Introduction
Non-small-cell lung cancer (NSCLC) accounts for ~85%
of all lung cancers (1). NSCLC is
the most common cause of cancer-associated mortality worldwide,
with<15% of patients surviving beyond five years, due to the
lack of early diagnosis and effective treatment methods (2,3).
Chemotherapy and radiotherapy have been widely used in the
treatment of advanced NSCLC; however, the outcome remains
unsatisfactory, with low long-term survival rates, therefore a more
effective and safer therapy for NSCLC is required (4). With advances in the understanding of
the molecular pathways associated with NSCLC progression, targeted
therapies that are designed to interfere with cancer cell growth
and survival, have potential as novel NSCLC therapeutics.
Survivin, a member of the Inhibitor of Apoptosis
Protein (IAP) family (5), has been
identified as a promising therapeutic target in cancers, as it has
been shown to be overexpressed in a wide range of tumors, including
stomach (6), colorectal (7), lung (8), breast (9), pancreatic (10), ovarian (11), prostate cancers (12), and melanoma (13). In addition to its role in the
inhibition of apoptosis, survivin has a critical role in the
regulation of cell division, by inducing exit from G1
checkpoint arrest and subsequent entry into the S phase (14). Previous studies have demonstrated
that survivin expression is low in normal cells, but is high in
proliferating cancer cells and during cancer angiogenesis (15). These results suggest that survivin
may be a suitable target for anticancer therapy.
Survivin expression is closely associated with the
sensitivity of cancer cells to chemotherapy, survival time, and
prognosis (16). Inhibition of
survivin expression may induce apoptosis, inhibit cancer growth,
and enhance the sensitivity of cancer cells to chemotherapy and
radiotherapy (17). Previous
studies have reported on the transfection of lung cancer cells with
small interfering (si)RNA specifically targeting survivin. Chen
et al (18) reported that
downregulation of survivin expression by RNA interference (RNAi) in
A549 cells, markedly decreased the invasive and metastatic
capabilities of the cells, suppressed proliferation, decelerated
the rate of growth, reduced the number of clones on soft agar and
decreased the capacity of reconstituted basement membrane
penetration and migration. However, this study did not investigate
the responses in an in vivo model.
In the present study, the effects of RNAi targeting
survivin were examined, on cell proliferation, cell cycle
distribution and cell apoptosis in the A549 human NSCLC cell line
in vitro and on tumor growth in a lung cancer xenograft
in vivo.
Materials and methods
Cell culture
The A549 human NSCLC and HEK 293T human embryonic
kidney cells were purchased from the Cell Bank of Type Culture
Collection of Chinese Academy of Sciences, Shanghai Institute of
Cell Biology, Chinese Academy of Sciences (Shanghai, China). The
A549 and HEK 293T cells were cultured in RPMI-1640 medium
(Invitrogen Life Technologies, Carlsbad, CA, USA), supplemented
with heat-inactivated 10% fetal bovine serum (FBS) (Biochrom AG,
Berlin, Germany) at 37°C, in a humidified atmosphere containing 5%
CO2.
Construction of survivin small hairpin
(sh)RNA-containing lentivirus and infection
The pshRNA-H1-Luc lentivector (System Biosciences,
Mountain View, CA, USA) used in the present study was designed to
coexpress luciferase, cloned from the Copepod. The shRNA sequence
targeting the human survivin gene was as follows: Sense,
5′-GGCTGGCTTCATCCACTGC-3′, and has previously been shown to
efficiently downregulate human survivin expression (18). The shRNA sequence used for the
scrambled negative control (NC) was: Sense,
5′-AATTCTCCGAACGTGTCACGT-3′. This sequence did not target any known
gene product and had no significant sequence homology with human
gene sequences, which is essential for determining the effects of
shRNA delivery. Pairs of complementary oligonucleotides containing
these sequences were synthesized (Invitrogen Life Technolgies) and
cloned into the pshRNA-H1-Luc lentivector. The pshRNA-H1-Luc
lentivectors containing the shRNA sequences and the pPACK Packaging
Plasmid Mix (System Biosciences, Shanghai, China) were
cotransfected into HEK 293T producer cells using
Lipofectamine® 2000 (Invitrogen Life Technologies). The
viral supernatants were harvested following 48 h, and the titers
were determined with serial dilutions of concentrated
lentivirus.
