-2 in epithelial ovarian tumors and its relation
to vascular endothelial growth factor and p53 expression. Int J
Gynecol Cancer. 16(Suppl 1): 247–253. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stetler-Stevenson WG and Yu AE: Proteases
in invasion: matrix metalloproteinases. Semin Cancer Biol.
11:143–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nguyen DX, Bos PD and Massaué J:
Metastasis: from dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu X, Shen A and Wang Zh: Expressions and
significances of COX-2, VEGF, MMP-9 in epithelial ovarian carcinoma
tissues. Basic Clin Onco. 23:67–469, (In Chinese).
|
|
19
|
Symowicz J, Adley BP, Woo MM, et al:
Cyclooxygenase-2 functions as a downstream mediator of
lysophosphatidic acid to promote aggressive behavior in ovarian
carcinoma cells. Cancer Res. 65:2234–2242. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leung E, McArthur D, Morris A, et al:
Cyclooxygenase-2 inhibition prevents migration of colorectal cancer
cells to extracellular matrix by down-regulation of matrix
metalloproteinase-2 expression. Dis Colon Rectum. 51:342–347. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan MR, Chuang LY and Hung WC:
Non-steroidal anti-inflammatory drugs inhibit matrix
metalloproteinase-2 expression via repression of transcription in
lung cancer cells. FEBS Lett. 508:365–368. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li T, Lu J and Zhong Y:
Lentivirus-mediated shRNA interference targeting cyclooxygenase-2
inhibits growth of human non-small cell lung cancer. J BUON.
18:908–14. 2013.PubMed/NCBI
|
|
23
|
Liu Z, Wu XZ, Song R, et al: RNAi-mediated
knockdown of cyclooxygenase 2 inhibits the growth and migration of
SaOS2 human osteosarcoma cells. Zhonghua Yi Xue Za Zhi. 93:1028–31.
2013.(In Chinese).
|
|
24
|
Zhang L, Wu YD, Li P, et al: Effects of
cyclooxygenase-2 on human esophageal squamous cell carcinoma. World
J Gastroenterol. 17:4572–80. 2011. View Article : Google Scholar : PubMed/NCBI
|