Quantitative polymerase chain reaction
(qPCR)
The A549 and HEK 293T cells were harvested for RNA
extraction 48 h after shRNA treatment. The RNA was extracted using
the RNeasy Mini kit, with the RNase-free DNase Set to remove any
contaminating genomic DNA (Qiagen, Valencia, CA, USA). The RNA was
reverse-transcribed into cDNA using the Primescript™ RT reagent
kit, according to the manufacturer’s instructions (Takara Bio Inc.,
Otsu, Japan). The qPCR assays were performed using the
SYBR® Green Real-Time PCR Master Mix (Toyobo Co., Ltd.,
Osaka, Japan) and qPCR amplification equipment (ABI 7900 Fast
System; Applied Biosystems Life Technologies, Foster City, CA, USA)
using the following specific primers: Survivin forward,
5′-AGAACTGGCCCTTCTTGGAGG-3′, and reverse,
5′-CTTTTTATGTTCCTCTATGGGGTC-3′; GAPDH forward,
5′-TGTGGGCATCAATGGATTTGG-3′, and reverse,
5′-ACACCATGTATTCCGGGTCAAT-3′. The PCR conditions were used as
follows: Pre-denaturing at 95°C for 5 min, followed by 40 cycles of
denaturation at 95°C for 10 sec, annealing/extension at 60°C for 15
sec, and a final extension at 72°C for 10 min. The specificity of
the amplification was checked using a melting curve analysis. The
expression of the genes of interest were determined by
normalization of the cycle threshold (Ct) of the target genes to
that of the control GAPDH. The 2−ΔΔCT method was used to
calculate the relative abundance of the target gene expression
levels, generated by Rotor-Gene 6000 Series Software 1.7 (Qiagen,
Hilden, Germany). Each sample was performed in triplicate.
Western blotting
The cells were lysed in 100 μl
radioimmunoprecipitation assay buffer. The proteins were then
quantified using the bicinchoninic acid kit (Pierce Biotrchnology
Inc., Rockford, IL, USA). A total of 50 μg of protein samples were
loaded and separated by 8–15% SDS-PAGE and transferred onto
nitrocellulose membranes (Santa Cruz Biotechnology, Inc., Dallas,
TX, USA). The membranes were blocked in tris-buffered saline (TBS),
containing 5% skim milk and 0.1% Tween® 20 for 2 h at
room temperature, and then incubated with the following antibodies:
Mouse anti-Caspase-3 monoclonal antibody (1:500 dilution), mouse
anti-Caspase-8 monoclonal antibody (1:500 dilution), rabbit
anti-survivin polyclonal antibody (1:1,000 dilution; Cell Signaling
Technologies, Danvers, MA, USA) and mouse anti-GAPDH monoclonal
antibody (1:1,000 dilution; Ambion Life Technologies, Carlsbad, CA,
USA) for 2 h. The membranes were then incubated with anti-rabbit or
anti-mouse secondary horseradish peroxidase conjugated antibodies
(1:10,000 dilutions; GE Healthcare Life Sciences, Uppsala, Sweden)
for 2 h. The protein bands were visualized using an Enhanced
Chemiluminescence reagent (GE Healthcare, Vélizy-Villacoublay,
France).
Measurement of A549 cell viability by MTT
assay
To measure the effects of downregulation of survivin
expression by shRNA, on cell proliferation, an MTT assay was
performed. A total of 5 × 104 A549 cells/ml were added
to a 96-well plate (100 μl/well). In the blank control wells, 100
μl of medium alone was added. Following a 24 h culture, the cells
were transfected with a lentivirus expressing an shRNA targeting
survivin. The cells were divided into three groups: Normal control,
negative control (NC), and survivin shRNA groups. There were eight
wells of cells cultured for each group. Following a 48 h culture,
20 μl MTT (5 mg/ml) was added to each well, followed by an
incubation at 37°C for 48 h. The plates were then centrifuged at
2,000 × g for 10 min. The supernatants were removed, and 200 μl
dimethyl sufoxide was added to each well, followed by agitation for
10 min. The absorbance was measured at 570 nm using a microplate
reader (Molecular Devices, Sunnyvale, CA, USA), and the inhibition
of growth was calculated. The mean proliferation of cells without
any treatment was expressed as 100%.
Cell colony formation assay
The cell suspensions containing shRNA
(1×104 diluted in 0.33% low-melting agarose) were
overlaid on a bottom 0.5% agar layer (3 ml) in a 60-mm dish. The
cells were incubated at 37°C for 14 days, and the medium was
refreshed every three days. Following the incubation, the colonies
were washed twice with phosphate-buffered saline (PBS), fixed with
ice-cold methanol for 30 min and stained with giemsa (Dingguo
Biotechnology, Co., Ltd., Beijing, China) for 10 min. The visible
colonies were counted.
Cell cycle analysis
To determine the cell cycle distribution, 5 ×
105 A549 cells were plated in 60-mm dishes and treated
with NC or survivin shRNA for 48 h. Following treatment, the cells
were collected by trypsinization, fixed in 70% ethanol, and
maintained at −20°C overnight for fixation. The cells were then
washed in PBS, resuspended in 1 ml of PBS containing 100 μg/ml
RNase and 40 μg/ml propidium iodide (PI) (Merck, Whitehouse
Station, NJ, USA) and incubated in the dark for 30 min at room
temperature. The distribution of the cells in the cell cycle phases
were analyzed from the DNA histogram using a FACSCaliber™ flow
cytometer and CellQuest™ software (BD Biosciences, San Jose, CA,
USA).
Detection of apoptosis
The A549 cells were cultured in six-well plates in
RPMI-1640, containing 10% FBS medium, and were treated with NC or
survivin shRNA for 48 h. The cover slips were washed three times
with PBS (pH = 7.2) and single cell suspensions were fixed in 1%
PBS. The cells were stained with 100 μg/ml acridine orange and 100
μg/ml ethidium bromide for 1 min. The cells were observed under a
fluorescence microscope (Olympus Corporation, Tokyo, Japan). A
minimum number of 200 cells were counted and the percentage of
apoptotic cells was determined. At the molecular level, caspase-3
and caspase-8 protein expression levels were detected by western
blotting, which provided an additional indicator of apoptosis.
Antitumor effects of survivin shRNA in
vivo
All animal experiments were performed in accordance
with institutional guidelines, following a protocol approved by the
Ethics Committees of the Disease Model Research Center, the First
Hospital of Jilin University (Changchun, China). Female BALB mice,
~6–7 weeks old, were purchased from the Institute of Laboratory
Animal Science, Jilin University (Changchun, China) and maintained
under specific pathogen-free conditions and provided with food and
water ad libitum. One week prior to the experiment the mice
were fed with a normal pellet diet.
The A549 cells in the exponential growth phase were
harvested and single-cell suspensions (2×106 cells/100
μl PBS) were injected subcutaneously into the right dorsal flank of
the mice. The size of the tumors were measured every 2–3 days, and
the tumor volume was calculated as 0.5236 × width2 ×
length. When the tumors had grown to an average volume of 75
mm3, the mice were randomly divided into survivin shRNA,
NC and control groups (n=10/group) and treated with survivin shRNA
or NC shRNA, plus PBS, in a total volume of 20 μl (10 μl virus plus
10 μl PBS) once a week for 21 days. When the control mice began to
succumb to the tumors, the mice in all treatment groups were
sacrificed. After the mice had been sacrificed, the tumors were
removed and directly embedded in an optimal cutting temperature
compound and frozen at −80°C.
Statistical analysis
All data are expressed as the means ± standard
deviation. The statistical analyses between two samples was
performed using a student’s t test. A statistical comparison of
more than two groups was performed using a one-way analysis of
variance, followed by a Tukey post hoc test. Graphpad Prism
5.0 software (GraphPad Software, San Diego, CA, USA) was used for
all statistical analyses. A P<0.05 was considered to indicate a
statistically significant difference.
Results
Survivin gene silencing efficiency in
A549 NSCLC cells
Previously validated survivin shRNA sequences
(18), were cloned into the
pshRNA-H1-Luc lentivector and transfected into HEK 293T cells. The
silencing efficiency of the lentiviruses was determined at both the
mRNA and protein expression levels in the A549 cells. A shRNA
sequence presenting no homology with mRNA databases, was used as a
negative control. A qPCR and western blot analyses were performed
to detect the mRNA and protein expression levels of survivin, three
days post-transfection. There was no significant inhibition of
survivin mRNA expression levels in the NC and control groups.
However, the mRNA expression levels of survivin in the survivin
shRNA group were significantly downregulated, as compared with the
NC and control groups (Fig. 1A,
P<0.01).
There was also no significant inhibition in the
protein expression levels of survivin in the NC and control groups,
whereas the band density was markedly decreased in the survivin
shRNA group, as compared with the NC and control groups (Fig. 1B, P<0.01). These results suggest
that silencing survivin expression with shRNA significantly
decreased the expression levels of survivin in NSCLC cells.
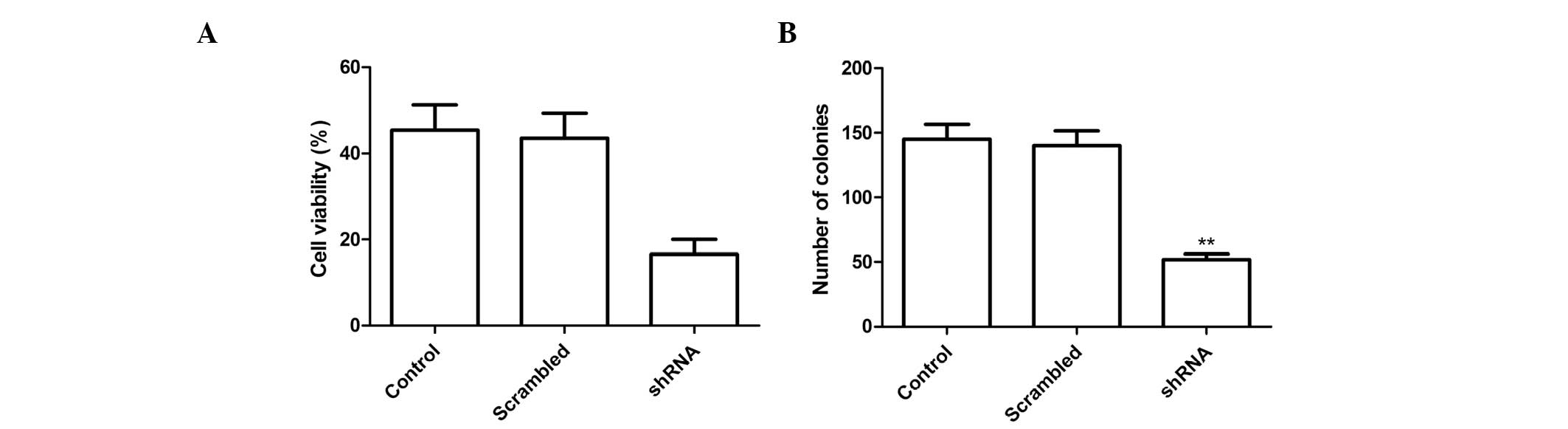
Effects of survivin shRNA on cell
proliferation and colony formation
Following transfection of the cells with survivin
shRNA, the effects of silencing survivin expression were determined
on tumor cell growth in vitro. The anti-proliferative
effects of survivin expression silencing on A549 tumor cells were
examined using an MTT assay.
Transfection of A549 cells with shRNA targeting
survivin inhibited cell proliferation, as compared with the control
and NC groups (Fig. 2A,
P<0.01). The effects of silencing survivin expression on NSCLC
cell colony formation ability were also assessed. Silencing
survivin expression reduced the number of tumor cell colonies, as
compared with the NC and control groups (Fig. 2B; P<0.01).
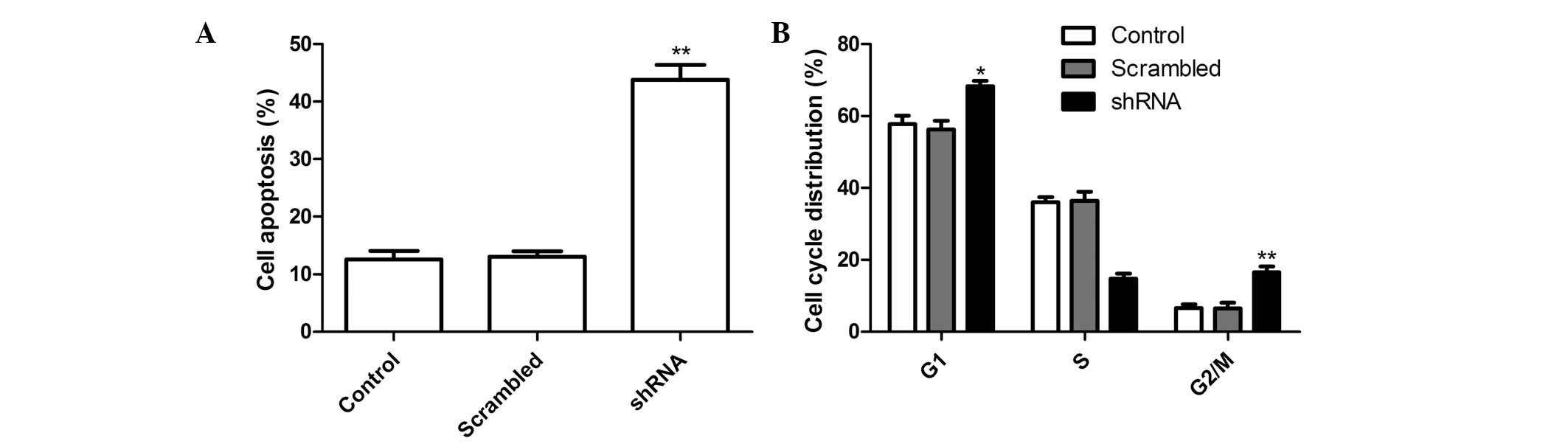
Effects of survivin shRNA on cell
apoptosis and cell cycle distribution
To investigate whether silencing survivin expression
may induce apoptosis, the percentage of apoptotic cells was
determined following treatment with shRNA targeting survivin. The
A549 cells treated with survivin shRNA had a significantly higher
rate of apoptosis, as compared with the control and NC groups
(Fig. 3A; P<0.01).
Flow cytometry was performed to assess the effects
of survivin shRNA on the progression of the cell cycle in A549
cells. A comparison of the cell cycle distribution of the survivin
shRNA group with the control and NC groups, indicated that the
survivin shRNA group had a significantly higher proportion of cells
in G1 and G2 phases, and fewer cells in S
phase (Fig. 3B; P<0.05). There
were no significant differences between the control and the NC
groups.
Effects of survivin shRNA on caspase-3
and caspase-8 expression
To determine the effects on apoptosis following
silencing survivin expression, the protein expression levels of
caspase-3 and caspase-8 were analyzed by western blotting.
Silencing survivin expression markedly increased the expression
levels of caspase-3 and caspase-8, in A549 cells, as compared with
the control and NC groups (Fig.
4).
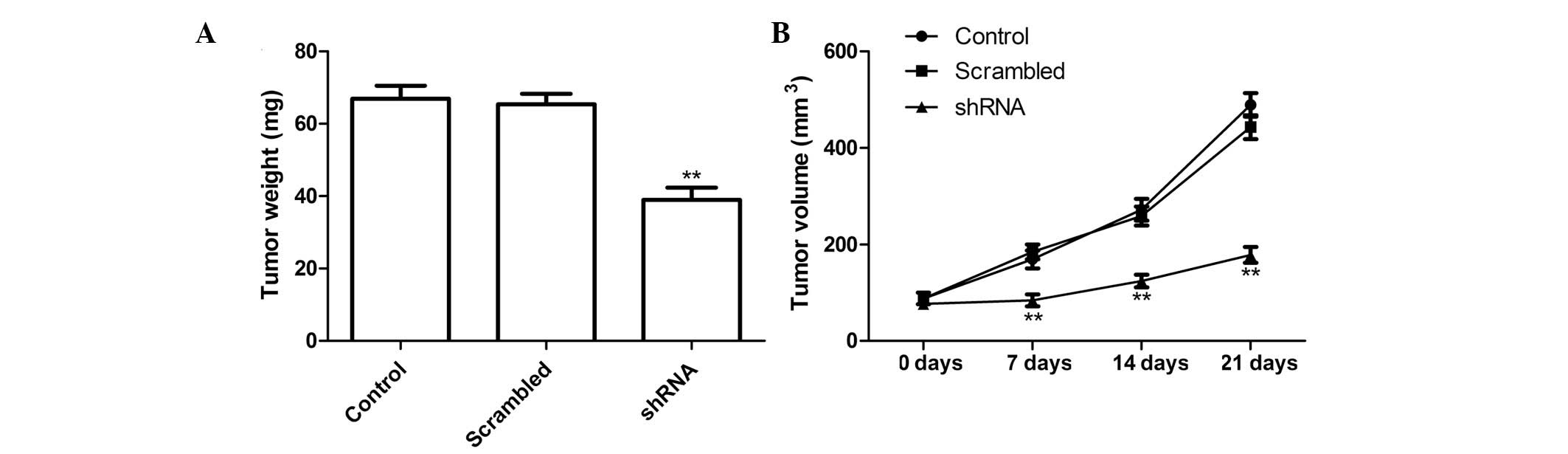
Survivin shRNA slows tumor growth in a
murine xenograft model
The effects of survivin shRNA were also investigated
on tumor growth in mice with lung cancer xenografts. There were no
significant differences in tumor volume pre-treatment, and the mice
from all three groups had clearly evident tumors at 21 days. The
tumor volume was significantly reduced in the survivin shRNA group
on days 7, 14 and 21 (Fig. 5A;
P<0.001 for all).
Three days after the end of the treatment, the mice
were sacrificed, and the weight of the tumors were measured. The
tumor weight was significantly less in the survivin shRNA group, as
compared with the control and NC groups (Fig. 5B; P<0.001). These results
demonstrate that silencing survivin expression may suppress tumor
growth of NSCLC in vivo.
Discussion
RNAi is a promising approach for antitumor therapy
that allows in vivo silencing of essential genes for tumor
progression. RNAi provides an alternative to traditional small
molecule therapies, and has generated a great deal of interest in
treating cancer. However, success of this treatment method depends
on the identification of a gene which is expressed universally in
cancer cells, but not in normal cells. Survivin may be a potential
candidate molecule to target for antitumor therapy. Extensive
studies have shown that survivin is upregulated in various human
cancers, including lung cancer (6–13),
but not in normal prostatic tissues. In addition, a previous study
showed that elevated survivin expression is associated with
chemoresistance (19–21). Therefore, targeted suppression of
survivin expression may be a potential therapeutic strategy for
lung cancer. In the present study, silencing survivin expression in
A549 NSCLC cells using a specific shRNA resulted in a decreased
cell growth and increased apoptotic rate, these findings are
consistent with previous reports (18).
Survivin, a member of the IAP family, has previously
been reported to be associated with the cell cycle (15). Numerous studies have shown that
survivin protein is synthesized and expressed at high levels during
the G2/M phase of the cancer cell division cycle,
effectively supporting the active cell growth process (22–25);
therefore, inhibition of survivin may preferentially block cell
division. The present study showed that silencing survivin
expression resulted in a significantly higher proportion of cells
in G1 and G2 phases fewer cells in S phase,
and more apoptotic cells, which may help to block cell
division.
Previous studies have shown that an increased
expression of survivin in cancer tissues is associated with a short
survival time and poor prognosis. Silencing of survivin expression
has been demonstrated to induce apoptosis, inhibit the growth of
cancer cells, and enhance the sensitivity of cancer cells to
chemotherapy and/or radiotherapy (11,18,26).
These findings suggest that survivin may be a potential target for
the diagnosis and treatment of various types of cancer. The results
of the present study showed that downregulation of survivin
expression, using an RNA silencing approach, in A549 NSCLC cells
significantly suppressed the proliferation and colony forming
ability of the cells, and induced tumor apoptosis in vitro,
and suppressed tumor growth of NSCLC in vivo. These results
further confirm that inhibition of survivin expression may suppress
tumor growth.
In conclusion, the present study demonstrated that
silencing survivin expression may significantly suppress the
proliferation and cell cycle distribution in vitro, and
tumor growth in vivo of NSCLC. These results suggest that
shRNA targeting survivin may have therapeutic potential for the
treatment of NSCLC.
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2009. CA Cancer J Clin. 59:225–249. 2009. View Article : Google Scholar
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
3
|
Reungwetwattana T, Weroha SJ and Molina
JR: Oncogenic pathways, molecularly targeted therapies, and
highlighted clinical trials in non-small-cell lung cancer (NSCLC).
Clin Lung Cancer. 13:252–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roy M, Luo YH, Ye M and Liu J: Nonsmall
cell lung cancer therapy: insight into multitargeted small-molecule
growth factor receptor inhibitors. Biomed Res Int.
2013:9647432013.PubMed/NCBI
|
|
5
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu CD, Altieri DC and Tanigawa N:
Expression of a novel antiapoptosis gene, survivin, correlated with
tumor cell apoptosis and p53 accumulation in gastric carcinomas.
Cancer Res. 58:1808–1812. 1998.PubMed/NCBI
|
|
7
|
Kawasaki H, Altieri DC, Lu CD, et al:
Inhibition of apoptosis by survivin predicts shorter survival rates
in colorectal cancer. Cancer Res. 58:5071–5074. 1998.PubMed/NCBI
|
|
8
|
Monzó M, Rosell R, Felip E, et al: A novel
anti-apoptosis gene: Re-expression of survivin messenger RNA as a
prognosis marker in non-small-cell lung cancers. J Clin Oncol.
17:2100–2104. 1999.PubMed/NCBI
|
|
9
|
Tanaka K, Iwamoto S, Gon G, et al:
Expression of survivin and its relationship to loss of apoptosis in
breast carcinomas. Clin Cancer Res. 6:127–134. 2000.PubMed/NCBI
|
|
10
|
Liggins C, Orlicky DJ, Bloomquist LA and
Gianani R: Developmentally regulated expression of survivin in
human pancreatic islets. Pediatr Dev Pathol. 6:392–397. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xing J, Jia CR, Wang Y, Guo J and Cai Y:
Effect of shRNA targeting survivin on ovarian cancer. J Cancer Res
Clin Oncol. 138:1221–1229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krajewska M, Krajewski S, Banares S, et
al: Elevated expression of inhibitor of apoptosis proteins in
prostate cancer. Clin Cancer Res. 9:4914–4925. 2003.PubMed/NCBI
|
|
13
|
McKenzie JA and Grossman D: Role of the
apoptotic and mitotic regulator survivin in melanoma. Anticancer
Res. 32:397–404. 2012.PubMed/NCBI
|
|
14
|
Wheatley SP and McNeish IA: Survivin: a
protein with dual roles in mitosis and apoptosis. Int Rev Cytol.
247:35–88. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Altieri DC: Survivin and IAP proteins in
cell-death mechanisms. Biochem J. 430:199–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xing J, Jia CR, Wang Y, Guo J and Cai Y:
Effect of shRNA targeting survivin on ovarian cancer. J Cancer Res
Clin Oncol. 138:1221–1229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhen HN, Li LW, Zhang W, et al: Short
hairpin RNA targeting survivin inhibits growth and angiogenesis of
glioma U251 cells. Int J Oncol. 31:1111–1117. 2007.PubMed/NCBI
|
|
18
|
Chen XQ, Yang S, Kang MQ, et al: Survivin
expression in human lung cancer and the influence of its
downregulation on the biological behavior of human lung cancer
cells. Exp Ther Med. 3:1010–1014. 2012.PubMed/NCBI
|
|
19
|
Virrey JJ, Guan S, Li W, et al: Increased
survivin expression confers chemoresistance to tumor-associated
endothelial cells. Am J Pathol. 173:575–585. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang M, Mukherjee N, Bermudez RS, et al:
Adenovirus-mediated inhibition of survivin expression sensitizes
human prostate cancer cells to paclitaxel in vitro and in vivo.
Prostate. 64:293–302. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang H, Fu JH, Hu Y, et al: Influence of
siRNA targeting survivin on chemosensitivity of H460/cDDP lung
cancer cells. J Int Med Res. 36:734–747. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamazaki H, Takagi S, Hoshino Y, Hosoya K
and Okumura M: Inhibition of survivin influences the biological
activities of canine histiocytic sarcoma cell lines. PLoS One.
8:e798102013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamanaka K, Nakahara T, Yamauchi T, et al:
Antitumor activity of YM155, a selective small-molecule survivin
suppressant, alone and in combination with docetaxel in human
malignant melanoma models. Clin Cancer Res. 17:5423–5431. 2011.
View Article : Google Scholar
|
|
24
|
Chen J, Pise-Masison CA, Shih JH, et al:
Markedly additive antitumor activity with the combination of a
selective survivin suppressant YM155 and alemtuzumab in adult
T-cell leukemia. Blood. 121:2029–2037. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Asanuma K, Tsuji N, Endoh T, Yagihashi A
and Watanabe N: Survivin enhances Fas ligand expression via
up-regulation of specificity protein 1-mediated gene transcription
in colon cancer cells. J Immunol. 172:3922–3929. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamamoto H, Ngan CY and Monden M: Cancer
cells survive with survivin. Cancer Sci. 99:1709–1714. 2008.
View Article : Google Scholar : PubMed/NCBI
|